The Effect and Mechanism of AQDS Promoting Anaerobic Cr(VI) Bio-Reduction Under a Sulfate-Rich Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Sludge Acclimation
2.2. Experimental Procedure
2.2.1. Effect of AQDS on Cr(VI) Bio-Reduction
2.2.2. Effect of AQDS on the SO42− Interference During the Cr(VI) Bio-Reduction
2.3. Chemical and Statistical Analysis
3. Results and Discussion
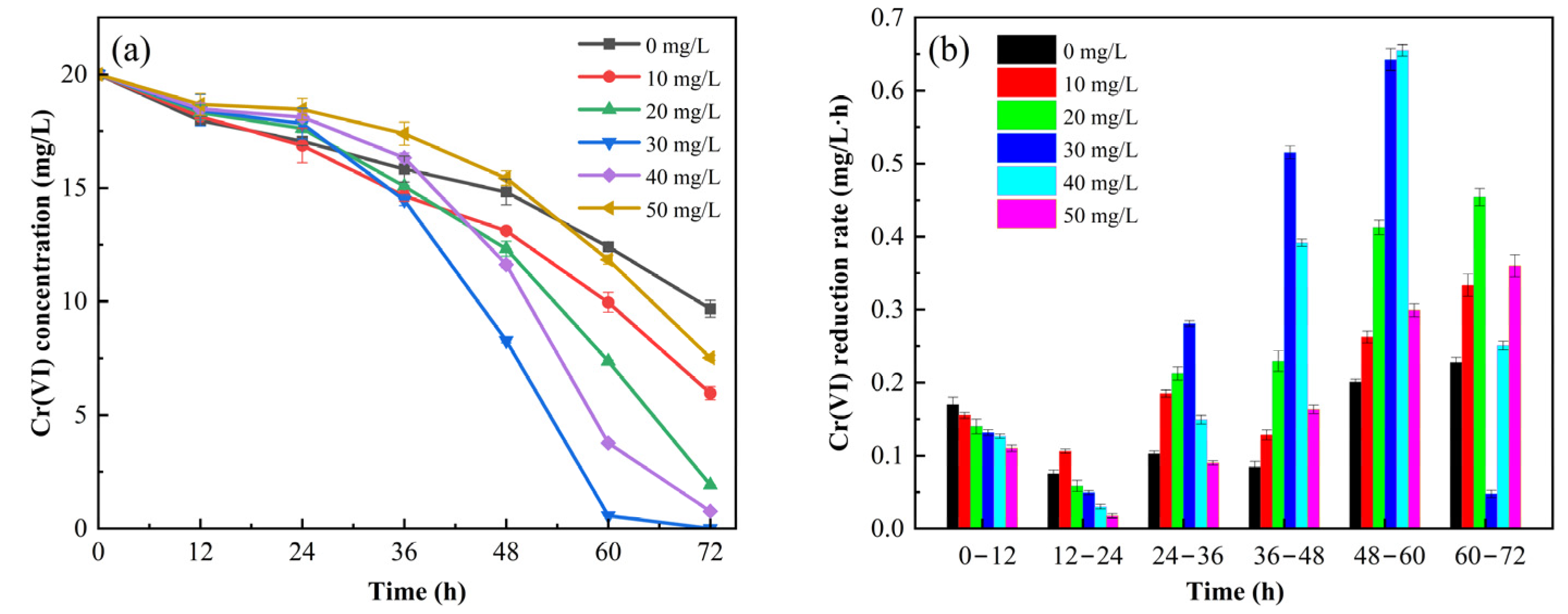
3.1. The Effect of Strengthening Cr(VI) Reduction by AQDS
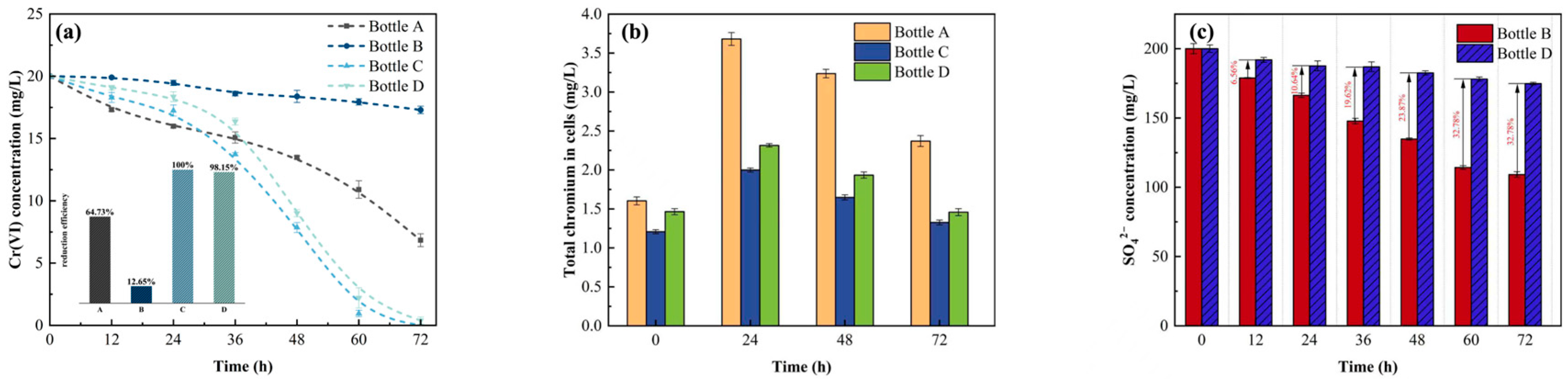
3.2. The Effect of AQDS on Chromium Reduction in Sulfate-Rich Environments
3.3. Effects of AQDS and SO42− on ROS
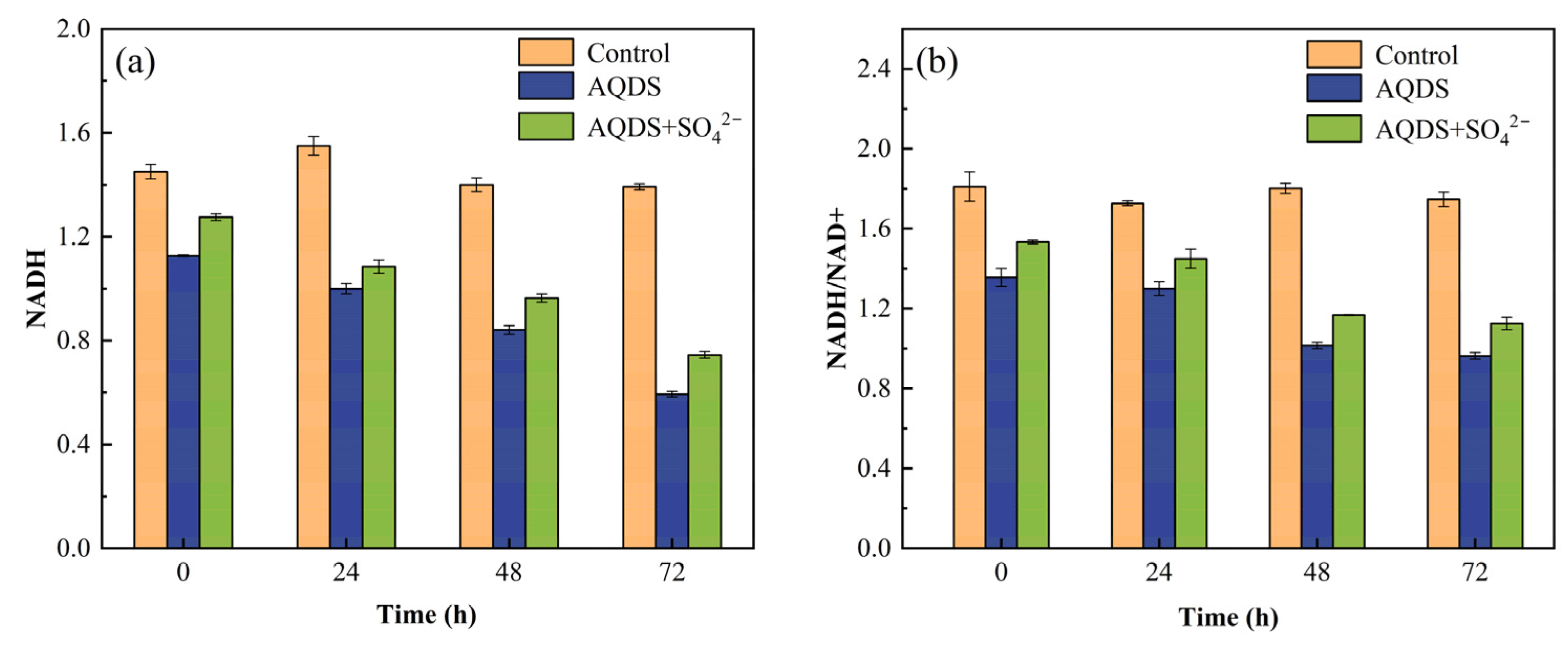
3.4. Effects of AQDS and SO42− on NADH
3.5. Possible Mechanism and Limitations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AQDS | anthraquinone-2,6-disulfonate |
| ROS | reactive oxygen species |
| SRB | sulfate-reducing bacteria |
| TCOD | Total Chemical Oxygen Demand |
| SCOD | Soluble Chemical Oxygen Demand |
| TSS | Total Suspended Solids |
| VSS | Volatile Suspended Solids |
| NADH | Nicotinamide Adenine Dinucleotide (reduced form) |
| NAD+ | Nicotinamide Adenine Dinucleotide (oxidized form) |
References
- He, D.; Zheng, M.; Ma, T.; Li, C.; Ni, J. Interaction of Cr(VI) Reduction and Denitrification by Strain Pseudomonas Aeruginosa PCN-2 under Aerobic Conditions. Bioresour. Technol. 2015, 185, 346–352. [Google Scholar] [CrossRef] [PubMed]
- More, A.G.; Gupta, S.K. Removal of Chromium from Electroplating Industry Wastewater Using Bioelectrochemical System: Kinetic Study and Statistical Analysis. J. Hazard. Toxic Radioact. Waste 2021, 25, 04020069. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Zhao, N.; Pang, R.; Zhao, C.; Deng, Y.; Yang, D.; Jiang, H.; Wu, Z.; Qiu, R. A Novel Biochar-Based 3D Composite for Ultrafast and Selective Cr(VI) Removal in Electroplating Wastewater. Biochar 2024, 6, 46. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A Review of Chemical, Electrochemical and Biological Methods for Aqueous Cr(VI) Reduction. J. Hazard. Mater. 2012, 223–224, 1–12. [Google Scholar] [CrossRef]
