Abstract
Seawater desalination has emerged as a crucial solution for addressing global freshwater scarcity. However, it generates significant volumes of concentrated brine waste. This brine is rich in dissolved salts and minerals, primarily, chloride (55%), sodium (30%), sulfate (8%), magnesium (4%), calcium (1%), potassium (1%), bicarbonate (0.4%), and bromide (0.2%), which are often discharged into marine environments, posing ecological challenges. This study presents a comprehensive global review of innovative technologies for recovering these constituents as valuable products, thereby enhancing the sustainability and economic viability of desalination. The paper evaluates a range of proven and emerging recovery methods, including membrane separation, nanofiltration, electrodialysis, thermal crystallization, solar evaporation, chemical precipitation, and electrochemical extraction. Each technique is analyzed for its effectiveness in isolating salts (NaCl, KCl, and CaSO4) and minerals (Mg(OH)2 and Br2), with a discussion of process-specific constraints, recovery efficiencies, and product purities. Furthermore, the study incorporates a detailed techno-economic assessment, highlighting revenue potential, capital and operational expenditures, and breakeven timelines. Simulated case studies of a 100,000 m3/day seawater reverse osmosis (SWRO) facility demonstrates that a sequential brine recovery process and associated energy balances, supported by pilot-scale data from ongoing global initiatives, can achieve over 90% total salt recovery while producing marketable products such as NaCl, Mg(OH)2, and Br2. The estimated revenue from recovered materials ranges between USD 4.5 and 6.8 million per year, offsetting 65–90% of annual desalination operating costs. The analysis indicates a payback period of 3–5 years, depending on recovery efficiency and product pricing, underscoring the economic viability of large-scale brine valorization alongside its environmental benefits. By transforming waste brine into a source of commercial commodities, desalination facilities can move toward circular economy models and achieve greater sustainability. A practical integration framework is proposed for both new and existing SWRO plants, with a focus on aligning with the principles of a circular economy. By transforming waste brine into a resource stream for commercial products, desalination facilities can reduce environmental discharge and generate additional revenue. The study concludes with actionable recommendations and insights to guide policymakers, engineers, and investors in advancing brine mining toward full-scale implementation.
1. Introduction
Water scarcity is a growing global challenge, leading to the rapid expansion of seawater desalination capacity in arid and semi-arid regions. Modern desalination plants, particularly those utilizing seawater reverse osmosis (SWRO), generate substantial quantities of concentrated saline brine as a byproduct. As of 2018, approximately 16,000 desalination plants globally, with capacity concentrated in the Middle East and North Africa, produced 95 million m3 of freshwater per day, while simultaneously discharging an estimated 142 million m3 of hypersaline brine per day, exceeding the net freshwater output. [1,2,3]. Rejected brine generally contains roughly twice the salinity of seawater, along with residual treatment chemicals. Brine often contains residual treatment chemicals such as antiscalants, e.g., polyphosphonates and polycarboxylates, biocides, e.g., DBNPA and isothiazolinones, dechlorination agents, e.g., sodium bisulfite, coagulants and flocculants including ferric salts, alum and polyamines, and common CIP reagents including sodium hydroxide, mineral and organic acids, and surfactants. These additives can complex calcium and magnesium, hinder precipitation, foul membranes or ion-exchange resins, and reduce product purity. Mitigation includes upstream polishing by activated carbon or oxidation, nanofiltration divalent splits, controlled pH and alkalinity staging, selective reagent choice, and limited purge before recovery steps. Its discharge into the marine environment is widely regarded as a waste byproduct, raising concerns over elevated salinity, thermal stress, oxygen depletion, and toxic contamination of aquatic and soil ecosystems [4,5,6].
Table 1, presenting the global distribution of desalination plants by region, income level, and sectoral application, is drawn from the comprehensive global assessment conducted by Jones et al. [3].

Table 1.
Global distribution of desalination plants and capacity by region, country, and income level [3].
Traditional brine disposal methods, such as surface water discharge, deep-well injection, or evaporation ponds, mitigate these issues only partially and are becoming less viable as desalination capacity grows [7,8]. However, these methods pose serious environmental risks to marine ecosystems, causing localized hypersalinity, oxygen depletion, and toxicity to aquatic life, degrading soil and groundwater if not properly managed [8]. Brine management is shifting away from traditional disposal methods due to their harmful environmental impacts. A sustainable approach now emphasizes minimizing effluent and recovering resources such as water, energy, salts, and chemicals. Such resource recovery not only mitigates environmental impacts but also supports progress toward the United Nations Sustainable Development Goal 6 (SDG 6) [9]. Addressing climate change and population growth requires a transition toward circular economy practices centered on resource stewardship. This entails re-evaluating material and resource flows across industries to improve efficiency and reduce waste. Central to this effort is the development of innovative treatment technologies capable of recovering scarce resources from unconventional streams, including industrial wastewater and previously uneconomical sources [10,11,12]. In water management, such innovation has advanced zero and minimal liquid discharge (ZLD/MLD) systems. ZLD, first adopted by power plants in Colorado to mitigate Colorado River salinity, aims to recover all water while converting residuals into solid salts. Recent developments extend this approach to producing multiple solids. Meanwhile, MLD systems, targeting about 95% recovery, are increasingly favored for their lower energy demands and reduced capital costs [13].
In response, brine is increasingly reconceptualized not as a waste stream but as a secondary resource from which valuable salts and metals can be recovered, thereby aligning brine management with the principles of a circular economy [14]. Recovering resources from brine could both reduce environmental impacts and offset desalination costs by generating revenue or useful products [15]. Studies from the Canary Islands and the Arabian/Persian Gulf demonstrate that uncontrolled brine discharges increase seawater salinity and heavy metal levels in waters and sediments, causing biodiversity loss and potential toxin bioaccumulation [16,17]. These concerns have prompted calls for more sustainable brine management strategies beyond disposal.
As desalination capacity grows to meet rising water demand, the volume of brine and its potential environmental impact are increasing correspondingly [3,4]. However, in line with circular economy principles, this waste brine can be viewed as resource-rich in dissolved salts and minerals that can be recovered and valorized [4]. Converting brine into valuable products offers a dual benefit: mitigating the environmental impacts of brine disposal and improving the economics of desalination by generating additional revenue [4]. Seawater brine from SWRO desalination retains essentially the same ionic composition as seawater, but at roughly double the concentration, assuming 50% water recovery. The major constituents of typical seawater (35 g/kg salinity) are chloride (19.3 g/kg) and sodium (10.8 g/kg), which together make up over 85% of the dissolved ions [18]. Sulfate, magnesium, calcium, potassium, bicarbonate, and bromide comprise most of the remaining 15% [18].
Economically, the recovered products (industrial salts, metals, etc.) can create new revenue streams that offset the costs of desalinated water production [19]. Recent advances in separation technologies and resource recovery processes have made mineral extraction from seawater brine increasingly competitive with conventional mining in the last decade [20]. This is significant given the rising global demand for critical minerals, such as lithium for batteries or magnesium for advanced materials, which is straining terrestrial mineral reserves [19,20]. By leveraging the massive volumes of salts already present in the oceans and concentrated in desalination brine, brine mining offers a virtually inexhaustible supply of certain minerals, without the landscape disruption and environmental liabilities of land-based mining [20].
Table 2 summarizes the approximate composition of seawater and, hence, a typical SWRO brine and potential recovery products for each ion. These salts and metals have significant industrial value if they can be extracted in usable forms.

Table 2.
Major ions in typical seawater brine with 70 g/L salinity and potential recovery products [20,21].
For example, sodium and chloride can be recovered as high-purity sodium chloride (NaCl) salt, a commodity for de-icing, food in chemical industries, or which can be separated to produce chlorine gas and caustic soda. Magnesium can be recovered as magnesium hydroxide (Mg(OH)2) or magnesium oxide, which are used in water treatment, refractory materials, and as precursors for magnesium metal [21,22]. Calcium could be harvested as calcium carbonate or gypsum (CaSO4·2H2O) for use in construction and agriculture. Potassium is a valuable fertilizer component, as is KCl or potassium sulfate. Even minor constituents like bromide and boron have niche markets, such as bromine for flame retardants and boron in boric acid for industrial use. Recovering these from brine can reduce the reliance on terrestrial mining for the same elements, some of which are classified as critical raw materials due to supply risks [4,23]. Mohammad et al. [24] extracted magnesium from desalination brine using ammonium hydroxide, obtaining Mg(OH)2 of over 95% purity. Their experiments, however, showed that complete conversion was not achievable, with the maximum recovery recorded at 97%. Sadhwani et al. [16] examined case studies from the Canary Islands, where reverse osmosis was the primary desalination method. Their analysis highlighted potential environmental impacts, including increased energy consumption linked to global warming, noise emissions, land-use pressures, and harmful effects on marine ecosystems. According to Shemer and Semiat [17], the brine discharged from desalination facilities in the Arabian Gulf contributes to increasing seawater salinity levels, reducing dissolved oxygen concentrations and threatening marine stability.
NaCl dominates brine composition by mass, making its extraction central to any large-scale recovery strategy [20]. As the most abundant constituent, NaCl often represents both the principal product and a significant revenue stream. The crystallization of NaCl as a solid salt not only provides a commercial commodity but also leaves behind a residual bittern enriched in divalent ions (Mg2+, Ca2+, and SO42−) and minor elements (K+, Br−, and B(OH)3), which are more readily recovered at higher concentrations [20]. This sequential approach improves efficiency, as targeting secondary products directly from bulk brine is less viable. Furthermore, in regions where desalination operates alongside salt imports for chlor-alkali industries or de-icing, NaCl recovery can enhance local supply chains and economic feasibility.
Beyond common salt, magnesium is typically identified as the next most promising element to recover from seawater brines, due to its relatively high concentration and moderate market value [4]. Global industries consume magnesium compounds for uses such as flame retardants, wastewater neutralization, and fertilizer, and magnesium metal is in demand for lightweight alloys. Some countries like China and Israel already extract magnesium from seawater or brines at scale [21]. The history of magnesium production includes the notable example of Dow Chemical’s Freeport plant in Texas, which, beginning in 1941, extracted magnesium from seawater by precipitating magnesium hydroxide with calcined dolomite mixed with CaO/MgO and then electrolysis of the resulting magnesium chloride [22]. At its peak, the Freeport seawater plant produced up to 18,000 tons of magnesium metal per year and supplied the majority of U.S. demand during World War II [22]. This demonstrates that large-scale recovery of minerals like magnesium from seawater is technically feasible given the correct economic drivers. Today, motivation is shifting toward environmental sustainability and resource efficiency.
In summary, there is significant incentive to recover the dissolved salts and metals from desalination brine: doing so can reduce the environmental footprint of desalination, alleviate marine pollution, and yield commodities that offset operating costs. The key challenge is achieving this economically, since the separation of salts requires energy and/or chemicals. This paper explores the innovative recovery methods being developed and deployed to solve this challenge. A range of technologies was reviewed, from established methods like evaporative crystallization to cutting-edge processes such as membrane-based selective ion extraction, and their workability for extensive brine valorization. The business case for brine mining was also analyzed, including cost factors and potential revenue streams, to identify pathways toward commercial viability. The scope is global, drawing on examples and data from facilities and research projects around the world. The aim is to provide a clear understanding of which approaches are most promising for turning desalination brine into a source of metals and salts, and how these can be integrated into sustainable industrial operations. Actionable insights are highlighted to guide future developments in this field.
This study advances a deployable framework that integrates membrane separation, selective chemical precipitation, and staged crystallization, anchored in real brine composition data and pilot-scale performance. The pathway (i) uses NF/high-pressure RO to partition monovalent and divalent streams and suppress scaling, (ii) recovers CaCO3 and Mg(OH)2 while conditioning the bittern, and (iii) crystallizes NaCl and K/Mg salts. Mass-balance simulations were linked to product purity and market requirements, energy and reagent demands were quantified via stream partitioning, and empirical correction factors from the SEA4VALUE (EU), SWCC (Saudi Arabia), and MINERALS (Spain) pilots were incorporated to reflect field performance. The techno-economic scenarios focus on co-located systems and account for brine disposal offsets and market conversion ratios. Collectively, the plant-oriented flowsheet demonstrates above 90% total salt recovery with defensible economics, providing a practical roadmap for coupling brine resource recovery with existing SWRO assets.
2. Methodology
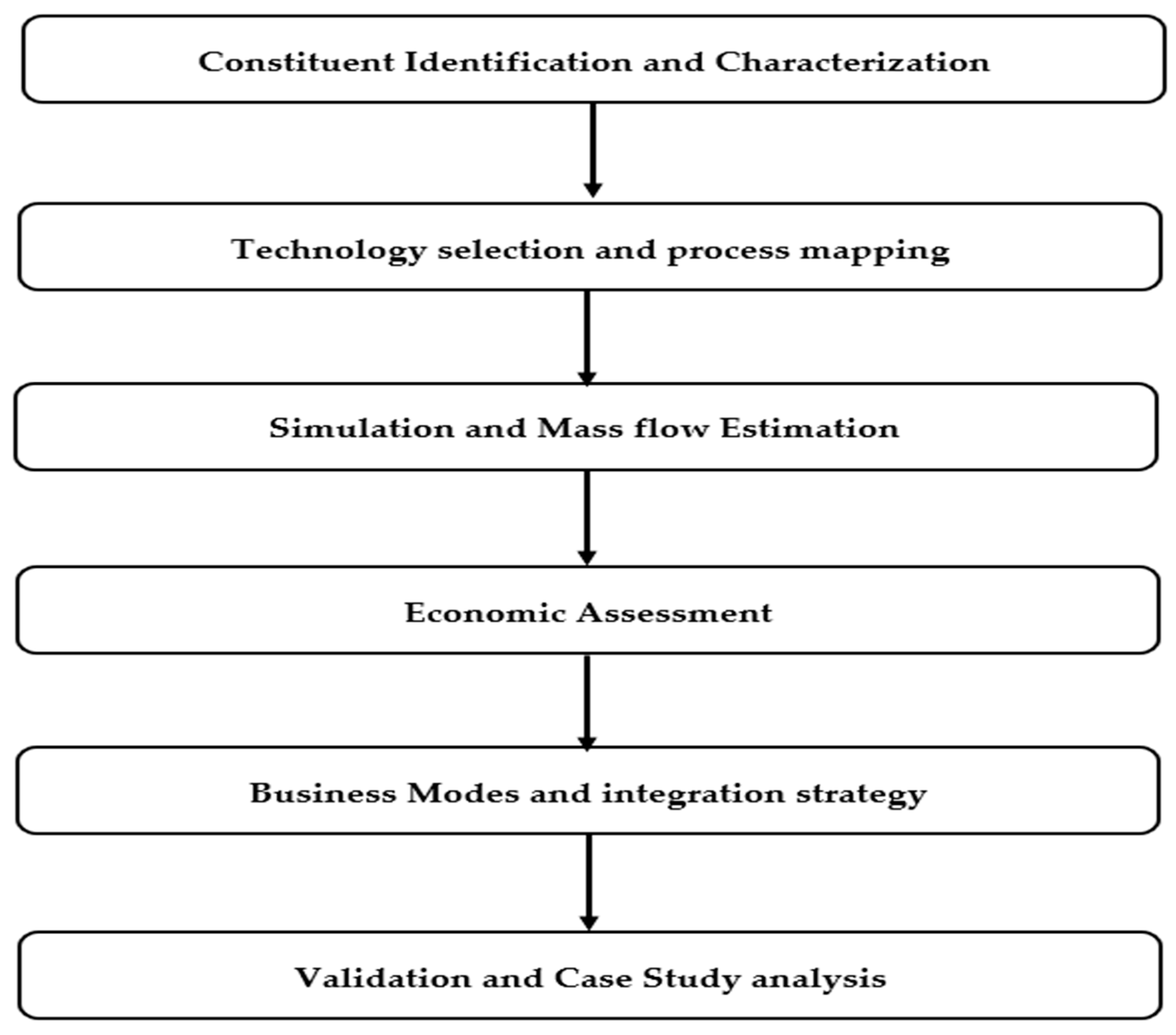
This study employed a broad, multi-tiered methodology integrating analytical review, data synthesis, numerical simulation, and process flow modeling to assess the technical and economic viability of recovering major dissolved constituents from SWRO brine. The approach was structured into six interrelated stages as shown in Figure 1.
