Effects of Polyculture Patterns in Ponds on Water Quality and Intestinal Flora of Penaeus monodon
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Sample
2.1.2. Experimental Supplies and Instruments
2.2. Methods
2.2.1. Extraction of Intestinal Bacteria from P. monodon
2.2.2. Extraction of Bacteria from Water Samples of Mixed Culture Pond
2.2.3. Extraction, PCR Amplification and Purification of Total DNA of the Sample and Sequence Determination
2.2.4. Water Quality Index Testing
2.2.5. Data Processing
3. Results
3.1. Water Quality Index Data
3.2. High-Throughput Sequencing Data
3.3. OTU Cluster Analysis
3.3.1. OTU Clustering and Annotation Analysis
3.3.2. Analysis of OTU Petal Diagram and Wayne Diagram
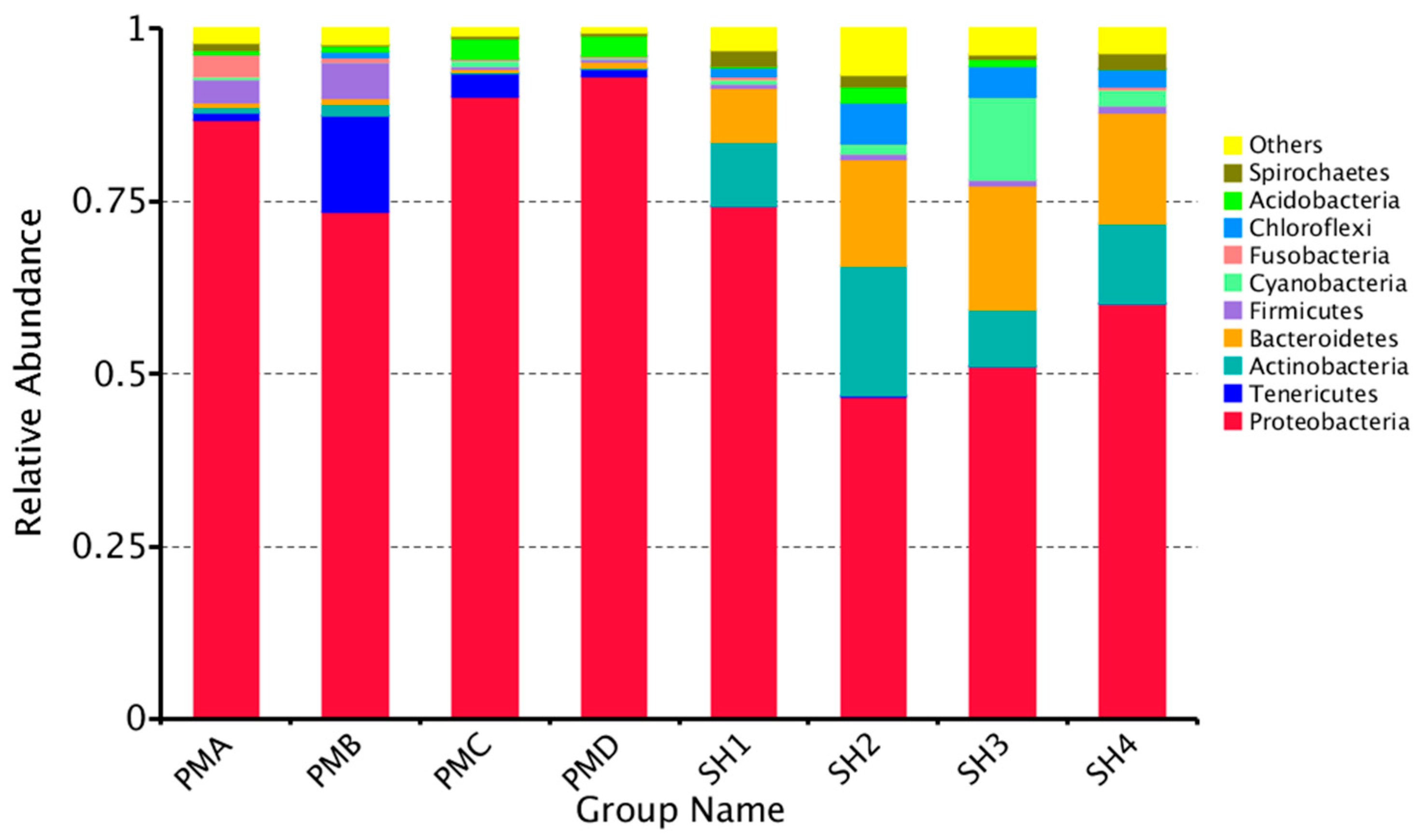
3.4. Analysis of Flora Structure and Abundance
3.4.1. Microflora Structure Under Phyla Classification Level
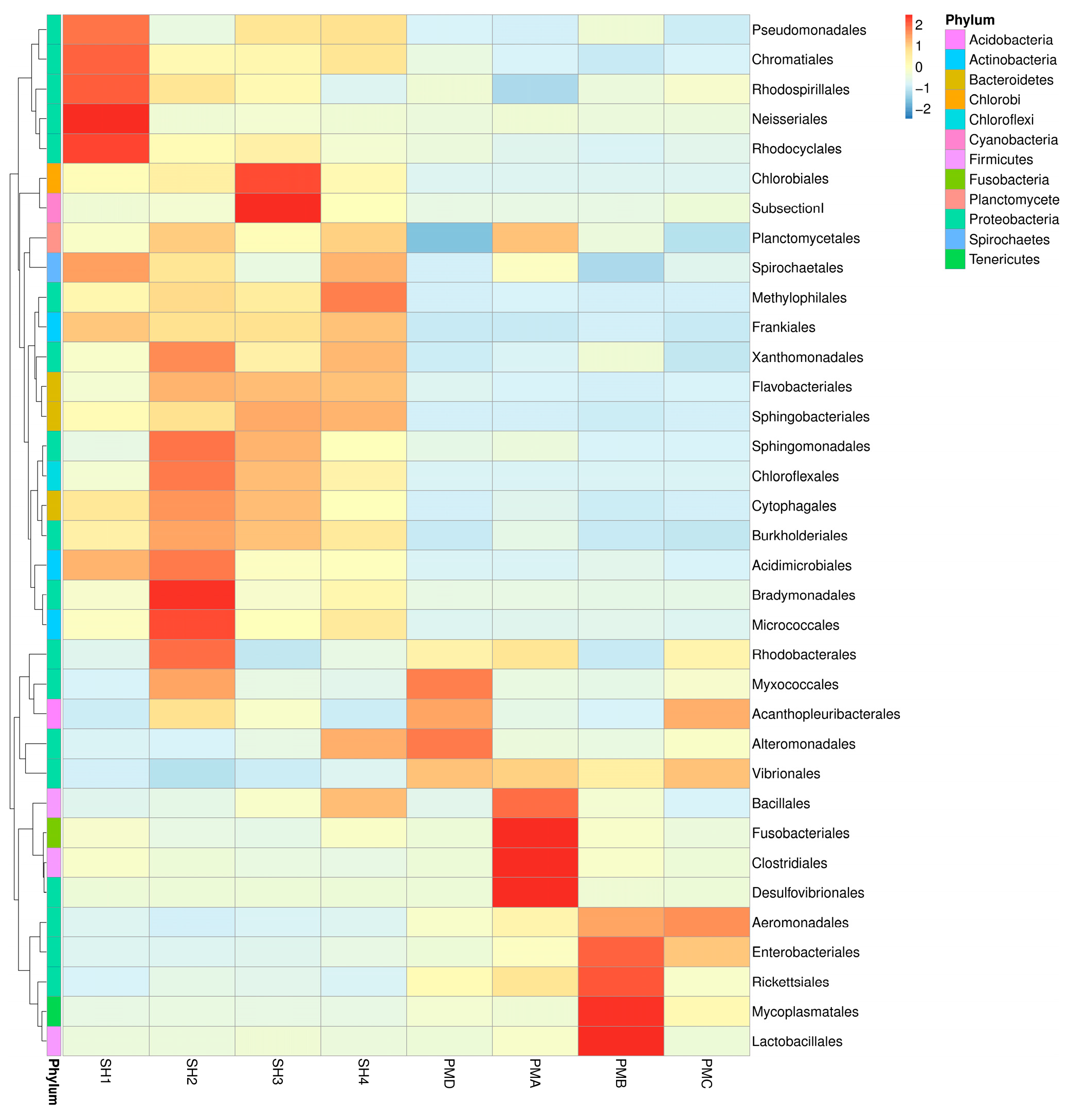
3.4.2. Flora Structure at the Order Classification Level
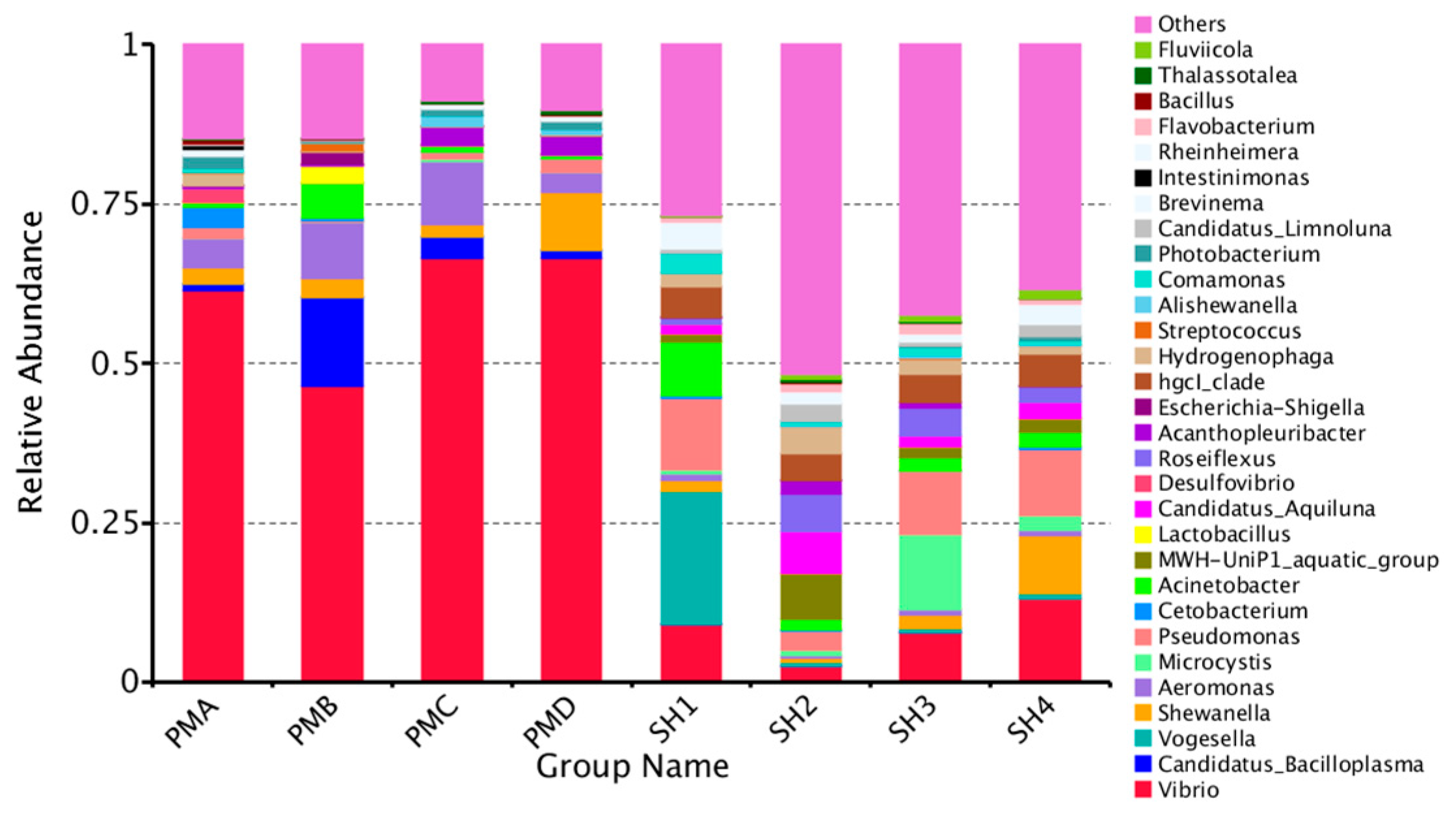
3.4.3. Microflora Structure at Genus Classification Level
3.5. Analysis of Flora Diversity and Similarity
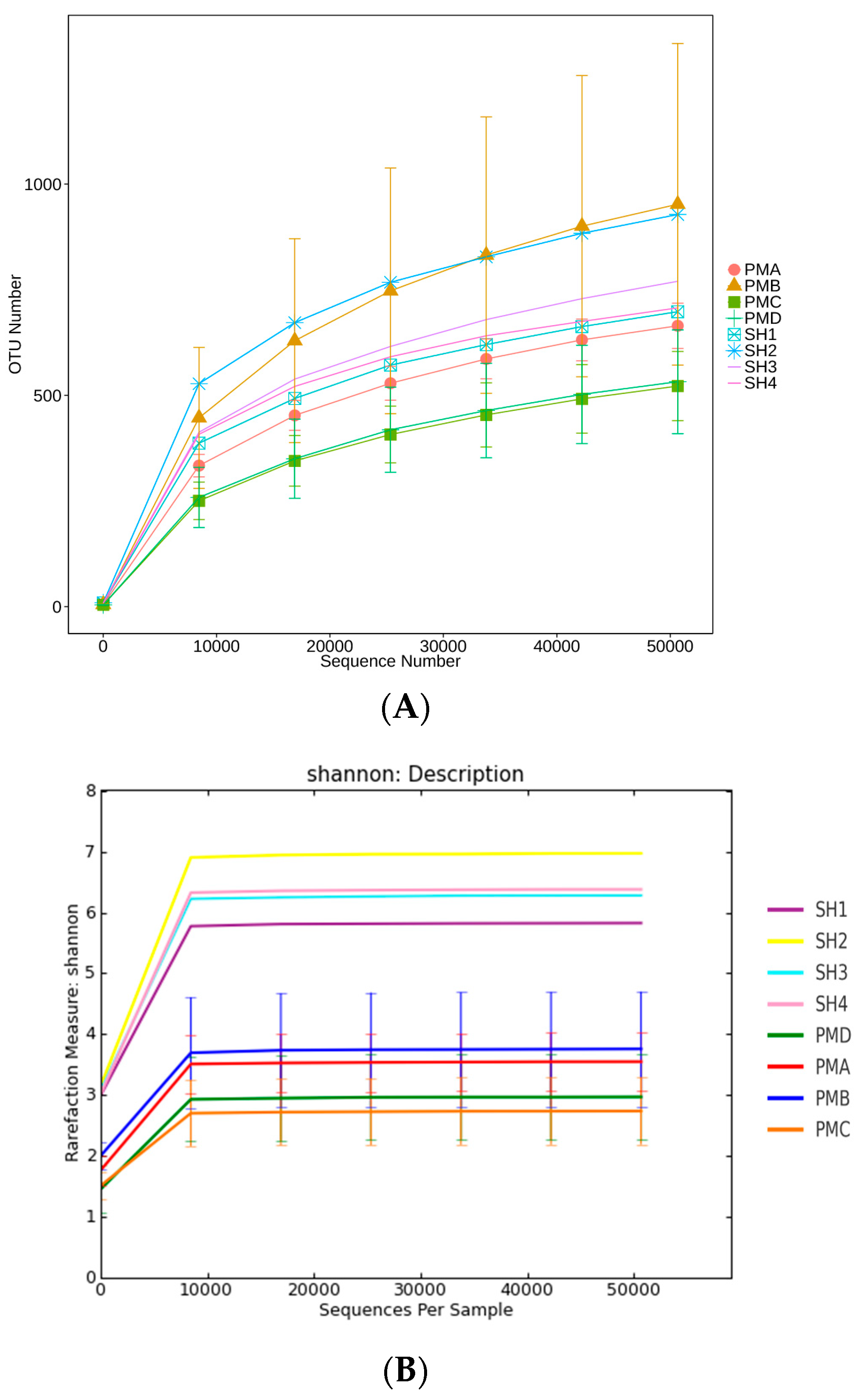
3.5.1. Alpha Diversity Index Analysis
3.5.2. Alpha Diversity Curve Analysis
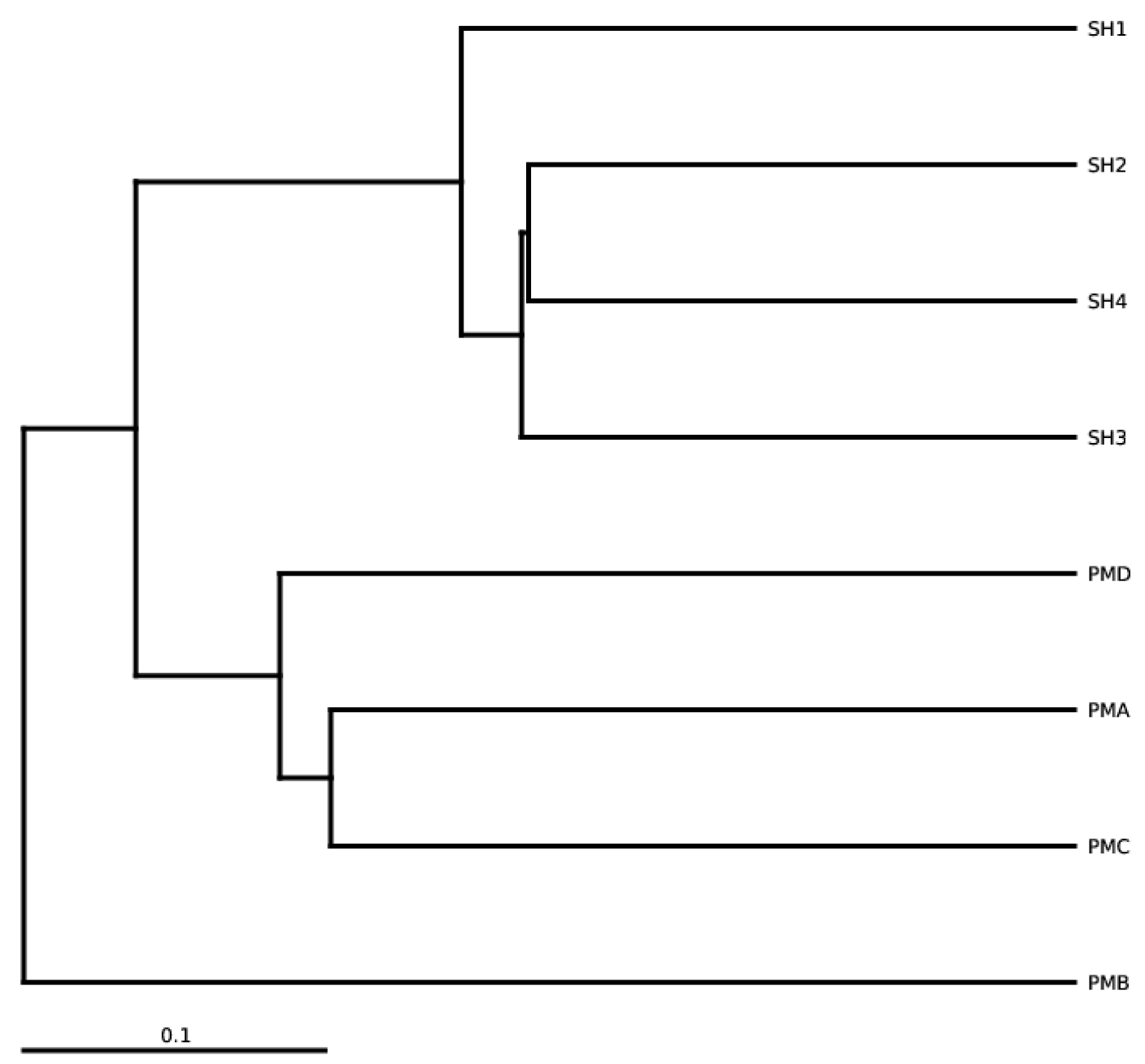
3.5.3. Species Similarity Analysis
4. Discussion
4.1. Water Quality Index Data Analysis
4.2. OTU Cluster Analysis of Prawn Gut and Its Aquaculture Water Body
4.3. Analysis and Discussion on the Structure of Intestinal Flora of Penaeus Prawn and Its Aquaculture Water
4.4. Analysis of Microbial Diversity in Prawn Intestine and Its Aquaculture Water
4.5. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- Zeng, S.; He, J.; Huang, Z. The intestine microbiota of shrimp and its impact on cultivation. Appl. Microbiol. Biotechnol. 2024, 108, 362. [Google Scholar] [CrossRef]
- Asmild, M.; Hukom, V.; Nielsen, R.; Nielsen, M. Is economies of scale driving the development in shrimp farming from Penaeus monodon to Litopenaeus vannamei? The case of Indonesia. Aquaculture 2024, 579, 740178. [Google Scholar] [CrossRef]
