Diversity and Activity of Bacterioplankton in Shallow Lakes During Cyanobacterial Blooms
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Lakes
2.2. Sampling Methods
2.3. Physiological Properties of Bacterioplankton Populations
2.4. Sample Preparation and Bacterial DNA Extraction
2.5. PCR Amplification and Sequencing
2.6. Bioinformatics and Statistical Analysis
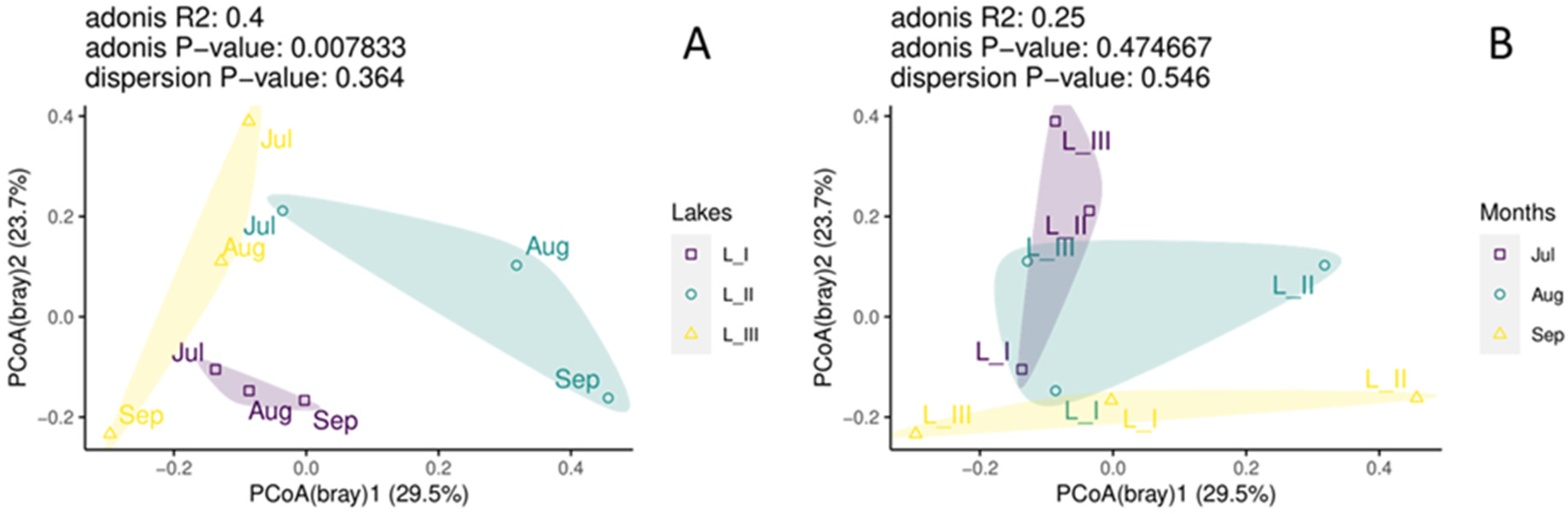
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feresin, E.G.; Arcifa, M.S.; Sampaio da Silva, L.H.; Esguicero, A.L.H. Primary productivity of the phytoplankton in a tropical Brazilian shallow lake: Experiments in the lake and in mesocosms. Acta Limnol. Bras. 2010, 22, 384–396. [Google Scholar] [CrossRef]
- Padisák, J.; Reynolds, C.S. Shallow lakes: The absolute, the relative, the functional and the pragmatic. Hydrobiologia 2003, 506, 1–11. [Google Scholar] [CrossRef]
- Scheffer, M. Ecology of Shallow Lakes; Springer: Dordrecht, The Netherlands, 2004; pp. 76–121. [Google Scholar]
- Lindeman, R.L. The trophic-dynamic aspect of ecology. Ecology 1942, 23, 399–418. [Google Scholar] [CrossRef]
- Feng, C.; Jia, J.; Wang, C.; Han, M.; Dong, C.; Huo, B.; Li, D.; Liu, X. Phytoplankton and Bacterial Community Structure in Two Chinese Lakes of Different Trophic Status. Microorganisms 2019, 7, 621. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.J.; Jones, S.E.; Eiler, A.; McMahon, K.D.; Bertilsson, S. A Guide to the Natural History of Freshwater Lake Bacteria. Microbiol. Mol. Biol. Rev. 2011, 75, 14–49. [Google Scholar] [CrossRef] [PubMed]
- Tandon, K.; Yang, S.H.; Wan, M.T.; Yang, C.C.; Baatar, B.; Chiu, C.Y.; Tsai, J.W.; Liu, W.C.; Tang, S.L. Bacterial Community in Water and Air of Two Sub-Alpine Lakes in Taiwan. Microbes Environ. 2018, 33, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, T.; Chen, S.; Yang, X.; Lv, K.; Sekar, R. Abundance and diversity of bacteria in oxygen minimum drinking water reservoir sediments studied by quantitative PCR and pyrosequencing. Microb. Ecol. 2015, 69, 618–629. [Google Scholar] [CrossRef]
- Miks, M.H.; Warminska-Radyko, I. Selected fluorescent techniques in the research of the physiological state and viability of bacteria cells in food. Med. Weter. 2008, 64, 623–627. [Google Scholar]