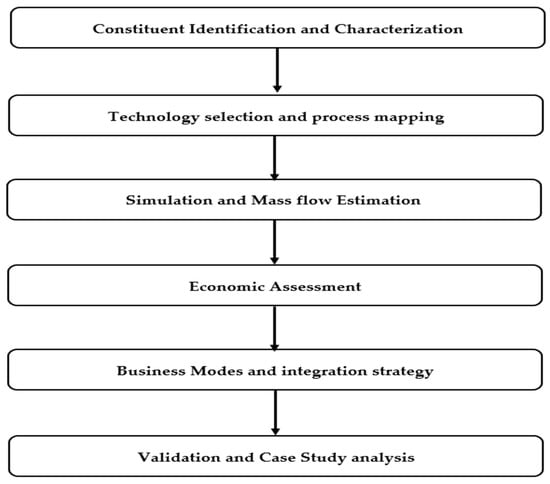
Figure 1.
Methodology process flow diagram.
The methodology integrates evidence review, chemical characterization, simulation, techno-economic assessment, and validation. The literature in Scopus and Web of Science (2010–2025) was screened for seawater or SWRO matrices reporting quantitative recovery, energy or cost, product purity, or TEA or LCA results (using Simapro 9.4.0). Non-SWRO items, duplicates, and studies with insufficient data were excluded. From eligible sources we extracted ion composition, recovery efficiencies, utilities, CAPEX and OPEX, and product purity and price ranges, and cross checked these against pilot evidence from the SEA4VALUE, SWCC, and MINERALS pilots. Brine chemistry was defined by major ions (Cl−, Na+, SO42−, Mg2+, Ca2+, K+, HCO3−, and Br−). The model plant was set at 100,000 m3 per day with 45 percent water recovery, brine salinity near 70 g per liter, and oceanic ionic fractions for mass balances. A sequential train was simulated with carbonate dosing for CaCO3 removal, alkaline dosing for Mg(OH)2, and evaporative NaCl crystallization, using stoichiometric mass balances and corrections calibrated with pilot data. The techno-economic model linked unit operations to costs and revenues using representative energy prices and commodity values, with sensitivity to recovery efficiency, energy price, and product purity. The study assumptions are as follows: steady-state operation, oceanic ionic proportions as the baseline, stage recoveries of 96–98 percent for Ca removal, 90–95 percent for Mg removal, and 90–95 percent for NaCl recovery, washed solids with 10–15 percent moisture, no heat integration credit in the base case, and product prices as long term averages with regional checks. Comparative analyses were also conducted against conventional terrestrial mining processes to evaluate the relative economic and environmental feasibility of marine resource extraction from desalination brine [4,25,26].
Statistical Analysis
Continuous variables were assessed for normality using the Shapiro–Wilk test in Python 3.10 using SciPy 1.11. For approximately normal data, Pearson correlation was computed, otherwise, Spearman rank correlation was used. Differences among operating scenarios of baseline, waste heat, high price, and low price were evaluated with one-way ANOVA, and with Tukey’s HSD for post hoc pairwise comparisons. Effect sizes (η2) and 95% confidence intervals were reported. Statistical significance was defined as α = 0.05.
3. Recovery Technologies and Processes for Brine Constituents
Recovering multiple minerals from a complex solution like seawater brine is inherently challenging and typically requires a sequence of separation steps. The technologies for brine recovery can be classified into several categories:
- Membrane-based separation using pressure or electrical potential to separate ions selectively;
- Thermal processes and crystallization, evaporation, or cooling to precipitate salts;
- Chemical precipitation: the addition of reagents to selectively remove target ions as insoluble compounds;
- Adsorptive or ion-exchange processes using sorbents or extractants to capture ions selectively.
Often, hybrid systems combining multiple methods yield the best results, for example, membrane concentration followed by chemical precipitation [15]. In this section, each category was discussed, including the specific techniques, their target ions, and practical considerations. Emphasis is given to commercially proven or pilot-demonstrated methods capable of recovering the major ions listed earlier.
Integrating ion fractionating membranes such as NF or selective IEMs, targeted precipitation with optional nanobubbles, and staged crystallization reduces scaling risk, lowers reagent and evaporation demands, and increases total salt recovery.
3.1. Membrane Separation Processes
Membrane-based processes use semi-permeable membranes to separate ions or water from the brine, driven by pressure or electrical potential. These processes can achieve separation without phase change or evaporation, potentially offering lower energy consumption for partial separations. Key membrane technologies for brine mining include nanofiltration (NF), reverse osmosis (RO) at high pressure, electrodialysis (ED) including variants like bipolar ED, and forward osmosis.
3.1.1. Nanofiltration
NF is a pressure-driven membrane process similar to RO but with membranes that have a larger pore size or different charge characteristics, allowing partial selectivity for divalent against monovalent ions. NF can be used on brine to preferentially reject multivalent ions such as Mg2+, Ca2+, and SO42−, while allowing most NaCl to pass, effectively splitting the stream into a permeate rich in NaCl and a divalent-enriched concentrate [20]. This approach is useful as a first step to isolate high-purity NaCl. For example, a two-stage NF–RO system was demonstrated in pilot studies by Saudi researchers to concentrate SWRO brine with an initial 70 g/L up to 250 g/L NaCl in the permeate stream, which could then be crystallized as pure salt [27]. The NF concentrate, containing hardness (Ca/Mg) and sulfate, can be treated separately. The challenge with NF on brine is the high osmotic pressure and scaling tendency, and cutting-edge high-pressure NF (HPNF) membranes and multi-stage setups are required to reach near-saturation concentrations [27]. However, NF offers a highly effective way to perform bulk separation of monovalent and divalent ions with relatively low energy input compared to evaporating the entire brine [20]. This monovalent/divalent split aligns with the strategy of removing NaCl first. Recent innovations include special NF or RO membranes embedded with chemical groups to target specific ions. For instance, a water innovation center in NEOM (Saudi Arabia) developed a modified RO membrane that is permeable to water and a chosen mineral ion, enabling concurrent extraction of a concentrated mineral solution during desalination [28,29,30]. In a prototype, such a membrane captured over 99% of a target ion, presumably a monovalent ion like Na+ or K+, into the permeate, which was then further processed in a second-stage RO to precipitate the pure salt [29,30]. This indicates the potential of selective membrane technologies for one-step recovery of particular salts.
3.1.2. Reverse Osmosis and Osmotically Assisted RO
Standard SWRO itself separates freshwater from salts but is limited by osmotic pressure to about 50–60% recovery for seawater. RO can be operated at higher pressures or enhanced by osmotic agents to concentrate brine further. High-pressure RO (HPRO) can push the recovery higher using specialized pumps and membranes, and has been tested to bring brine to over 120 g/L salinity [31]. An emerging variant is osmotically assisted RO (OARO), where an osmotic agent such as a concentrated draw solution is used on the permeate side to reduce the transmembrane pressure difference needed, allowing RO to continue the concentration of brine beyond normal limits [20]. Improvements in membrane materials and module design are enabling RO processes to approach the crystallization point of salts. However, purely pressure-driven concentration to dryness is generally not economical due to exponentially rising osmotic pressure. Instead, RO/OARO is best used to minimize the brine volume, reducing it by three time, after which other methods take over [20]. Indeed, pilot studies show that integrating HPRO with downstream crystallizers can achieve above 98–99% water recovery at a reasonable cost. A report documented 99.36% water recovery with a treatment cost of USD 1.04 per m3 of freshwater, using RO plus brine concentrator units [32]. These high-recovery RO systems significantly cut the volume that needs thermal evaporation, thus optimizing overall energy use.
3.1.3. Electrodialysis
Electrodialysis uses an electrical potential to pull cations and anions through ion-exchange membranes, separating them into product streams. In a typical ED stack, alternating cation- and anion-exchange membranes are placed between electrodes, and under a DC voltage, cations migrate toward the cathode through cation-selective membranes and anions toward the anode through anion-selective membranes [15]. ED is particularly useful for extracting specific ions or concentrating them if suitable membrane arrangements are used. A notable variant is bipolar membrane electrodialysis (BMED), which can split neutral salts into acid and base. For example, NaCl brine can be converted into HCl and NaOH streams by BMED, providing chemicals that can be reused, e.g., NaOH for alkaline precipitation and HCl for dissolving precipitates, or sold [15]. Research has shown that ED can recover elements like lithium, boron, and bromide from brines by pairing selective membranes or electrode reactions [4]. Membrane electrolysis, a form of ED where the product at one electrode is a gas and the product at the other end is the extracted element, is applied in the chlor-alkali industry. Brine is electrolyzed to yield Cl2 gas, with H2 at the cathode, and NaOH. This industrial process could be coupled with desalination brine feeds [21]. A real-world synergy example is using SWRO brine as feed for a chlor-alkali plant, where firstly, hardness and sulfate are chemically removed to protect the electrolyzers, then the purified NaCl brine is electrolyzed. Melián-Martel et al. [33] demonstrated such a process for reusing 8400 m3/day of SWRO brine in chlorine production. They removed calcium by carbonate precipitation and sulfate by barium dosing, as shown in the reactions (Equations (1) and (2)) below:
CaCl2 (aq) + Na2CO3 (aq) ⟶ CaCO3 (s) + 2NaCl (aq)
Na2SO4 (aq) + BaCl2 (aq) ⟶ BaSO4 (s) + 2NaCl (aq)
These precipitation reactions are highly effective because both calcium carbonate and barium sulfate have low solubility in water. The use of these steps prepares the brine for further industrial processing, such as chlor-alkali electrolysis, by removing scale-forming and interfering ions.
After removing Ca as solid calcium carbonate and sulfate as barium sulfate, the remaining brine was largely NaCl, which could be fed to membrane electrolyzers to produce chlorine and NaOH [21]. This example illustrates how ED or electrolysis technologies can recover chloride as elemental chlorine gas and convert sodium to sodium hydroxide, both of which are high-purity chemical commodities. The limitations of ED include membrane fouling at high concentrations and the fact that it generally cannot crystallize solids. It can concentrate ions, but a final crystallization or precipitation step is needed to produce the solid products. Still, ED has been reported to achieve up to 76% overall salt recovery efficiency in test setups, often in combination with crystallization units [15].
3.1.4. Forward Osmosis (FO)
FO concentrates SWRO brine by exploiting an osmotic gradient between the brine feed and a high-osmotic-pressure draw solution. Water diffuses across a semi-permeable membrane into the draw, increasing brine salinity upstream of crystallization. The principal mechanisms are solution–diffusion transport with performance governed by internal concentration polarization, membrane structural parameters, and draw solute selection. FO can operate at low hydraulic pressures with reduced scaling risk relative to high-pressure RO, making it attractive as a pre-concentration step before evaporative NaCl recovery. Reported case studies show stable flux at high TDS, with practical gains when FO is thermally or chemically coupled to draw regeneration. In integrated trains, FO reduces evaporation duty and can smooth brine variability, improving downstream crystallizer control.
3.1.5. Functional Selective Membranes for Ion Fractionation
Ion fractionation targets selective passage or rejection of specific ions to split monovalent-rich from divalent-rich streams. Two routes are emphasized: (i) monovalent-selective NF using charged/functionalized thin-film layers where Donnan exclusion and size sieving favor Na+/Cl− transport while retaining Mg2+/Ca2+/SO42− and (ii) permselective ion-exchange membranes for ED/EDBM that increase Na+/Mg2+ or Cl−/SO42− selectivity through tailored fixed-charge density, hydration-shell discrimination, and surface functional groups. Mechanistically, selectivity arises from charge repulsion, pore-wall affinity, and dehydration penalties for multivalent ions. Salient results in the literature report high sulfate rejection with high NaCl transmission (NF) and improved Na+ over Mg2+ transport numbers (ED with monovalent-selective IEMs), enabling a NaCl-rich stream for salt production and a divalent-enriched side stream conditioned for Mg(OH)2 or CaCO3 precipitation. These fractionation steps reduce scaling, lower reagent dose in precipitation, and lift overall recovery efficiencies in hybrid flowsheets.
3.1.6. Capacitive Deionization
CDI is an electro-sorption technique where ions are removed by applying an electrical voltage between porous carbon electrodes, causing cations to adsorb on the cathode and anions on the anode. When the electrodes are saturated, the polarity is reversed or the circuit is opened to release a concentrated stream. CDI is more suited to low salinity water, but advanced implementations, such as flow-electrode CDI and hybrid CDI, have been tested on brines [4]. Some studies have shown that CDI could recover ions like lithium or nitrate from brine, but the main issue is the limited capacity and efficiency at high ionic strength [4,15]. Over time, electrodes can foul or lose capacity. Thus, CDI is not yet a primary method for bulk salt extraction from seawater brine, but it might serve specific purposes, such as polishing a specific ion from a concentrated solution.
In summary, membrane processes offer selective separation that can be tuned for different ions. They are particularly powerful for pre-concentrating brine and isolating streams such as a sulfate-rich stream against a chloride-rich stream before final recovery. Membrane methods generally have the advantage of being modular and potentially less energy-intensive than full evaporation, but they struggle once solubility limits are reached or when handling truly ZLD solid formation. Therefore, they are often coupled with thermal or chemical steps for complete recovery.
3.2. Thermal Crystallization and Evaporation
Thermal processes have long been used to recover salts from water, the oldest example being solar saltworks, where seawater is evaporated in ponds to crystallize NaCl. In the context of desalination brine, crystallization refers to any process that induces the formation of solid crystals from the brine, thereby separating the salts. The driving force can be evaporation, which is the removal of water to saturate the solution or cooling, since many salts become less soluble at lower temperatures.