- Vaiopoulou, E.; Gikas, P. Regulations for Chromium Emissions to the Aquatic Environment in Europe and Elsewhere. Chemosphere 2020, 254, 126876. [Google Scholar] [CrossRef]
- Mukhopadhyay, B.; Sundquist, J.; Schmitz, R.J. Removal of Cr(VI) from Cr-Contaminated Groundwater through Electrochemical Addition of Fe(II). J. Environ. Manag. 2007, 82, 66–76. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of Heavy Metals from Aqueous Solution Using Carbon-Based Adsorbents: A Review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.-J.; Tseng, W.-L. Effective Adsorption of Chromium(VI)/Cr(III) from Aqueous Solution Using Ionic Liquid Functionalized Multiwalled Carbon Nanotubes as a Super Sorbent. J. Mater. Chem. A 2015, 3, 7044–7057. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, S.; Ai, P.; Li, C.; Ye, F.; Gan, J.; Xu, J.; Su, L.; Chen, Q.; Chen, J.; et al. Adsorption of Cr(VI) Ions from Wastewater Using Water-Based Polyacrylic Resin. Sci. Rep. 2025, 15, 35645. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K.S.M. Recent Bioreduction of Hexavalent Chromium in Wastewater Treatment: A Review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- Ukhurebor, K.E.; Aigbe, U.O.; Onyancha, R.B.; Nwankwo, W.; Osibote, O.A.; Paumo, H.K.; Ama, O.M.; Adetunji, C.O.; Siloko, I.U. Effect of Hexavalent Chromium on the Environment and Removal Techniques: A Review. J. Environ. Manag. 2021, 280, 111809. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Han, H.; Zhu, J.; Zhou, J. Navigating Challenges in Electroplating Wastewater Management: A Study on Pollutant Removal Characteristics and Economic Impacts by Physicochemical Treatment. Colloids Surf. C Environ. Asp. 2025, 3, 100060. [Google Scholar] [CrossRef]
- Zhu, F.; He, S.; Liu, T. Effect of pH, Temperature and Co-Existing Anions on the Removal of Cr(VI) in Groundwater by Green Synthesized nZVI/Ni. Ecotoxicol. Environ. Saf. 2018, 163, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.-L.; Zhong, L.; Dong, Q.-Y.; Yang, S.-L.; Shen, W.-W.; Zhu, Q.-S.; Lai, C.-Y.; Luo, A.-C.; Tang, Y.; Zhao, H.-P. The Effect of Electron Competition on Chromate Reduction Using Methane as Electron Donor. Environ. Sci. Pollut. Res. 2018, 25, 6609–6618. [Google Scholar] [CrossRef]
