Abstract
The Black Sea is a semi-enclosed basin subject to intense anthropogenic pressures and transboundary pollution, making reliable and comparable monitoring data essential for large-scale environmental assessments. However, national practices differ considerably, hindering data integration and coordinated reporting under international frameworks. This study, conducted within the Horizon 2020 project “Advancing Black Sea Research and Innovation to Co-develop Blue Growth within Resilient Ecosystems” (BRIDGE-BS), evaluated pollutant surveillance methodologies with a focus on heavy metals and priority organic contaminants (polycyclic aromatic hydrocarbons, polychlorinated biphenyls, organochlorine pesticides). Standard Operating Procedures (SOPs) were collected from institutions across Black Sea countries and systematically compared for water, sediment, and biota matrices. The analysis revealed shared reliance on internationally recognized techniques but also heterogeneity in sediment fraction selection, digestion and extraction conditions, instrumental approaches, and quality assurance/quality control (QA/QC) documentation. To complement this assessment, an intercalibration (IC) exercise was organized through the QUASIMEME proficiency testing scheme, accompanied by a follow-up structured questionnaire sent to participant institutions. While individual results remain confidential, collective feedback highlighted common challenges in calibration, blank correction, certified reference materials (CRMs) availability, digestion variability, instrument maintenance, and the reporting of uncertainty and detection limits. Together, these findings confirm that harmonization in the Black Sea requires not only improved comparability of laboratory methods but also the future alignment of assessment methodologies, including indicators and thresholds, to support coherent, basin-wide environmental evaluations under regional conventions and EU directives.
1. Introduction
The Black Sea is a semi-enclosed basin bordered by six countries and subject to complex geopolitical, ecological, and environmental pressures. It is recognized as one of the most degraded regional seas in Europe due to a combination of eutrophication, toxic contamination, and sediment and litter inputs, many of which originate from upstream river basins and diffuse land-based sources [1,2,3,4,5]. These transboundary impacts require an integrated and consistent observation framework to accurately assess environmental status, identify pollution sources, and support evidence-based marine management.
Several sea-wide frameworks, such as the Black Sea Integrated Monitoring and Assessment Program (BSIMAP) under the Black Sea Commission, have promoted collaborative ecological assessments. However, discrepancies persist in national monitoring practices, particularly concerning chemical pollutant analysis. Variations in sampling, preservation, extraction, instrumental techniques, and QA/QC procedures hinder the comparability and usability of data across institutions and jurisdictions. These discrepancies challenge coordinated assessment and reporting under international mandates such as the Marine Strategy Framework Directive (MSFD), the Water Framework Directive (WFD), and the Bucharest Convention.
Over the past decade, cross-border initiatives such as EC DG ENV project “MSFD Guiding Improvements in the Black Sea Integrated Monitoring System” (MISIS), EU-UNDP project “Improving Environmental Monitoring in the Black Sea” (EMBLAS) (https://emblasproject.org/), and ENI CBC Black Sea Basin Project “Assessing the vulnerability of the Black Sea marine ecosystem to human pressures” (ANEMONE) (https://www.anemoneproject.eu/) [6] have worked to improve assessment capacity and foster better methodological alignment. These projects contributed significantly to developing sub-basin datasets, improving access to equipment and reference materials, and raising awareness on the importance of quality assurance. Despite these advances, differences in analytical methods, matrix treatment, and detection thresholds persist, particularly in heavy metals (HMs) and persistent organic pollutants (POPs) methods, limiting the cross-border interoperability of results.
The Horizon 2020 project “Advancing Black Sea Research and Innovation to Co-develop Blue Growth within Resilient Ecosystems” (BRIDGE-BS) (2021–2025) (https://bridgeblacksea.org/) builds on this legacy with a broader, transdisciplinary approach. BRIDGE-BS aims to support science-based governance of the Black Sea through innovative research and improved assessment tools. Its Work Package 1 (WP1) focuses on harmonizing evaluation strategies across multiple disciplines, including pollution, biodiversity, ecosystem health, and marine litter. Within WP1, Task 1.3 targeted, among others, the review and comparison of Standard Operating Procedures (SOPs) for chemical pollution surveillance, namely heavy metals (HMs) and organic contaminants (POPs) such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and organochlorine pesticides (OCPs).
The rationale for standardization is well established. Since the 1990s, European marine monitoring programs have recognized that variations in laboratory practices, even when nominally applying the same methods, lead to substantial discrepancies in data quality and interpretation. Such variations undermine ecosystem health assessment, obscure trends, and can misinform policy or regulatory action. As highlighted in early European QA frameworks [7,8], effective marine environmental protection depends not only on equipment or capacity, but also on shared protocols, traceable QA/QC frameworks, and interlaboratory performance testing. This is particularly critical for HMs and POPs, which, due to their persistence, toxicity, and potential for bioaccumulation, are priority substances under the Water Framework Directive (WFD) “Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for Community action in the field of water policy”, Marine Strategy Framework Directive (MSFD) “Directive 2008/56/EC of the European Parliament and of the Council establishing a framework for community action in the field of marine environmental policy”, and regional seas conventions (HELCOM “Convention on the Protection of the Marine Environment of the Baltic Sea Area”, OSPAR “Convention for the Protection of the Marine Environment of the North-East Atlantic”, Barcelona Convention “Convention for the Protection of the Marine Environment and the Coastal Region of the Mediterranean”, Bucharest Convention “Convention on the Protection of the Black Sea Against Pollution”).
