Ecotoxicological Assessment of Perfluorooctane Sulfonate and Perfluorooctanoic Acid Following Biodegradation: Insights from Daphnia magna Toxicity and Yeast Estrogen Screen Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Biodegradation Process
2.3. Chemical Analysis
2.4. The Recombinant Yeast Estrogen Screen (YES)
2.4.1. Sample Preparation
2.4.2. The Recombinant Yeast Estrogen Assay
2.5. Daphnia magna Acute Toxicity Test (Water Flea)
2.6. Hazard Classification for Natural Waters
2.7. Statistical Analysis
3. Results
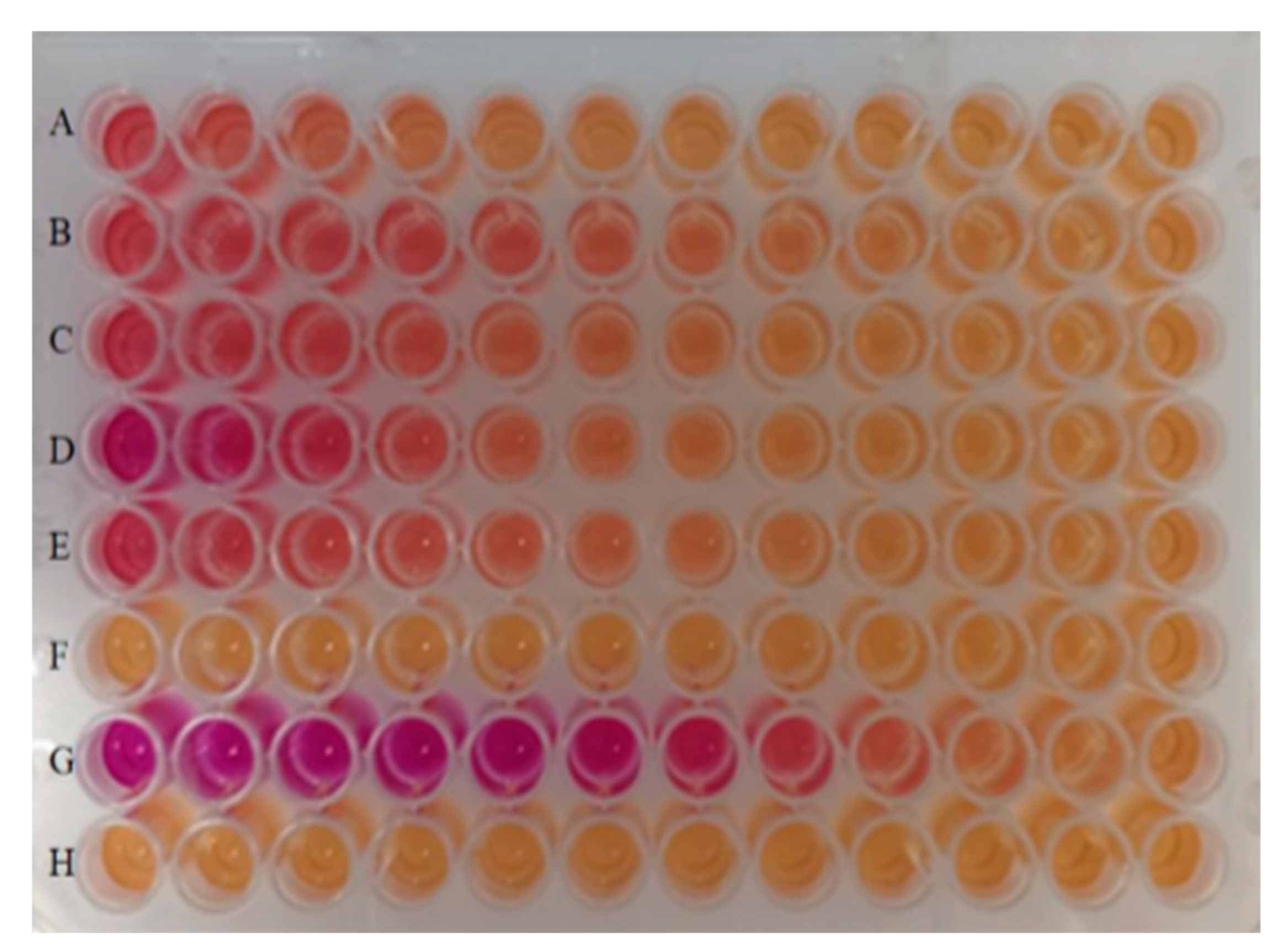
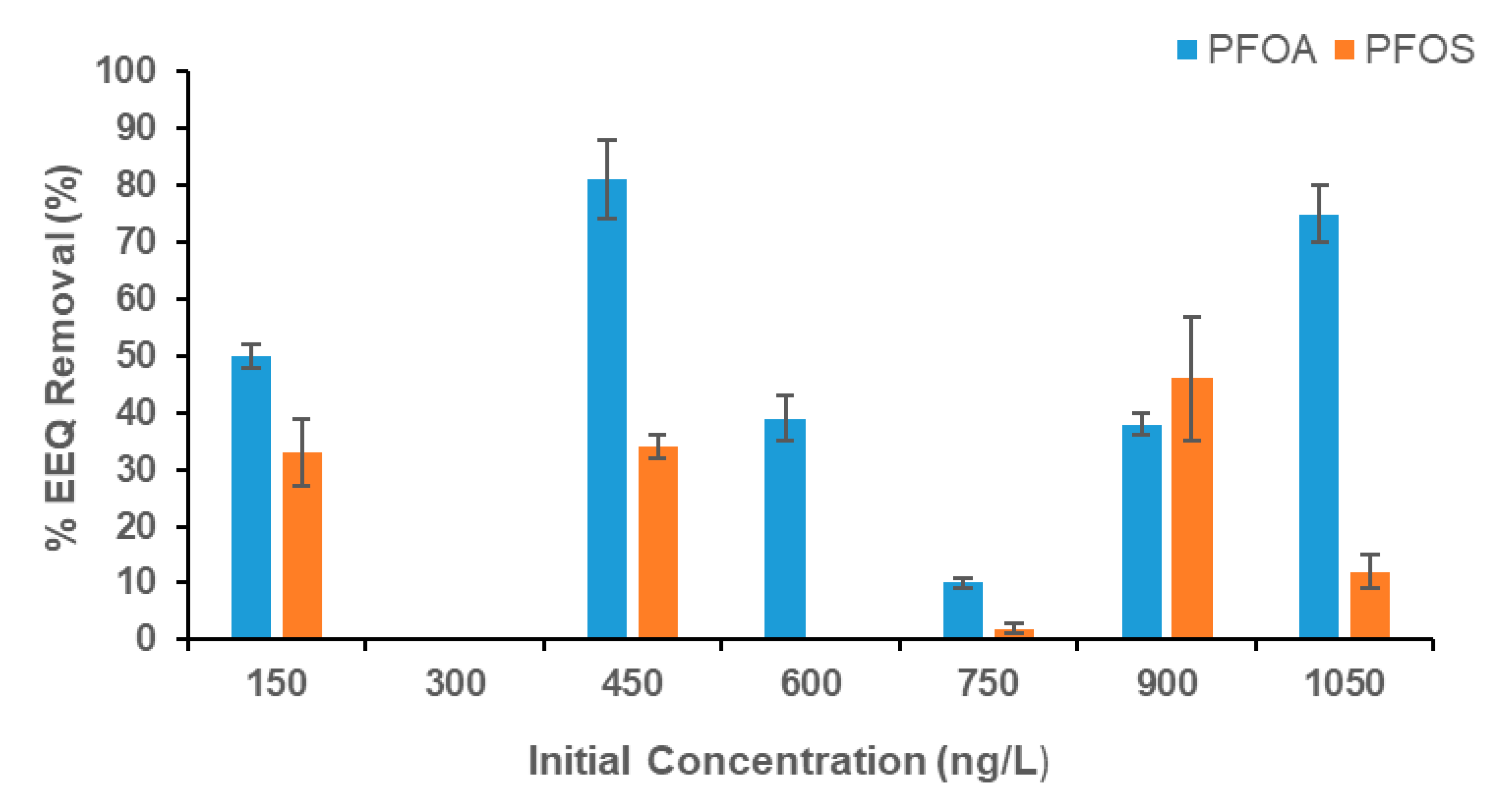
3.1. Results of the Recombinant Yeast Estrogen Assay
3.2. Daphnia magna Screening Assays
4. Discussion
4.1. Reduction in Estrogenic Activity During the YES Bioassay
4.2. Acute Toxicity Assessment Using Daphnia magna
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; De Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ozaki, N.; KarimiDermani, B.; Razmi, E.; Kasmuri, N. Occurrence of per-and polyfluoroalkyl substances in aquatic environments and their removal by advanced oxidation processes. Chemosphere 2023, 330, 138666. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per-and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- Adetunla, A.; Afolalu, S.; Jen, T.-C.; Ogundana, A. The roles of surfactant in tribology applications of recent technology; an overview. E3S Web Conf. 2023, 391, 01021. [Google Scholar] [CrossRef]
- Lenka, S.P.; Kah, M.; Padhye, L.P. A review of the occurrence, transformation, and removal of poly-and perfluoroalkyl substances (PFAS) in wastewater treatment plants. Water Res. 2021, 199, 117187. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per-and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef]
