Behavioral Responses of Unio tumidus Freshwater Mussels to Neonicotinoid Pesticide Contamination
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Model Organism
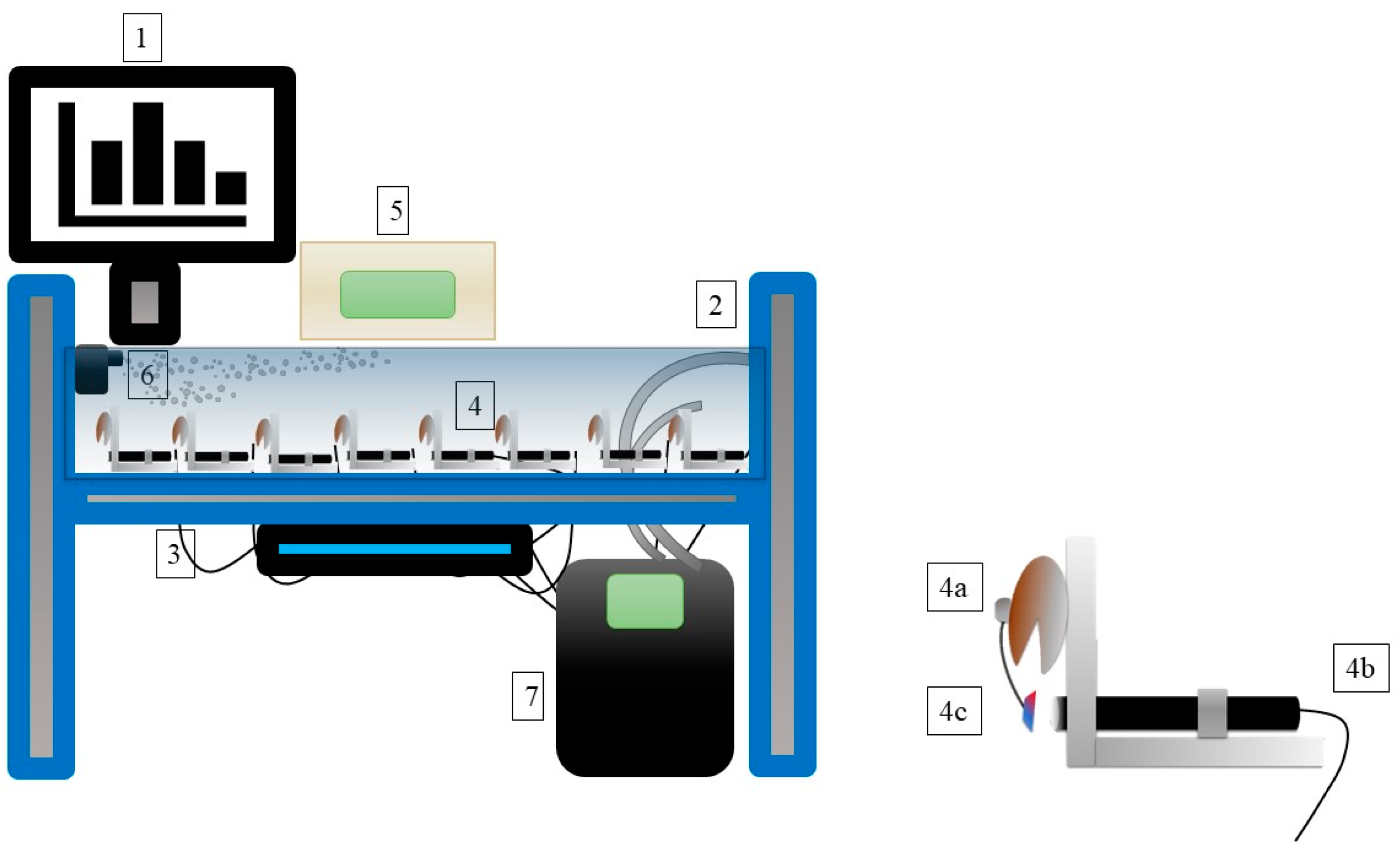
2.2. Construction of the Monitoring System
2.3. Pesticides Tested
2.4. Experimental Setup and Behavioral Assessment
3. Results
3.1. Analysis for 5 min Intervals
3.2. Analysis for Hourly Intervals
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goh, M.S.; Lam, S.D.; Yang, Y.; Naqiuddin, M.; Addis, S.N.K.; Yong, W.T.L.; Luang-InMa, V.; Sonne, C.N.L. Omics technologies used in pesticide residue detection and mitigation in crop. J. Hazard. Mater. 2021, 420, 126624. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, P. Pesticides in agriculture and environment: Impacts on human health. In Contaminants in Agriculture and Environment: Health Risks and Remediation; Agro Environ Media: Haridwar, India, 2019; pp. 77–91. [Google Scholar] [CrossRef]
- Pathiratne, A.; Kroon, F.J. Using species sensitivity distribution approach to assess the risks of commonly detected agricultural pesticides to Australia’s tropical freshwater ecosystems. Environ. Toxicol. Chem. 2016, 35, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Nowell, L.H.; Moran, P.W.; Schmidt, T.S.; Norman, J.E.; Nakagaki, N.; Shoda, M.E.; Mahler, B.J.; Van Metre, P.C.; Stone, W.W.; Sandstrom, M.W.; et al. Complex mixtures of dissolved pesticides show potential aquatic toxicity in a synoptic study of Midwestern U.S. streams. Sci. Total Environ. 2014, 613–614, 1469–1488. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.A.; El Nemr, A. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Gupta, S.; Gajbhiye, V.T.; Gupta, R.K. Soil Dissipation and Leaching Behavior of a Neonicotinoid Insecticide Thiamethoxam. Bull. Environ. Contam. Toxicol. 2008, 80, 431–437. [Google Scholar] [CrossRef]
- Tišler, T.; Jemec, A.; Mozetic, B.; Trebše, P. Hazard identification of imidacloprid to aquatic environment. Chemosphere 2009, 76, 907–914. [Google Scholar] [CrossRef]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple Routes of Pesticide Exposure for Honey Bees Living Near Agricultural Fields. PLoS ONE 2012, 7, e29268. [Google Scholar] [CrossRef]