- Fan, C.; Guo, C.; Chen, W.; Tao, L.; Yao, Q.; Lu, G.; Shen, Y.; Dang, Z. Chromate and Phosphate Adsorption on Schwertmannite: Competition, Mobilization and Mechanisms. Colloids Surf. Physicochem. Eng. Asp. 2023, 658, 130691. [Google Scholar] [CrossRef]
- Qian, J.; Wei, L.; Liu, R.; Jiang, F.; Hao, X.; Chen, G.-H. An Exploratory Study on the Pathways of Cr (VI) Reduction in Sulfate-Reducing Up-Flow Anaerobic Sludge Bed (UASB) Reactor. Sci. Rep. 2016, 6, 23694. [Google Scholar] [CrossRef]
- Hu, K.; Xu, D.; Chen, Y. An Assessment of Sulfate Reducing Bacteria on Treating Sulfate-Rich Metal-Laden Wastewater from Electroplating Plant. J. Hazard. Mater. 2020, 393, 122376. [Google Scholar] [CrossRef]
- Chang, I.S.; Kim, B.H. Effect of Sulfate Reduction Activity on Biological Treatment of Hexavalent Chromium [Cr(VI)] Contaminated Electroplating Wastewater under Sulfate-Rich Condition. Chemosphere 2007, 68, 218–226. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, M.; Gao, N.; Chu, W.; Zhao, L.; Wang, Q. Enhanced Dissimilatory Perchlorate Reduction in the Presence of Humic Acids or 2,6-Anthraquinone Disulfonate as Quinone Redox Mediators. Chem. Eng. J. 2019, 357, 75–83. [Google Scholar] [CrossRef]
- Whisonant, M.D.; Belt, S.M.; Meeker, A.E.; Weber, C.J.; Simoska, O. Electrochemical Study of Quinone-Mediated Extracellular Electron Transfer in Escherichia Coli during Glucose Oxidation Metabolism. ACS Electrochem. 2025, 1, 338–350. [Google Scholar] [CrossRef]
- Pat-Espadas, A.M.; Razo-Flores, E.; Rangel-Mendez, J.R.; Cervantes, F.J. Direct and Quinone-Mediated Palladium Reduction by Geobacter Sulfurreducens: Mechanisms and Modeling. Environ. Sci. Technol. 2014, 48, 2910–2919. [Google Scholar] [CrossRef]