- Kunacheva, C.; Tanaka, S.; Fujii, S.; Boontanon, S.K.; Musirat, C.; Wongwattana, T.; Shivakoti, B.R. Mass flows of perfluorinated compounds (PFCs) in central wastewater treatment plants of industrial zones in Thailand. Chemosphere 2011, 83, 737–744. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K.M.; Li, X.Y. The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 2015, 119, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Zacs, D.; Bartkevics, V. Trace determination of perfluorooctane sulfonate and perfluorooctanoic acid in environmental samples (surface water, wastewater, biota, sediments, and sewage sludge) using liquid chromatography–Orbitrap mass spectrometry. J. Chromatogr. A 2016, 1473, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Bundschuh, M. Fate and effects of poly-and perfluoroalkyl substances in the aquatic environment: A review. Environ. Toxicol. Chem. 2014, 33, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.Y.; Aris, A.Z. Revisiting the “forever chemicals”, PFOA and PFOS exposure in drinking water. NPJ Clean Water 2023, 6, 57. [Google Scholar] [CrossRef]
- Wang, N.; Jagani, R.; Nwobodo, N.; Ma, J. Toxicity of environmentally relevant concentration of PFAS chemicals in Lumbriculus variegatus (Oligochaeta, Lumbriculidae)—A multi-bioindicator study. Ecotoxicol. Environ. Saf. 2023, 268, 115722. [Google Scholar] [CrossRef] [PubMed]
- Flaws, J.; Damdimopoulou, P.; Patisaul, H.B.; Gore, A.; Raetzman, L.; Vandenberg, L.N. Plastics, EDCs and Health; Endocrine Society: Washington, DC, USA, 2020. [Google Scholar]
- Li, M. Toxicity of perfluorooctane sulfonate and perfluorooctanoic acid to plants and aquatic invertebrates. Environ. Toxicol. Int. J. 2009, 24, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Winquist, A. PFAS and cancer, a scoping review of the epidemiologic evidence. Environ. Res. 2021, 194, 110690. [Google Scholar] [CrossRef]