Recent sea-wide assessments reinforce these concerns. The Interreg VB Adriatic–Ionian (ADRION) Programme project HarmoNIA—”Harmonization and Networking for contaminant assessment in the Ionian and Adriatic Seas” (ADRION 340, 2018–2020) (https://harmonia.adrioninterreg.eu/), which reviewed chemical assessment strategies across the Adriatic–Ionian region, identified widespread heterogeneity in sample preparation, sediment grain size selection, extraction techniques, and QA documentation, even among countries operating under shared EU and Barcelona Convention frameworks [9,10]. Only a small fraction of pollutants was consistently measured across institutions, and major differences were found in how data were normalized, quality-flagged, and reported. These findings echo the challenges now faced in the Black Sea context.
Harmonization efforts in BRIDGE-BS addressed these challenges by:
- Documenting and comparing SOPs for water, sediment, and biota across Black Sea institutions, identifying similarities, differences, and key parameters affecting data comparability;
- Validating SOPs through intercalibration (IC) exercises with QUASIMEME, focusing on trace metals and PAHs in sediments;
- Promoting shared QA/QC frameworks incorporating certified reference materials, detection limit alignment, and standardized metadata;
- Recommending adoption of harmonized protocols within Black Sea Integrated Monitoring and Assessment Programme (BSIMAP) (https://blackseacommission.org/_bsimap_description.php) to ensure coherent transboundary assessments.
This paper synthesizes methodological comparison and IC findings, providing practical insights for advancing coordinated pollutant monitoring in the Black Sea. By embedding harmonized protocols and regular IC participation into large-scale practice, the comparability, quality, and policy relevance of environmental data can be significantly enhanced. In the framework of BRIDGE-BS, SOPs were collected from national institutions in Romania, Ukraine, Georgia, and Türkiye and reviewed across all major steps: sampling, preservation, extraction, instrumental analysis, and QA/QC. The IC exercise provided a practical evaluation of analytical performance for selected trace metals and PAHs in sediments, highlighting strengths and gaps in regional capacity.
These efforts contribute to a broader European legacy of methodological alignment and analytical validation. By localizing these principles to the Black Sea setting, the findings from BRIDGE-BS offer practical insights and targeted recommendations for advancing the harmonization of pollutant evaluation practices in support of cross-border ecological evaluation and transboundary cooperation.
2. Materials and Methods
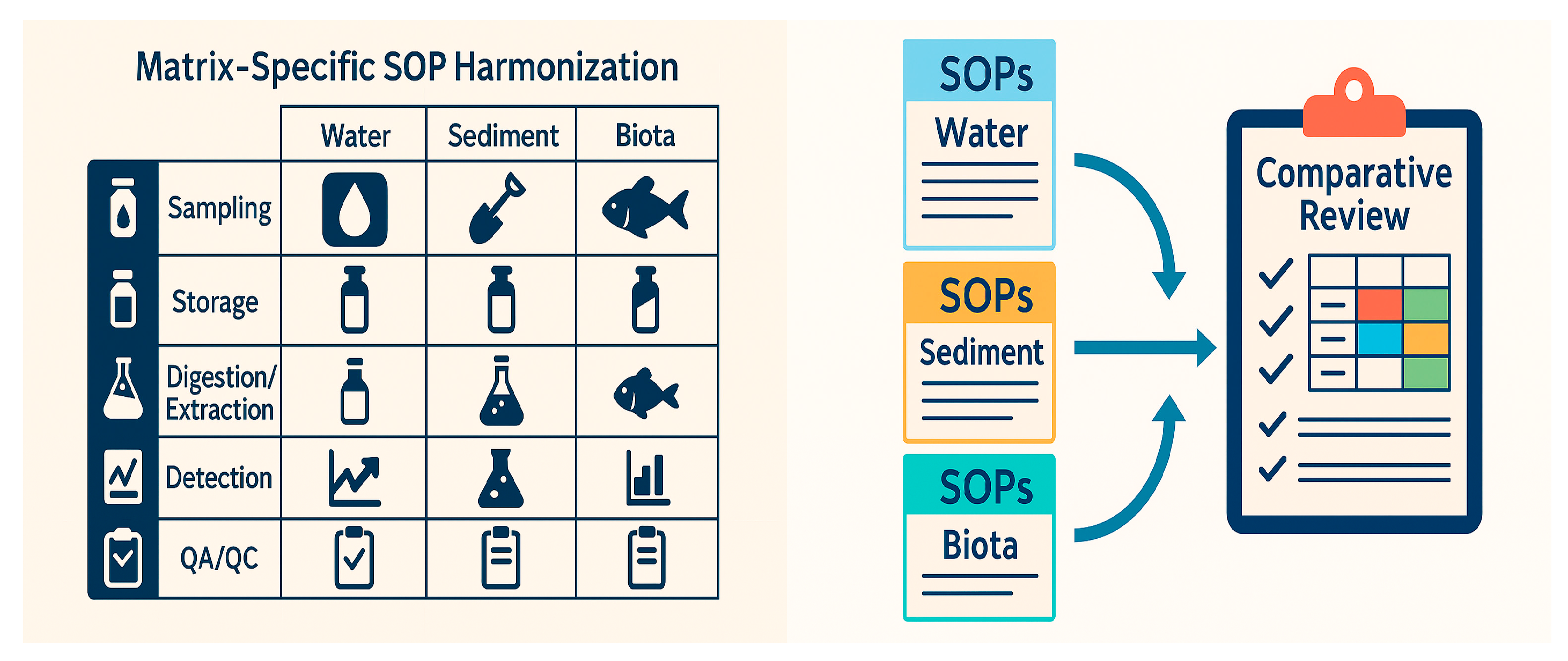
2.1. SOP Collection and Comparative Review
As part of BRIDGE-BS WP1, Task 1.3, Standard Operating Procedures (SOPs) for HMs and priority organic contaminants (PAHs, PCBs, OCPs) were collected from several institutions from four Black Sea coastal countries (Romania, Ukraine, Georgia, and Türkiye). Contributors included the National Institute for Marine Research and Development “Grigore Antipa” (NIMRD, Romania), the National Institute for Marine Geology and Geoecology (GeoEcoMar, Romania), the Ukrainian Scientific Centre of Ecology of the Sea (UkrSCES, Ukraine), Ivane Javakhishvili Tbilisi State University (TSU, Georgia), and the Scientific and Technological Research Council of Türkiye. Marmara Research Center (TÜBİTAK MRC, Türkiye), Sinop University (SNU, Türkiye), and Istanbul University—Institute of Marine Sciences and Management (IU-IMSM, Türkiye). The participating organizations included national research institutes and academic laboratories engaged in national or sub-basin monitoring programs.