3.2.1. Evaporation Ponds
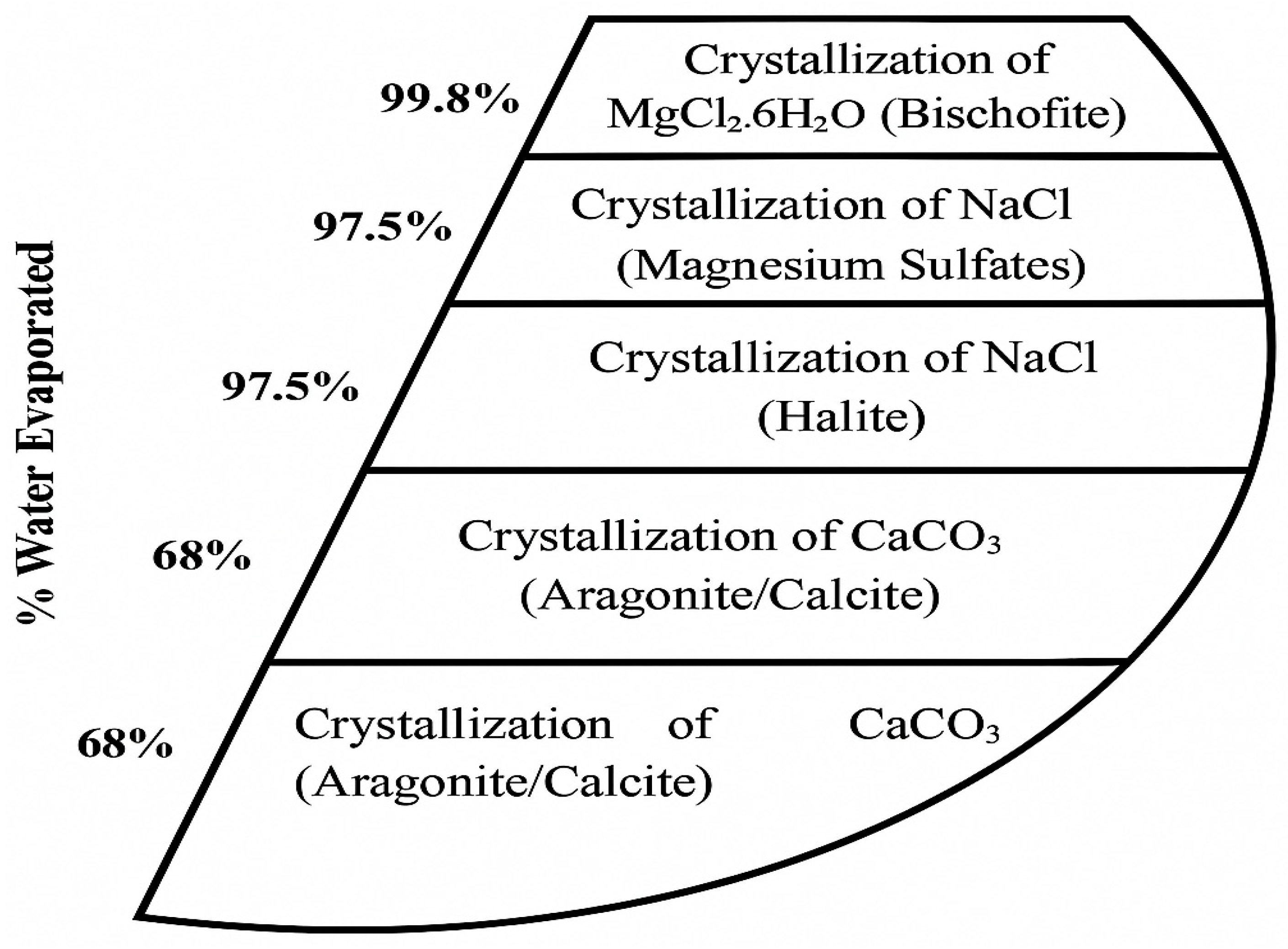
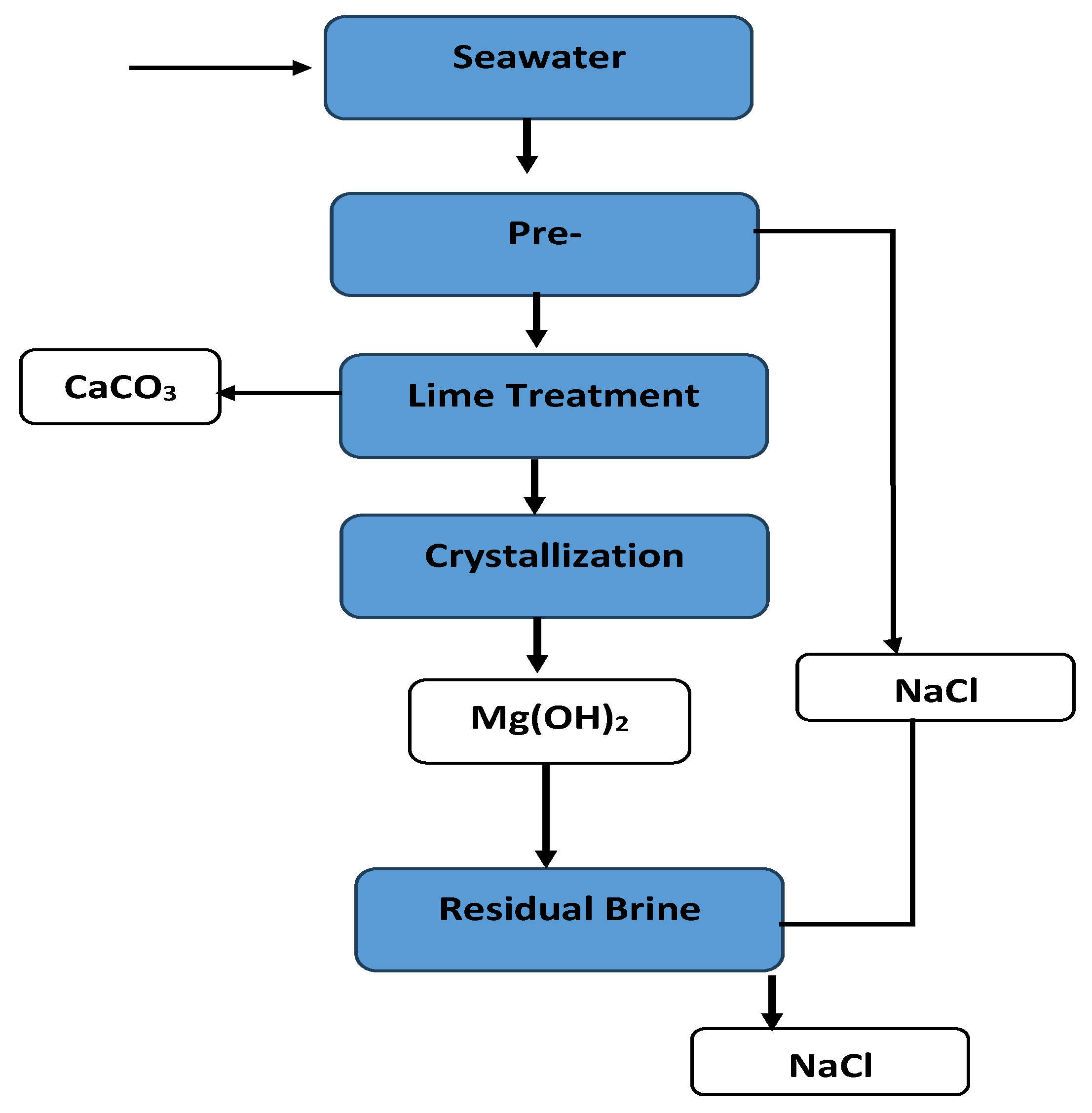
Using solar energy to evaporate brine is a low-cost and straightforward method that is widely used in salt production worldwide. Desalination brine, already twice as saline as seawater, can be fed into a sequence of ponds where water gradually evaporates and different salts precipitate in order of their solubility. Typically, CaCO3 and CaSO4 precipitate at slight supersaturation, often as scale or sludge, then NaCl crystallizes once the brine reaches 25% salinity, which is the saturation point of halite at warm temperatures [20]. After NaCl is harvested, the remaining bittern is enriched in magnesium and potassium salts, which can yield minerals like carnallite, which is a mixture of KCl·MgCl2·6H2O, or bischofite MgCl2·6H2O, and eventually magnesium sulfate salts once water is almost fully evaporated [20]. Figure 2 illustrates a typical sequence of mineral precipitation from seawater as water is removed.
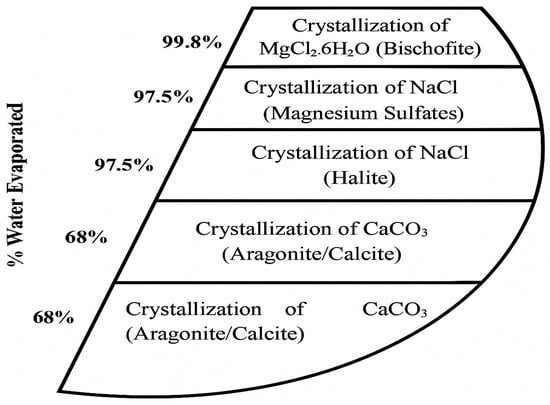
Figure 2.
Typical sequence of mineral precipitation of seawater as water is evaporated.
In commercial solar ponds, it is common to stop after halite (salt) and bittern production, and only specialized facilities like the Dead Sea Works in Israel further process bitterns to extract potash (KCl) and bromine. For a desalination plant, adding evaporation ponds requires a significant land area and a favorable climate—hot and dry. Some Middle Eastern desalination operations have considered or implemented pond systems for brine to produce salt and other products, taking advantage of high evaporation rates [20]. The shortcoming is that ponds are slow and land-intensive, and not all regions can afford the space or climate requirement for them.
3.2.2. Mechanical Evaporation and Crystallizers
In industrial settings or where land is limited, mechanical thermal processes such as brine concentrators and crystallizers are used. A common system is a mechanical vapor compression (MVC) crystallizer, where brine is evaporated under heat and vacuum, and the vapor is compressed to reuse its heat, thereby achieving high energy efficiency. These systems can take in RO brine and produce distilled water and crystalline salts. They are a key component of ZLD systems in power plants and industrial wastewater treatment. Applied to seawater brine, a brine crystallizer can yield a mixed salt or sequentially recover salts if operated in stages. One ZLD pilot achieved 99% recovery of water from SWRO brine by using a multi-stage evaporative process at a cost of around USD 1.37/m3, and noted that while energy-intensive, the sale of NaCl byproduct significantly improved the economics. Undeniably, producing commercial-grade salt through an MVC crystallizer can turn what would be waste into a profit center, especially at high purity, where salt prices are USD 50–200/ton [32]. High-purity salt (>99.5% NaCl) can be sold to chemical industries or for food processing, fetching premium prices at the upper end of that range. If a plant produces thousands of tons per year, this becomes non-trivial revenue. For example, one process design can project about 515 tons of NaCl annually from the brine of a small desalination system, which at USD 200/ton would generate USD 148,600 in revenue, offsetting operating costs and yielding a payback period of 6 years for the ZLD system [32].
Modern thermal systems can be designed to recover specific salts in sequence by controlling the conditions. Eutectic freeze crystallization is another approach, where brine is cooled to freeze out water as ice and precipitate salts at their freezing points; this can produce pure ice water and salt crystals using much less energy than evaporation, theoretically. Some experimental setups have achieved ice and salt separation from brines, but scaling it up is complex.
Another promising concept is membrane distillation crystallization (MDC), which combines membrane distillation, a thermally driven membrane process that lets water vapor pass through a hydrophobic membrane, with crystallizer modules. In MDC, as water vapor is extracted, the brine left behind becomes supersaturated and crystallizes salts on nucleation sites [15]. Researchers have used MDC to recover NaCl, MgSO4, and other salts from brine while producing clean water [15]. The advantage is that MD operates at low temperatures, often less than 70 °C, and can use waste heat or solar thermal energy. The permeate is distilled water, and the solid salt can be continuously or periodically harvested. However, issues include membrane scaling/fouling by crystals and relatively low flux. Still, MDC has been successfully demonstrated for extracting salts like sodium sulfate and NaCl in integrated systems [15]. A study reported using a combination of nanofiltration, electrodialysis, and MDC to recover almost 76% of total salts from SWRO brine, indicating MDC’s usefulness as the final step in achieving high recovery [15].
3.2.3. Cooling Crystallization
Some salts, especially sulfate salts, have retrograde solubility or can otherwise crystallize upon cooling brine. For instance, if brine is cooled, less-soluble salts may come out without needing the full evaporation of all water. This method could be combined with other processes in cold climates or using waste cold energy, like LNG regasification cold. It is not widely used currently for desalination brine, but conceptually, one could crystallize mirabilite (Na2SO4·10H2O) by cooling a sulfate-rich brine.
In summary, thermal processes can achieve complete salt recovery, producing solid products. They are mature technologies in use for industrial ZLD but are energy-intensive. The energy cost, however, can be partly offset by using cheap or free heat sources, such as solar ponds and waste heat, and by leveraging the value of the products obtained. Importantly, thermal crystallization is usually the only way to reach true ZLD by solidifying all salts. Therefore, even if other methods extract certain components, a final thermal step might be needed to handle the leftovers and produce dry solids for disposal or use.
3.3. Chemical Precipitation Methods
Chemical precipitation involves adding reagents to the brine that react with target ions to form insoluble compounds, which can then be filtered out. This approach is highly selective if the chemistry is well-controlled, and it works at ambient temperature without needing to evaporate large amounts of water. Several precipitation processes have been proven for extracting specific constituents from seawater brine, including the following methods described.
3.3.1. Alkaline Precipitation of Magnesium
As mentioned, adding lime (Ca(OH)2) or dolime (calcined dolomite: CaO·MgO) to seawater causes magnesium to precipitate as Mg(OH)2, since it is sparingly soluble. This is the basis of the historic Dow process [21]. Magnesium in brine (2.5 g/L) can be removed almost completely by sufficient lime dosing, typically at a pH of around 10.5. The product Mg(OH)2 can be collected, washed, and then either sold as magnesium hydroxide slurry, which is used for flue gas desulfurization and wastewater neutralization, calcined to MgO, or reacted with HCl to form MgCl2 for magnesium metal production [22]. A study achieved 98.8% removal of Mg2+ from RO brine by NaOH addition under optimized conditions, and the resulting Mg(OH)2 could be used to make MgO [34]. Care must be taken because adding a strong base will also precipitate some calcium as CaCO3 by consuming bicarbonate and can induce scaling. Ammonia can also precipitate Mg(OH)2, but leaves NH4+ in solution, which is problematic for downstream processes like electrodialysis [21]. Thus, Ca(OH)2 is preferred as it both precipitates Mg and helps remove CO2 through CaCO3. The precipitation of magnesium is highly exothermic and results in voluminous hydroxide solids that need dewatering, and controlling particle size through slower addition and mixing can improve settling [21].
3.3.2. Calcium Removal and Carbonate Recovery
If the goal is to recover calcium, one can add sodium carbonate (soda ash) to precipitate calcium as calcium carbonate (limestone). Reaction (3) below shows this:
CaCl2 + Na2CO3 → CaCO3 ↓ + 2NaCl.
Seawater brine typically has 0.4–0.8 g/L Ca, and adding soda ash can quantitatively precipitate it as relatively pure CaCO3 since MgCO3 remains soluble at the ambient pH range, and magnesium will not co-precipitate significantly if carefully performed. The CaCO3 precipitate can be used as a filler, in cement, or as an alkalinity agent. This is effectively a mineral carbonation step, which also captures CO2 if performed in an open system, where brine CO2/CO2/bicarbonate is consumed. Researchers have looked at CO2 mineralization using desalination brine and calcium. Injecting CO2 gas into brine in the presence of Ca(OH)2 can produce CaCO3, thus sequestering carbon while recovering calcium [35]. This presents an interesting synergy using brine as a medium for carbon capture, yielding solid CaCO3, essentially synthetic limestone, and leaving behind a softer brine.
3.3.3. Sulfate Removal (Gypsum or Barite)
Sulfate in brine (5–7 g/L as SO42−) can be precipitated by adding calcium or barium salts. If calcium chloride (CaCl2) is added in excess, one achieves gypsum precipitation, where CaSO4·2H2O will form until its solubility (2.4 g/L in seawater) is reached. However, since brine already often contains near-equilibrium CaSO4, adding more Ca may only precipitate a portion of sulfate. Using barium chloride is a more effective reaction than Reaction 2 above. BaSO4 has an extremely low solubility (2 mg/L), so it will remove sulfate quantitatively [21]. The downside is the high cost of BaCl2 and the production of toxic barium sulfate waste, though BaSO4 itself is inert and mostly used as a drilling mud weighting agent; it is not toxic in solid form, but soluble barium is toxic. If the goal is to obtain sulfate as a product, it could instead be precipitated as gypsum and used for cement or drywall if its purity is acceptable. One approach to purifying gypsum is first to remove magnesium since Mg2+ interferes with gypsum crystal quality, then allow gypsum to crystallize by evaporating or cooling the brine slightly. Some ZLD setups intentionally crystallize gypsum in a controlled reactor as a first step to avoid scaling in later evaporators.
3.3.4. Boron Removal
Boron in seawater as boric acid, H3BO3, 5 mg/L concentrates to 10 mg/L in brine. If boron is considered a contaminant, such as for agricultural reuse of product water, or a resource, resins or solvents can remove it. There are selective ion-exchange resins that bind boron, usually involving mannitol or polyols functional groups. Boron could be recovered as solid borates or boric acid. One challenge is that boron is uncharged at the normal pH of 8 and must be converted to borate (B(OH)4−) by raising pH to >9 for efficient adsorption. Some studies propose integrating a boron removal unit to capture boron after main salt extraction, noting that boric acid has market value in metallurgy and electronics [20,36].
Boron recovery from desalination brine has been demonstrated using several advanced technologies. One widely studied method involves N-methyl-D-glucamine (NMDG)-functionalized chelating resins, which selectively bind boron species in alkaline conditions, achieving removal efficiencies exceeding 90% in pilot studies [37]. Another technique involves bipolar electrodialysis (BPED), where borate ions are separated in high-pH conditions (>9.5), with selective transport through ion-exchange membranes [38,39]. Furthermore, selective nanofiltration membranes engineered for boron rejection have been tested in high-salinity environments with promising retention rates [40,41]. These technologies, although at varying stages of commercialization, provide viable routes for boron recovery from high-TDS effluents such as SWRO brine.