- Murínová, S.; Dercová, K. Response Mechanisms of Bacterial Degraders to Environmental Contaminants on the Level of Cell Walls and Cytoplasmic Membrane. Int. J. Microbiol. 2014, 2014, 873081. [Google Scholar] [CrossRef]
- Ramamurthy, T.; Ghosh, A.; Pazhani, G.P.; Shinoda, S. Current Perspectives on Viable but Non-Culturable (VBNC) Pathogenic Bacteria. Front. Public Health 2014, 2, 103. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef]
- Wagley, S.; Morcrette, H.; Kovacs-Simon, A.; Yang, Z.R.; Power, A.; Tennant, R.K.; Love, J.; Murray, N.; Titball, R.W.; Butler, C.S. Bacterial dormancy: A subpopulation of viable but non-culturable cells demonstrates better fitness for revival. PLoS Pathog. 2021, 17, e1009194. [Google Scholar] [CrossRef]
- Samuel, P.S.; Lopez, J.V. Diversity fluctuations of the microbial community during annual Microcystis blooms within Lake Okeechobee, FL. Front. Water 2025, 7, 1678547. [Google Scholar] [CrossRef]
- Nusch, E.A. Comparison of different methods for chlorophyll and phaeopigment. Arch. Hydrobiol. 1980, 14, 14–16. [Google Scholar]
- Carlson, R.E.; Simpson, J. Coordinator’s Guide to Volunteer Lake Monitoring Methods. N. Am. Lake Manag. Soc. 1996, 96, 305. [Google Scholar]
- Dembowska, E. Phytoplankton of shallow lakes in the Iławskie Lake District. Limnol. Pap. 2008, 3, 19–31. [Google Scholar] [CrossRef]
- Eisenhofer, R.; Minich, J.J.; Marotz, C.; Cooper, A.; Knight, R.; Weyrich, L.S. Contamination in Low Microbial Biomass Microbiome Studies: Issues and Recommendations. Trends Microbiol. 2019, 27, 105–117. [Google Scholar] [CrossRef]
- Reynoso, G.; Smith, M.R.; Holmes, C.P.; Keelan, C.R.; McGrath, S.E.; Alvarez, G.H.; Coceano, M.A.; Eldridge, K.A.; Fried, H.I.; Gilbert, N.E.; et al. Bacterial community structure and response to nitrogen amendments in Lake Shenandoah (VA, USA). Water Sci. Technol. 2019, 80, 675–684. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.X.; Huang, L. ImageGP: An Easy-to-Use Data Visualization Web Server for Scientific Researchers. iMeta 2022, 1, e5. [Google Scholar] [CrossRef]
- Cook, K.V.; Li, C.; Cai, H.; Krumholz, L.R.; Hambright, K.D.; Paerl, H.W.; Steffen, M.M.; Wolson, A.E.; Burford, M.A.; Grossart, H.-P.; et al. The global Microcystis interactome. Limnol. Oceanogr. 2020, 66, 194–207. [Google Scholar] [CrossRef]