- Orsetti, S.; Laskov, C.; Haderlein, S.B. Electron Transfer between Iron Minerals and Quinones: Estimating the Reduction Potential of the Fe(II)-Goethite Surface from AQDS Speciation. Environ. Sci. Technol. 2013, 47, 14161–14168. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, G.; Du, Y.; Li, J.; Si, Y. The Role of Electron Shuttle Enhances Fe(III)-Mediated Reduction of Cr(VI) by Shewanella Oneidensis MR-1. World J. Microbiol. Biotechnol. 2019, 35, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-M.; Wang, L.; Chen, L.; Tian, L.-J.; Zhu, T.-T.; Wu, Q.-Z.; Hu, Y.-R.; Zheng, L.-R.; Li, W.-W. AQDS Activates Extracellular Synergistic Biodetoxification of Copper and Selenite via Altering the Coordination Environment of Outer-Membrane Proteins. Environ. Sci. Technol. 2022, 56, 13786–13797. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Hu, Y.; Du, J.; Chung, H.Y.; Kim, K.; Lee, G.; Choi, W. Photooxidation of Natural and Waste Cr(III)-Bearing Hydroxides in Water and Ice: Cr(III)-O2 Complex-Mediated Oxidation and the Effect of Natural Organic Matter. Environ. Sci. Technol. 2025, 59, 19502–19512. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, J.; Zheng, H.; Wu, X.; Wang, Y.; Liu, M.; Xiang, S.; Cao, L.; Ruan, R.; Liu, Y. Characterization of Additional Zinc Ions on the Growth, Biochemical Composition and Photosynthetic Performance from Spirulina Platensis. Bioresour. Technol. 2018, 269, 285–291. [Google Scholar] [CrossRef]
- Gong, Y.; Werth, C.J.; He, Y.; Su, Y.; Zhang, Y.; Zhou, X. Intracellular versus Extracellular Accumulation of Hexavalent Chromium Reduction Products by Geobacter Sulfurreducens PCA. Environ. Pollut. 2018, 240, 485–492. [Google Scholar] [CrossRef]
- Su, Y.; Zheng, X.; Chen, A.; Chen, Y.; He, G.; Chen, H. Hydroxyl Functionalization of Single-Walled Carbon Nanotubes Causes Inhibition to the Bacterial Denitrification Process. Chem. Eng. J. 2015, 279, 47–55. [Google Scholar] [CrossRef]
- Limbach, L.K.; Wick, P.; Manser, P.; Grass, R.N.; Bruinink, A.; Stark, W.J. Exposure of Engineered Nanoparticles to Human Lung Epithelial Cells: Influence of Chemical Composition and Catalytic Activity on Oxidative Stress. Environ. Sci. Technol. 2007, 41, 4158–4163. [Google Scholar] [CrossRef]
- Tang, M.; Ma, S.; Yao, S.; Lu, F.; Yang, Y. Enhanced Denitrification Performance and Extracellular Electron Transfer for Eletrotrophic Bio-Cathode Mediated by Anthraquinone-2, 6-Disulfonate (AQDS) at Low Temperature. Int. J. Hydrogen Energy 2024, 84, 215–223. [Google Scholar] [CrossRef]