- Emmanuel, A.; Wei, Y.; Ramzan, M.N.; Yang, W.; Zheng, Z. Dynamics of Bacterial Communities and Their Relationship with Nutrients in a Full-Scale Shrimp Recirculating Aquaculture System in Brackish Water. Animals 2025, 15, 1400. [Google Scholar] [CrossRef]
- Zoqratt, M.Z.H.M.; Eng, W.W.H.; Thai, B.T.; Austin, C.M.; Gan, H.M. Microbiome Analysis of Pacific White Shrimp Gut and Rearing Water from Malaysia and Vietnam. PeerJ 2018, 6, e5826. [Google Scholar] [CrossRef]
- Abakari, G.; Wu, X.; He, X.; Fan, L.; Luo, G. Bacteria in Biofloc Technology Aquaculture Systems: Roles and Mediating Factors. Rev. Aquac. 2022, 14, 1260–1284. [Google Scholar] [CrossRef]
- Holt, C.C.; Bass, D.; Stentiford, G.D.; van der Giezen, M. Understanding the Role of the Shrimp Gut Microbiome in Aquaculture. J. Invertebr. Pathol. 2021, 186, 107387. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, L.; Mai, G.; Zhang, H.; Yang, J.; Deng, D.; Ma, Y. Dynamics of the gut microbiota in developmental stages of Litopenaeus vannamei reveal its association with body weight. Sci. Rep. 2019, 9, 5182. [Google Scholar] [CrossRef]
- Imaizumi, K.; Sakamoto, R.; Inoue, Y.; Yamada, T.; Wada, M. Analysis of Microbiota in the Stomach and Midgut of Two Penaeid Shrimps during Probiotic Feeding. Sci. Rep. 2021, 11, 9936. [Google Scholar] [CrossRef] [PubMed]
- Cardona, E.; Gueguen, Y.; Magré, K.; Lorgeoux, B.; Piquemal, D.; Pierrat, F.; Noguier, F.; Saulnier, D. Bacterial Community Characterization of Water and Intestine of the Shrimp Litopenaeus stylirostris in a Biofloc System. BMC Microbiol. 2016, 16, 157. [Google Scholar] [CrossRef]
- Angthong, P.; Uengwetwanit, T.; Uawisetwathana, U.; Chaitongsakul, P.; Karoonuthaisiri, N. Investigating Host–Gut Microbial Relationship in Penaeus monodon upon Exposure to Vibrio harveyi. Aquaculture 2023, 567, 739252. [Google Scholar] [CrossRef]
- Cornejo-Granados, F.; Lopez-Zavala, A.A.; Gallardo-Becerra, L.; Poot-Hernández, A.C.; Sánchez-Flores, A. Host genome drives the microbiota enrichment of beneficial microbes in shrimp: Exploring the hologenome perspective. Anim. Microbiome 2025, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Ko, H.T.; Wu, P.L.; Kumar, R.; Wang, H.C.; Lu, H.P. Gut microbiota of Pacific white shrimp (Litopenaeus vannamei) exhibits distinct responses to pathogenic and non-pathogenic Vibrio parahaemolyticus. Microbiol Spectr. 