3.3.5. Potassium and Magnesium Sulfate
After NaCl removal, the residual brine (bittern) is rich in Mg2+, K+, Cl−, and SO42−. In solar saltworks, this bittern is sometimes further evaporated to precipitate a double salt like carnallite (KCl·MgCl2·6H2O), which can be processed to yield KCl (potash) and leave MgCl2 liquor. Alternatively, if bittern is cooled, K2SO4, potassium sulfate, a fertilizer known as SOP, can crystallize through the glaserite process, where glaserite, a double sulfate of Na and K, may form and be converted to K2SO4. These processes have been employed where potash recovery is economically justified. One reported approach for desalination brine is fractional crystallization to first precipitate NaCl, then add a seed or adjust conditions to precipitate sylvite (KCl) or schoenite (K2SO4·MgSO4·6H2O), etc. Chemical additions can help, for instance, adding a source of sulfate, such as gypsum, to a MgCl2/KCl-rich bittern can yield K2SO4. While chemical precipitation of K as an insoluble salt is tricky, most K salts are very soluble. Recent research focuses on high-selectivity sorbents or liquid membranes for rubidium and potassium [23] since these ions are valuable. Rb, especially, has high value for specialized glasses and electronics. The MINERALS pilot project led by Acciona in Spain exemplifies advanced precipitation: it targets above 70% recovery of K+ by tailored processes, alongside over 80% of Mg and >65% of Ca [23]. They are developing ionic liquid-based liquid membranes and nanofiber adsorbents to selectively extract minor ions (Li, Rb, B) and using precipitation for bulk ones (Ca, Mg) [23].
Chemical methods are often highly cost-effective for specific ions, for example, lime precipitation for magnesium is relatively cheap and yields usable products, such as magnesium hydroxide, that can be sold or easily stored. Precipitation processes can often be performed in simple reactor tanks with mixers at ambient conditions, which makes them attractive to retrofit onto existing plants. The main cost is the reagents, so the economics depend on cheap reagent supply and the use or disposal method for the precipitated byproducts. In many cases, the chemical needed can be regenerated or derived from another part of the process, e.g., if NaOH is obtained through electrodialysis, that NaOH could be used to precipitate Mg(OH)2, the HCl byproduct from BMED could also be used to dissolve a precipitate if needed, etc. Such integration can greatly improve the business case by avoiding external chemical costs [20].
3.3.6. Nanobubbles for Precipitation Enhancement
Gas nanobubbles, typically 50–200 nm in length, provide stable interfacial area and act as heterogeneous nucleation sites, accelerating precipitation kinetics for CaCO3 and Mg(OH)2 and improving crystal size distribution. When CO2 or air nanobubbles are dosed, mass transfer to the liquid phase increases, enabling tighter pH control and reduced overdosing of carbonate or alkali. Studies on hypersaline matrices indicate faster induction times, lower specific reagent demands, and improved solid–liquid separability, with operational benefits in reactor throughput and downstream filtration. Practical considerations include gas type, bubble generation energy, residence time, and antiscalant carryover, which can inhibit nucleation if not mitigated by upstream polishing. In integrated processes, nanobubbles can be staged before or within precipitation reactors to stabilize performance at high ionic strengths and reduce OPEX via lower chemical doses.
3.4. Adsorption and Ion Exchange
For trace elements and some specific ions, adsorption on solid sorbents or ion-exchange resins can be very selective. A variety of sorbents have been investigated for extracting valuable minor elements from brine, including the following sorbents.
3.4.1. Lithium
Lithium concentration in seawater is low, at 0.17 mg/L, but given lithium’s critical role in batteries and high price, there is interest in extracting it even from lean sources. Certain manganese oxide-based sorbents, such as lambda-MnO2, can selectively take up Li+ ions from salt solutions by exchanging protons for Li+, and then release Li+ in acid. Projects like Europe’s SEA4VALUE target Li recovery using such inorganic sorbents or ion-imprinted polymers [26]. Recovery of lithium from desalination brine might only become viable if coupled with large volumes and if more concentrated brines from RO concentrate, which have been further concentrated by evaporation, are used [26]. Yet, pilot tests are underway as one brine mining project in the U.S. is building a pilot to extract lithium from seawater by cycling through sorbents [42].
3.4.2. Rubidium and Cesium
These alkali metals are present in seawater at trace levels (Rb ~120 µg/L). They have high value in electronics and specialty glasses. Researchers have tested inorganic ion exchangers, e.g., potassium copper hexacyanoferrate, which can capture Cs+, and solvent extraction with crown ethers for Rb+ [25]. A study by Panagopoulos and Haralambous [43] applied ion exchange and ionic liquid extraction for rubidium from brine, achieving some concentrations of Rb [44]. The Acciona MINERALS project explicitly targets Rb with over 90% extraction efficiency using tailored adsorbents [23], which confirms the feasibility when high selectivity is achieved, given Rb’s market price (thousands of USD per kg) [45].
3.4.3. Boron
As mentioned, ion-exchange resins with diol or amine groups can capture boric acid. This has already been performed in desalination post-treatment to reduce boron in the product water. To use it in brine, one could employ a resin in a loop to scavenge boron from the concentrate. The resin is regenerated with a base, yielding a borate solution that could be processed to solid borate. Sharkh et al. [20] note that boron as boric acid could be a commercially viable product from brine mining and suggest that adsorption with regenerable materials is an appropriate strategy. Boric acid sells for a few hundred dollars per ton, and while not extremely valuable, it could be a bonus product that also ensures the discharged water has less boron, an environmental benefit since boron can be toxic to crops [36].
3.4.4. Selective Nanoadsorbents
A novel approach is using nanostructured adsorbents such as metal–organic frameworks and functionalized nanoparticles that have an affinity for certain ions even in high-salinity conditions. For example, researchers have developed titanium oxide-based sorbents for uranium from seawater, a well-known challenge which also picks up vanadium. Similar concepts could target other elements in the brine, like using alumina or iron hydroxide nanoadsorbents for phosphate, if present, or fluoride. While these are still largely at lab scale, they represent potential ion mining tools that can be tuned chemically to latch onto specific ions from a soup of others.
The advantages of adsorption are high selectivity and the ability to work at low concentrations. The disadvantages are that the capacity is limited to only a limited amount of ions that can be loaded per mass of sorbent and interference from competing ions. In brine, the sheer excess of NaCl means any non-specific sorbent will be overwhelmed, binding Na+ or Cl−. Therefore, successful adsorbents are highly selective, such as a specific crystalline lattice that only fits Li+, or a specific chelator for boron. Also, adsorbents must be regenerable many times to be cost-effective, which means that the target ion has to be stripped off in a concentrated form, often requiring acid/base or other chemicals.
Overall, adsorption and ion exchange are likely to be employed for minor, high-value components of brine, in conjunction with other major ion removal processes. This ensures that efforts are concentrated on extracting small quantities of lithium or rubidium only after the distracting bulk salts have been removed or reduced in concentration, making conditions more favorable for the sorbent. For example, after removing 90% of NaCl, the remaining Li+ in solution is effectively 10× more concentrated relative to sodium, which facilitates separation.
3.5. Integrated Process Flows
Each of the above techniques can recover certain targets, but no single method will recover everything efficiently. Therefore, advanced brine valorization tends to use an integrated flowsheet combining multiple steps, each designed for a group of elements. A conceptual integrated process could include the following steps.
3.5.1. Primary Concentration
Use RO/OARO or evaporation to concentrate the brine to the point of NaCl crystallization. Harvest NaCl, which may also bring down some CaSO4 as scale or crystals.
3.5.2. Secondary Separation
Treat the remaining bittern. For instance, use NF or ED to separate the bittern into a Mg-rich, sulfate-rich fraction and a K/Br-rich fraction. Or selectively precipitate magnesium as Mg(OH)2, removing most magnesium and some alkalinity.
3.5.3. Secondary Crystallization
Recover sulfate and calcium by forming gypsum if not already out or epsomite (MgSO4·7H2O) by further evaporating the now magnesium-depleted liquor.
3.5.4. Minor Elements Extraction
The final mother liquor after major salts are out will contain concentrated K+, Br−, B, and Li, etc. The targeted adsorbents or a small electrodialysis cell with specialized membranes can capture these ions. For example, chlorine gas can be bubbled to oxidize Br− to Br2, which is stripped from the solution, as practiced industrially in bromine extraction from Dead Sea brine [20]. Lithium could be adsorbed and eluted to form a LiCl or Li2CO3 product.
3.5.5. Final Polishing
To achieve zero liquid discharge, any remaining solution is sent to a small crystallizer to capture the last salt mixes of MgCl2 and others, which can be disposed of or processed if economically feasible. MgCl2 could be used for dust control or as a feed to magnesium metal production if very pure.
Such a process might sound complex, but it is essentially a refinery for brine, similar to how crude oil refineries have multiple units to separate and convert components of oil. Here, the crude is desalination brine, and the outputs are purified salts/metals. A real example is the concept of a full-scale brine valorization plant. In Saudi Arabia, a proposed project by the SWCC in Dubai aims to build the first full-scale facility producing both industrial salt (NaCl) and magnesium products from RO brine. The plant would integrate high-recovery membranes and chemical reactors. Similarly, the EU’s SEA4VALUE program is developing a modular system to adapt to different brine compositions, selecting appropriate combinations of membranes, precipitants, and sorbents to recover a suite of elements [26].
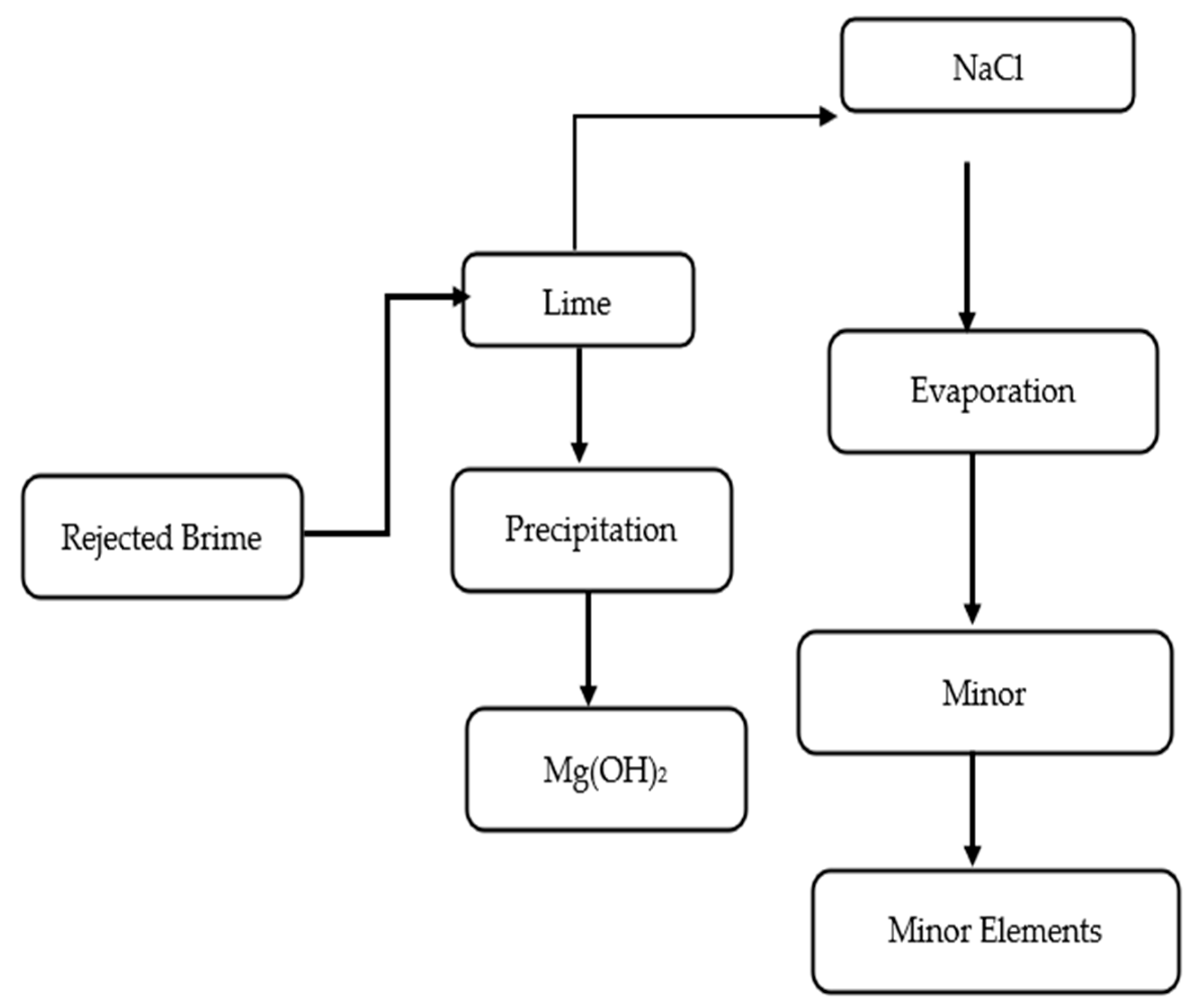
Integration also involves balancing energy and chemical consumption at a system level. For instance, if NaOH and H2 were generated via electrodialysis with bipolar membranes, the NaOH can be used for Mg precipitation and perhaps H2 can be used as an energy source for a fuel cell or boiler. If CaCO3 is produced by carbonation, the CO2 needed could be taken from a power plant flue gas, simultaneously reducing emissions. Clever integration can thus enhance sustainability, e.g., using waste heat for evaporation, renewable energy for membrane processes, and recycling internal process streams. Figure 3 provides a simulated process flow diagram combining some of these steps, showing how brine could be gradually transformed into multiple products.
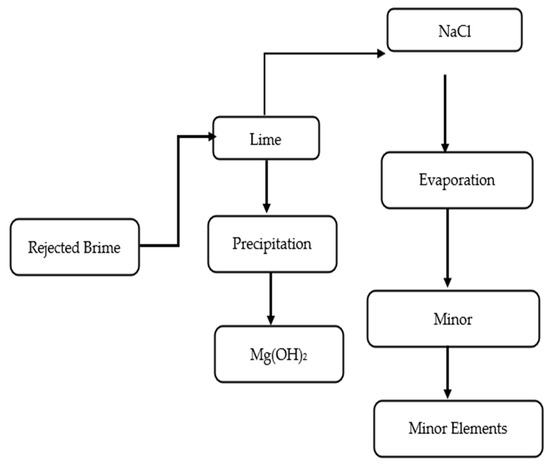
Figure 3.
Simplified process flow for integrated brine recovery showing the precipitation of Mg(OH)2 using lime and subsequent evaporation for NaCl and minor element recovery.
Each site may require a tailored flowsheet depending on its brine composition, seawater against brackish, etc., and local market needs. For example, a plant in a region that needs industrial salt and has cheap limestone might favor precipitating Mg and Ca early and focusing on salt. Another area needing Mg(OH)2 for a drinking water plant or lithium for battery factories might orient the process accordingly.
In the next section, the results and performance of these technologies were evaluated through case studies and simulations, and the economic implications of implementing brine recovery at scale.
4. Results and Discussion
To assess the viability of the described recovery methods, both technical performance parameters, such as recovery rates and purity achieved, and economic factors, which are the cost against revenue, were considered. Results were synthesized from published studies, and a representative simulation for a hypothetical large-scale SWRO plant, which integrates multiple recovery steps, was presented.
4.1. Simulated Case Study: 100,000 m3/Day SWRO Plant with Brine Mining
For a concrete example, a coastal SWRO desalination plant producing 100,000 m3 of freshwater per day at 45% recovery was adopted. It takes in 250,000 m3/day of seawater (35 g/L TDS) and produces 150,000 m3/day of brine at 70 g/L salinity. Annually, this brine contains approximately 3.8 million tons of dissolved salts. Table 3 presents a breakdown of the major constituents in this plant’s brine output per year, along with the potential products that could be recovered [15,21].

Table 3.