- Ankley, G.T.; Cureton, P.; Hoke, R.A.; Houde, M.; Kumar, A.; Kurias, J.; Lanno, R.; McCarthy, C.; Newsted, J.; Salice, C.J. Assessing the ecological risks of per-and polyfluoroalkyl substances: Current state-of-the science and a proposed path forward. Environ. Toxicol. Chem. 2021, 40, 564–605. [Google Scholar] [CrossRef]
- Mhadhbi, L.; Rial, D.; Pérez, S.; Beiras, R. Ecological risk assessment of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in marine environment using Isochrysis galbana, Paracentrotus lividus, Siriella armata and Psetta maxima. J. Environ. Monit. 2012, 14, 1375–1382. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel treatment technologies for PFAS compounds: A critical review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef]
- Nzeribe, B.N.; Crimi, M.; Mededovic Thagard, S.; Holsen, T.M. Physico-chemical processes for the treatment of per-and polyfluoroalkyl substances (PFAS): A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 866–915. [Google Scholar] [CrossRef]
- Leusch, F.D.L.; De Jager, C.; Levi, Y.; Lim, R.; Puijker, L.; Sacher, F.; Tremblay, L.A.; Wilson, V.S.; Chapman, H.F. Comparison of five in vitro bioassays to measure estrogenic activity in environmental waters. Environ. Sci. Technol. 2010, 44, 3853–3860. [Google Scholar] [CrossRef]
- Brion, F.; De Gussem, V.; Buchinger, S.; Hollert, H.; Carere, M.; Porcher, J.-M.; Piccini, B.; Féray, C.; Dulio, V.; Könemann, S. Monitoring estrogenic activities of waste and surface waters using a novel in vivo zebrafish embryonic (EASZY) assay: Comparison with in vitro cell-based assays and determination of effect-based trigger values. Environ. Int. 2019, 130, 104896. [Google Scholar] [CrossRef]
- Ebert, D. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia; National Library of Medicine: Bethesda, MD, USA, 2005.