2023, 11, e0118023. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gao, Y.; Wang, C.; Zeng, Y.; Lai, Z. Proportions of Pacific white shrimp, Litopenaeus vannamei, gut microbiota from ambient microbiota increased with aquaculture process. J. World Aquac. Soc. 2023, 54, 982–993. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Khanjani, M.; Mozanzadeh, M.; Gisbert, E.; Hoseinifar, S. Probiotics, prebiotics, and synbiotics in shrimp aquaculture: Their effects on growth performance, immune responses, and gut microbiome. Aquac. Rep. 2024, 38, 102362. [Google Scholar] [CrossRef]
- Hou, D.; Huang, Z.; Zeng, S.; Liu, J.; Wei, D.; Yan, Q. Intestine Bacterial Community Composition of Shrimp Varies under Low- and High-Salinity Culture Conditions. Front. Microbiol. 2020, 11, 589164. [Google Scholar] [CrossRef]
- Cicala, F.; Lago-Lestón, A.; Gomez-Gil, B.; Escobedo-Bonilla, C.M.; Martínez-Porchas, M.; Rojas, R. Gut microbiota shifts in the giant tiger shrimp, Penaeus monodon, during the postlarvae, juvenile, and adult stages. Aquac. Int. 2020, 28, 1421–1433. [Google Scholar] [CrossRef]
- Raza, B.; Zheng, Z.; Zhu, J.; Yang, W. A Review: Microbes and Their Effect on Growth Performance of Litopenaeus vannamei (White Leg Shrimps) during Culture in Biofloc Technology System. Microorganisms 2024, 12, 1013. [Google Scholar] [CrossRef]
- Shan, X.Y.; Li, K.Y.; Stadler, P.; Borbor, M.; Reyes, G.; Solórzano, R.; Chamorro, E.; Bayot, B.; Cordero, O.X. Microbiome determinants of productivity in aquaculture of whiteleg shrimp. Appl Environ. Microbiol. 2025, 91, e02420-24. [Google Scholar] [CrossRef]
- Lin, X.Y.; Shih, Y.J.; Zhang, X.J.; Cai, Y.S.; Zhou, X.W.; Chen, J.S. Perspective on intestinal microbiota temporal changes of herbal additives treated shrimp in a natural aquaculture setting. Front. Mar. Sci. 2024, 11, 1332585. [Google Scholar] [CrossRef]