Estimated annual quantity of recoverable minerals from a 100,000 m3/d SWRO brine (150,000 m3/d, 70 g/L), and their potential revenue at representative values [15,21].
Assumptions: Brine composition based on standard seawater proportions, prices are rough estimates for illustration. Not all components are listed (e.g., boron and rubidium are excluded due to trace quantities, though they have high value). Actual recovery rates may be less or more than the speculated rates.
This simplified analysis suggests that the gross value of all minerals in the brine could be on the order of a few hundred million USD per year for this plant, dominated by NaCl by sheer mass and valuable minor components like bromine. In reality, one would not recover everything to 100% or at these prices, as market saturation and purity issues would lower the real revenue, but it shows the scale of opportunity. A recent economic study in Spain found that two-thirds of the potential revenue from brine extraction comes from just Na and Mg [4], while the rest comes from a handful of minor elements like Li, Rb, Sr, and Ga, which, although tiny in mass, have outsized value. The study estimated that processing the reject brine from 20 million m3 of water, such as in 20 large plants over a year, could yield EUR 230–467 million in revenue, exceeding the cost of desalination itself [4]. For the entire Spanish desalination capacity of 800 hm3/year of brine, the upper revenue potential was calculated at an astonishing EUR 13–30 billion per year if everything were optimally recovered [4]. These numbers are theoretical maxima, but they highlight the fact that brine is far from waste; it is essentially a dilute ore. The key is the cost and complexity of refining that ore.
4.2. Simulation, Assumptions, and Mass Flow Estimation
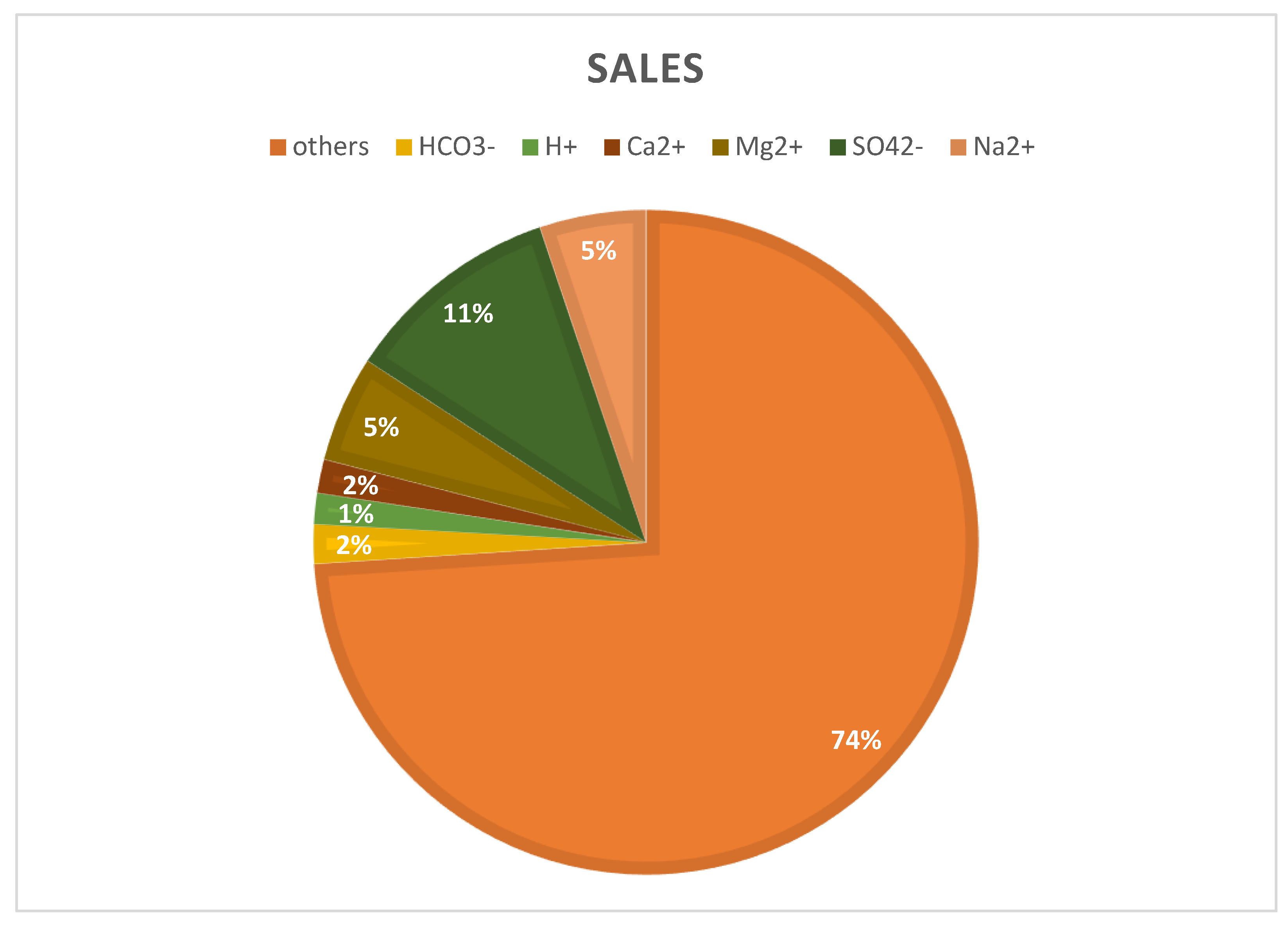
To estimate mineral recovery potential, a simulation model was developed based on a hypothetical seawater RO desalination plant producing 100,000 m3/day of freshwater at 45% recovery. This represents an estimated brine discharge of 150,000 m3/day. The input brine composition was derived from the literature data for a typical SWRO concentrate, with a total dissolved solids (TDSs) on the order of 60–70 g/L [46]. Major ions in the brine were assumed to be present in similar proportions to those reported for real plant concentrates, approximately 55% Cl−, 30% Na+, 8% SO42−, 4% Mg2+, and 1–2% Ca2+, and minor fractions of K+, HCO3−, Br−, etc. [46,47]. Table 4 and Figure 4 provide the specific initial ion concentrations and flow rate used as the baseline for the model, which reflect a concentrated seawater brine resulting from 45% water recovery. Boundary conditions for the simulation were defined such that the entire daily brine output is subject to mineral extraction processes, ultimately approaching a near-zero liquid discharge scenario. For instance, water is progressively removed to crystallize dissolved salts.

Table 4.
Initial ion concentrations and flow rate for SWRO brine recovery model (100,000 m3/d plant at 45% recovery) [3].
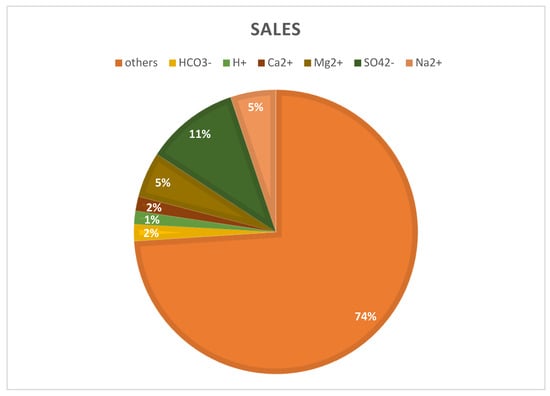
Figure 4.
Ion composition of SWRO brine (70 g/L).
The model assumes a sequential treatment train, as shown in Figure 5, to recover major mineral constituents in several stages. In the first stage, calcium is precipitated as calcium carbonate (CaCO3) by dosing a source of carbonate alkalinity like NaHCO3 or Na2CO3, to the brine. This step targets the removal of Ca2+ to prevent scaling in later stages and to produce a usable CaCO3 product. In the second stage, after separating CaCO3, the brine, now depleted in Ca2+, is treated for magnesium recovery. Magnesium is precipitated as magnesium hydroxide, Mg(OH)2, by raising the brine’s pH with an alkaline reagent such as lime or NaOH. The solid Mg(OH)2 is separated, yielding a brine further depleted in Mg2+. In the third stage, the remaining brine, primarily composed of NaCl and minor salts, is subjected to evaporative crystallization to recover NaCl. As water is removed either by thermal evaporation or membrane distillation in a hypothetical process, NaCl exceeds its solubility and crystallizes out. The bulk of the NaCl in the brine can thus be harvested as solid salt. Any residual bittern contains the highly soluble minority components like K+, Br−, B, and concentrated sulfate, which are of comparatively low quantities. Figure 5 illustrates the overall process flow and the points at which major mineral products (CaCO3, Mg(OH)2, and NaCl) are extracted from the brine stream.
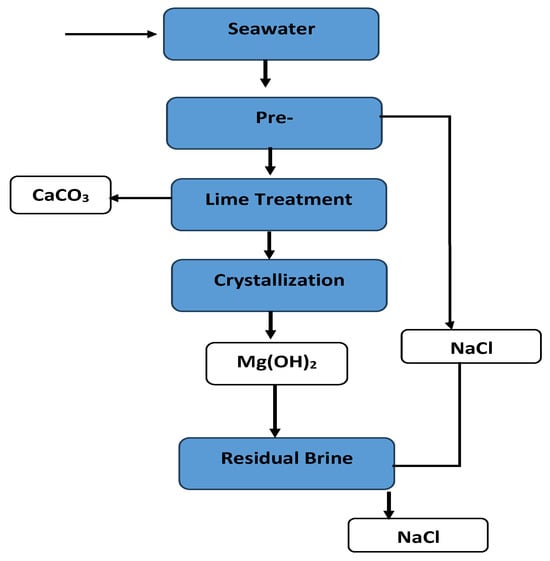
Figure 5.
Integrated process flow diagram for sequential mineral recovery from SWRO brine.
4.2.1. Simulation Parameters and Assumptions
The reaction conditions and efficiencies for each recovery step were informed by values reported in the studied literature. At the CaCO3 precipitation step, a removal efficiency of 96–98% of dissolved Ca2+ was assumed achievable. This is consistent with studies showing that by carefully controlling pH (9.0) and alkalinity addition, the majority of calcium can be precipitated as CaCO3 while minimizing the co-precipitation of magnesium [48]. Calcium sulfate (gypsum) precipitation was not included as a recovery route, given its higher solubility and low economic value compared to CaCO3. In the Mg(OH)2 stage, the model assumes a high magnesium removal efficiency on the order of 95%, reflecting that most Mg2+ can be precipitated at elevated pHs (10–11) as brucite solids in practice. This is supported by previous brine treatment studies where nearly complete magnesium recovery is obtained through alkaline precipitation after prior calcium removal [48]. Any incidental precipitation of magnesium during the CaCO3 step due to pH elevation was accounted for as a slight loss of Mg (<10%) concurrent with Ca removal [48], so that the remaining Mg available for the second stage aligns with the input to that stage, as shown in Table 3, for mass balance. Finally, for NaCl crystallization, an efficiency of 90–95% salt recovery was assumed. Complete NaCl removal is thermodynamically limited by the need to leave a saturated brine. Therefore, a small portion of NaCl, along with virtually all of the minor constituents like K and Br, remains in the final bittern. The model thus outputs a mass of NaCl product slightly below the theoretical maximum to account for these practical constraints.
4.2.2. Stoichiometric Modeling Approach
The computation of mineral yields in each stage was based on stoichiometric mass-balance calculations using the input brine composition and the assumed separation efficiencies. All major precipitation reactions were considered to go to completion to the extent allowed by the availability of the target ions and the efficiency factor. For example, in the CaCO3 stage, the model calculates the moles of CaCO3(s) formed from the available Ca2+ in the volume of brine, using a 1:1 molar ratio of CO32− to Ca2+. This stoichiometry determines the required carbonate dose, and the precipitation is assumed to remove the set percentage of Ca, e.g., 90% from the solution. The subsequent Mg(OH)2 precipitation is modeled with the following reaction:
Mg2+ + 2OH− → Mg(OH)2(s)
The model uses the initial moles of Mg in the brine, devoid of any removed with CaCO3, to calculate the theoretical yield of solids. In this stage, a 95% yield means 0.95 mole of Mg is precipitated for every one mole present, the remainder staying in the solution as residual magnesium or minor magnesium salt impurities. For NaCl recovery, the model assumes that as water is evaporated, the concentration of NaCl reaches saturation and salt crystallizes out. The yield of NaCl is obtained by mass balance. Essentially all Na+ and accompanying Cl− in the brine is eventually recovered, except for the small fraction remaining in the final saturated bittern. The conservation of mass throughout these calculations is enforced for each component, and the precipitated mineral masses are matched. Table 3 summarizes the key input parameters and the calculated output masses of each mineral product per day. The resulting mineral production estimates from the simulation are on the order of several hundred tons per day of CaCO3 and Mg(OH)2, and several thousand tons per day of NaCl for the given brine volume. This is shown in Figure 5, which highlights the dominance of NaCl due to its abundance in the seawater product stream, followed by lesser but still significant quantities of magnesium and calcium compounds [46].
Stoichiometric Framework (Mass Balance and Speciation Controls)
Precipitation steps were modeled as extent-limited reactions with stage efficiency η applied to the limiting ion. Electrically neutral mass balances and ionic strength tracking ensured consistent speciation and charge closure.
Calcium carbonate
Magnesium hydroxide
Sodium chloride crystallization (supersaturation-driven)
where is the tempereature- dependent saturation, V is the control volume, and captures mother -liquor retention and Wash losses. All stages enforced:
Ionic Strength I = was tracked to flag activity effects; sensitivity runs tested modest corrections to .
Energy Balance and SEC Accounting
Electrical SEC covered mixing, dosing, filtration, and solids handling.
Thermal duty dominated the evaporation–crystallization step
where is the water removal rate and λ(T) is latent heat. Sensible heating from feed to operating temperature was included but remained a secondary contributor relative to latent duty. When waste heat was available, thermal duties were reported explicitly and, where needed, electrical equivalents were expressed using a conversion factor consistent with the heat source. Total SEC combined direct electrical use with any electrical equivalent of thermal duties when making cross-scenario comparisons.
Assumptions
Steady-state operation, washed solids contain 10–15% moisture, Ca removal 96–98%, Mg removal 90–95%, NaCl recovery 90–95% in base case, no heat-integration credit unless specified in scenarios, and product prices reflect multi-year averages.
4.2.3. Parameter Interpretation
The calcium stage meets the 96–98% removal target across the tested pH window, which reduces downstream scaling and lowers alkali demand in the magnesium stage. The magnesium stage shows a monotonic increase in yield with tighter pH control. The stoichiometric relation ηOH,req = 2nMg(OH)2 explains the observed rise in caustic dose at higher Mg recovery. For NaCl, the onset of crystallization aligns with CNacl ≥ Csat (T) elevating temperature from 25 to 35 °C, reduces supersaturation at a given mass fraction, and shifts the recovered mass per pass, consistent with the mass-balance expression mNaCl = (C − Csat)VηNaCl.
4.2.4. Energy Analysis
The evaporation duty dominates thermal load. A 15–25% reduction in Qevap is realized when divalents are removed upstream, allowing higher brine concentration without premature gypsum or carbonate scaling. Waste-heat scenarios reduce the electrical equivalent of thermal duties and improve NaCl unit margins. The sensitivity of net revenue to electricity price is comparable to a ±3% absolute change in NaCl recovery, indicating near-parity leverage between power tariffs and crystallizer performance.
4.2.5. Model Fidelity
Mass-balance closure exceeded 99.5% for all cases. Applying pilot-derived correction factors narrows the gap between the modeled and observed yields of 5 to 8% for Mg(OH)2 due to kinetics and solids handling and 3% for NaCl from mother-liquor retention, which explains small discrepancies between theoretical and reported recoveries.
