Mechanistic Insights into the Phenomenon of Ammonia-Only Removal in Sulfate-Rich Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Bioreactor
2.2. Batch Test Equipment
2.3. Inoculated Sludge and Test Water Quality
2.4. Experimental Procedures
2.5. Batch Experiments
- (1)
- Batch Test 1 examined oxygen as a potential electron acceptor. The influent contained only ammonia nitrogen, and no deoxygenation was applied. Ammonia nitrogen removal was monitored and analyzed.
- (2)
- Batch Test 2 investigated bicarbonate as a potential electron acceptor. The influent contained ammonia nitrogen and bicarbonate (supplied as NaHCO3). After adding the influent, the reactor was deoxygenated to keep dissolved oxygen below 0.1 mg/L. By gradually increasing bicarbonate concentrations and monitoring effluent NH4+-N and HCO3− in real time, we evaluated whether bicarbonate participated in ammonia oxidation and if its concentration impacted ammonia nitrogen removal.
2.6. Activity Measurement
2.7. Sample Collection and Analysis Methods
3. Results and Discussion
3.1. USBR Operation Status
3.2. Batch Experiments
3.2.1. Possibility Analysis of Oxygen as Electron Acceptor
3.2.2. Possibility Analysis of Bicarbonate as Electron Acceptor
3.3. Microbial Community Analysis
3.3.1. Microbial Diversity Analysis
3.3.2. Evolution of Microbial Communities
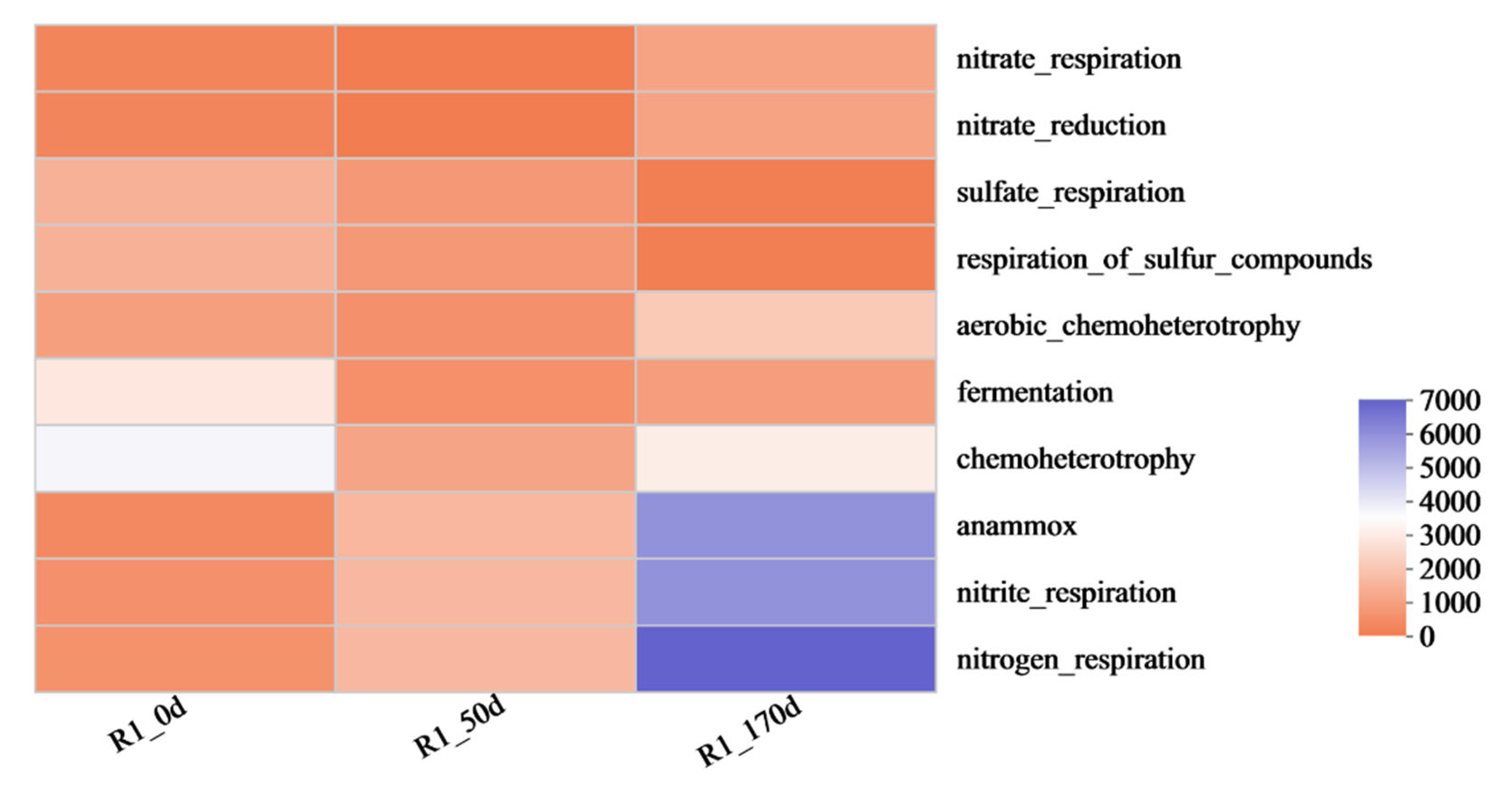
3.3.3. Analysis of Microbial Metabolic Pathway Characteristics
4. Conclusions
- (1)
- The reactor consistently achieved high NH4+-N removal, while SO42−-S concentrations showed no significant decrease and SRB declined rapidly, indicating that SO42−-S did not serve as an electron acceptor for NH4+-N conversion.