- Nuyttens, D.; Devarrewaere, W.; Verbovenb, P.; Foqu’ea, D. Pesticide-laden dust emission and drift from treated seeds during seed drilling: A review. Pest Manag. Sci. 2013, 69, 564–575. [Google Scholar] [CrossRef]
- Tufi, S.; Stel, J.M.; de Boer, J.; Lamoree, M.H.; Leonards, P.E. Metabolomics to explore imidacloprid-induced toxicity in the central nervous system of the freshwater snail Lymnaea stagnalis. Environ. Sci. Technol. 2015, 49, 14529–14536. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.H.; Sadhu, A.; Ghosh, S.; Saha, N.C.; Mossotto, C.; Pastorino, P.; Saha, S.; Faggio, C. Evaluating the Impact of Neonicotinoids on Aquatic Non-target Species: A comprehensive Review. Environ. Toxicol. Pharmacol. 2025, 113, 1382–6689. [Google Scholar] [CrossRef] [PubMed]
- Oehlmann, J.; Schulte-Oehlmann, U. Chapter 17 Molluscs as bioindicators. In Trace Metals and Other Contaminants in the Environment; Bioindicators and Biomonitors; Elsevier: Amsterdam, The Netherlands, 2003; Volume 6, pp. 577–635. [Google Scholar] [CrossRef]
- Beiras, R. Chapter 17—Marine Pollution Monitoring Programs. In Marine Pollution: Sources, Fate and Effects of Pollutants in Coastal Ecosystems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 293–311. [Google Scholar] [CrossRef]
- Bae, M.J.; Park, Y.S. Biological early warning system based on the responses of aquatic organisms to disturbances: A review. Sci. Total Environ. 2014, 466, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Chmist, J.; Szoszkiewicz, K. Choice of the most useful biological early warning system, based on ahp and rembrandt analysis. Acta Sci. Pol. Form. Circumiectus 2018, 17, 95–102. [Google Scholar] [CrossRef]
- Chmist, J.; Szoszkiewicz, K.; Drożdżyński, D. Behavioural responses of Unio tumidus freshwater mussel to pesticide contamination. Arch. Environ. Contam. Toxicol. 2019, 77, 432–442. [Google Scholar] [CrossRef]
- Mbah, J.T.; Chmist-Sikorska, J.; Szoszkiewicz, K.; Czekała, W. The effects of inflow of agricultural biogas digestate on bivalves’ behavior. Environ. Sci. Pollut. Res. 2021, 28, 67385–67393. [Google Scholar] [CrossRef]
- Salánki, J.; Farkas, A.; Kamardina, T.; Rózsa, K.S. Molluscs in biological monitoring of water quality. Toxicol. Lett. 2006, 140, 403–410. [Google Scholar] [CrossRef]