- Nevin, K.P.; Lovley, D.R. Potential for Nonenzymatic Reduction of Fe(III) via Electron Shuttling in Subsurface Sediments. Environ. Sci. Technol. 2000, 34, 2472–2478. [Google Scholar] [CrossRef]
- Doong, R.-A.; Chiang, H.-C. Transformation of Carbon Tetrachloride by Thiol Reductants in the Presence of Quinone Compounds. Environ. Sci. Technol. 2005, 39, 7460–7468. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, R.; Huang, T.; Wu, F. The Characteristics and Two-Step Reaction Model of p-Nitroacetophenone Biodegradation Mediated by Shewanella Decolorationis S12 and Electron Shuttle in the Presence/Absence of Goethite. Environ. Technol. 2014, 35, 3116–3123. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Tian, T.; Zhou, J. Effects of Quinoid Redox Mediators on the Activity of Anammox Biomass. Bioresour. Technol. 2014, 152, 116–123. [Google Scholar] [CrossRef]
- Chen, H.; Jin, R.; Liu, G.; Tian, T.; Gu, C.; Zhou, J.; Xing, D. Effects of Sludge Lysate for Cr(VI) Bioreduction and Analysis of Bioaugmentation Mechanism of Sludge Humic Acid. Environ. Sci. Pollut. Res. 2019, 26, 5065–5075. [Google Scholar] [CrossRef]
- Pattanapipitpaisal, P.; Mabbett, A.N.; Finlay, J.A.; Beswick, A.J.; Paterson-Beedle, M.; Essa, A.; Wright, J.; Tolley, M.R.; Badar, U.; Ahmed, N.; et al. Reduction of Cr(VI) and Bioaccumulation of Chromium by Gram Positive and Gram Negative Microorganisms Not Previously Exposed to CR-Stress. Environ. Technol. 2002, 23, 731–745. [Google Scholar] [CrossRef]
- Novotnik, B.; Zuliani, T.; Ščančar, J.; Milačič, R. Inhibition of the Nitrification Process in Activated Sludge by Trivalent and Hexavalent Chromium, and Partitioning of Hexavalent Chromium between Sludge Compartments. Chemosphere 2014, 105, 87–94. [Google Scholar] [CrossRef]
- Yu, L.; Ju, C.; Jing, K.; Wang, Z.; Niyazi, S.; Wang, Q. The Role of Anthraquinone-2-Sulfonate on Intra/Extracellular Electron Transfer of Anaerobic Nitrate Reduction. J. Environ. Manag. 2023, 333, 117455. [Google Scholar] [CrossRef]