- (2)
- Batch experiments showed distinct roles for oxygen and bicarbonate. Using oxygen as a potential electron acceptor, NH4+-N removal was notable, with low levels of NO2−-N and NO3−-N, indicating that trace DO and ROS-derived oxygen were key factors. Conversely, under strictly anaerobic conditions with ample HCO3−, NH4+-N removal dropped sharply, and no NO2−-N or NO3−-N were detected, suggesting that HCO3− mainly functions as an inorganic carbon source rather than an electron acceptor.
- (3)
- Microbial community succession confirmed the non-sulfate-dependent pathway. Over time, Proteobacteria, Chloroflexi, and Planctomycetota became dominant. Facultative anaerobes likely mediated short-term nitrification under microaerobic conditions, while anammox bacteria served as a secondary nitrite-consuming pathway. Functional predictions further indicated an enrichment of nitrogen metabolism, whereas sulfur metabolism remained consistently low.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, Y.; Wang, X.; Bao, X.; Lam, K.L. Life Cycle Assessment of Ammonium Sulfate Recovery from Urban Wastewater. Blue-Green Syst. 2024, 6, 90–99. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Wang, P.; Feng, Z. Cleaner Production of Monosodium Glutamate in China. J. Clean. Prod. 2018, 190, 452–461. [Google Scholar] [CrossRef]
- Yang, D.; Jia, X.; Dang, M.; Han, F.; Shi, F.; Tanikawa, H.; Klemeš, J.J. Life Cycle Assessment of Cleaner Production Measures in Monosodium Glutamate Production: A Case Study in China. J. Clean. Prod. 2020, 270, 122126. [Google Scholar] [CrossRef]
- Chen, H.; Wang, H.; Yu, G.; Xiong, Y.; Wu, H.; Yang, M.; Chen, R.; Yang, E.; Jiang, C.; Li, Y.-Y. Key Factors Governing the Performance and Microbial Community of One-Stage Partial Nitritation and Anammox System with Bio-Carriers and Airlift Circulation. Bioresour. Technol. 2021, 324, 124668. [Google Scholar] [CrossRef]
- James, S.N.; Vijayanandan, A. Recent Advances in Simultaneous Nitrification and Denitrification for Nitrogen and Micropollutant Removal: A Review. Biodegradation 2023, 34, 103–123. [Google Scholar] [CrossRef]
- Kosgey, K.; Zungu, P.V.; Bux, F.; Kumari, S. Biological Nitrogen Removal from Low Carbon Wastewater. Front. Microbiol. 2022, 13, 968812. [Google Scholar] [CrossRef]