- Kubera, Ł.; Donderski, W. Distribution and activity of benthic bacteria in four lakes in the Bory Tucholskie National Park (Poland). Aquat. Microb. Ecol. 2017, 79, 127–135. [Google Scholar] [CrossRef]
- Visser, P.M.; Verspagen, J.M.H.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.P.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef]
- Buchan, A.; LeCleir, G.R.; Gulvik, C.A.; González, J.M. Master recyclers: Features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol. 2014, 12, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Doucette, G.J.; Green, D.H. Relationships Between Bacteria and Harmful Algae. In Ecology of Harmful Algae; Granéli, E., Turner, J.T., Eds.; Ecological Studies 189; Springer: Berlin/Heidelberg, Germany, 2006; pp. 243–255. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.H.; Cho, D.H. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Skerratt, J.H.; Bowman, J.; Hallegraeff, G.; James, G.; Nichols, P.D. Algicidal bacteria associated with blooms of a toxic dinoflagellate in a temperate Australian estuary. Mar. Ecol. Prog. Ser. 2002, 244, 1–15. [Google Scholar] [CrossRef]
- Doucette, G.J.; McGovern, E.R.; Babinchak, J.A. Algicidal bacteria active against Gymnodinium breve (Dinophyceae). I. Bacterial isolation and characterization of killing activity. J. Phycol. 1999, 35, 1447–1454. [Google Scholar] [CrossRef]
- Dziallas, C.; Grossart, H.P. Microbial interactions with the cyanobacterium Microcystis aeruginosa and their dependence on temperature. Mar. Biol. 2016, 159, 2389–2398. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, C.; Xu, S.; Zheng, X.; Lv, P.; Wang, C.; Wang, D.; Zhuang, X. Spatiotemporal changes of bacterial communities during a cyanobacterial bloom in a subtropical water source reservoir ecosystem in China. Sci. Rep. 2022, 12, 14573. [Google Scholar] [CrossRef]
- Mankiewicz-Boczek, J.; Font-Nájera, A. Temporal and functional interrelationships between bacterioplankton communities and the development of a toxigenic Microcystis bloom in a lowland European reservoir. Sci. Rep. 2022, 12, 19332. [Google Scholar] [CrossRef]
- Woodhouse, J.N.; Ziegler, J.; Grossart, H.P.; Neilan, B.A. Cyanobacterial Community Composition and Bacteria–Bacteria Interactions Promote the Stable Occurrence of Particle-Associated Bacteria. Front. Microbiol. 2018, 9, 777. [Google Scholar] [CrossRef]