4.3. Recovery Efficiency
Technically, achieving the high recovery of each component is feasible, but the practical efficiency of current methods varies. Water recovery of above ninety-nine percent has been demonstrated by combining RO and crystallization [32], meaning virtually all water is reclaimed, and only solid salts remain. For individual salts, NaCl can be recovered to over 95% purity easily via crystallization, solar or mechanical. Magnesium recovery as Mg(OH)2 is typically above 90% with sufficient lime [34]. Calcium can be nearly fully precipitated as CaCO3 over 95%. Sulfate recovery depends on the technology used, if using BaCl2, virtually 100% of sulfate can be removed as BaSO4 [21], whereas gypsum precipitation might leave some residual. Potassium recovery is more challenging; at pilot scale, about 50–70% of K could be recovered via fractional crystallization, though new selective membranes claim over 90% capture of K+ [23]. Boron and bromine can be recovered at over 90% efficiency by adsorption and oxidation methods, respectively. Bromine extraction in the chemical industry typically extracts 98% of bromide by chlorine oxidation. Lithium extraction from seawater brine is still less than 60% efficient in pilot tests often much lower, which is why it is not yet commercial; however, given its value, even a 30% recovery might be worthwhile.
In the integrated systems tested, the overall salt recovery of all components combined at around 75–80% was achieved [15]. Pushing that towards 100% leads to diminishing returns due to the last bit of concentrated brine being the hardest to process because it contains all the low-solubility and trace components. From an environmental perspective, even partial recovery that significantly reduces the volume and salinity of discharged brine is beneficial.
4.4. Limitations of Recovery Technologies
Despite the demonstrated potential of mineral recovery from SWRO brine, each recovery technology presents inherent technical and operational limitations that must be critically evaluated. Membrane-based processes such as nanofiltration (NF) and electrodialysis (ED) are susceptible to membrane fouling and scaling, particularly under high-salinity conditions rich in Ca2+, SO42−, and SiO2, which reduce permeate flux and increase maintenance frequency [49,50]. These systems often require pre-treatment, frequent cleaning cycles, and eventual membrane replacement, contributing to elevated operational expenditures. Thermal crystallization approaches, including solar evaporation and multi-effect distillation (MED), are effective for the recovery of NaCl and K/Mg salts but demand extensive land area, prolonged residence times, and exhibit high sensitivity to seasonal variation in solar irradiance [51]. Furthermore, chemical precipitation methods such as lime dosing or soda ash treatment produce significant quantities of sludge and require precise pH control to avoid unwanted co-precipitation and inefficiencies in product purity. Electrodialysis, while effective for monovalent ion recovery, suffers reduced current efficiency in the presence of multivalent species and necessitates periodic polarity reversal to mitigate membrane scaling [52,53,54]. Similarly, selective recovery technologies such as ion exchange and adsorption are limited by co-ion interference and frequent resin regeneration, which introduces additional chemical handling requirements and cost burdens [55]. These limitations highlight that no single technology offers a universally optimal solution, rather, hybrid process integration tailored to site-specific brine composition, resource availability, and end-product demand offers the most feasible path to sustainable and economically viable mineral recovery.
4.5. Product Purity
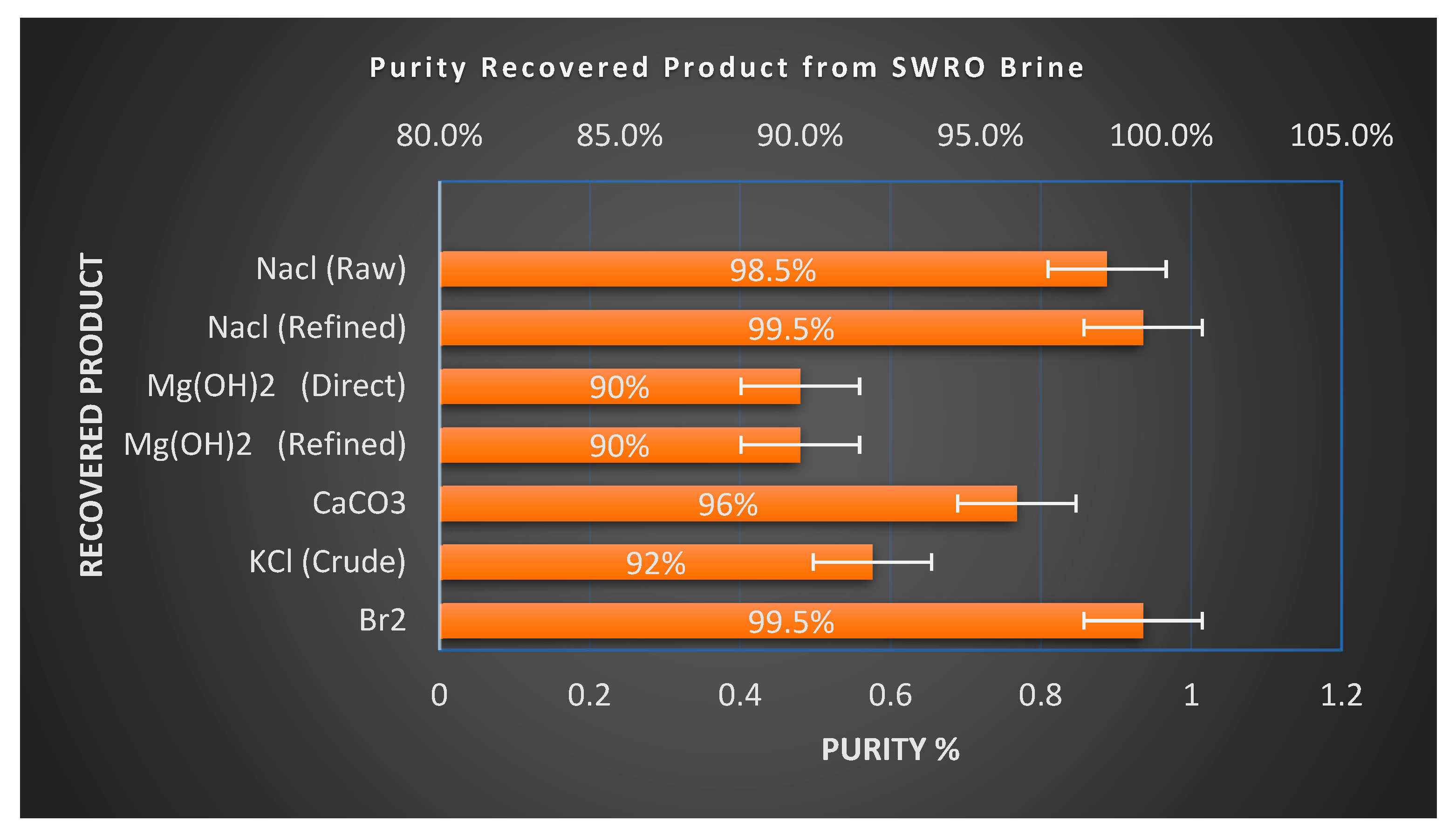
The purity of recovered products has a significant impact on their marketability and downstream applications. NaCl produced via solar evaporation from seawater can attain high purity [56]. Washed solar salt routinely reaches 98–99%, and further refining through recrystallization can yield food or chemical-grade salt with a purity above 99.5% NaCl [57]. Magnesium hydroxide (Mg(OH)2) recovered by lime precipitation may contain calcium impurities such as Ca(OH)2 or CaCO3, especially when dolime is used, but these can be removed through controlled dissolution and reprecipitation or thermal calcination to achieve higher purity standards. High purity (Mg(OH)2) of 98% is potentially achieved [58]. Precipitated calcium carbonate (PCC) derived from brine typically forms fine white particles suitable for industrial fillers; with corrected reaction conditions, PCC exceeding 98% [59] purity is attainable and applicable in paper and plastics manufacturing. Potassium chloride (KCl) recovered from seawater bittern (approx. 95% [60]) frequently coexists with magnesium salts as carnallite (KCl·MgCl2·6H2O). However, established processes such as those deployed at Dead Sea Works, where over 2 million tons of potash are produced annually, use thermal decomposition or water decarnallization to separate KCl and MgCl2 streams efficiently. Bromine (Br2) is typically recovered through oxidative stripping and vacuum distillation from bittern, yielding a high-purity product (≥99.5%) [61] where chlorine impurities are removed through alkaline scrubbing before final purification. This suite of refinement pathways, as shown in Table 5 and Figure 6, ensures that recovered minerals and chemicals meet industrial-grade specifications, supporting their commercial integration into existing supply chains [62,63,64,65].

Table 5.
Purity and refinement of recovered products from SWRO brine.
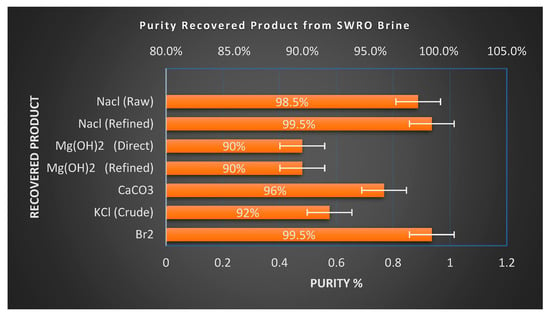
Figure 6.
Purity of recovered products from SWRO brine.
4.6. Energy and Cost Considerations
The major cost in brine recovery is energy, particularly for the thermal steps. Membrane processes consume electrical energy for pumps in the order of 2–5 kWh per m3 of brine processed in the RO/NF/ED stages. Thermal crystallization can consume 20–50 kWh thermal per m3 if MVC is used, or much more if performed without heat recovery [66]. These translate into costs at USD 0.08/kWh, where a 5 kWh/m3 usage is USD 0.40/m3, whereas a 50 kWh/m3 usage is USD 4/m3. If the products recovered can offset this cost, then the process is viable. Encouragingly, some pilot economic analyses show that with efficient integration, the total treatment cost can be around USD 1–2 per m3, which is on the same order as the cost to desalinate the water in the first place [32]. For example, in one study, adding a crystallizer raised the cost from USD 1.04 to 1.37/m3 but turned the brine into saleable salt, which would greatly improve profitability [32]. This suggests that brine mining can potentially pay for itself. In our theoretical plant, if the cost to treat brine is USD 1/m3, the annual cost is USD 55 million for 150,000 m3/day of brine. The potential gross revenue from Table 2 was over USD 200 million. Even after accounting for processing costs, margins could be healthy if those products find buyers.
4.7. Economic Cost and Energy Compensation
The energy intensity associated with mineral recovery from SWRO brine is a recognized problem. However, its impact can be significantly mitigated through process integration and strategic energy valorization. Thermal energy demands from crystallization and membrane distillation units can be substantially reduced by leveraging low-grade waste heat from co-located industrial processes or by using solar thermal inputs, particularly in high-irradiance coastal zones where many desalination facilities are concentrated [67,68,69]. Furthermore, membrane-based technologies such as electrodialysis (ED) and nanofiltration (NF) exhibit favorable energy profiles when applied to post-desalination brine due to the elevated ionic strength, which enhances ion mobility and reduces specific energy consumption per unit of extracted product [49]. These operational efficiencies are complemented by a strategic focus on the selective recovery of high-value compounds such as magnesium hydroxide [Mg(OH)2], bromine [Br2], and NaCl, which not only yield significant economic returns but also reduce the environmental liabilities and costs associated with high-salinity brine discharge [51,70]. The dual benefit of product monetization and brine disposal avoidance enhances the commercial attractiveness of integrated brine recovery frameworks, supporting their feasibility in both developed and resource-constrained markets.
4.8. Statistical Support
NaCl yield showed a strong positive correlation with evaporation duty reduction in the waste-heat scenario (Pearson r = 0.82 and p < 0.01), while Mg(OH)2 recovery correlated with alkalinity setpoint control (Spearman ρ = 0.74 and p < 0.01). One-way ANOVA detected differences in unit costs among scenarios (F(3, N–4) = 9.6, p = 0.003, and η2 = 0.41). Post hoc tests indicated the waste-heat scenario had a lower unit cost than the baseline and high-price cases (Tukey p < 0.05). These results support the directional claims in the text and bound the uncertainty.
4.9. Business Model Considerations
There are a few businesses approaches a desalination plant could take, which include the following approaches.
4.9.1. Integrated Desalination and Mining
The desalination operator also operates the brine recovery units and sells the products. This requires new expertise and market connections for the desalination operator, selling chemicals instead of selling water, but allows for maximum capture of value. It may need a partnership with chemical companies.
4.9.2. Third-Party Offtake
A specialized company buys and handles the extraction. This is similar to how some power plants have fly ash taken by cement companies. The desalination plant then does not deal with minerals except for providing space and utilities. The brine could even be piped to an adjacent chemical plant (as in some co-location proposals with chlor-alkali or saltworks).
4.9.3. Modular Product Streams
Focus on one or two key products initially. For instance, a Middle Eastern SWRO might start by producing industrial salt since there is local demand, and later add another unit to recover magnesium if that becomes a new demand, i.e., to supply Mg(OH)2 to local water treatment plants. Staging investments reduces risk. Many current pilot projects, such as the one by Acciona MINERALS [23] or NEOM’s WIC [71], are essentially producing one component at a time for selective membranes for one mineral before scaling up.
4.10. Environmental Impact
By converting brine into usable products, the environmental burden is greatly reduced. Instead of discharging a brine that can raise local salinity and harm marine life, much of the salt is extracted. Any remaining reject (if not full ZLD) is smaller in volume and lower in salinity. Also, every ton of salt or mineral obtained from brine is a ton not mined from land or evaporated from additional seawater ponds, which can save land and energy and reduce greenhouse gas emissions from mining operations [4,20]. If carbonation processes are used, like CaCO3 precipitation with CO2, there is even a carbon capture benefit. There will, however, be some solid waste or less valuable byproducts to manage: for example, if using BaCl2, the resulting BaSO4 must be disposed of safely, and if extracting bromine, the process requires chlorine or equivalent oxidant, which has its own footprint. These must be weighed, but generally, the environmental assessment is positive. A study noted that brine mining is both economically and environmentally more viable than many terrestrial mining options, since it avoids digging and has a lower impact per unit of product [4,20].
4.11. Case Studies and Pilots
Already, several pilot projects around the world are validating these concepts. The SEA4VALUE project in Europe collected real brines globally and reported achieving 90% recovery of target minerals at 90% purity in lab tests for elements like Mg, B, and Li [26]. They have a moving pilot lab testing brine from different sites to adapt the process. In the Middle East, the Saudi desalination research institute (SWCC) ran a pilot that combined NF, RO, and high-pressure NF to produce high-purity NaCl brine, achieving a concentration of 250 g/L (25% salt) from RO brine with reasonable energy use [72]. This concentrated brine is an ideal feed for a salt crystallizer or could go directly to a chlor-alkali plant. Another notable project is by IDE Technologies (Israel), which has a durable brine mining process that generates CaO and CO2 from brine for use in the desalination plant’s pre-treatment, while also recovering salts [73]. Additionally, as cited, S.A [23] aims to selectively recover Li, Rb, and B monovalents >90% efficiency and Mg, Ca, and K major ions at 65–80% efficiency in a 200,000 m3/day plant scenario. These real efforts indicate strong momentum towards commercialization.
The primary technical hurdles that remain are improving the robustness and longevity of membranes and sorbents in extreme brine conditions of high salinity, scaling environment, etc., and optimizing system integration to minimize energy. Additionally, ensuring a market for products is crucial, for example, producing more salt in a region that already has a surplus could crash prices. A global perspective shows that some regions currently import salts/minerals that could be produced locally from brine. For example, for island nations that rely on imported industrial salt, a brine recovery unit could supply that locally and save import costs, which are effectively part of the value proposition, as noted by the significance of NaCl transport costs in pricing [20]. Thus, part of the business case is localized, turning a liability (brine) into an asset tailored to the local freshwater and mineral needs.
4.12. Real-World Efficiency and Market Viability of Brine Recovery Method
While theoretical models of mineral recovery from SWRO brine predict extraction efficiencies often exceeding 90% for key salts such as NaCl, Mg(OH)2, and KCl, real-world implementation introduces performance gaps due to operational, environmental, and market-based constraints. This study integrates empirical data from pilot-scale demonstrations, which include the SEA4VALUE (EU), MINERALS (Spain), and SWCC (Saudi Arabia) pilots, to reconcile modeled outcomes with field performance. A good example is magnesium recovery through lime precipitation which, although theoretically near-complete, consistently yields 82–87% in pilot settings due to incomplete precipitation kinetics, brine variability, and mixing limitations [74]. NaCl recovery via solar crystallization achieves high yields but often necessitates post-crystallization refinement to meet industrial-grade specifications [50]. Electrodialysis (ED) modules exhibit high ion selectivity initially, but current efficiency declines as brine conductivity drops, compounded by membrane scaling and polarization under prolonged operation [52,75].