- Li, G.; Yu, Y.; Li, X.; Jia, H.; Ma, X.; Opoku, P.A. Research Progress of Anaerobic Ammonium Oxidation (Anammox) Process Based on Integrated Fixed-film Activated Sludge (IFAS). Environ. Microbiol. Rep. 2024, 16, e13235. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, T.; Wang, Z.; Meng, J.; Zheng, M. Toward Energy Neutrality: Novel Wastewater Treatment Incorporating Acidophilic Ammonia Oxidation. Environ. Sci. Technol. 2023, 57, 4522–4532. [Google Scholar] [CrossRef]
- Arora, A.S.; Nawaz, A.; Qyyum, M.A.; Ismail, S.; Aslam, M.; Tawfik, A.; Yun, C.M.; Lee, M. Energy Saving Anammox Technology-Based Nitrogen Removal and Bioenergy Recovery from Wastewater: Inhibition Mechanisms, State-of-the-Art Control Strategies, and Prospects. Renew. Sustain. Energy Rev. 2021, 135, 110126. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, L.; Zhang, J.; Li, J.; Peng, Y. Enhancing Nitrogen Removal through Directly Integrating Anammox into Mainstream Wastewater Treatment: Advantageous, Issues and Future Study. Bioresour. Technol. 2022, 362, 127827. [Google Scholar] [CrossRef]
- Fdz-Polanco, F.; Fdz-Polanco, M.; Fernandez, N.; Urueña, M.A.; Garcia, P.A.; Villaverde, S. New Process for Simultaneous Removal of Nitrogen and Sulphur under Anaerobic Conditions. Water Res. 2001, 35, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Strous, M.; Kuenen, J.G.; Jetten, M.S. Key Physiology of Anaerobic Ammonium Oxidation. Appl. Environ. Microbiol. 1999, 65, 3248–3250. [Google Scholar] [CrossRef] [PubMed]
- Marietou, A.; Kjeldsen, K.U.; Glombitza, C.; Jørgensen, B.B. Response to Substrate Limitation by a Marine Sulfate-Reducing Bacterium. ISME J. 2022, 16, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Rikmann, E.; Mandel, A.; Tomingas, M.; Tenno, T.; Loorits, L.; Vabamäe, P.; Mandel, A.; Raudkivi, M.; Daija, L.; Kroon, K.; et al. Sulfate-Reducing Anammox for Sulfate and Nitrogen Containing Wastewaters. Desalin. Water Treat. 2016, 57, 3132–3141. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Xie, G.-J.; Xing, D.-F.; Liu, B.-F.; Ding, J.; Cao, G.-L.; Ren, N.