- Le, Q.-A.V.; Sekhon, S.S.; Lee, L.; Ko, J.H.; Min, J. Daphnia in water quality biomonitoring-“omic” approaches. Toxicol. Environ. Health Sci. 2016, 8, 1–6. [Google Scholar] [CrossRef]
- Payne, J.; Rajapakse, N.; Wilkins, M.; Kortenkamp, A. Prediction and assessment of the effects of mixtures of four xenoestrogens. Environ. Health Perspect. 2000, 108, 983–987. [Google Scholar] [CrossRef][Green Version]
- Brix, R.; Noguerol, T.-N.; Piña, B.; Balaam, J.; Nilsen, A.J.; Tollefsen, K.E.; Levy, W.; Schramm, K.-W.; Barceló, D. Evaluation of the suitability of recombinant yeast-based estrogenicity assays as a pre-screening tool in environmental samples. Environ. Int. 2010, 36, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Gayda, G.; Stasyuk, N.; Smutok, O.; Gonchar, M.; Sibirny, A.A. Yeast-Based Biosensors for Clinical Diagnostics, Food Control, and Environmental Safety. In Biotechnology of Yeasts and Filamentous Fungi; Springer: Cham, Switzerland, 2025; pp. 405–435. [Google Scholar]
- Routledge, E.J.; Sumpter, J.P. Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ. Toxicol. Chem. Int. J. 1996, 15, 241–248. [Google Scholar] [CrossRef]
- Dhooge, W.; Arijs, K.; D’Haese, I.; Stuyvaert, S.; Versonnen, B.; Janssen, C.; Verstraete, W.; Comhaire, F. Experimental parameters affecting sensitivity and specificity of a yeast assay for estrogenic compounds: Results of an interlaboratory validation exercise. Anal. Bioanal. Chem. 2006, 386, 1419–1428. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Pratt, L.A.; Watnick, P.I.; Newman, D.K.; Weaver, V.B.; Kolter, R. [6] Genetic approaches to study of biofilms. Methods Enzymol. 1999, 310, 91–109. [Google Scholar]
- Aneck-Hahn, N.H.; Bornman, M.S.; De Jager, C. Preliminary assessment of oestrogenic activity in water sources in Rietvlei Nature Reserve, Gauteng, South Africa. Afr. J. Aquat. Sci. 2008, 33, 249–254. [Google Scholar] [CrossRef]
- De Jager, C.; Aneck-Hahn, N.H.; Barnhoorn, I.E.J.; Bornman, M.S.; Pieters, R.; Van Wyk, J.H.; Van Zijl, C. The Compilation of a Toolbox of Bio-Assays for Detection of Estrogenic Activity in Water. WRC K5/1816. 2011. Available online: https://www.wrc.org.za/wp-content/uploads/mdocs/1816-1-101.pdf (accessed on 4 November 2025).
- U.S. Environmental Protection Agency (USEPA). National Recommended Water Quality Criteria: 2002; EPA-822-Z-02-001; U.S. Environmental Protection Agency: Washington, DC, USA, 2002. Available online: https://www.epa.gov/sites/default/files/2018-12/documents/national-recommended-hh-criteria-2002.pdf (accessed on 4 November 2025).
- Slabbert, J.L. Methods for Direct Estimation of Ecological Effect Potential (DEEEP); Water Research Commission: Pretoria, South Africa, 2004. [Google Scholar]
- OECD. Test No. 202: Daphnia Sp. Acute Immobilisation Test; OECD: Paris, France, 2004. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2005.