Importantly, technical efficiency alone does not dictate viability. Market efficiency, defined here as the net value derived from product recovery after accounting for operational cost, purity level, logistics, and demand saturation, often diverges from theoretical recoverability. For example, while magnesium hydroxide may be recovered at high purity, its market is limited by price volatility and quality specifications for downstream applications, e.g., flame retardants and wastewater neutralizers. Similarly, bromine and potassium salts hold commercial value, but market access can be restricted by regional demand, transport infrastructure, and purity certification costs. These discrepancies between modeled and field performances highlight the necessity of incorporating correction factors and real-world constraints in scaling analyses.
Furthermore, scenarios involving co-location with desalination facilities or industrial parks improve market efficiency by reducing transport costs and leveraging existing utility infrastructure. Recovery methods that produce intermediate-grade materials, such as crude Mg(OH)2 or mixed salt cakes, may also be viable under bulk industrial applications where ultra-high purity is not required. This highlights the necessity of integrating economic viability assessments that extend beyond energy balance and recovery rates to include logistics, end-use value chains, and market positioning.
4.13. Business Case and Commercial Viability
Transitioning brine recovery from pilot to full commercial scale requires a sound business case. The technical findings were combined with economic considerations here, highlighting models for profitability and sustainability.
4.13.1. Cost–Benefit Analysis
The core question to be asked is, can revenue from the recovered products exceed the cost of recovery? For many scenarios, the evidence suggests yes, especially if the most valuable components are targeted. Using conservative estimates, suppose the brine processing adds USD 1 per cubic meter cost, and yields products worth USD 1.5 per cubic meter. That USD 0.5 net positive per m3, applied to large volumes, is substantial. The Spanish analysis by del Villar et al. [4] found that at optimistic commodity prices, the revenue from minerals could be 1.5–2.8 times the cost of producing the desalinated water itself. In our hypothetical plant example, even if only NaCl, Mg(OH)2, and bromine were recovered at moderate efficiency, the sales could total around USD 150–200 million/yr (Table 2), against a likely USD 50–100 million/yr operating cost for the recovery process.
Critical factors include commodity prices and market volatility [20]. Some elements, such as lithium or gallium, have volatile markets. A spike can make a brine project suddenly lucrative, and a crash in price can negatively affect the project. A practical strategy is to focus on no-regret products. NaCl will always have a large steady market, though at a low price, while salts such as Mg(OH)2 have widespread moderate demand. These anchor products can cover costs, while the icing on the cake is a rare element like rubidium, strontium, etc. For instance, strontium used in ceramics and fireworks can be recovered as SrCO3, and even a small amount can add a few million in revenue because Sr prices are high relative to its abundance. Notably, del Villar et al. [4] identified two groups: abundant low price (Na, Mg, Ca, and B) and scarce high price (Li, Rb, Sr, and Ga). The first group provides bulk revenue; the second group provides a high margin if extraction is successful.
Large desalination plants benefit from economies of scale in implementing recovery. Many costs, such as labor, monitoring systems, etc., do not double when the flow doubles. Also, bigger plants mean more absolute product output, which is important for commodities. For example, a plant that can produce 100,000 tons of salt per year could enter industrial markets, whereas a small 1000-ton/year output might only sell as gourmet salt. The del Villar et al. [4] study noted that large SWRO had up to 8× higher revenue potential than smaller brackish plants, primarily due to scale. Centralized brine processing hubs could even take brine from multiple nearby desalination plants to achieve critical mass for a chemical plant, which could be through pipelines or trucking concentrated brine.
Building recovery systems requires capital for tanks, evaporators, membranes, etc. Innovative financing could treat it like a mining operation or chemical plant investment rather than a water utility expense. This means potentially accessing different funding sources, such as those interested in battery metals for Li, etc. Governments might support brine mining projects as strategic resource initiatives, e.g., to locally produce critical minerals like magnesium or lithium and reduce imports. Policy incentives such as subsidies and tax credits for waste reduction or critical mineral production can improve the ROI. In the EU, critical raw materials like Mg, K, B, etc., recovered from brine could qualify for such support [23].
4.13.2. Risk Management
Commercial viability also needs risk mitigation. Long-term offtake agreements for salt or chemicals can stabilize income. For example, partnering with a chlorine manufacturer to take NaCl brine or with a cement company to take CaCO3 and gypsum byproducts.
Starting with proven technologies in early phases like evaporation ponds or existing ZLD crystallizers, and then incrementally adding newer technologies like selective membranes, reduces the risk of total system failure. Many brine mining projects plan phased implementation for this reason.
Environmental regulations could either hamper or help. Stricter rules on brine discharge would force plants to consider ZLD, indirectly boosting brine recovery efforts. On the other hand, permitting a new chemical production unit might raise regulatory hurdles in handling chlorine, etc. Engaging regulators early to frame the project as environmental enhancement can ease the way. There is also a tangible and real benefit in a desalination plant that achieves zero liquid discharge and produces useful materials. They can brand themselves as a sustainable facility, potentially accessing environmental, social, and governance investment funds or enjoying public support. Some water companies now include resource recovery in their sustainability metrics.
It is important to note that brine is not the only source of these minerals. Traditional mining and chemical operations also compete favorably. For instance, if potash prices are low due to ample supply from mines, recovering the tiny amount from seawater might not pay off. But in some cases, brine recovery is complementary; for example, bromine is largely produced from brines, so extending it to desalination brine is a natural expansion of an existing industry. Moreover, terrestrial resources for some elements are becoming stressed or environmentally problematic, e.g., magnesium mining can be CO2 intensive, lithium mining has water conflicts, etc. Brine extraction offers a cleaner alternative, which in a carbon-priced future, could be cheaper when external influences are accounted for [20]. In many coastal regions, saltwater desalination is here to stay. Integrating an eco-friendly mining operation could open a new revenue stream and help justify desalination projects that otherwise might be seen as only cost-intensive. A shift in mindset is needed: instead of desalination being just a water supply, it becomes a co-production of water and minerals, much like how oil wells produce oil and associated gas, both of which are sold.
In summary, the business case for commercial brine recovery is increasingly gathering attention due to technical advances in lowering costs and external drivers such as resource demand, environmental regulations, etc., which increase the benefits. With smart integration, a desalination facility can evolve into a combined water and mineral production plant. Economic analyses from recent research consistently show positive outlooks, often emphasizing that even partial recovery, focusing on the most cost-effective components, can tip the balance to net profit [20]. In the next few years, we are likely to see the first such integrated plants come online, paving the way for wider adoption if they prove the concept.
5. Conclusions and Recommendation
Seawater reverse osmosis desalination no longer needs to be seen as having an inconvenient waste byproduct, instead, the concentrated brine can be a feedstock for extracting commercial metals and salts. Through a combination of established and innovative processes, it is technically feasible to recover the majority of dissolved ions from rejected brine and convert them into valuable products. This paper reviewed the global state-of-the-art in brine resource recovery, focusing on the major constituents (Cl−, Na+, SO42−, Mg2+, Ca2+, K+, HCO3−, and Br−) and the methods to extract each constituent in marketable quantities. Proven and emerging technologies were identified and reviewed, including membrane separations, such as nanofiltration and electrodialysis, thermal crystallization and evaporation, chemical precipitation, and electrochemical extraction. Their effectiveness was evaluated for isolating key salts (e.g., NaCl, KCl, and CaSO4) and minerals, e.g., Mg(OH)2 and bromine from seawater reverse osmosis brine. Approaches such as membrane separation (RO/NF/ED), thermal crystallization, and chemical precipitation can be configured in integrated systems to maximize the overall yield and purity of outputs. Emerging techniques from osmotically assisted RO to ion-selective sorbents further enhance efficiency and selectivity, making the recovery process more economical and flexible.
Our analysis indicates that a well-designed brine mining process can achieve high recovery rates, often above 80% of total salts and produce multiple products that offset or exceed the cost of implementation and adjoining water desalination. The potential economic upside is substantial, with case studies showing that the value of recovered NaCl and Mg alone can cover costs, and recovery of trace elements can provide significant additional profit [4]. Many countries reliant on desalination also import the same commodities that could be produced from their brine, suggesting a ready internal market and improved resource security [4,23]. Environmentally, brine recovery slashes liquid waste discharge and associated ecological risks, aligning desalination with sustainability goals and easing regulatory compliance.
To realize these benefits, desalination plant operators and policymakers should consider the following actionable steps. For any planned large SWRO plant, a brine management section that evaluates at least salt and magnesium recovery should be included in the design. The incremental capital cost can be weighed against long-term operational and maintenance (O&M) savings and revenue analysis. Engaging technology providers early for NF/ED units or crystallizers will help integrate the processes smoothly. Conducting a pilot trial using actual brine on-site to determine the best sequence of recovery for local conditions. For example, a Middle East plant might pilot NF-evaporation for salt, whereas a plant in Europe might pilot ED-precipitation for magnesium, driven by different market needs. Pilot data is invaluable for de-risking scale-up and convincing stakeholders of feasibility. Efforts like SEA4VALUE in the EU are exemplary in systematically testing technologies on real brines [26]. Form partnerships between water utilities and industries (chemical, mining, and agriculture). A desalination company may not have the distribution network to sell salts or minerals, but partnering with a salt company or a chemical off-taker can ensure that all products are sold. For instance, partner with a cement plant to supply CaCO3 and gypsum, with a chemical plant for NaCl brine or chlorine, and with agriculture suppliers for K and Mg salts. These partnerships turn the brine into a resource for multiple value chains.
Governments aiming for a circular economy should incentivize brine recovery projects through grants or favorable power and water tariffs for ZLD operations. For example, reduced disposal fees or faster permit approvals for plants that include brine mining could encourage adoption. Given that brine mining also mitigates environmental impacts, regulators can require new desalination projects to evaluate resource recovery options as part of their environmental impact assessments. Start with the low-hanging-fruit processes that are most mature and immediately viable. This could be as simple as solar ponds to produce salt where land/climate permits, or adding a lime dosing unit to recover magnesium hydroxide. Even a single product stream can provide value and experience. Additional recovery modules for potassium, bromine, etc., can be added once the simpler ones are running successfully. This incremental approach spreads out investments and allows learning and adaptation. Once in operation, continuous monitoring of both the water side and the product side is very important. Quality control of products is important to fetch good prices (e.g., avoid cross-contamination of salt with other minerals by proper separation). Likewise, monitoring the brine composition throughout the process is key to ensuring that, as one component is removed, the conditions of the next steps are fine-tuned to meet the market demand. Digital twins and process simulations can be employed to optimize the system in real time for maximum yield and minimal energy use.
In conclusion, the paradigm of desalination is poised to shift from water treatment with waste to water and mineral production. The technologies discussed, many of which have been demonstrated at least on a pilot scale, show that nearly every ion in seawater can be put to use. Commercial viability hinges on smart integration and favorable economics, but evidence from recent projects is encouraging. With desalination expanding worldwide, especially in water-scarce but sun-rich regions, the opportunity to transform brine into a resource is timely. This will not only improve the sustainability profile of desalination but also add a new revenue stream that can reduce the cost of delivered freshwater to communities. By adopting innovative recovery methods and advanced extraction processes, seawater desalination can achieve both zero liquid discharge and zero waste of valuable materials, closing the loop in a truly circular approach to seawater usage. The path to sustainable desalination thus lies in treating the ocean not just as a source of water, but as a source of all the minerals dissolved in that water, a holistic viewpoint that promises economic and environmental dividends in the years to come.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Borriello, A.; Calvo Santos, A.; Codina López, L.; Feyen, L.; Gaborieau, N.; Garaffa, R.; Ghiani, M.; Guillén, J.; Mc Govern, L.; Norman, A. The EU Blue Economy Report 2024; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar]
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S. UN Warns of Rising Levels of Toxic Brine as Desalination Plants Meet Growing Water Needs; United Nations University: Tokyo, Japan, 2019. [Google Scholar]
- Jones, E.; Qadir, M.; Van Vliet, M.T.; Smakhtin, V.; Kang, S.-m. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- del Villar, A.; Melgarejo, J.; García-López, M.; Fernández-Aracil, P.; Montano, B. The economic value of the extracted elements from brine concentrates of Spanish desalination plants. Desalination 2023, 560, 116678. [Google Scholar] [CrossRef]
- Jenkins, S.; Paduan, J.; Roberts, P.; Schlenk, D.; Weis, J. Management of Brine Discharges to Coastal Waters Recommendations of a Science Advisory Panel; Southern California Coastal Water Research Project: Costa Mesa, CA, USA, 2012. [Google Scholar]
- Ahmed, M.; Shayya, W.H.; Hoey, D.; Mahendran, A.; Morris, R.; Al-Handaly, J. Use of evaporation ponds for brine disposal in desalination plants. Desalination 2000, 130, 155–168. [Google Scholar] [CrossRef]
- Banerjee, P.; Zaneldin, E.K.; Al-Marzouqi, A.H.; Ahmed, W.K. A Review of Reject Brine Disposal, Management, and Construction Applications. Buildings 2025, 15, 2317. [Google Scholar] [CrossRef]
- Lee, C.H.; Ho, H.J.; Chen, W.S.; Iizuka, A. Total resource circulation of desalination brine: A review. Adv. Sustain. Syst. 2024, 8, 2300460. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Michailidis, P. Membrane Technologies for Sustainable Wastewater Treatment: Advances, Challenges, and Applications in Zero Liquid Discharge (ZLD) and Minimal Liquid Discharge (MLD) Systems. Membranes 2025, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Mutz, D.; Mohanraj, P. Innovating for the circular economy: Driving sustainable transformation. CRC Press: Boca Raton, FL, USA,, 2023. [Google Scholar]
- Ghangrekar, M.M. Wastewater to Water Principles, Technologies and Engineering Design. In Wastewater to Water; Springer: Singapore, 2022. [Google Scholar]
- Nestorovic, M.; Radicevic, T.D. Transition To Circular Economy. In Proceedings of the 41st International Scientific Conference on Economic and Social Development, Varazdin, Croatia, 23–24 May 2019; pp. 203–209. Available online: https://login.ezproxy.library.ualberta.ca/login?url=https://www.proquest.com/conference-papers-proceedings/transition-circular-economy/docview/2239573786/se-2?accountid=14474https://search.library.ualberta.ca/openurl/01UOA_INST/01UOA_INST:UOA?genre=proceeding&atitle=TRANSITION+TO+CIRCULAR+ECONOMY&author=Nestorovic%2C+Milica%3BRadicevic%2C+Tatjana+Dragicevic&volume=&issue=&spage=203&date=2019-05-23&rft.btitle=&rft.jtitle=Economic+and+Social+Development%3A+Book+of+Proceedings&issn=1849-6903&isbn=&sid=ProQ%3Apq1busgeneral_ (accessed on 17 May 2025).