- Jatuyosporn, T.; Wongpanya, R.; Boonchuen, P.; Rungrassamee, W.; Karoonuthaisiri, N. White Spot Syndrome Virus Impact on the Expression of Vago and Gut Microbiome of the Black Tiger Shrimp (Penaeus monodon). Sci Rep. 2023, 13, 27906. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zeng, S.; Xiong, J. Microecological Koch’s postulates reveal that intestinal microbiota dysbiosis contributes to shrimp white feces syndrome. Microbiome 2022, 8, 32. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Z.; Zhang, Y.; Chen, X.; Zeng, S. Postlarval Shrimp-Associated Microbiota and Underlying Ecological Processes over AHPND Progression. Microorganisms 2025, 13, 720. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Luo, X.; Guo, L.; Zhang, J.; Wang, Q.; Xu, Z. Profile of the Gut Microbiota of Pacific White Shrimp under Industrial Indoor Farming System. Appl. Microbiol. Biotechnol. 2024, 108, 225–239. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Gai, C.; Zhang, J.; Li, Y. WSSV Infection in the Gut Microbiota of the Black Tiger Shrimp Penaeus monodon. Fishes 2025, 10, 440. [Google Scholar] [CrossRef]
- Qi, J.; Deng, L.; Song, Y.; Qi, W.; Hu, C. Nutrient Thresholds Required to Control Eutrophication: Does It Work for Natural Alkaline Lakes? Water 2022, 14, 2674. [Google Scholar] [CrossRef]
- Wu, F.; Fang, Y.; Feng, M.; Xie, Z.; Zhu, L.; Feng, J. Developing Ecological Thresholds for Nitrogen and Phosphorus in the Haihe River Basin in China. Int. J. Environ. Res. Public Health 2022, 19, 16951. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Mir, T.U.; Rahayu, F.; Suhara, C.; Anjli, A.; Chopra, C.; Singh, R.; Prakash, A.; El Messaoudi, N.; et al. Eco-friendly and safe alternatives for the valorization of shrimp farming waste. Environ. Sci. Pollut. Res. 2024, 31, 38960–38989. [Google Scholar] [CrossRef] [PubMed]
- Lo, L.S.H.; Xu, Z.; Lee, S.S.; Lau, W.K.; Qiu, J.W.; Liu, H.; Qian, P.Y.; Cheng, J. How elevated nitrogen load affects bacterial community structure and nitrogen cycling services in coastal water. Front Microbiol. 2022, 13, 1062029. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shi, X.; Wang, J.; Zheng, X.; Qian, X.; Xie, S.; Tang, J. Elevated water nutrient levels promote co-occurrence of sediment microbiota in white-leg shrimp (Litopenaeus vannamei) culture ponds. Aquac. Rep. 2025, 45, 103121. [Google Scholar] [CrossRef]