- OECD. Test No. 211: Daphnia magna Reproduction Test; OECD: Paris, France, 2002. [Google Scholar]
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nalecz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ. Toxicol. Int. J. 2003, 18, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Genthe, B.; Steyn, M. Health Risk Assessment Protocol for Endocrine Disrupting Chemicals; WRC Project No. KV 206/08; Water Research Commission: Pretoria, South Africa, 2008; ISBN 978-1-77005-686-2. Available online: https://www.wrc.org.za/wp-content/uploads/mdocs/KV-206-08.pdf (accessed on 4 November 2025).
- Jobling, S.; Casey, D.; Rodgers-Gray, T.; Oehlmann, J.; Schulte-Oehlmann, U.; Pawlowski, S.; Baunbeck, T.; Turner, A.P.; Tyler, C.R. Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent. Aquat. Toxicol. 2003, 65, 205–220. [Google Scholar] [CrossRef]
- Jarošová, B.; Bláha, L.; Giesy, J.P.; Hilscherová, K. What level of estrogenic activity determined by in vitro assays in municipal waste waters can be considered as safe? Environ. Int. 2014, 64, 98–109. [Google Scholar] [CrossRef]
- Kjeldsen, L.S.; Bonefeld-Jørgensen, E.C. Perfluorinated compounds affect the function of sex hormone receptors. Environ. Sci. Pollut. Res. 2013, 20, 8031–8044. [Google Scholar] [CrossRef] [PubMed]
- Benninghoff, A.D.; Lefevre, M.; Hintze, K.J.; Ward, R.E.; Broadbent, J.R. Fighting Cancer with Functional Foods: New Approaches to Investigate the Interactions of Dietary Bioactive Chemicals and the Gut Microbiome. J. Dev. Sustain. Agric. 2015, 10, 34–54. [Google Scholar]
- Liu, J.; Avendaño, S.M. Microbial degradation of polyfluoroalkyl chemicals in the environment: A review. Environ. Int. 2013, 61, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, H.; Lin, Y.; Qin, X.; Zhang, Y.; Geng, X.; Kannan, K. Distribution of poly-and perfluoroalkyl substances in matched samples from pregnant women and carbon chain length related maternal transfer. Environ. Sci. Technol. 2013, 47, 7974–7981. [Google Scholar] [CrossRef] [PubMed]
- Wielsøe, M.; Long, M.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere 2015, 129, 239–245. [Google Scholar] [CrossRef]
- Florentin, A.; Deblonde, T.; Diguio, N.; Hautemaniere, A.; Hartemann, P. Impacts of two perfluorinated compounds (PFOS and PFOA) on human hepatoma cells: Cytotoxicity but no genotoxicity? Int. J. Hyg. Environ. Health 2011, 214, 493–499. [Google Scholar] [CrossRef]
- Komissarova, E.V.; Saha, S.K.; Rossman, T.G. Dead or dying: The importance of time in cytotoxicity assays using arsenite as an example. Toxicol. Appl. Pharmacol. 2005, 202, 99–107. [Google Scholar] [CrossRef]
- Henry, N.D.; Fair, P.A. Comparison of in vitro cytotoxicity, estrogenicity and anti-estrogenicity of triclosan, perfluorooctane sulfonate and perfluorooctanoic acid. J. Appl. Toxicol. 2013, 33, 265–272. [Google Scholar] [CrossRef]
- Sonthithai, P.; Suriyo, T.; Thiantanawat, A.; Watcharasit, P.; Ruchirawat, M.; Satayavivad, J. Perfluorinated chemicals, PFOS and PFOA, enhance the estrogenic effects of 17β-estradiol in T47D human breast cancer cells. J. Appl. Toxicol. 2016, 36, 790–801. [Google Scholar] [CrossRef]
- Zhang, A.; Li, Y.; Chen, L. Distribution and seasonal variation of estrogenic endocrine disrupting compounds, N-nitrosodimethylamine, and N-nitrosodimethylamine formation potential in the Huangpu River, China. J. Environ. Sci. 2014, 26, 1023–1033. [Google Scholar] [CrossRef]