- Yang, C.; Hou, X.; Wu, D.; Chang, W.; Zhang, X.; Dai, X.; Du, H.; Zhang, X.; Igarashi, Y.; Luo, F. The characteristics and algicidal mechanisms of cyanobactericidal bacteria. World J. Microbiol. Biotechnol. 2020, 36, 188. [Google Scholar] [CrossRef] [PubMed]
- Menezes, C.; Churro, C.; Dias, E. Risk Levels of Toxic Cyanobacteria in Portuguese Recreational Freshwaters. Toxins 2017, 9, 327. [Google Scholar] [CrossRef]
- Liu, L.; Lv, X.; Li, W.; Chen, L.; Li, Y.; Lin, B. Relationship between bacterial community and its environmental in Zuohai Lake, China. Acta Microbiol. Sin. 2015, 55, 1177–1189. [Google Scholar]
- Wang, M.; Ateia, M.; Hatano, Y.; Miyanaga, K.; Yoshimura, C. Novel fluorescence-based method for rapid quantification of live bacteria in river water and treated wastewater. Environ. Sci. Adv. 2022, 1, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Perliński, P.; Mudryk, Z.J.; Zdanowicz, M.; Kubera, Ł. Abundance of Live and Dead Bacteriopsammon Inhabiting Sandy Ecosystems of Recreational Marine Beaches of the Southern Baltic Sea. Microb. Ecol. 2023, 86, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Haglund, A.L.; Lantz, P.; Törnblom, E.; Tranvik, L. Depth distribution of active bacteria and bacterial activity in lake sediment. FEMS Microbiol. Ecol. 2003, 46, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lew, S.; Glińska-Lewczuk, K.G.; Lew, M. The effects of environmental parameters on the microbial activity in peat-bog lakes. PLoS ONE 2019, 14, e0224441. [Google Scholar] [CrossRef]
- Louati, I.; Pascault, N.; Debroas, D.; Bernard, C.; Humbert, J.-F.; Leloup, J. Structural Diversity of Bacterial Communities Associated with Bloom-Forming Freshwater Cyanobacteria Differs According to the Cyanobacterial Genus. PLoS ONE 2015, 10, e0146866. [Google Scholar] [CrossRef]
- Klein, A.M.; Bohannan, B.J.M.; Jaffe, D.A.; Levin, D.A.; Green, J.L. Molecular Evidence for Metabolically Active Bacteria in the Atmosphere. Front. Microbiol. 2016, 7, 772. [Google Scholar] [CrossRef]
- Raina, J.B. The Life Aquatic at the Microscale. mSystems 2018, 3, e00150-17. [Google Scholar] [CrossRef]