- Savorelli, F.; Manfra, L.; Croppo, M.; Tornambè, A.; Palazzi, D.; Canepa, S.; Trentini, P.L.; Cicero, A.M.; Faggio, C. Fitness Evaluation of Ruditapes philippinarum Exposed to Ni. Biol. Trace Elem. Res. 2017, 177, 384–393. [Google Scholar] [CrossRef]
- Le Bris, H.; Maffart, P.; Bocquené, G.; Buchet, V.; Galgani, F.; Blanc, G. Laboratory study on the effect of dichlorvos on two commercial bivalves. Aquaculture 1995, 138, 139–144. [Google Scholar] [CrossRef]
- Almeshal, W.; Takács, A.; Aradi, L.; Sandil, S.; Dobosy, P.; Záray, G. Comparison of Freshwater Mussels Unio tumidus and Unio crassus as Biomonitors of Microplastic Contamination of Tisza River (Hungary). Environments 2022, 9, 122. [Google Scholar] [CrossRef]
- Sow, M.; Durrieu, G.; Briollais, L.; Ciret, P.; Massabuau, J.C. Water quality assessment by means of HFNI valvometry and high-frequency data modeling. Environ. Monit. Assess. 2011, 182, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.T.; Beggel, S.; Auerswald, K.; Stoeckle, B.C.; Geist, J. Establishing mussel behavior as a biomarker in ecotoxicology. Aquat. Toxicol. 2016, 170, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaya, Y.; Bódis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biol. Rev. 2017, 92, 572–607. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.; Bertilsson, A.; Rydgard, M. Spatial distribution and age structure of the freshwater unionid mussels Anodonta anatina and Unio tumidus: Implications for environmental monitoring. Hydrobiologia 2013, 711, 61–70. [Google Scholar] [CrossRef]
- Drewek, A.; Lubawy, J.; Domek, P.; Polak, J.; Słocińska, M.; Dzięgelewska, A.; Klimaszyk, P. Behavioral and Biochemical Effects of Glyphosate-Based Herbicide Roundup on Unionid Mussels: Are Mussels Good Indicators of Water Pollution with Glyphosate-Based Pesticides? Water 2024, 16, 1882. [Google Scholar] [CrossRef]
- Chmist, J.; Szoszkiewicz, K. Attempt at assessment of Unio tumidus bivalve mollusks suitability for monitoring water iron content. Environ. Prot. 2017, 39, 39–43. [Google Scholar]
- Alhassan, A.B.; Aljahdali, M.O. Behavioural and Biochemical Responses of Freshwater Bivalve Anodonta marginata Exposed to Dichlorvos. Water 2024, 16, 3572. [Google Scholar] [CrossRef]