- Huang, S.; Jaffé, P.R. Defluorination of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) by Acidimicrobium sp. strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419. [Google Scholar] [CrossRef]
- Kwon, B.G.; Lim, H.-J.; Na, S.-H.; Choi, B.-I.; Shin, D.-S.; Chung, S.-Y. Biodegradation of perfluorooctanesulfonate (PFOS) as an emerging contaminant. Chemosphere 2014, 109, 221–225. [Google Scholar] [CrossRef]
- Wen, B.; Zhang, H.; Li, L.; Hu, X.; Liu, Y.; Shan, X.; Zhang, S. Bioavailability of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in biosolids-amended soils to earthworms (Eisenia fetida). Chemosphere 2015, 118, 361–366. [Google Scholar] [CrossRef]
- Ochoa-Herrera, V.; Field, J.A.; Luna-Velasco, A.; Sierra-Alvarez, R. Microbial toxicity and biodegradability of perfluorooctane sulfonate (PFOS) and shorter chain perfluoroalkyl and polyfluoroalkyl substances (PFASs). Environ. Sci. Process. Impacts 2016, 18, 1236–1246. [Google Scholar] [CrossRef]
- Olaniyan, L.W.B.; Okoh, A.I. Determination and ecological risk assessment of two endocrine disruptors from River Buffalo, South Africa. Environ. Monit. Assess. 2020, 192, 750. [Google Scholar] [CrossRef]
- Cao, L.-Y.; Ren, X.-M.; Guo, L.-H. Estrogen-related receptor γ is a novel target for lower-chlorinated polychlorinated biphenyls and their hydroxylated and sulfated metabolites. Environ. Pollut. 2019, 254, 113088. [Google Scholar] [CrossRef]
- Qu, C.; Ma, J.; Zhang, Y.; Han, C.; Huang, L.; Shen, L.; Li, H.; Wang, X.; Liu, J.; Zou, W. Estrogen receptor variant ER-α36 promotes tamoxifen agonist activity in glioblastoma cells. Cancer Sci. 2019, 110, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Marzan, L.W.; Barua, P.; Akter, Y.; Mannan, A.; Hossain, A.; Ali, Y. Molecular investigation on clinopathological, genetic and biochemical changes in Channa punctata infected with internal parasites and subjected to metals pollution in Chittagong. Bangladesh. J. Biomol. Res. Ther. 2014, 3, 113. [Google Scholar]
- Park, M.-Y.; Choi, H.-Y.; Kim, J.-D.; Lee, H.-S.; Ku, S.-K. 28 Days repeated oral dose toxicity test of aqueous extracts of mahwangyounpae-tang, a polyherbal formula. Food Chem. Toxicol. 2010, 48, 2477–2482. [Google Scholar] [CrossRef]
- Weltens, R.; Vanermen, G.; Tirez, K.; Robbens, J.; Deprez, K.; Michiels, L. Screening tests for hazard classification of complex waste materials–Selection of methods. Waste Manag. 2012, 32, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Koçbaş, F.; Oral, R. Daphnia magna as a test species for toxicity evaluation of municipal wastewater treatment plant effluents on freshwater cladoceran in Turkey. Turk. J. Fish. Aquat. Sci. 2015, 15, 619–624. [Google Scholar] [CrossRef]
- Zhang, J.; Naveed, H.; Chen, K.; Chen, L. Toxicity of Per-and Polyfluoroalkyl Substances and Their Substitutes to Terrestrial and Aquatic Invertebrates—A Review. Toxics 2025, 13, 47. [Google Scholar] [CrossRef]
- Logeshwaran, P.; Sivaram, A.K.; Surapaneni, A.; Kannan, K.; Naidu, R.; Megharaj, M. Exposure to perfluorooctanesulfonate (PFOS) but not perflurorooctanoic acid (PFOA) at ppb concentration induces chronic toxicity in Daphnia carinata. Sci. Total Environ. 2021, 769, 144577. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Kim, Y.; Oh, S.; Ahn, B.; Jo, H.; Choi, K. Toxicity of perfluorooctane sulfonic acid and perfluorooctanoic acid on freshwater macroinvertebrates (Daphnia magna and Moina macrocopa) and fish (Oryzias latipes). Environ. Toxicol. Chem. 2008, 27, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Zhang, J.; Chen, Y.; Wang, L.; Wang, M.; Xiong, D.; Sun, Y. Combined effects of PFOS and PFOA on zebrafish (Danio rerio) embryos. Arch. Environ. Contam. Toxicol. 2013, 64, 668–675. [Google Scholar] [CrossRef]