- Touliabah, H.E.S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A Review of Microalgae- and Cyanobacteria-Based Biodegradation of Organic Pollutants. Molecules 2022, 27, 1141. [Google Scholar] [CrossRef]
- Søndergaard, M.; Danielsen, M. Active bacteria (CTC+) in temperate lakes: Temporal and cross-system variations. J. Plankton Res. 2001, 23, 1195–1206. [Google Scholar] [CrossRef]
- Proctor, L.M.; Souza, A.C. Method for enumeration of 5-cyano-2,3-ditoyl tetrazolium chloride (CTC)-active cells and cell-specific CTC activity of benthic bacteria in riverine, estuarine and coastal sediments. J. Microbiol. Methods 2001, 43, 213–222. [Google Scholar] [CrossRef]
- Le, V.V.; Kang, M.; Ko, S.R.; Jeong, S.; Park, C.-Y.; Lee, J.J.; Choi, I.-C.; Oh, H.-M.; Ahn, C.-Y. Dynamic response of bacterial communities to Microcystis blooms: A three-year study. Sci. Total Environ. 2023, 902, 165888. [Google Scholar] [CrossRef]
- Giani, A.; Taranu, Z.E.; von Rückert, G.; Eaves, I.G. Comparing key drivers of cyanobacteria biomass in temperate and tropical systems. Harmful Algae 2020, 97, 101859. [Google Scholar] [CrossRef] [PubMed]
- Mousa, I.E.; Emara, I.K.; Farfour, S.A.; Eldourghamy, A.S. Microbial profile and its changing rates of Lake Burullus, Egypt as wastewater receiving body. Water Environ. J. 2018, 32, 67–74. [Google Scholar] [CrossRef]
- Reichwaldt, E.S.; Ghadouani, A. Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: Between simplistic scenarios and complex dynamics. Water Res. 2012, 46, 1372–1393. [Google Scholar] [CrossRef]
- Rösel, S.; Allgaier, M.; Grossart, H.P. Long-Term Characterization of Free-Living and Particle-Associated Bacterial Communities in Lake Tiefwaren Reveals Distinct Seasonal Patterns. Microbiol. Aquat. Syst. 2012, 64, 571–583. [Google Scholar] [CrossRef]
- Lefler, F.W.; Barbosa, M.; Zimba, P.V.; Smyth, A.R.; Berthold, D.E.; Laughinghouse, H.D. Spatiotemporal diversity and community structure of cyanobacteria and associated bacteria in the large shallow subtropical Lake Okeechobee (Florida, United States). Front. Microbiol. 2023, 14, 1219261. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Alsanea, A.; Barber, M.; Goel, R. High-throughput DNA sequencing reveals the dominance of pico- and other filamentous cyanobacteria in an urban freshwater Lake. Sci. Total Environ. 2019, 661, 465–480. [Google Scholar] [CrossRef]