- Stara, A.; Pagano, M.; Albano, M.; Savoca, S.; Di Bella, G.; Albergamo, A.; Koutkova, Z.; Sandova, M.; Velisek, J.; Fabrello, J.; et al. Effects of long-term exposure of Mytilus galloprovincialis to thiacloprid: A multibiomarker approach. Environ. Pollut. 2021, 289, 117892. [Google Scholar] [CrossRef]
- Pisa, L.; Goulson, D.; Yang, E.-C.; Gibbons, D.; Sánchez-Bayo, F.; Mitchell, E.; Aebi, A.; Sluijs, J.; MacQuarrie, C.J.K.; Giorio, C.; et al. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: Impacts on organisms and ecosystems. Environ. Sci. Pollut. Res. 2021, 28, 11749–11797. [Google Scholar] [CrossRef]
- Raby, M.; Nowierski, M.; Perlov, D.; Zhao, X.; Hao, C.; Poirier, D.G.; Sibleya, P.K. Acute Toxicity of 6 Neonicotinoid Insecticides to Freshwater Invertebrates. Environ. Toxicol. Chem. 2018, 37, 1430–1445. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Goka, K.; Hayasaka, D. Contamination of the Aquatic Environment with Neonicotinoids and its Implication for Ecosystems. Front. Environ. Sci. 2016, 4, 71. [Google Scholar] [CrossRef]
- Schmidt, T.S.; Miller, J.L.; Mahler, B.J.; Metre, P.C.; Nowell, L.H.; Sandstrom, M.W.; Carlisle, D.M.; Moran, P.W.; Bradley, P.M. Ecological consequences of neonicotinoid mixtures in streams. Sci. Adv. 2022, 8, eabj8182. [Google Scholar] [CrossRef]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental Risks and Challenges Associated with Neonicotinoid Insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D. Sub-lethal effects of chlorinated hydrocarbons on bivalve. Mar. Pollut. Bull. 1975, 6, 20–24. [Google Scholar] [CrossRef]
- Wang, W.-X.; Rainbow, R.S. Subcellular Partitioning and the Prediction of Cadmium Toxicity to Aquatic Organisms. Environ. Chem. 2006, 3, 395–399. [Google Scholar] [CrossRef]
- Hamlet, S.A.; Bensoltane, S.; Djekoun, M.; Yassi, F.; Berrebbah, H. Histological changes and biochemical parameters in the hepatopancreas of terrestrial gastropod Helix aspersa as biomarkers of neonicotinoid insecticide exposure. Afr. J. Biotechnol. 2012, 11, 16277–16283. [Google Scholar] [CrossRef]