-Q. Sulfate Dependent Ammonium Oxidation: A Microbial Process Linked Nitrogen with Sulfur Cycle and Potential Application. Environ. Res. 2021, 192, 110282. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, Y.; Kong, L.; Shoukat, A. Efficient Nitrogen Removal and Elemental Sulfur Recovery through Sulfide-Driven Partial Denitrification Coupled with Anammox: Strategies Based on N/S Ratio and HRT. J. Environ. Manage. 2025, 376, 124464. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, T.; Zhou, J. Effect of Dissolved Oxygen on Elemental Sulfur Generation in Sulfide and Nitrate Removal Process: Characterization, Pathway, and Microbial Community Analysis. Appl. Microbiol. Biotechnol. 2016, 100, 2895–2905. [Google Scholar] [CrossRef]
- Rikmann, E.; Zekker, I.; Tomingas, M.; Tenno, T.; Menert, A.; Loorits, L.; Tenno, T. Sulfate-Reducing Anaerobic Ammonium Oxidation as a Potential Treatment Method for High Nitrogen-Content Wastewater. Biodegradation 2012, 23, 509–524. [Google Scholar] [CrossRef]
- Li, J.; Sui, Q.; Zuo, F.; Yang, Y.; Chen, M.; Wei, Y. Insight into Nitrogen Removal through Sulfate Reducing Anaerobic Ammonia Oxidation Coupled with Sulfur Cycle: A Comparative Study on Inorganic Organic Conditions. J. Environ. Manag. 2025, 373, 123566. [Google Scholar] [CrossRef]
- Wu, T.; Ding, J.; Zhong, L.; Sun, H.-J.; Pang, J.-W.; Zhao, L.; Bai, S.-W.; Ren, N.-Q.; Yang, S.-S. Sulfate-Reducing Ammonium Oxidation: A Promising Novel Process for Nitrogen and Sulfur Removal. Sci. Total Environ. 2023, 893, 164997. [Google Scholar] [CrossRef]
- Sabumon, P.C. Effect of Potential Electron Acceptors on Anoxic Ammonia Oxidation in the Presence of Organic Carbon. J. Hazard. Mater. 2009, 172, 280–288. [Google Scholar] [CrossRef]
- Helton, A.M.; Ardón, M.; Bernhardt, E.S. Thermodynamic Constraints on the Utility of Ecological Stoichiometry for Explaining Global Biogeochemical Patterns. Ecol. Lett. 2015, 18, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Wanyan, D.; Li, X.; Huang, Y. Biological Conversion Pathways of Sulfate Reduction Ammonium Oxidation in Anammox Consortia. Front. Environ. Sci. Eng. 2020, 14, 38. [Google Scholar] [CrossRef]