- Li, H.; Gu, S.; Wang, L.; Shi, W.; Jiang, Q.; Wan, X. Dynamic Changes of Environment and Gut Microbial Community of Litopenaeus vannamei in Greenhouse Farming and Potential Mechanism of Gut Microbial Community Construction. Fishes 2024, 9, 155. [Google Scholar] [CrossRef]
- Zhao, Q.; Wei, C.; Dou, J.; Sun, Y.; Zeng, Q.; Bao, Z. Molecular and Physiological Responses of Litopenaeus vannamei to Nitrogen and Phosphorus Stress. Antioxidants 2025, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Huang, J.; Wang, Y.; Zhang, J. Characterization of Bacterial Community in Intestinal and Rearing Water of Penaeus monodon Differing Growth Performances in Outdoor and Indoor Ponds. Aquac. Res. 2020, 51, 4376–4387. [Google Scholar] [CrossRef]
- Angthong, P.; Uengwetwanit, T.; Arayamethakorn, S.; Chaitongsakul, P.; Karoonuthaisiri, N.; Rungrassamee, W. Bacterial Analysis in the Early Developmental Stages of the Black Tiger Shrimp (Penaeus monodon). Sci. Rep. 2020, 10, 4896. [Google Scholar] [CrossRef]
- Garibay-Valdez, E.; Martínez-Córdova, L.R.; López-Torres, M.A.; Almendariz-Tapia, F.J.; Martínez-Porchas, M.; Calderón, K. The Implication of Metabolically Active Vibrio spp. in the Digestive Tract of Litopenaeus vannamei for Its Post-Larval Development. Sci. Rep. 2020, 10, 11428. [Google Scholar] [CrossRef]
- Kawasaki, M.; Lo, T.H.W.; Lazzarotto, V.; Briggs, M.; Smullen, R.P.; Barnes, A.C. Biodiversity of the Intestinal Microbiota of Black Tiger Prawn (Penaeus monodon) Increases with Age and Is Only Transiently Impacted by Major Ingredient Replacement in the Diet. Aquac. Rep. 2022, 22, 100948. [Google Scholar] [CrossRef]
- Thomas, R.; Fukamizo, T.; Suginta, W. Bioeconomic Production of High-Quality Chitobiose from Chitin Food Wastes Using an In-House Chitinase from Vibrio campbellii. Bioresour. Bioprocess. 2022, 9, 86. [Google Scholar] [CrossRef]
- Gao, N.; Shu, Y.; Wang, Y.; Liu, J.; Zhang, M. Acute Ammonia Causes Pathogenic Dysbiosis of Shrimp Gut Biofilms. Int. J. Mol. Sci. 2024, 25, 2614. [Google Scholar] [CrossRef]