- Wang, N.; Ivey, C.D.; Ingersoll, C.G.; Brumbaugh, W.G.; Alvarez, D.; Hammer, E.J.; Bauer, C.R.; Augspurger, T.; Raimondo, S.; Barnhart, M.C. Acute sensitivity of a broad range of freshwater mussels to chemicals with different modes of toxic action. Environ. Toxicol. Chemistry 2016, 36, 786–796. [Google Scholar] [CrossRef]
- Dzierżyńska, A.; Białończyk, Ł.; Jermacz, Ł.; Zielska, J.; Kobak, J. What scares a mussel? Changes in valve movement pattern as an immediate response of a byssate bivalve to biotic factors. Hydrobiologia 2019, 841, 65–77. [Google Scholar] [CrossRef]
- Robson, A.; Wilson, R.; de Leaniz, C.G. Mussels Xexing their muscles: A new method for quantifying bivalve behaviour. Mar. Biol. 2007, 151, 1195–1204. [Google Scholar] [CrossRef]
- Reynolds, A.; Cody, E.; Giltrap, M.; Chambers, G. Toxicological and Biomarker Assessment of Freshwater Zebra Mussels (Dreissena polymorpha) Exposed to Nano-Polystyrene. Toxics 2024, 12, 774. [Google Scholar] [CrossRef]
- Dondero, F.; Negri, A.; Boatti, L.; Marsano, F.; Mignone, F.; Viarengo, A. Transcriptomic and proteomic effects of a neonicotinoid insecticide mixture in the marine mussel (Mytilus galloprovincialis, Lam.). Sci. Total Environ. 2010, 408, 3775–3786. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Terao, T.; Hisatomi, H.; Kawasaki, H.; Arakawa, R. Evaluation of river pollution of neonicotinoids in Osaka City (Japan) by LC/MS with dopant-assisted photoionization. J. Environ. Monit. 2012, 14, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Kerner, M.; Flach, H.; Dietmann, P.; Kühl, M.; Kühl, S.J. The impact of the insecticide acetamiprid on the embryogenesis of the aquatic model organism Xenopus laevis. Environ. Toxicol. Pharmacol. 2023, 103, 104278. [Google Scholar] [CrossRef] [PubMed]
- Gacem, H.; Bendali-Saoudi, F.; Serradj, N.; Houmani, M.; Soltani, N. Risk assessment of the neonicotinoid insecticide acetamiprid on two non-target species, Daphnia magna Straus, 1820 (Crustacea, Cladocera) and Plea minutissima Leach, 1817 (Insecta, Heteroptera). Appl. Ecol. Environ. Res. 2023, 21, 2. [Google Scholar] [CrossRef]