- van de Graaf, A.A.; de Bruijn, P.; Robertson, L.A.; Jetten, M.S.M.; Kuenen, J.G. Autotrophic Growth of Anaerobic Ammonium-Oxidizing Micro-Organisms in a Fluidized Bed Reactor. Microbiology 1996, 142, 2187–2196. [Google Scholar] [CrossRef]
- Rahman, H. Analytical Applications of Permanganate as an Oxidant in the Determination of Pharmaceuticals Using Chemiluminescence and Spectrophotometry: A Review. Curr. Anal. Chem. 2020, 16, 670–686. [Google Scholar] [CrossRef]
- Guo, J.; Jing, H.; Hu, B.; Ju, Z.; Hui, W. General Administration of Environmental Protection of the People’s Republic of China. In Water and Wastewater Monitoring and Analysis Method, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Wu, Y.; Liang, Q.; Wang, H.; Zhang, L.; Zeng, W.; Cao, Y.-A.; Liao, J.; Liang, Z.; Liang, Q. Hydraulic Retention Time Optimization Achieved Unexpectedly High Nitrogen Removal Rate in Pilot-Scale Anaerobic/Aerobic/Anoxic System for Low-Strength Municipal Wastewater Treatment. Bioresour. Technol. 2024, 393, 130128. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, T.; Zhao, Y.; Chen, X.; Ni, S.-Q. Uncovering the Co-Metabolic Flux of Ammonia Thiosulfate/Sulfate in Sulfate-Reducing Ammonia Oxidation. Chem. Eng. J. 2025, 505, 159225. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Cema, G. Influence of Temperature and pH on the Anammox Process: A Review and Meta-Analysis. Chemosphere 2017, 182, 203–214. [Google Scholar] [CrossRef]
- Stumpe, J.M.; Vlek, P.L.G. Acidification Induced by Different Nitrogen Sources in Columns of Selected Tropical Soils. Soil Sci. Soc. Am. J. 1991, 55, 145–151. [Google Scholar] [CrossRef]
- Chung, J.; Amin, K.; Kim, S.; Yoon, S.; Kwon, K.; Bae, W. Autotrophic Denitrification of Nitrate and Nitrite Using Thiosulfate as an Electron Donor. Water Res. 2014, 58, 169–178. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Yuan, Y.; Li, X.; Liu, F. Study on the Biotransformation of Sulfate Ammonia in Anaerobic Conditions. Chin. J. Environ. Sci. 2013, 34, 4356–4361. [Google Scholar] [CrossRef]
- Liu, F.; Huang, Y.; Yuan, Y.; Li, X.; Zhang, C.; Zhang, L. Study of anaerobic sulfate-reducing ammonium oxidation reaction. Chin. J. Environ. Eng. 2015, 9, 699–704. [Google Scholar]