- Xiong, J.-B.; Sha, H.-N.; Chen, J. Updated Roles of the Gut Microbiota in Exploring Shrimp Etiology, Polymicrobial Pathogens, and Disease Incidence. Zool. Res. 2024, 45, 910–923. [Google Scholar] [CrossRef]
- Lv, X.; Li, S.; Yu, Y.; Zhang, X.; Li, F. Crustin Defense against Vibrio parahaemolyticus Infection by Regulating Intestinal Microbial Balance in Litopenaeus vannamei. Mar. Drugs 2023, 21, 130. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-T.; Huang, W.-T.; Wu, P.-L.; Kumar, R.; Wang, H.-C.; Lu, H.-P. Low Salinity Stress Increases the Risk of Vibrio parahaemolyticus Infection and Gut Microbiota Dysbiosis in Pacific White Shrimp. BMC Microbiol. 2024, 24, 275. [Google Scholar] [CrossRef]
- Mat Deris, Z.; Iehata, S.; Gan, H.M.; Ikhwanuddin, M.; Najiah, M.; Asaduzzaman, M. Understanding the Effects of Salinity and Vibrio harveyi on the Gut Microbiota Profiles of Litopenaeus vannamei. Front. Mar. Sci. 2022, 9, 974217. [Google Scholar] [CrossRef]
- Shi, C.E.; Xia, M.; Li, R.; Mu, C.; Zhang, L.; Liu, L.; Wang, C. Vibrio alginolyticus infection induces coupled changes of bacterial community and metabolic phenotype in the gut of swimming crab. Aquaculture 2019, 499, 251–259. [Google Scholar] [CrossRef]
- Thompson, J.; Weaver, M.A.; Lupatsch, I.; Shields, R.J.; Plummer, S.; Coates, C.J.; Rowley, A.F. Antagonistic activity of lactic acid bacteria against pathogenic vibrios and their potential use as probiotics in shrimp (Penaeus vannamei) culture. Front. Mar. Sci. 2022, 9, 807989. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Lu, J.; Qiu, Q.; Chen, J.; Xiong, J. Quantifying the importance of external and internal sources to the gut microbiota in juvenile and adult shrimp. Aquaculture 2021, 531, 735910. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Huang, L.; Dong, P.; Hou, D.; Chen, H.; Lu, Z.; Zhang, D. Fine-scale succession patterns and assembly mechanisms of bacterial community of Litopenaeus vannamei larvae across the developmental cycle. Microbiome 2020, 8, 106. [Google Scholar] [CrossRef]