- Dai, Y.; Yang, Y.; Wu, Z.; Feng, Q.; Xie, S.; Liu, Y. Spatiotemporal variation of planktonic and sediment bacterial assemblages in two plateau freshwater lakes at different trophic status. Appl. Microbiol. Biotechnol. 2015, 100, 4161–4175. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, H.; Zhang, Y.; Li, D.; Gao, Y.; Li, H.; Duan, L.; Zhang, X.; Liu, F.; Xu, J.; et al. Heterogeneous bacterial communities affected by phytoplankton community turnover and microcystins in plateau lakes of Southwestern China. Sci. Total Environ. 2023, 903, 166303. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Deng, L.J.; Lou, K.; Zhang, T.; Yang, H.-M.; Shi, Y.-W.; Lin, Q. Molecular characterization of the planktonic microorganisms in water of two mountain brackish lakes. J. Basic Microbiol. 2014, 54, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, N.; Albanese, D.; Capelli, C.; Boscaini, A.; Pindo, M.; Donati, C. Diversity and Cyclical Seasonal Transitions in the Bacterial Community in a Large and Deep Perialpine Lake. Microb. Ecol. 2018, 76, 125–143. [Google Scholar] [CrossRef]
- Woodhouse, J.N.; Kinsela, A.S.; Collins, R.N.; Bowling, L.C.; Honeyman, G.L.; Holliday, J.K.; Neilan, B.A. Microbial communities reflect temporal changes in cyanobacterial composition in a shallow ephemeral freshwater lake. ISME J. 2016, 10, 1337–1351. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Liu, L.; Fan, Y.; Li, L.; Yang, Y.; Lu, Z.; Zhang, X. Annual periodicity in planktonic bacterial and archaeal community composition of eutrophic Lake Taihu. Sci. Rep. 2015, 5, 15488. [Google Scholar] [CrossRef]
- Ma, J.; Brookes, J.D.; Qin, B.; Paerl, H.W.; Gao, G.; Wu, P.; Zhang, W.; Deng, J.; Zhu, G.; Zhang, Y.; et al. Environmental factors controlling colony formation in blooms of the cyanobacteria Microcystis spp. in Lake Taihu, China. Harmful Algae 2014, 31, 136–142. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [CrossRef]
- Wan, L.; Chen, X.; Deng, Q.; Yang, L.; Li, X.; Zhang, J.; Song, C.; Zhou, Y.; Cao, X. Phosphorus strategy in bloom-forming cyanobacteria (Dolichospermum and Microcystis) and its role in their succession. Harmful Algae 2019, 84, 46–55. [Google Scholar] [CrossRef]