- Prachakittikul, P.; Wantawin, C.; Noophan, P.; Boonapatcharoen, N. ANAMMOX-like Performances for Nitrogen Removal from Ammonium-Sulfate-Rich Wastewater in an Anaerobic Sequencing Batch Reactor. J. Environ. Sci. Health A 2016, 51, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Zhang, L.; Zhao, Q.; Peng, Y. The Effect of Biomass Accumulation on the Sulfur Oxidation Pathway and the Synergy of Microorganisms in Desulfurization Reactors under Different pH Conditions. J. Hazard. Mater. 2022, 432, 128638. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Fu, K.; Xu, R.; Guan, T.; Huo, A.; Zhang, R.; Li, X.; Qiu, F.; Zhang, Y. Achieving Partial Nitrification and Denitrification Coupled with Simultaneous Partial Nitrification, Anammox, and Denitrification (PND-SNAD) by the Inhibition of Sulfide to Accomplish Stabilized Nitrogen Removal. Environ. Res. 2025, 278, 121630. [Google Scholar] [CrossRef]
- Lawson, C.E.; Nuijten, G.H.L.; de Graaf, R.M.; Jacobson, T.B.; Pabst, M.; Stevenson, D.M.; Jetten, M.S.M.; Noguera, D.R.; McMahon, K.D.; Amador-Noguez, D.; et al. Autotrophic and Mixotrophic Metabolism of an Anammox Bacterium Revealed by in Vivo 13C and 2H Metabolic Network Mapping. ISME J. 2021, 15, 673–687. [Google Scholar] [CrossRef]
- González-Cabaleiro, R.; Curtis, T.P.; Ofiţeru, I.D. Bioenergetics Analysis of Ammonia-Oxidizing Bacteria and the Estimation of Their Maximum Growth Yield. Water Res. 2019, 154, 238–245. [Google Scholar] [CrossRef]
- Lombard, M.; Fontecave, M.; Touati, D.; Nivière, V. Reaction of the Desulfoferrodoxin from Desulfoarculus baarsii with Superoxide Anion: Evidence for a superoxide reductase activity. Biol. Chem. 2000, 275, 115–121. [Google Scholar] [CrossRef]
- Brioukhanov, A.; Netrusov, A.; Sordel, M.; Thauer, R.K.; Shima, S. Protection of Methanosarcina barkeri against Oxidative Stress: Identification and Characterization of an Iron Superoxide Dismutase. Arch. Microbiol. 2000, 174, 213–216. [Google Scholar] [CrossRef]
- Dos Santos, W.G.; Pacheco, I.; Liu, M.-Y.; Teixeira, M.; Xavier, A.V.; LeGall, J. Purification and Characterization of an Iron Superoxide Dismutase and a Catalase from the Sulfate-Reducing Bacterium Desulfovibrio gigas. J. Bacteriol. 2000, 182, 796–804. [Google Scholar] [CrossRef]