- Vajargah, M.F.; Namin, J.I.; Mohsenpour, R.; Yalsuyi, A.M.; Prokić, M.D.; Faggio, C. Histological effects of sublethal concentrations of insecticide Lindane on intestinal tissue of grass carp (Ctenopharyngodon idella). Vet. Res. Commun. 2021, 45, 373–380. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A. Effect of thiacloprid on early life stages of common carp (Cyprinus carpio). Chemosphere 2018, 194, 481–487. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.; Waswani, H.; Prasad, M.; Ranjan, R. Impact of pesticides on the ecosystem. In Agrochemicals in Soil and Environment: Impacts and Remediation; Springer Nature: Singapore, 2022; pp. 157–181. [Google Scholar] [CrossRef]
- Stehle, S.; Ovcharova, V.; Wolfram, J.; Bub, S.; Herrmann, L.Z.; Petschick, L.L.; Schulz, R. Neonicotinoid Insecticides in Global Agricultural Surface Waters—Exposure, Risks and Regulatory Challenges. Sci. Total Environ. 2023, 867, 161383. [Google Scholar] [CrossRef]

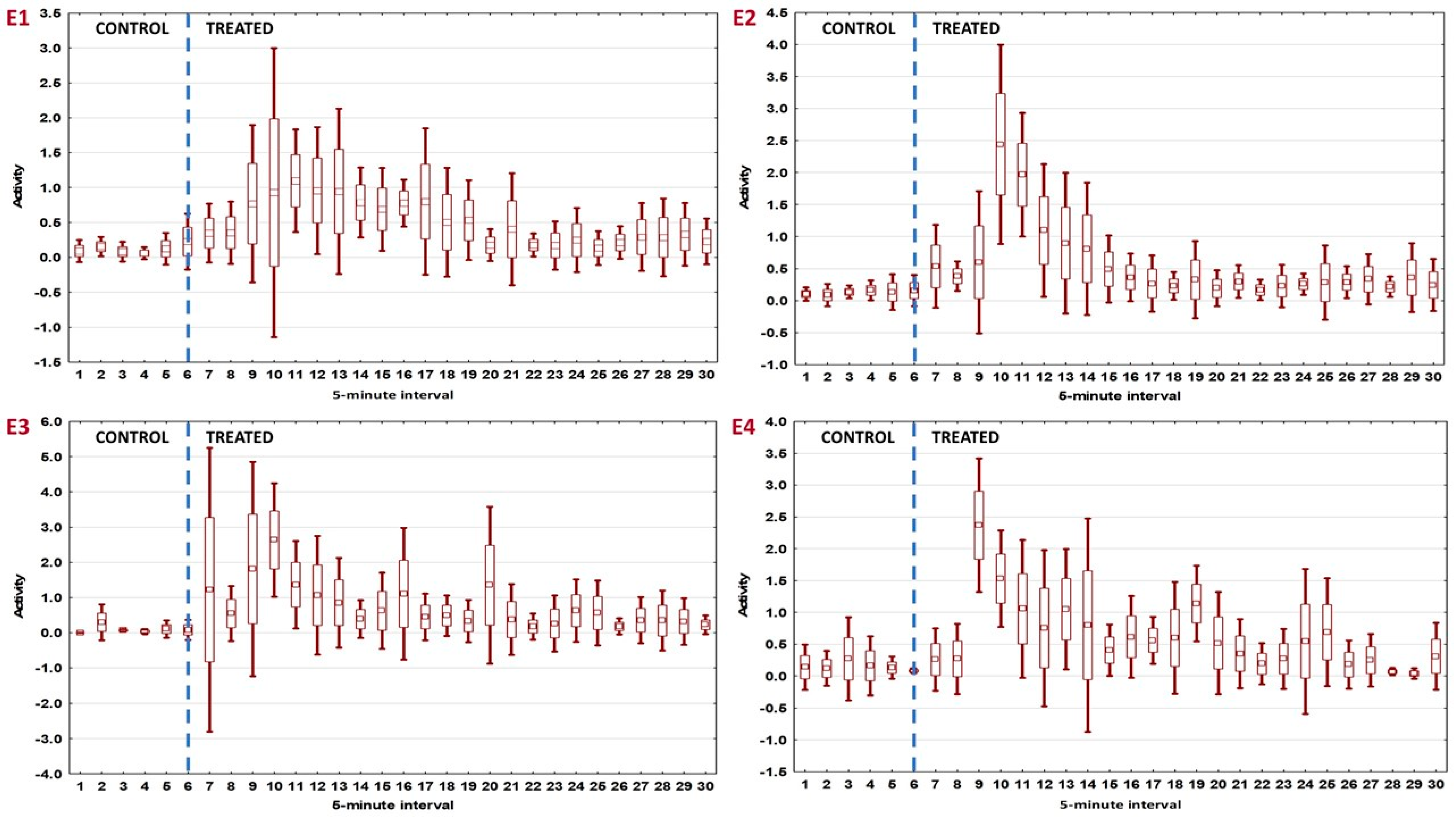
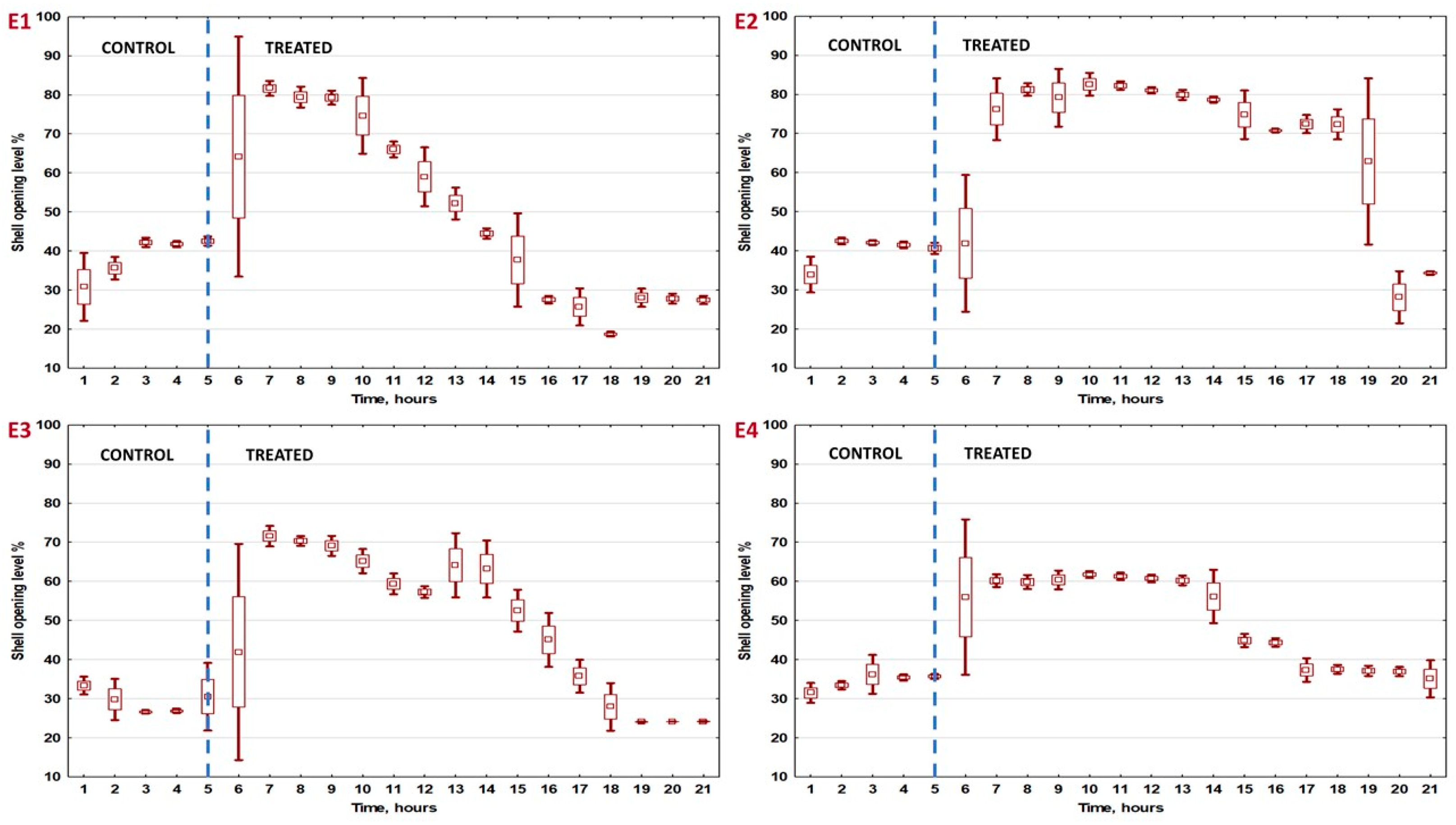

| Interval Number | Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Opening (%) | Activity | Opening (%) | Activity | Opening (%) | Activity | Opening (%) | Activity | |
| 1 | 43.16 | 0.09 | 43.75 | 0.09 | 43.16 | 0.00 | 43.09 | 0.14 |
| 2 | 43.00 | 0.15 | 43.72 | 0.14 | 42.82 | 0.30 | 43.16 | 0.12 |
| 3 | 43.43 | 0.08 | 43.43 | 0.16 | 43.13 | 0.07 | 42.79 | 0.27 |
| 4 | 43.32 | 0.06 | 42.97 | 0.13 | 43.38 | 0.03 | 42.46 | 0.16 |
| 5 | 42.54 | 0.12 | 43.03 | 0.16 | 43.45 | 0.10 | 42.62 | 0.13 |
| 6 | 41.72 | 0.23 | 42.78 | 0.53 | 43.58 | 0.08 | 43.07 | 0.08 |
| 7 | 39.17 | 0.35 | 38.76 | 0.38 | 35.64 | 1.22 | 42.69 | 0.26 |
| 8 | 37.55 | 0.35 | 38.35 | 0.60 | 34.43 | 0.54 | 41.33 | 0.27 |
| 9 | 44.09 | 0.77 | 40.48 | 2.44 | 41.89 | 1.81 | 46.51 | 2.37 |
| 10 | 51.22 | 0.93 | 55.10 | 1.97 | 58.68 | 2.63 | 53.62 | 1.53 |
| 11 | 69.68 | 1.10 | 67.76 | 1.10 | 75.88 | 1.36 | 61.74 | 1.05 |
| 12 | 73.32 | 0.96 | 71.83 | 0.90 | 77.10 | 1.06 | 66.17 | 0.75 |
| 13 | 73.72 | 0.95 | 72.06 | 0.81 | 76.29 | 0.85 | 69.03 | 1.05 |
| 14 | 73.81 | 0.79 | 78.48 | 0.49 | 77.11 | 0.39 | 71.65 | 0.80 |
| 15 | 74.75 | 0.69 | 79.22 | 0.36 | 77.83 | 0.62 | 71.45 | 0.41 |
| 16 | 76.37 | 0.78 | 79.08 | 0.27 | 77.04 | 1.11 | 70.92 | 0.61 |
| 17 | 77.67 | 0.80 | 80.14 | 0.23 | 79.16 | 0.45 | 70.50 | 0.56 |
| 18 | 78.61 | 0.50 | 81.77 | 0.33 | 78.81 | 0.48 | 69.32 | 0.60 |
| 19 | 79.20 | 0.53 | 81.85 | 0.19 | 79.66 | 0.33 | 65.97 | 1.14 |