- Ahn, C.Y.; Chung, A.S.; Oh, H.M. Rainfall, phycocyanin, and N:P ratios related to cyanobacterial blooms in a Korean large reservoir. Hydrobiologia 2002, 474, 117–124. [Google Scholar] [CrossRef]
- Muluye, T.; Fetahi, T.; Engdaw, F.; Mohammed, A. Cyanotoxins in African waterbodies: Occurrence, adverse effects, and potential risk to animal and human health. Environ. Geochem. Health 2023, 45, 7519–7542. [Google Scholar] [CrossRef]
- Salazara-Torresa, A.; Robles, D.; Reyes, A.; Santa-Maria, M.C.; Venail, P. Evaluation of planktonic cyanobacteria in Peruvian freshwater lentic water bodies: Prevalence and regulatory framework to aid policy making. Environ. Monit. Assess. 2023, 195, 852. [Google Scholar] [CrossRef]
- Xie, M.; Ren, M.; Yang, C.; Yi, H.; Li, Z.; Li, T.; Zhao, J. Metagenomic Analysis Reveals Symbiotic Relationship among Bacteria in Microcystis-Dominated Community. Sec. Aquat. Microbiol. 2016, 7, 56. [Google Scholar] [CrossRef]
- Brunberg, A.K. Contribution of bacteria in the mucilage of Microcystis spp. (Cyanobacteria) to benthic and pelagic bacterial production in a hypereutrophic lake. FEMS Microbiol. Ecol. 1999, 29, 13–22. [Google Scholar] [CrossRef]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shen, H.; Shi, P.; Chen, J.; Ni, L.; Xie, P. Experimental evidence for the role of heterotrophic bacteria in the formation of Microcystis colonies. J. Appl. Phycol. 2016, 28, 1111–1123. [Google Scholar] [CrossRef]
- Shen, H.; Niu, Y.; Xie, P.; Tao, M.; Yang, X. Morphological and physiological changes in Microcystis aeruginosa as a result of interactions with heterotrophic bacteria. Freshw. Biol. 2011, 56, 1065–1080. [Google Scholar] [CrossRef]
- Fuster, M.; Ruiz, T.; Lamarque, A.; Coulon, M.; Legrand, B.; Sabart, M.; Latour, D.; Mallet, C. Cyanosphere Dynamic During Dolichospermum Bloom: Potential Roles in Cyanobacterial Proliferation. Microb. Ecol. 2024, 87, 3. [Google Scholar] [CrossRef]
- Te, A.H.; Kok, J.W.K.; Luo, R.; You, L.; Sukarji, N.H.; Goh, K.C.; Sim, Z.Y.; Zhang, D.; He, Y.; Gin, K.Y.-H. Coexistence of Synechococcus and Microcystis Blooms in a Tropical Urban Reservoir and Their Links with Microbiomes. Environ. Sci. Technol. 2023, 57, 1613–1624. [Google Scholar] [CrossRef]