- Anjali, G.; Sabumon, P. Development of Simultaneous Partial Nitrification, Anammox and Denitrification (SNAD) in a Non-Aerated SBR. Int. Biodeterior. Biodegrad. 2017, 119, 43–55. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, J.-M.; Sun, Y.-J.; Liu, X.-T.; Zhang, X.-R.; Wang, Y.; Chen, Y.; Jin, R.-C.; Zhang, Q.-Q. Revealing the Combined Effect of Hydroxylamine and Hydrazine on Nitrogen Removal Performance of Completely Autotrophic Nitrogen Removal over Nitrite (CANON) Process. Bioresour. Technol. 2025, 418, 131964. [Google Scholar] [CrossRef]
- Fernández, I.; Dosta, J.; Fajardo, C.; Campos, J.; Mosquera-Corral, A.; Méndez, R. Short- and Long-Term Effects of Ammonium and Nitrite on the Anammox Process. J. Environ. Manag. 2012, 95, S170–S174. [Google Scholar] [CrossRef]
- Ye, W.; Yan, J.; Yan, J.; Lin, J.-G.; Ji, Q.; Li, Z.; Ganjidoust, H.; Huang, L.; Li, M.; Zhang, H. Potential Electron Acceptors for Ammonium Oxidation in Wastewater Treatment System under Anoxic Condition: A Review. Environ. Res. 2024, 252, 118984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cui, L.; Wang, H.; Liang, J. Study of Sulfate-Reducing Ammonium Oxidation Process and Its Microbial Community Composition. Water Sci. Technol. 2019, 79, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Prodan, A.; Tremaroli, V.; Brolin, H.; Zwinderman, A.H.; Nieuwdorp, M.; Levin, E. Comparing Bioinformatic Pipelines for Microbial 16S rRNA Amplicon Sequencing. PLoS ONE 2020, 15, e0227434. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J., Jr.; Stumm, W. Mechanism of Hydrogen Ion Buffering in Natural Waters. J. AWWA 1963, 55, 1553–1578. [Google Scholar] [CrossRef]
- Zhu, J. Validation on Nitrogen Removal Performance of Sulfate-Reducing Ammonia Oxidation. Master’s Thesis, China University of Mining and Technology , Xuzhou, China, 2022. [Google Scholar]
- Esposti, M.D.; Romero, E.M. The Functional Microbiome of Arthropods. PLoS ONE 2017, 12, e0176573. [Google Scholar] [CrossRef]
- Jong, T.; Parry, D. Parry Microbial Sulfate Reduction under Sequentially Acidic Conditions in an Upflow Anaerobic Packed Bed Bioreactor. Water Res. 2006, 40, 2561–2571. [Google Scholar] [CrossRef]