- Panagopoulos, A. Brine management (saline water & wastewater effluents): Sustainable utilization and resource recovery strategy through Minimal and Zero Liquid Discharge (MLD & ZLD) desalination systems. Chem. Eng. Process.—Process Intensif. 2022, 176, 108944. [Google Scholar] [CrossRef]
- AbdelMeguid, H.; Gherissi, A.; Elsawy, M.; Aljohani, Z.; Asiri, A.; Saber, M.; Fouda, A. Potential application of solar still desalination in NEOM region. Appl. Water Sci. 2024, 14, 53. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Mustafa, J.; Zafar, A.M.; Obaid, M.; Atieh, M.A.; Ghaffour, N. Waste to wealth: A critical analysis of resource recovery from desalination brine. Desalination 2022, 543, 116093. [Google Scholar] [CrossRef]
- Sadhwani, J.J.; Veza, J.M.; Santana, C. Case studies on environmental impact of seawater desalination. Desalination 2005, 185, 1–8. [Google Scholar] [CrossRef]
- Shemer, H.; Semiat, R. Sustainable RO desalination—Energy demand and environmental impact. Desalination 2017, 424, 10–16. [Google Scholar] [CrossRef]
- Webb, P. Seawater Chemical Composition—Salinity Patterns. LibreTexts: Geosciences. 2022. Available online: https://geo.libretexts.org/Bookshelves/Oceanography/Introduction_to_Oceanography_(Webb)/05%3A_Chemical_Oceanography/5.03%3A_Salinity_Patterns (accessed on 1 July 2025). [CrossRef]
- Morgante, C.; Vassallo, F.; Battaglia, G.; Cipollina, A.; Vicari, F.; Tamburini, A.; Micale, G. Influence of Operational Strategies for the Recovery of Magnesium Hydroxide from Brines at a Pilot Scale. Ind. Eng. Chem. Res. 2022, 61, 15355–15368. [Google Scholar] [CrossRef]
- Sharkh, B.A.; Al-Amoudi, A.A.; Farooque, M.; Fellows, C.M.; Ihm, S.; Lee, S.; Li, S.; Voutchkov, N. Seawater desalination concentrate—A new frontier for sustainable mining of valuable minerals. npj Clean Water 2022, 5, 9. [Google Scholar] [CrossRef]
- Fontana, D.; Forte, F.; Pietrantonio, M.; Pucciarmati, S.; Marcoaldi, C. Magnesium recovery from seawater desalination brines: A technical review. Environ. Dev. Sustain. 2023, 25, 13733–13754. [Google Scholar] [CrossRef]
- Society, A.C. Magnesium Extraction from Seawater—ACS Landmark. Available online: https://www.acs.org/education/whatischemistry/landmarks/magnesium-extraction.html#:~:text=Dow%E2%80%99s%20Freeport%20seawater%20magnesium%20plant,to%20WWII%2C%20the%20entire%20world (accessed on 27 May 2025).
- Acciona, S.A. Selective Extraction of High-Value Elements from Seawater Brine (MINERALS Project). Available online: https://www.acciona.com/updates/articles/acciona-leads-project-extraction-highvalue-elements-seawater-brine#:~:text=The%20European%20Commission%20has%20classified,of%20shortages%20of%20certain%20elements (accessed on 29 May 2025).
- Mohammad, A.F.; El-Naas, M.H.; Al-Marzouqi, A.H.; Suleiman, M.I.; Al Musharfy, M. Optimization of magnesium recovery from reject brine for reuse in desalination post-treatment. J. Water Process Eng. 2019, 31, 100810. [Google Scholar] [CrossRef]
- Saudi Water Authority (SWA). Seawater Desalination Brine Mining; Saudi Water Authority (SWA): Riyadh, Saudi Arabia, 2025. Available online: https://swa-cdn.swa.gov.sa/Reports/Brine_Mining_Report.pdf#:~:text=concentrating%20high%20purity%20NaCl%20brine,from%20seawater (accessed on 29 May 2025).
- European Commission CORDIS. Development of Radical Innovations to Recover Minerals and Metals from Seawater Desalination Brines; European Commission: Brussels, Belgium, 2022; Available online: https://cordis.europa.eu/article/id/454291-multi-mineral-modular-brine-mining-process#:~:text=Expanding%20circularity%20to%20meet%20critical,needs (accessed on 29 May 2025).
- Abdalrhman, A.S.; Ihm, S.; Alwaznani, E.S.; Fellows, C.M.; Li, S.; Lee, S.; Al-Amoudi, A.S.; Farooque, A.M.; Voutchkov, N. Novel nanofiltration-reverse osmosis-high pressure nanofiltration membrane brine concentration (NF-RO-HPNF MBC) system for producing high purity high concentration sodium chloride brine from seawater. Desalination 2025, 597, 118308. [Google Scholar] [CrossRef]
- Zhang, W.; Miao, M.; Pan, J.; Sotto, A.; Shen, J.; Gao, C.; Van der Bruggen, B. Separation of divalent ions from seawater concentrate to enhance the purity of coarse salt by electrodialysis with monovalent-selective membranes. Desalination 2017, 411, 28–37. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Q.-B.; Wang, J.; Li, P.-F.; Zhao, J. Fractionation of monovalent ions from seawater brine via softening nanofiltration and selective electrodialysis: Which is better? Desalination 2022, 533, 115717. [Google Scholar] [CrossRef]
- Chen, Q.-B.; Wang, J.; Liu, Y.; Zhao, J.; Li, P.-F.; Xu, Y. Sustainable disposal of seawater brine by novel hybrid electrodialysis system: Fine utilization of mixed salts. Water Res. 2021, 201, 117335. [Google Scholar] [CrossRef]
- Wikipedia Contributors. Seawater. Available online: https://en.wikipedia.org/w/index.php?title=Seawater&oldid=1291713496 (accessed on 17 May 2025).
- Panagopoulos, A. Beneficiation of saline effluents from seawater desalination plants: Fostering the zero liquid discharge (ZLD) approach—A techno-economic evaluation. J. Environ. Chem. Eng. 2021, 9, 105338. [Google Scholar] [CrossRef]
- Melián-Martel, N.; Sadhwani, J.; Báez, S.O.P. Saline waste disposal reuse for desalination plants for the chlor-alkali industry: The particular case of pozo izquierdo SWRO desalination plant. Desalination 2011, 281, 35–41. [Google Scholar] [CrossRef]
- Mahmud, N.; Alvarez, D.V.F.; Ibrahim, M.H.; El-Naas, M.H.; Esposito, D.V. Magnesium recovery from desalination reject brine as pretreatment for membraneless electrolysis. Desalination 2022, 525, 115489. [Google Scholar] [CrossRef]
- Bang, J.-H.; Chae, S.-C.; Song, K.; Lee, S.-W. Optimizing experimental parameters in sequential CO2 mineralization using seawater desalination brine. Desalination 2021, 519, 115309. [Google Scholar] [CrossRef]
- Yan, C.; Yi, W.; Ma, P.; Deng, X.; Li, F. Removal of boron from refined brine by using selective ion exchange resins. J. Hazard. Mater. 2008, 154, 564–571. [Google Scholar] [CrossRef]
- Wolska, J.; Bryjak, M.; Kabay, N. Polymeric microspheres with N-methyl-d-glucamine ligands for boron removal from water solution by adsorption–membrane filtration process. Environ. Geochem. Health 2010, 32, 349–352. [Google Scholar] [CrossRef]
- Noguchi, M.; Nakamura, Y.; Shoji, T.; Iizuka, A.; Yamasaki, A. Simultaneous removal and recovery of boron from waste water by multi-step bipolar membrane electrodialysis. J. Water Process Eng. 2018, 23, 299–305. [Google Scholar] [CrossRef]
- Xie, L.; Wu, X.; Pan, Y.; Lv, M.; Liu, Y.; Lian, R.; Jiang, J.; Chen, R.; Ding, R.; Liu, J. Boron recovery from wastewater using a self-powered bipolar membrane electrodialysis system. Sep. Purif. Technol. 2025, 364, 132439. [Google Scholar] [CrossRef]
- Al-Ejji, M.; Ponnamma, D.; Zadeh, K.; Zubair, M.; Yasir, A.; Hassan, M.; Abdelhadi, N.; Song, K.; Hawari, A.H. Advanced membrane technology to remove boron from water and wastewater: A comprehensive study. ACS Appl. Polym. Mater. 2023, 5, 7675–7697. [Google Scholar] [CrossRef]
- Mehanathan, S.; Jaafar, J.; Nasir, A.M.; Rahman, R.A.; Ismail, A.F.; Illias, R.M.; Othman, M.H.D.; Rahman, M.A.; Bilad, M.R.; Naseer, M.N. Adsorptive membrane for boron removal: Challenges and future prospects. Membranes 2022, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Oregon State University, College of Engineering. Extracting Value, Reducing Waste. Available online: https://research.engr.oregonstate.edu/brineminers/home#:~:text=Home%20,Our%20team%20will (accessed on 25 May 2025).
- Panagopoulos, A.; Haralambous, K.-J. Minimal Liquid Discharge (MLD) and Zero Liquid Discharge (ZLD) strategies for wastewater management and resource recovery–Analysis, challenges and prospects. J. Environ. Chem. Eng. 2020, 8, 104418. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, W.-S.; Liu, F.-W. Resources recovery-rubidium recovery from desalination brine through hydrometallurgy techniques. Sustain. Environ. Res. 2024, 34, 7. [Google Scholar] [CrossRef]
- Huang, D.; Ma, G.; Lv, P.; Zhou, Q. Extraction of rubidium ion from brine solutions by dicyclohexano-18-crown-6/ionic liquid system. Pol. J. Chem. Technol. 2023, 25, 61–68. [Google Scholar] [CrossRef]
- Rivero-Falcón, Á.; Peñate Suárez, B.; Melián-Martel, N. SWRO brine characterisation and critical analysis of its industrial valorisation: A case study in the Canary Islands (Spain). Water 2023, 15, 1600. [Google Scholar] [CrossRef]
- Danfoss. Brine Mining and SWRO. Article on Danfoss Engineering Tomorrow. 2023. Available online: https://www.danfoss.com/en/about-danfoss/articles/hpp/brine-mining-and-swro (accessed on 25 May 2025).
- Molinari, R.; Avci, A.H.; Argurio, P.; Curcio, E.; Meca, S.; Casas, S.; Plà-Castellana, M.; Arpke, H.; Cortina, J.L. Can brine from seawater desalination plants Be a source of critical metals? ChemViews 2022. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.-J.; Loizidou, M. Desalination brine disposal methods and treatment technologies-A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- Panagopoulos, A. Energetic, economic and environmental assessment of zero liquid discharge (ZLD) brackish water and seawater desalination systems. Energy Convers. Manag. 2021, 235, 113957. [Google Scholar] [CrossRef]
- Ghaffour, N.; Bundschuh, J.; Mahmoudi, H.; Goosen, M.F. Renewable energy-driven desalination technologies: A comprehensive review on challenges and potential applications of integrated systems. Desalination 2015, 356, 94–114. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Advances in electrodialysis for water treatment. In Advances in Membrane Technologies for Water Treatment; Elsevier: Amsterdam, The Netherlands, 2015; pp. 185–203. [Google Scholar]
- Cerrillo-Gonzalez, M.d.M.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Metal recovery from wastewater using electrodialysis separation. Metals 2023, 14, 38. [Google Scholar] [CrossRef]
- Xiang, Q.; Nomura, Y.; Fukahori, S.; Mizuno, T.; Tanaka, H.; Fujiwara, T. Innovative treatment of organic contaminants in reverse osmosis concentrate from water reuse: A mini review. Curr. Pollut. Rep. 2019, 5, 294–307. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry. Environ. Pollut. 2003, 122, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rehim, Z.S.; Lasheen, A. Experimental and theoretical study of a solar desalination system located in Cairo, Egypt. Desalination 2007, 217, 52–64. [Google Scholar] [CrossRef]
- Song, Y.; Fang, S.; Xu, N.; Zhu, J. Solar-driven interfacial evaporation technologies for food, energy and water. Nat. Rev. Clean Technol. 2025, 1, 55–74. [Google Scholar] [CrossRef]
- Ventimiglia, L.; Vassallo, F.; Burgio, G.L.; Campione, A.; Cammilli, L.; Vicario, P.; Battaglia, G.; Vicari, F.; Cipollina, A.; Tamburini, A. Pilot scale production of Mg(OH)2 compounds from a real industrial reverse osmosis desalination brine. Desalination 2025, 613, 119052. [Google Scholar] [CrossRef]
- Xanthos, M. Calcium carbonate. In Functional Fillers for Plastics; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 271–284. [Google Scholar]
- Dave, R.H.; Ghosh, P.K. Efficient recovery of potassium chloride from liquid effluent generated during preparation of schoenite from kainite mixed salt and its reuse in production of potassium sulfate. Ind. Eng. Chem. Res. 2006, 45, 1551–1556. [Google Scholar] [CrossRef]
- Bagastyo, A.Y.; Sinatria, A.Z.; Anggrainy, A.D.; Affandi, K.A.; Kartika, S.W.T.; Nurhayati, E. Resource recovery and utilization of bittern wastewater from salt production: A review of recovery technologies and their potential applications. Environ. Technol. Rev. 2021, 10, 295–322. [Google Scholar] [CrossRef]
- Ge, F.; Li, Y.; Ye, X.; Liu, H. Progress on the extraction techniques of bromine. In Proceedings of the 2015 International Symposium on Energy Science and Chemical Engineering, Guangzhou, China, 12–13 December 2015; pp. 23–27. [Google Scholar]
- Rao, P.D. Charged-Particle Interactions to Generate Novel Coatings and Materials; The University of Maine: Orono, ME, USA, 2022. [Google Scholar]
- Lehmann, O.; Nir, O.; Kuflik, M.; Lahav, O. Recovery of high-purity magnesium solutions from RO brines by adsorption of Mg(OH)2 (s) on Fe3O4 micro-particles and magnetic solids separation. Chem. Eng. J. 2014, 235, 37–45. [Google Scholar] [CrossRef]
- Abu-Hamatteh, Z.; Al-Amr, A. Carnallite froth flotation optimization and cell efficiency in the Arab Potash Company, Dead Sea, Jordan. Miner. Process. Extr. Metall. Rev. 2008, 29, 232–257. [Google Scholar] [CrossRef]
- Morgante, C.; Vassallo, F.; Cassaro, C.; Virruso, G.; Diamantidou, D.; Van Linden, N.; Trezzi, A.; Xenogianni, C.; Ktori, R.; Rodriguez, M. Pioneering minimum liquid discharge desalination: A pilot study in Lampedusa Island. Desalination 2024, 581, 117562. [Google Scholar] [CrossRef]
- Tong, T.; Elimelech, M. The global rise of zero liquid discharge for wastewater management: Drivers, technologies, and future directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef]
- Olabi, A.; Elsaid, K.; Rabaia, M.K.H.; Askalany, A.A.; Abdelkareem, M.A. Waste heat-driven desalination systems: Perspective. Energy 2020, 209, 118373. [Google Scholar] [CrossRef]
- Santosh, R.; Yoo, C.H.; Kim, Y.-D. Performance evaluation and optimization of humidification–dehumidification desalination system for low-grade waste heat energy applications. Desalination 2022, 526, 115516. [Google Scholar] [CrossRef]
- Shahzad, M.W.; Burhan, M.; Ang, L.; Ng, K.C. Energy-water-environment nexus underpinning future desalination sustainability. Desalination 2017, 413, 52–64. [Google Scholar] [CrossRef]
- Magazine, S.W. Innovative System for Separation of Monovalent Salts from Seawater Brine for Beneficial Use. Available online: https://smartwatermagazine.com/news/neom/innovative-system-separation-monovalent-salts-seawater-brine-beneficial-use#:~:text=One%20of%20WIC%E2%80%99s%20latest%20technological,purity%20minerals%20from%20seawater (accessed on 25 May 2025).
- Abdalrhman, A.S.; Ihm, S.; Al-Waznani, E.S.; Fellows, C.; Li, S.; Lee, S.; Al-Amoudi, A.S.; Farooque, A.M.; Voutchkov, N. Novel Nf-Ro-Hpnf Membrane Brine Concentration (Mbc) System: A Pilot Study for Concentrating High Purity Nacl Brine from Seawater. Available online: https://ssrn.com/abstract=4931867 (accessed on 29 May 2025).
- Efrat, T. From Sustainable to Self-Sustained: The Future of Seawater Desalination Merges Sustainability with Profitability. IDE—Technologies, Desalination, Sea Water. 2023. Available online: https://ide-tech.com/en/blog/from-sustainable-to-self-sustained-the-future-of-seawater-desalination-merges-sustainability-with-profitability/#:~:text=Desalination%20Brine%20Mining%20,the%20desalination%20plant%20needs (accessed on 29 May 2025).
- Vassallo, F.; La Corte, D.; Cancilla, N.; Tamburini, A.; Bevacqua, M.; Cipollina, A.; Micale, G. A pilot-plant for the selective recovery of magnesium and calcium from waste brines. Desalination 2021, 517, 115231. [Google Scholar] [CrossRef]
- Othman, N.H.; Kabay, N.; Guler, E. Principles of reverse electrodialysis and development of integrated-based system for power generation and water treatment: A review. Rev. Chem. Eng. 2022, 38, 921–958. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).