- Gin, K.Y.H.; Sim, Z.Y.; Goh, K.C.; Kok, J.W.K.; Te, S.H.; Tran, N.H.; Li, W.; He, Y. Novel cyanotoxin-producing Synechococcus in tropical lakes. Water Res. 2021, 192, 116828. [Google Scholar] [CrossRef] [PubMed]
- Sim, Z.Y.; Goh, K.C.; He, Y.; Gin, H.Y.H. Present and future potential role of toxin-producing Synechococcus in the tropical region. Sci. Total. Environ. 2023, 896, 165230. [Google Scholar] [CrossRef] [PubMed]

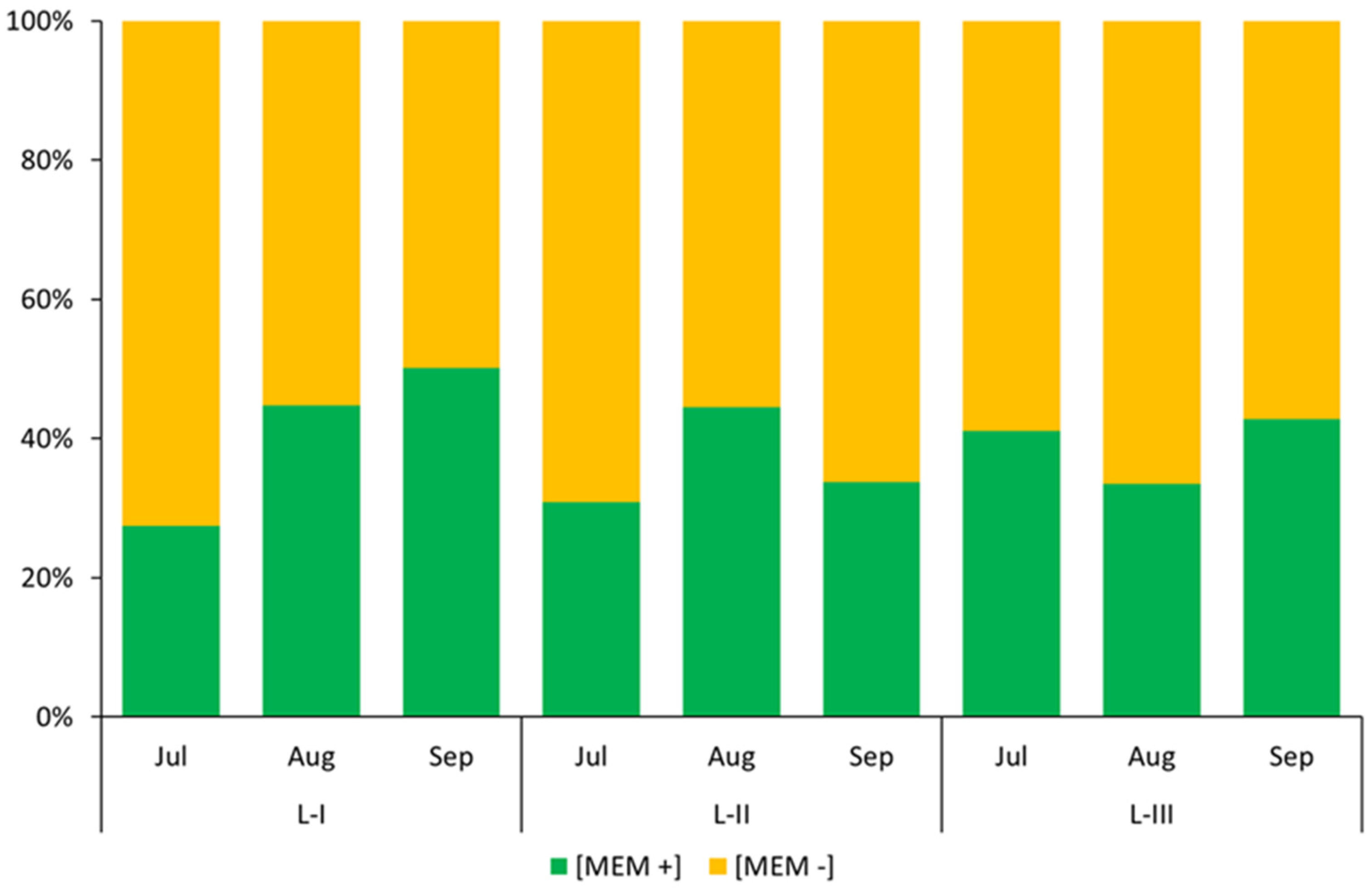
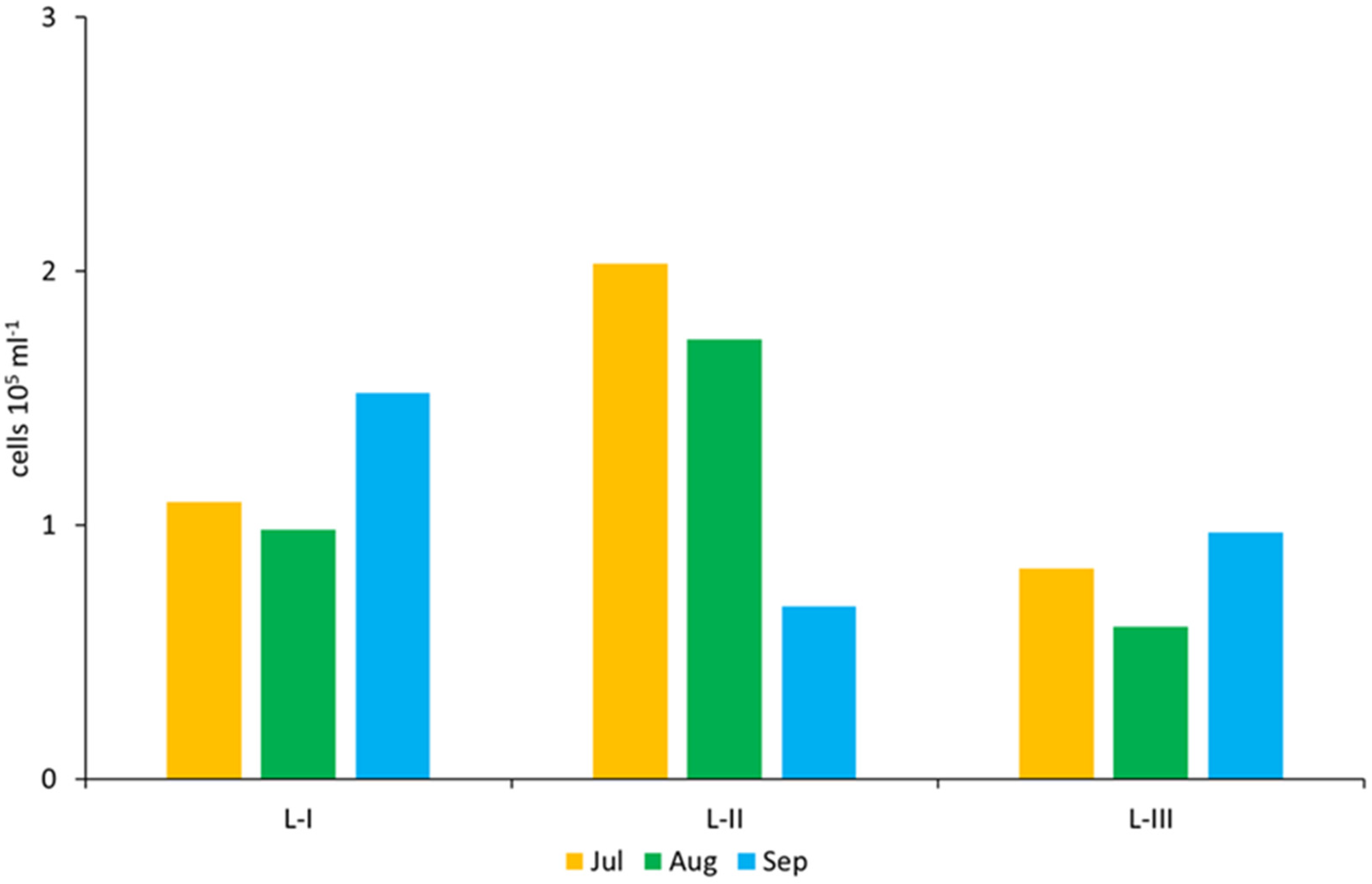

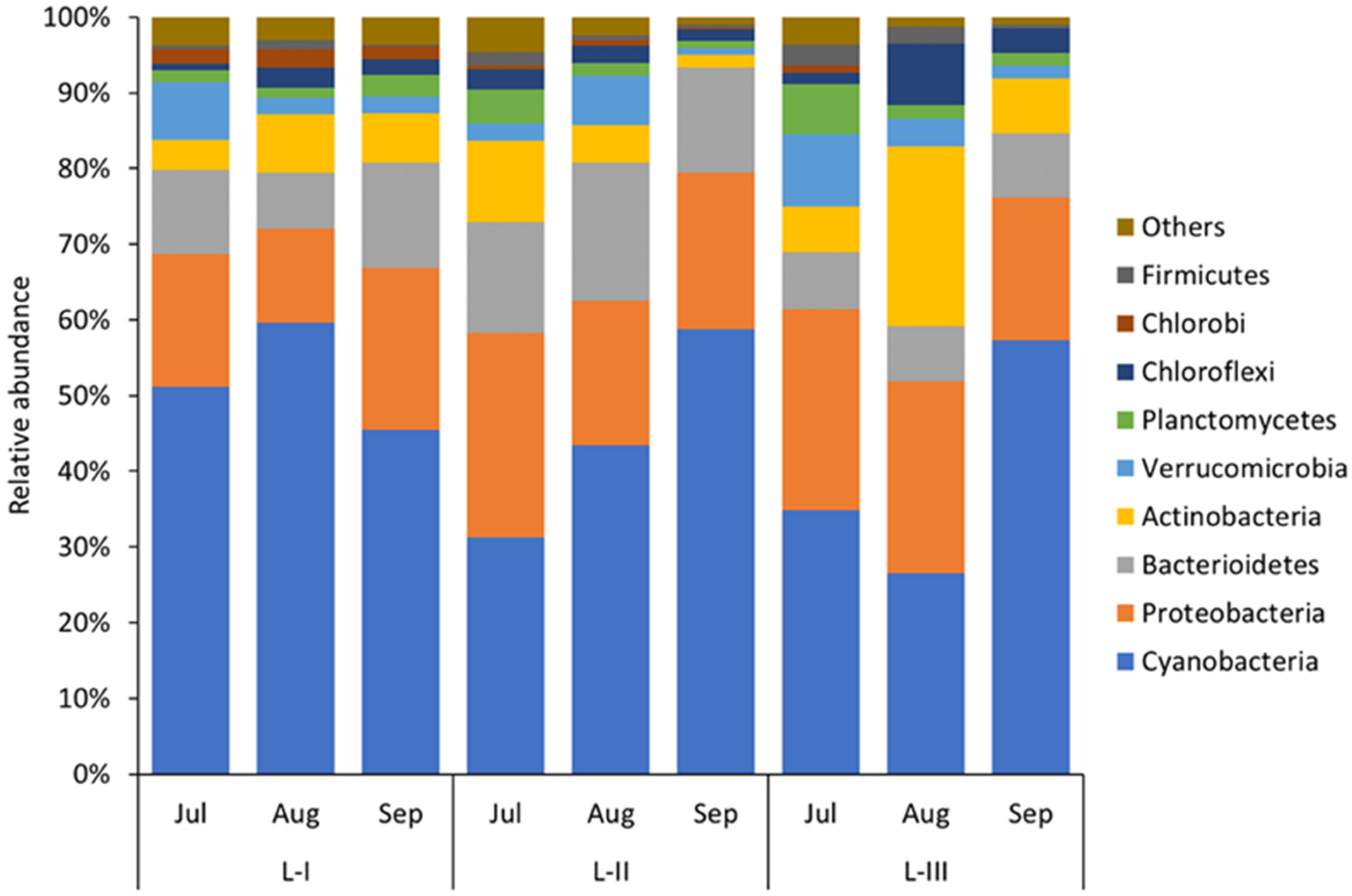
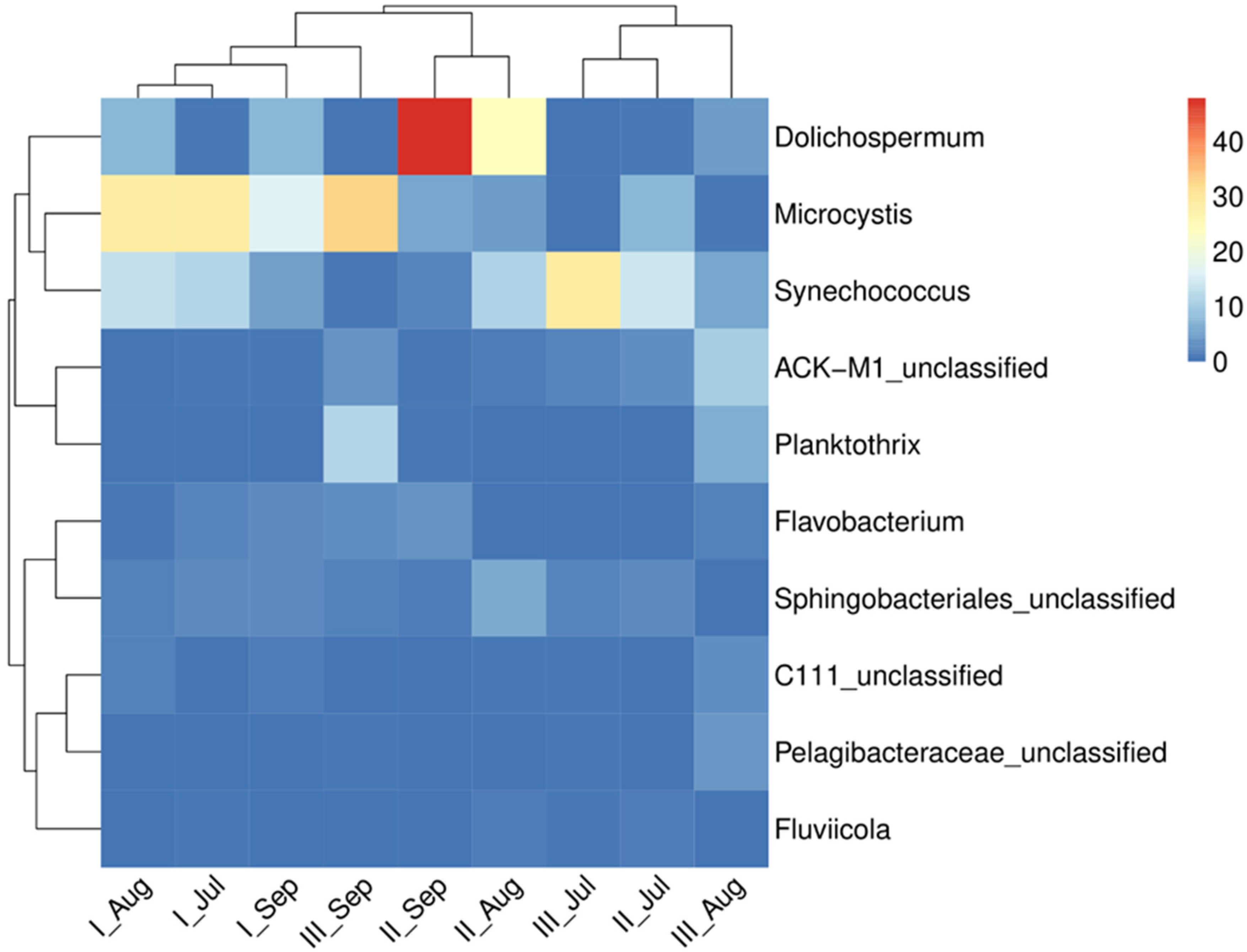
| L-I | L-II | L-III | |
|---|---|---|---|
| Location | 53°40′48″ N | 53°33′46″ N | 52°49′41″ N |
| 19°33′44″ E | 19°36′38″ E | 18°42′6″ E | |
| Surface [ha] | 86 | 20.2 | 30.7 |
| Volume [103 m3] | 1020 | 263 | 2.4 |
| Max. depth [m] | 2.1 | 2.4 | 3.7 |
| Type of catchment | forest | forest | agricultural |
| OTUs | 97% Similarity | ||||
|---|---|---|---|---|---|
| Shannon–Wiener | Simpson | InvSimpson | |||
| July | L-I | 343 | 3.540 | 0.888 | 8.929 |
| L-II | 457 | 4.446 | 0.963 | 27.520 | |
| L-III | 300 | 3.739 | 0.901 | 10.122 | |
| August | L-I | 254 | 3.372 | 0.889 | 9.022 |
| L-II | 324 | 3.365 | 0.917 | 12.078 | |
| L-III | 321 | 4.152 | 0.966 | 30.215 | |
| September | L-I | 260 | 3.800 | 0.941 | 17.078 |
| L-II | 264 | 2.693 | 0.749 | 3.986 | |
| L-III | 271 | 3.144 | 0.862 | 7.255 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankowiak, E.; Dembowska, E.; Małecka-Adamowicz, M.; Kubera, Ł. Diversity and Activity of Bacterioplankton in Shallow Lakes During Cyanobacterial Blooms. Water 2025, 17, 3376. https://doi.org/10.3390/w17233376
Jankowiak E, Dembowska E, Małecka-Adamowicz M, Kubera Ł. Diversity and Activity of Bacterioplankton in Shallow Lakes During Cyanobacterial Blooms. Water. 2025; 17(23):3376. https://doi.org/10.3390/w17233376
Chicago/Turabian StyleJankowiak, Emilia, Ewa Dembowska, Marta Małecka-Adamowicz, and Łukasz Kubera. 2025. "Diversity and Activity of Bacterioplankton in Shallow Lakes During Cyanobacterial Blooms" Water 17, no. 23: 3376. https://doi.org/10.3390/w17233376
APA StyleJankowiak, E., Dembowska, E., Małecka-Adamowicz, M., & Kubera, Ł. (2025). Diversity and Activity of Bacterioplankton in Shallow Lakes During Cyanobacterial Blooms. Water, 17(23), 3376. https://doi.org/10.3390/w17233376