- Hembrom, P.S.; Deepthi, M.; Kannoth, S.; Reeja, N.; Antony, G.; Grace, T. Amplicon sequencing reveals growth-associated microbial communities in black tiger shrimp (Penaeus monodon). Microb. Pathog. 2025, 205, 107636. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, J.; Xu, J. Warming-Driven Migration of Enterotypes Mediates Host Health and Disease Statuses in Ectotherm Litopenaeus vannamei. Commun. Biol. 2025, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Sha, H.; Lu, J.; Chen, J.; Xiong, J.B. Rationally designed probiotics prevent shrimp white feces syndrome via the probiotics–gut microbiome–immunity axis. npj Biofilms Microbiomes 2024, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Xing, Y.F.; Zheng, X.T.; Huang, J.H.; Qin, J.; Zhang, J.S. The effects of thermal stress on the morphology, physiological functions, gut microbiota, and metabolism of Penaeus vannamei under seawater and low-salinity conditions. Aquaculture 2025, 612, 743188. [Google Scholar] [CrossRef]
- Dong, P.; Guo, H.; Wang, Y.; Wang, R.; Chen, H.; Zhao, Y.; Zhang, D. Gastrointestinal microbiota imbalance is triggered by the enrichment of Vibrio in subadult Litopenaeus vannamei with acute hepatopancreatic necrosis disease. Aquaculture 2021, 533, 736199. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, J.; Dai, W.; Dong, C.; Qiu, Q.; Li, C. Integrating gut microbiota immaturity and disease-discriminatory taxa to diagnose the initiation and severity of shrimp disease. Environ. Microbiol. 2017, 19, 1490–1501. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, X. Core gut microbiota of shrimp function as a regulator to maintain immune homeostasis in response to WSSV infection. Microbiol. Spectr. 2022, 10, e02465-21. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Xu, C.; Wang, X.; Wang, S.; Zhao, Q.; Zhang, M.; Chen, L. Gut Microbiota and its Modulation for Healthy Farming of Pacific White Shrimp Litopenaeus vannamei. Rev. Fish. Sci. Aquac. 2018, 26, 381–399. [Google Scholar] [CrossRef]
- GB/T 35892-2018; Laboratory animal–Guideline for ethical review of animal welfare. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.