- Jeong, T.-Y.; Yuk, M.-S.; Jeon, J.; Kim, S.D. Multigenerational effect of perfluorooctane sulfonate (PFOS) on the individual fitness and population growth of Daphnia magna. Sci. Total Environ. 2016, 569, 1553–1560. [Google Scholar] [CrossRef]
- Ulhaq, M. The Toxicity of Perfluoroalkyl Acids in Zebrafish (Danio rerio). Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2013. [Google Scholar]
- Ulhaq, Z.S.; Tse, W.K.F. Perfluorohexanesulfonic acid (PFHxS) induces oxidative stress and causes developmental toxicities in zebrafish embryos. J. Hazard. Mater. 2023, 457, 131722. [Google Scholar] [CrossRef]
- Savoca, D.; Pace, A. Bioaccumulation, biodistribution, toxicology and biomonitoring of organofluorine compounds in aquatic organisms. Int. J. Mol. Sci. 2021, 22, 6276. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.; Yun, X.; Spooner, D.E.; Kurz, M.J.; McKenzie, E.R.; Sales, C.M. Exposure pathways and bioaccumulation of per-and polyfluoroalkyl substances in freshwater aquatic ecosystems: Key considerations. Sci. Total Environ. 2022, 822, 153561. [Google Scholar] [CrossRef]
- Boudreau, T.M.; Sibley, P.K.; Mabury, S.A.; Muir, D.G.C.; Solomon, K.R. Laboratory evaluation of the toxicity of perfluorooctane sulfonate (PFOS) on Selenastrum capricornutum, Chlorella vulgaris, Lemna gibba, Daphnia magna, and Daphnia pulicaria. Arch. Environ. Contam. Toxicol. 2003, 44, 307–313. [Google Scholar] [CrossRef]
- Seyoum, A.; Pradhan, A.; Jass, J.; Olsson, P.-E. Perfluorinated alkyl substances impede growth, reproduction, lipid metabolism and lifespan in Daphnia magna. Sci. Total Environ. 2020, 737, 139682. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.-C.; Plinsch, C.; Braeuning, A.; Buhrke, T. Activation of human nuclear receptors by perfluoroalkylated substances (PFAS). Toxicol. Vitr. 2020, 62, 104700. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs Jr, D.R.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
| Summary of Toxicity Test | |
| Test system | Daphnia test |
| Test species | Daphnia magna |
| Age of test organisms | Less than 48 h old |
| Trophic level | Acute toxicity |
| Test procedure | USEPA, 2002 [32] |
| Summary of test conditions for Daphnia magna acute toxicity test | |
| Test type | Static renewal |
| Water temperature | 20 °C ± 1 °C; or 25 °C ± 1 °C |
| Light qualityS | Ambient laboratory illumination |
| Photoperiod | 8 h dark: 16 h light |
| Feeding regime | Feed algae and commercial fish flakes while in holding prior to the test |
| Aeration | None |
| Size of test chamber | 50 mL |
| Volume of test sample | 25 mL |
| Number of test organisms per chamber | 5 |
| Number of replicate chambers | 4 |
| Total number of test organisms per sample | 20 |
| Control and dilution water | Moderately hard, reconstituted water |
| Test duration | 48 h |
| Effect measured | Percentage lethality (no movement on gentle prodding) calculated in relation to control |
| Test acceptability | 90% or greater survival in control |
| Interpretation | Lethality > 10% indicates toxicity if control lethality is ≤10% |
| Class | Description |
|---|---|
| CLASS I | No acute hazard—none of the tests shows effect |
| CLASS II | Slight acute hazard—a statistically significant percentage effect is reached in at least one test, but the effect level is below 50% |
| CLASS III | Acute hazard—the 50% effect level is reached or exceeded in at least one test, but the effect level is below 100%. |