| 20 | 80.76 | 0.18 | 82.97 | 0.30 | 77.78 | 1.35 | 65.94 | 0.52 |
| 21 | 81.17 | 0.40 | 83.29 | 0.17 | 79.49 | 0.37 | 66.08 | 0.35 |
| 22 | 81.84 | 0.18 | 84.03 | 0.23 | 79.09 | 0.18 | 67.14 | 0.19 |
| 23 | 82.26 | 0.17 | 84.24 | 0.26 | 78.36 | 0.26 | 67.05 | 0.27 |
| 24 | 82.30 | 0.25 | 84.50 | 0.28 | 77.91 | 0.62 | 67.26 | 0.55 |
| 25 | 82.44 | 0.13 | 85.05 | 0.29 | 77.66 | 0.56 | 67.10 | 0.69 |
| 26 | 82.55 | 0.21 | 85.14 | 0.33 | 77.89 | 0.18 | 67.94 | 0.18 |
| 27 | 81.94 | 0.29 | 85.59 | 0.22 | 77.04 | 0.36 | 68.11 | 0.25 |
| 28 | 81.82 | 0.29 | 86.32 | 0.36 | 76.70 | 0.34 | 68.18 | 0.07 |
| 29 | 81.89 | 0.33 | 86.10 | 0.24 | 76.99 | 0.32 | 68.36 | 0.04 |
| 30 | 81.34 | 0.23 | 85.53 | 0.22 | 76.45 | 0.22 | 67.98 | 0.31 |
| Experiment | Variable | SS | MS | SS | MS | F | p |
|---|---|---|---|---|---|---|---|
| 1 | Opening | 45,125 | 1556.1 | 235.9 | 1.97 | 791.6 | <0.01 |
| Activity | 14 | 0.5 | 14.3 | 0.12 | 4.2 | <0.01 | |
| 2 | Opening | 49,838 | 1718.6 | 358.2 | 2.98 | 575.7 | <0.01 |
| Activity | 40 | 1.4 | 11.5 | 0.10 | 14.7 | <0.01 | |
| 3 | Opening | 42,122 | 1452.57 | 554.5 | 4.62 | 314.3 | <0.01 |
| Activity | 51 | 1.77 | 53.3 | 0.44 | 4.0 | <0.01 | |
| 4 | Opening | 19,899 | 686.2 | 116.2 | 0.97 | 708.6 | <0.01 |
| Activity | 37 | 1.3 | 15.8 | 0.13 | 9.7 | <0.01 |
| Interval Number | Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Opening (%) | Activity | Opening (%) | Activity | Opening (%) | Activity | Opening (%) | Activity | |
| 1 | 30.79 | 0.23 | 33.90 | 0.40 | 33.33 | 0.24 | 31.46 | 0.18 |
| 2 | 35.59 | 0.16 | 42.48 | 0.22 | 29.79 | 0.26 | 33.41 | 0.15 |
| 3 | 42.18 | 0.12 | 42.05 | 0.18 | 26.58 | 0.04 | 36.20 | 0.26 |
| 4 | 41.76 | 0.13 | 41.51 | 0.15 | 26.84 | 0.05 | 35.41 | 0.12 |
| 5 | 42.53 | 0.11 | 40.62 | 0.17 | 30.49 | 0.15 | 35.66 | 0.14 |
| 6 | 64.16 | 0.75 | 41.89 | 0.57 | 41.94 | 0.77 | 55.97 | 0.93 |
| 7 | 81.63 | 0.27 | 76.20 | 0.45 | 71.55 | 0.58 | 60.16 | 0.29 |
| 8 | 79.42 | 0.32 | 81.22 | 0.30 | 70.31 | 0.28 | 59.82 | 0.22 |
| 9 | 79.26 | 0.34 | 79.13 | 0.74 | 69.08 | 0.25 | 60.35 | 0.27 |
| 10 | 74.61 | 0.29 | 82.59 | 0.32 | 65.16 | 0.31 | 61.75 | 0.17 |
| 11 | 66.01 | 0.22 | 82.25 | 0.15 | 59.32 | 0.28 | 61.28 | 0.18 |