| Sampling Phase | pH | NH3-N (mg·L−1) | NO2−-N (mg·L−1) | NO3−-N (mg·L−1) | PO43−-P (mg·L−1) | TN (mg·L−1) | TP (mg·L−1) |
|---|---|---|---|---|---|---|---|
| SH1 | 8.1 ± 0.02 a | 0.11 ± 0.01 d | 0.01 ± 0.02 a | 0.51 ± 0.02 c | 0.05 ± 0.012 c | 0.71 ± 0.02 d | 0.08 ± 0.05 c |
| SH2 | 7.8 ± 0.01 b | 0.25 ± 0.01 c | 0.02 ± 0.02 a | 0.75 ± 0.03 b | 0.11 ± 0.02 b | 1.34 ± 0.02 c | 0.15 ± 0.02 b |
| SH3 | 7.6 ± 0.04 bc | 0.33 ± 0.02 b | 0.02 ± 0.01 a | 1.18 ± 0.01 a | 0.27 ± 0.06 a | 1.53 ± 0.02 b | 0.31 ± 0.03 a |
| SH4 | 7.5 ± 0.02 c | 0.51 ± 0.02 a | 0.04 ± 0.02 a | 1.22 ± 0.02 a | 0.34 ± 0.07 a | 1.86 ± 0.02 a | 0.36 ± 0.02 a |
| Sample Names | Valid Sequence | Avg Len (nt) | Q20 | Q30 | GC% | Effective% |
|---|---|---|---|---|---|---|
| PMA | 84,067 | 253 | 99.12 | 98.23 | 52.90 | 88.77 |
| PMB | 85,093 | 253 | 99.29 | 98.58 | 52.32 | 89.15 |
| PMC | 84,922 | 253 | 99.34 | 98.65 | 52.94 | 92.32 |
| PMD | 84,456 | 253 | 99.30 | 98.59 | 53.10 | 90.51 |
| SH1 | 89,203 | 253 | 99.34 | 98.60 | 53.80 | 91.43 |
| SH2 | 84,365 | 253 | 99.34 | 98.61 | 53.57 | 94.68 |
| SH3 | 87,808 | 253 | 99.31 | 98.58 | 53.49 | 93.27 |
| SH4 | 79,681 | 253 | 99.31 | 98.57 | 53.33 | 90.74 |
| Sample Names | Total_tag | Taxon_Tag | Unique_Tag | OTU_num |
|---|---|---|---|---|
| PMA | 81,853 | 80,778 | 1069 | 784 |
| PMB | 75,182 | 73,775 | 1407 | 1119 |
| PMC | 79,882 | 79,326 | 555 | 638 |
| PMD | 81,857 | 81,259 | 596 | 651 |
| SH1 | 83,173 | 80,554 | 2617 | 812 |
| SH2 | 81,583 | 79,317 | 2266 | 1071 |
| SH3 | 83,688 | 81,546 | 2142 | 881 |
| SH4 | 73,983 | 71,725 | 2258 | 821 |
| Classification | PMA | PMB | PMC | PMD | SH1 | SH2 | SH3 | SH4 |
|---|---|---|---|---|---|---|---|---|
| Vibrio genus Candidatus_Bacilloplasma | 61.48% | 46.51% | 66.48% | 66.52% | 9.04% | 2.45% | 7.83% | 13.15% |
| Candidatus | 0.99% | 13.87% | 3.38% | 1.19% | 0.00% | 0.01% | 0.00% | 0.00% |
| Vogesella | 0.01% | 0.00% | 0.00% | 0.00% | 20.92% | 0.66% | 0.67% | 0.72% |
| Shewanella | 2.54% | 2.88% | 1.99% | 9.10% | 1.71% | 0.59% | 1.96% | 9.07% |
| Aeromonas spp. | 4.61% | 8.87% | 9.76% | 3.12% | 1.09% | 0.42% | 0.95% | 1.02% |
| Microcystis | 0.03% | 0.02% | 0.51% | 0.03% | 0.69% | 0.93% | 11.76% | 2.21% |
| Pseudomonas | 1.65% | 0.32% | 1.01% | 2.09% | 11.21% | 3.05% | 10.04% | 10.43% |
| Cetobacterium sporomonas | 3.27% | 0.44% | 0.16% | 0.20% | 0.36% | 0.03% | 0.01% | 0.46% |
| Acinetobacter Actinomyces | 0.70% | 5.46% | 0.94% | 0.54% | 8.39% | 1.71% | 2.11% | 2.17% |
| Others | 24.70% | 21.63% | 15.75% | 17.21% | 45.25% | 82.92% | 63.03% | 58.62% |
| Sample Group | Shannon | Simpson | chao1 | ACE | Goods_Coverage |
|---|---|---|---|---|---|
| PMA | 3.549 | 0.674 | 784.482 | 829.319 | 0.996 |
| PMB | 3.758 | 0.744 | 1192.509 | 1226.536 | 0.994 |
| PMC | 2.738 | 0.577 | 691.362 | 694.126 | 0.997 |
| PMD | 2.97 | 0.625 | 687.119 | 709.296 | 0.997 |
| SH1 | 5.825 | 0.94 | 915.686 | 900.221 | 0.996 |
| SH2 | 6.971 | 0.981 | 1114.667 | 1133.218 | 0.996 |
| SH3 | 6.279 | 0.967 | 1060.011 | 1036.474 | 0.995 |
| SH4 | 6.378 | 0.971 | 801.375 | 830.585 | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Fang, Z.; Yu, H.; Zhao, H.; Yang, Y.; Zhou, F.; Guo, Y.; Chen, C.; Zhao, L.; Tian, Y. Effects of Polyculture Patterns in Ponds on Water Quality and Intestinal Flora of Penaeus monodon. Water 2025, 17, 3194. https://doi.org/10.3390/w17223194
Sun X, Fang Z, Yu H, Zhao H, Yang Y, Zhou F, Guo Y, Chen C, Zhao L, Tian Y. Effects of Polyculture Patterns in Ponds on Water Quality and Intestinal Flora of Penaeus monodon. Water. 2025; 17(22):3194. https://doi.org/10.3390/w17223194
Chicago/Turabian StyleSun, Xueliang, Zhenzhen Fang, Hong Yu, Honghao Zhao, Yuanyuan Yang, Falin Zhou, Yongjun Guo, Chengxun Chen, Lin Zhao, and Yunchen Tian. 2025. "Effects of Polyculture Patterns in Ponds on Water Quality and Intestinal Flora of Penaeus monodon" Water 17, no. 22: 3194. https://doi.org/10.3390/w17223194
APA StyleSun, X., Fang, Z., Yu, H., Zhao, H., Yang, Y., Zhou, F., Guo, Y., Chen, C., Zhao, L., & Tian, Y. (2025). Effects of Polyculture Patterns in Ponds on Water Quality and Intestinal Flora of Penaeus monodon. Water, 17(22), 3194. https://doi.org/10.3390/w17223194