- Chen, B.; Song, J.; Zhang, J.; Ma, J.; Zhou, M.; Chen, W. The Potential Role of miR-450a-1-3p in Chromium-Associated Heart Rate Variability Reduction. Environ. Pollut. 2025, 373, 126117. [Google Scholar] [CrossRef]
- Gong, B.; Tan, Z.; Yang, X.; Liang, L.; Wu, P.; Li, Y. Induction of Zincophore Pseudopaline Secretion by Cr(VI) and Intracellular Formation of Granules from Nanocrystal Aggregation by Cr(III) in Pseudomonas aeruginosa. J. Environ. Manag. 2022, 323, 116201. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Wu, M.; He, T.; Li, C.; Liu, L.; Li, S.; Chang, Z.; Lang, D.; Du, W. The Dual Effect of Disodium Anthraquinone-2, 6-Disulfonate (AQDS) on the Cr (VI) Removal by Biochar: The Enhanced Electron Transfer and the Inhibited Adsorption. Chemosphere 2023, 343, 140245. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, X.; Ren, Y.; Xu, G.; Tang, J. Humic Analog AQDS Can Act as a Selective Inhibitor to Enable Anoxygenic Photosynthetic Bacteria to Outcompete Sulfate-reducing Bacteria under Microaerobic Conditions. J. Chem. Technol. Biotechnol. 2016, 91, 2103–2110. [Google Scholar] [CrossRef]
- Minderlein, S.; Blodau, C. Humic-Rich Peat Extracts Inhibit Sulfate Reduction, Methanogenesis, and Anaerobic Respiration but Not Acetogenesis in Peat Soils of a Temperate Bog. Soil Biol. Biochem. 2010, 42, 2078–2086. [Google Scholar] [CrossRef]
- Xu, H.; Tang, S.; Wang, Y.; Wu, M. Applying AQDS for In-Situ Sulfide Control in the Anaerobic Stage of Anaerobic/Anoxic/Oxic Process: Performance and Mechanism. Chem. Eng. J. 2025, 525, 169861. [Google Scholar] [CrossRef]
- Wakeel, A.; Xu, M.; Gan, Y. Chromium-Induced Reactive Oxygen Species Accumulation by Altering the Enzymatic Antioxidant System and Associated Cytotoxic, Genotoxic, Ultrastructural, and Photosynthetic Changes in Plants. Int. J. Mol. Sci. 2020, 21, 728. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yu, D.; Shi, M.; Zhang, Y.; Huang, T. Quinone-Mediated Microbial Goethite Reduction and Transformation of Redox Mediator, Anthraquinone-2,6-Disulfonate (AQDS). Geomicrobiol. J. 2017, 34, 27–36. [Google Scholar] [CrossRef]