| 12 | 58.98 | 0.23 | 80.99 | 0.14 | 57.27 | 0.34 | 60.74 | 0.18 |
| 13 | 52.17 | 0.23 | 79.86 | 0.13 | 64.10 | 0.48 | 60.23 | 0.11 |
| 14 | 44.48 | 0.16 | 78.65 | 0.11 | 63.14 | 0.54 | 56.14 | 0.18 |
| 15 | 37.70 | 0.31 | 74.77 | 0.23 | 52.50 | 0.47 | 44.86 | 0.20 |
| 16 | 27.53 | 0.14 | 70.69 | 0.08 | 45.01 | 0.40 | 44.38 | 0.13 |
| 17 | 25.67 | 0.19 | 72.40 | 0.16 | 35.72 | 0.33 | 37.32 | 0.11 |
| 18 | 18.69 | 0.09 | 72.32 | 0.37 | 27.89 | 0.24 | 37.49 | 0.11 |
| 19 | 28.03 | 0.19 | 62.85 | 0.11 | 24.05 | 0.00 | 37.10 | 0.19 |
| 20 | 27.79 | 0.18 | 28.10 | 0.16 | 24.12 | 0.00 | 36.93 | 0.20 |
| 21 | 27.44 | 0.18 | 34.28 | 0.14 | 24.15 | 0.00 | 35.05 | 0.27 |
| Experiment | Variable | SS | MS | SS | MS | F | p |
|---|---|---|---|---|---|---|---|
| 1 | Opening | 490,951 | 24,547.6 | 21,404.9 | 17.28 | 1420.9 | <0.01 |
| Activity | 23 | 1.1 | 64.1 | 0.05 | 22.0 | <0.01 | |
| 2 | Opening | 472,585 | 23,629.3 | 15,618.5 | 12.61 | 1874.5 | <0.01 |
| Activity | 35 | 1.8 | 164.1 | 0.132 | 13.3 | <0.01 | |
| 3 | Opening | 382,888 | 19,144.4 | 17,771.9 | 14.34 | 1334.7 | <0.01 |
| Activity | 52 | 2.6 | 185.7 | 0.15 | 17.2 | <0.01 | |
| 4 | Opening | 168,440 | 8422.0 | 8140.9 | 6.57 | 1281.8 | <0.01 |
| Activity | 35 | 1.8 | 104.3 | 0.08 | 21.0 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szostak, M.; Szoszkiewicz, K.; Achtenberg, K.; Drożdżyński, D. Behavioral Responses of Unio tumidus Freshwater Mussels to Neonicotinoid Pesticide Contamination. Water 2025, 17, 289. https://doi.org/10.3390/w17030289
Szostak M, Szoszkiewicz K, Achtenberg K, Drożdżyński D. Behavioral Responses of Unio tumidus Freshwater Mussels to Neonicotinoid Pesticide Contamination. Water. 2025; 17(3):289. https://doi.org/10.3390/w17030289
Chicago/Turabian StyleSzostak, Marta, Krzysztof Szoszkiewicz, Krzysztof Achtenberg, and Dariusz Drożdżyński. 2025. "Behavioral Responses of Unio tumidus Freshwater Mussels to Neonicotinoid Pesticide Contamination" Water 17, no. 3: 289. https://doi.org/10.3390/w17030289
APA StyleSzostak, M., Szoszkiewicz, K., Achtenberg, K., & Drożdżyński, D. (2025). Behavioral Responses of Unio tumidus Freshwater Mussels to Neonicotinoid Pesticide Contamination. Water, 17(3), 289. https://doi.org/10.3390/w17030289