| CLASS IV | High acute hazard—the 100% effect is reached in at least one test |
| CLASS V | Very high acute hazard—the 100% percentage effect is reached in all the tests |
| Initial Concentration (ng/L) | EEQ (ng/L) in the Influent | Final Concentration (ng/L) | EEQ (ng/L) in the Effluent | Toxicity |
|---|---|---|---|---|
| 150 | 1.94 ± 0.256 | 58 | 0.97 ± 0.034 | No |
| 300 | 0.72 ± 0.065 | 72 | 0.99 ± 0.108 | No |
| 450 | 1.21 ± 0.070 | 142 | 0.23 ± 0.029 | No |
| 600 | 0.84 ± 0.037 | 241 | 0.51 ± 0.063 | No |
| 750 | 0.99 ± 0.096 | 476 | 0.89 ± 0.041 | No |
| 900 | 1.06 ± 0.082 | 596 | 0.66 ± 0.027 | No |
| 1050 | 3.15 ± 0.056 | 702 | 0.84 ± 0.037 | No |
| Initial Concentration (ng/L) | EEQ (ng/L) in the Influent | Final Concentration (ng/L) | EEQ (ng/L) in the Effluent | Toxicity |
|---|---|---|---|---|
| 150 | 1.45 ± 0.038 | 62 | 0.97 ± 0.036 | No |
| 300 | 0.84 ± 0.065 | 139 | 1.16 ± 0.079 | No |
| 450 | 0.97 ± 0.0034 | 148 | 0.64 ± 0.039 | No |
| 600 | 0.43 ± 0.036 | 400 | 0.89 ± 0.041 | No |
| 750 | 1.08 ± 0.092 | 616 | 1.06 ± 0.096 | No |
| 900 | 1.96 ± 0.086 | 786 | 1.06 ± 0.079 | Yes |
| 1050 | 1.11 ± 0.134 | 811 | 0.98 ± 0.043 | No |
| Final Concentration (ng/L) | Hours | Mortality (%) | Toxicity Hazard Potential |
|---|---|---|---|
| Control | 24 | 0 | No acute hazard |
| 48 | 0 | ||
| 58 | 24 | 50 | Acute hazard |
| 48 | 50 | ||
| 72 | 24 | 50 | Acute hazard |
| 48 | 50 | ||
| 142 | 24 | 100 | Very high acute hazard |
| 48 | 100 | ||
| 241 | 24 | 100 | Very high acute hazard |
| 48 | 100 | ||
| 476 | 24 | 100 | Very high acute hazard |
| 48 | 100 | ||
| 596 | 24 | 100 | Very high acute hazard |
| 48 | 100 | ||
| 702 | 24 | 100 | Very high acute hazard |
| 48 | 100 |
| Final Concentration (ng/L) | Hours | Mortality (%) | Toxicity Hazard Potential |
|---|---|---|---|
| Control | 24 | 0 | No acute hazard |
| 48 | 0 | ||
| 62 | 24 | 100 | Very high acute hazard |
| 48 | 100 | ||
| 139 | 24 | 100 | Very high acute hazard |
| 48 | 100 | ||
| 148 | 24 | 100 | Very high acute hazard |
| 48 | 100 | ||
| 400 | 24 | 100 | Very high acute hazard |
| 48 | 100 | ||
| 616 | 24 | 100 | Very high acute hazard |
| 48 | 100 | ||
| 786 | 24 | 100 | Very high acute hazard |
| 48 | 100 | ||
| 811 | 24 | 100 | Very high acute hazard |
| 48 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kibambe, M.G.; Momba, M.N.B. Ecotoxicological Assessment of Perfluorooctane Sulfonate and Perfluorooctanoic Acid Following Biodegradation: Insights from Daphnia magna Toxicity and Yeast Estrogen Screen Assays. Water 2025, 17, 3368. https://doi.org/10.3390/w17233368
Kibambe MG, Momba MNB. Ecotoxicological Assessment of Perfluorooctane Sulfonate and Perfluorooctanoic Acid Following Biodegradation: Insights from Daphnia magna Toxicity and Yeast Estrogen Screen Assays. Water. 2025; 17(23):3368. https://doi.org/10.3390/w17233368
Chicago/Turabian StyleKibambe, Muyasu Grace, and Maggy Ndombo Benteke Momba. 2025. "Ecotoxicological Assessment of Perfluorooctane Sulfonate and Perfluorooctanoic Acid Following Biodegradation: Insights from Daphnia magna Toxicity and Yeast Estrogen Screen Assays" Water 17, no. 23: 3368. https://doi.org/10.3390/w17233368
APA StyleKibambe, M. G., & Momba, M. N. B. (2025). Ecotoxicological Assessment of Perfluorooctane Sulfonate and Perfluorooctanoic Acid Following Biodegradation: Insights from Daphnia magna Toxicity and Yeast Estrogen Screen Assays. Water, 17(23), 3368. https://doi.org/10.3390/w17233368