- Pal, A.; Bhattacharjee, S.; Saha, J.; Sarkar, M.; Mandal, P. Bacterial Survival Strategies and Responses under Heavy Metal Stress: A Comprehensive Overview. Crit. Rev. Microbiol. 2022, 48, 327–355. [Google Scholar] [CrossRef]
- Blacker, T.S.; Duchen, M.R. Investigating Mitochondrial Redox State Using NADH and NADPH Autofluorescence. Free Radic. Biol. Med. 2016, 100, 53–65. [Google Scholar] [CrossRef]
- Patgiri, A.; Skinner, O.S.; Miyazaki, Y.; Schleifer, G.; Marutani, E.; Shah, H.; Sharma, R.; Goodman, R.P.; To, T.-L.; Robert Bao, X.; et al. An Engineered Enzyme That Targets Circulating Lactate to Alleviate Intracellular NADH:NAD+ Imbalance. Nat. Biotechnol. 2020, 38, 309–313. [Google Scholar] [CrossRef]


| Component | Concentration (mg/L) | |
|---|---|---|
| Basic Materials | K2HPO4·3H2O | 750 |
| NaH2PO4·H2O | 400 | |
| NH4H2PO4 | 250 | |
| MgCl2·6H2O | 83 | |
| CaCl2·2H2O | 10 | |
| Trace Materials | FeCl2·4H2O | 2 |
| MnCl2·4H2O | 0.5 | |
| ZnCl2·7H2O | 0.05 | |
| AlCl3·6H2O | 0.09 | |
| CuCl2·2H2O | 0.03 | |
| CoCl2·6H2O | 2 | |
| H3BO3 | 0.05 | |
| NiCl2·6H2O | 0.05 | |
| Na2MoO4·2H2O | 0.03 | |
| NaSeO3·5H2O | 0.05 | |
| Na2WO4·2H2O | 0.05 | |
| Na2EDTA | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhao, L.; Huang, C.; Yao, D.; Wang, Y.; Wu, M. The Effect and Mechanism of AQDS Promoting Anaerobic Cr(VI) Bio-Reduction Under a Sulfate-Rich Environment. Water 2025, 17, 3287. https://doi.org/10.3390/w17223287
Wang Z, Zhao L, Huang C, Yao D, Wang Y, Wu M. The Effect and Mechanism of AQDS Promoting Anaerobic Cr(VI) Bio-Reduction Under a Sulfate-Rich Environment. Water. 2025; 17(22):3287. https://doi.org/10.3390/w17223287
Chicago/Turabian StyleWang, Zhujun, Liuzhu Zhao, Chunlin Huang, Duyang Yao, Yayi Wang, and Min Wu. 2025. "The Effect and Mechanism of AQDS Promoting Anaerobic Cr(VI) Bio-Reduction Under a Sulfate-Rich Environment" Water 17, no. 22: 3287. https://doi.org/10.3390/w17223287
APA StyleWang, Z., Zhao, L., Huang, C., Yao, D., Wang, Y., & Wu, M. (2025). The Effect and Mechanism of AQDS Promoting Anaerobic Cr(VI) Bio-Reduction Under a Sulfate-Rich Environment. Water, 17(22), 3287. https://doi.org/10.3390/w17223287