- Lu, Z.; Zhao, J.; Wu, Z.; Guo, T.; Wang, M.; Li, X.; Wan, D.; Du, Z.; He, Q. Nitrogen Removal Performance Functional Microbial Communities Evolution in a Continuous Up-Flow Fixed Bed Anammox System. J. Environ. Chem. Eng. 2024, 12, 113913. [Google Scholar] [CrossRef]
- Zheng, H.; Cao, X.; Liao, Y.; Feng, L.; Ji, F. Process Optimisation of a Packed Anaerobic Baffled Reactor-Deodorization Pool-Tidal Flow Constructed Wetland System for Synergistic Removal of Pollutants Odours from Rural Septic Tank Wastewater. J. Environ. Chem. Eng. 2025, 13, 118544. [Google Scholar] [CrossRef]



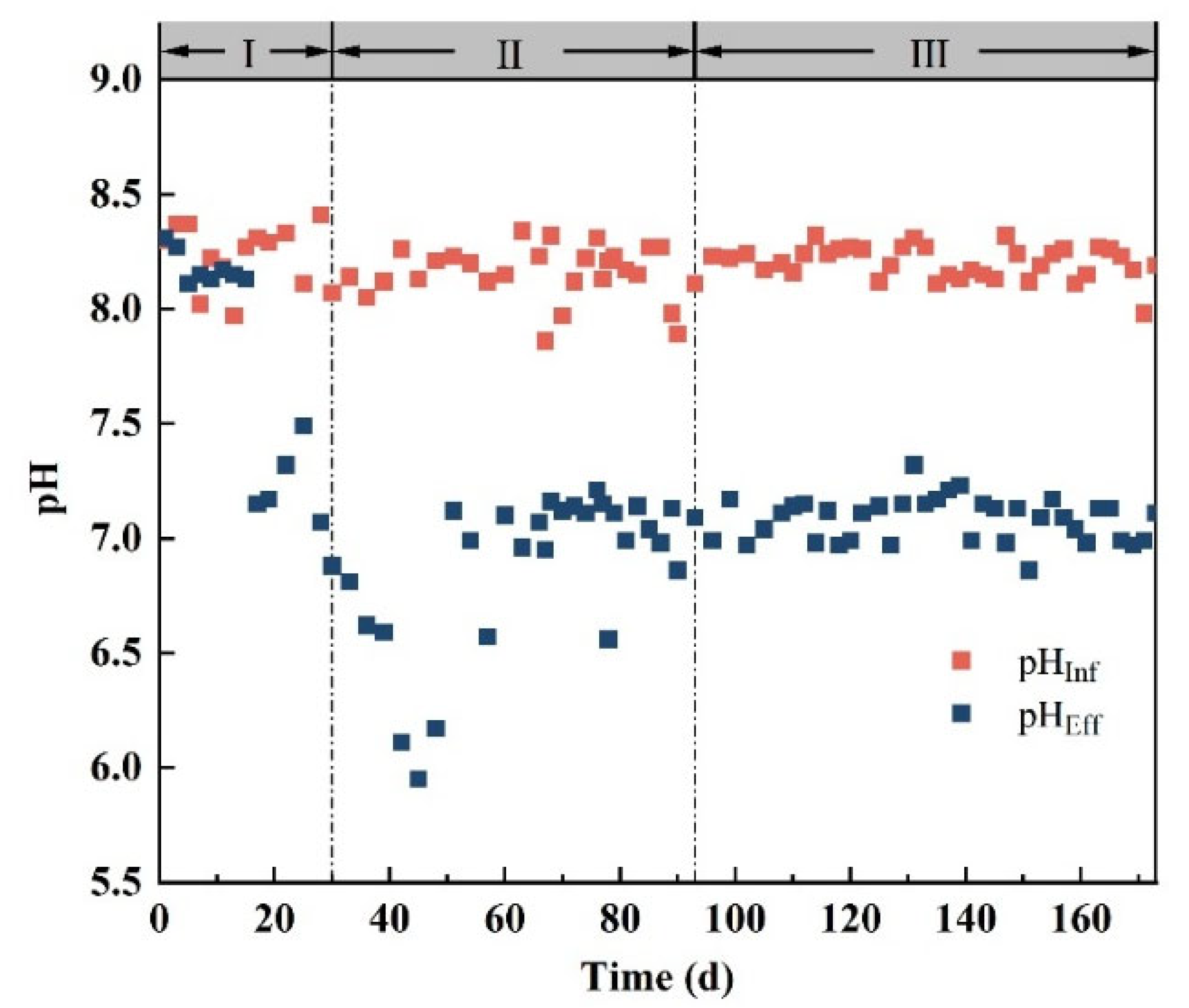



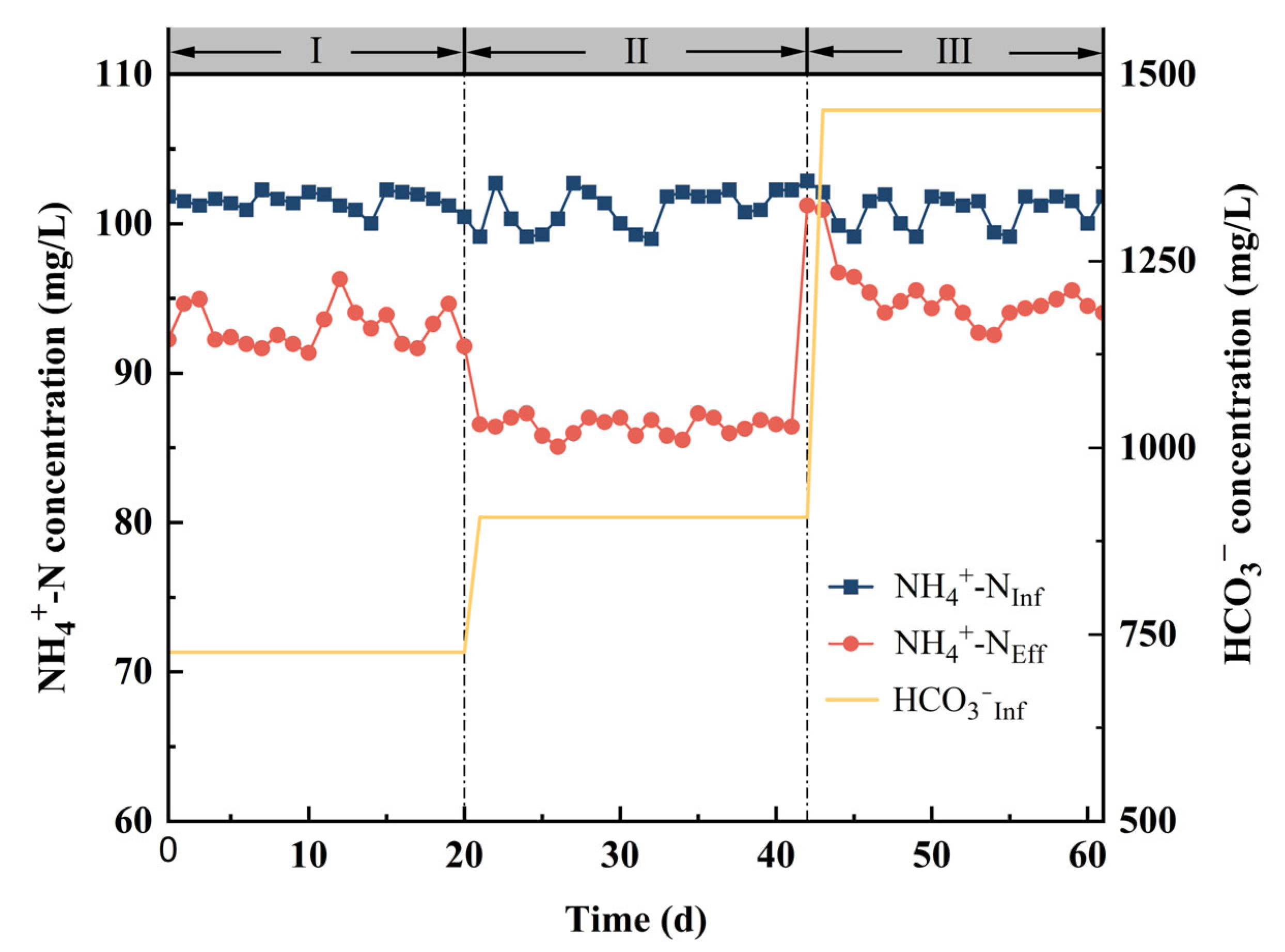
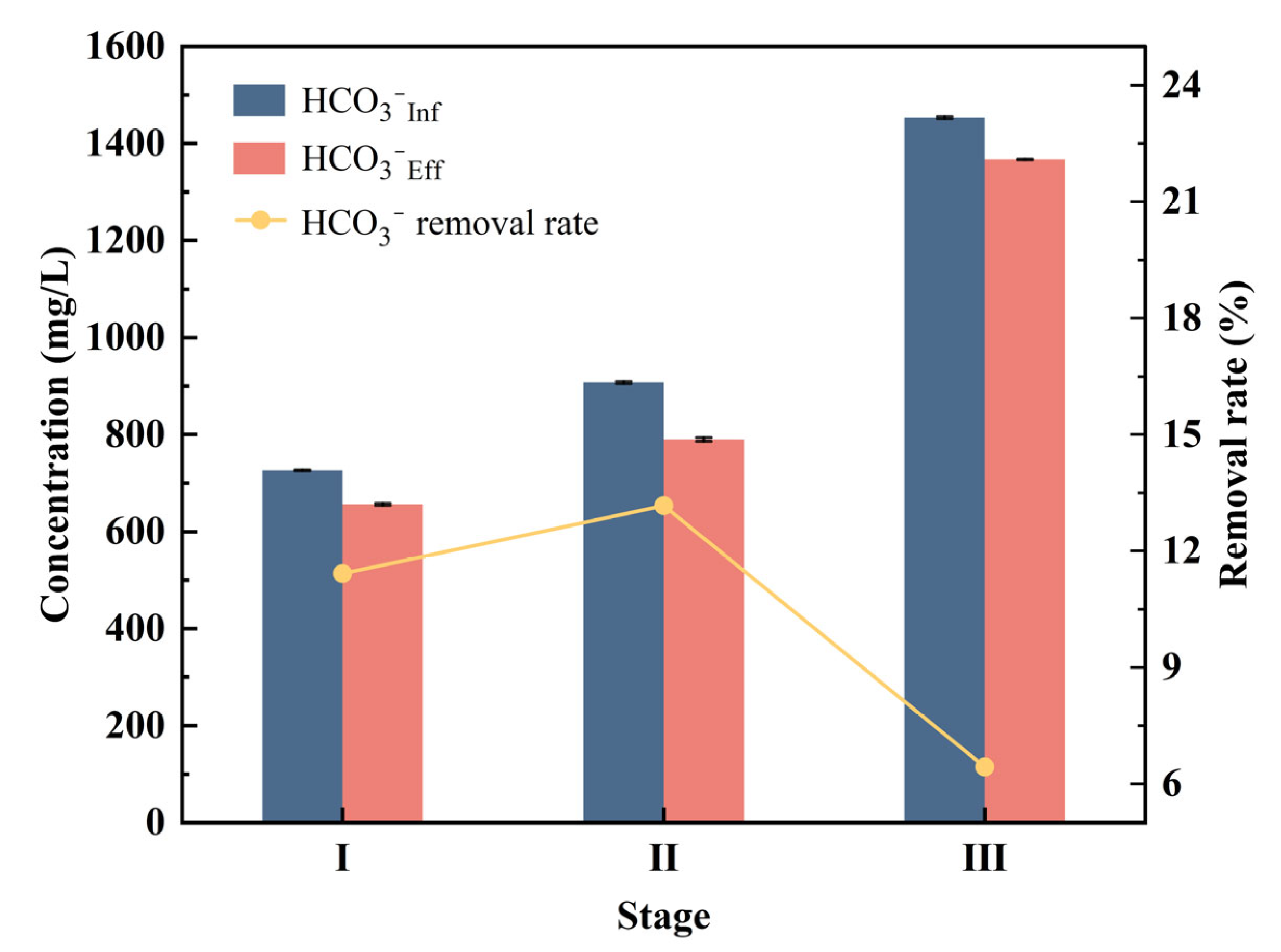




| Operating Days (d) | NH4+-N (mg/L) | SO42−-S (mg/L) | HRT (h) | HCO3− (mg/L) |
|---|---|---|---|---|
| 1~30 | 100 | 266.67 | 24 | 726.1 |
| 31~93 | 100 | 266.67 | 48 | 726.1 |
| 94~173 | 200 | 533.33 | 48 | 726.1 |
| Operating Days (d) | NH4+-N (mg/L) | HCO3− (mg/L) | DO (mg/L) | HRT (h) |
|---|---|---|---|---|
| 1~20 | 100 | 726 | <0.1 | 24 |
| 21~42 | 100 | 907 | <0.1 | 24 |
| 43~67 | 100 | 1452 | <0.1 | 24 |
| Samples | Richness Indices | Diversity Indices | Coverage | ||
|---|---|---|---|---|---|
| Shannon | Simpson | Ace | Chao | ||
| R1_0d | 4.831 | 0.036 | 1121.447 | 1121.447 | 0.999 |
| R1_50d | 5.191 | 0.019 | 1005 | 1005 | 1 |
| R1_170d | 5.156 | 0.020 | 978.06 | 978.06 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, F.; Cui, L.; Ren, F.; Gang, S. Mechanistic Insights into the Phenomenon of Ammonia-Only Removal in Sulfate-Rich Environments. Water 2025, 17, 3284. https://doi.org/10.3390/w17223284
Qiu F, Cui L, Ren F, Gang S. Mechanistic Insights into the Phenomenon of Ammonia-Only Removal in Sulfate-Rich Environments. Water. 2025; 17(22):3284. https://doi.org/10.3390/w17223284
Chicago/Turabian StyleQiu, Fan, Li Cui, Fangyuan Ren, and Siqi Gang. 2025. "Mechanistic Insights into the Phenomenon of Ammonia-Only Removal in Sulfate-Rich Environments" Water 17, no. 22: 3284. https://doi.org/10.3390/w17223284
APA StyleQiu, F., Cui, L., Ren, F., & Gang, S. (2025). Mechanistic Insights into the Phenomenon of Ammonia-Only Removal in Sulfate-Rich Environments. Water, 17(22), 3284. https://doi.org/10.3390/w17223284