Across BRIDGE-BS partners, we integrated laboratory SOPs per determinant group and matrix as follows. For HMs, five distinct SOPs informed the consolidated seawater method (laboratories from Romania and Türkiye), six SOPs informed the sediment method (laboratories from Romania, Türkiye, and Georgia), and four SOPs informed the biota method (laboratories from Romania and Türkiye). For PAHs, we incorporated three seawater SOPs and four SOPs each for sediment and biota, contributed by laboratories from Romania, Türkiye, and Ukraine. For OCPs and PCBs, the seawater, sediment, and biota methods each consolidated three SOPs from Romania, Türkiye, and Ukraine. Counts refer to distinct partner SOP submissions integrated in the final BRIDGE-BS methods for the specified matrix.
To ensure that information was reported in a consistent and comparable manner, partners were provided with both Word templates for detailed SOP descriptions (Appendix A.1) and Excel templates for structured reporting. Both capture the same core information, while the Excel files request finer detail at the determinand level. Collected fields include: method metadata (scope, matrix, determinand group, version/date, laboratory and contact), sampling design (field method and citation; fraction policy—dissolved/total with filter type and pore size for seawater; grain-size policy and sieve for sediments; species/tissue and biometric fields for biota), sample handling (container type, preservatives, storage and holding times), preparation (pre-treatment, digestion or extraction protocol, clean-up), analytical set-up (instrument, method reference), and validation/QA (calibration model and range, blanks, CRMs with codes and acceptance limits, spike/recovery policy, uncertainty, IC participation). The Excel templates additionally require per-analyte reporting of units, LOD/LOQ, basis of expression (dry vs. wet weight), normaliser where applicable, sediment core thickness and grain-size condition, and for biota, the species, size/weight, tissue. This structure ensures explicit reporting of digestion/extraction type, key QA/QC elements, and detection limits across matrices for transparent comparison and reproducible integration.
These formats allowed methodological information to be captured systematically across the main environmental matrices: seawater, sediment, and biota. The compiled protocols described the full chain of procedures, from sampling and conservation to sample preparation, data treatment, and quality assurance practices. Particular attention was paid to aspects most likely to influence comparability across institutions, such as sediment grain-size selection, the use of wet or dry weight for biota, or the treatment of dissolved versus total fractions in seawater.
All submitted SOPs were then reviewed and organized according to their methodological content. This process focused on how samples were collected, preserved, prepared, and analyzed, as well as on how quality control was implemented and documented. The comparative review made it possible to highlight where laboratories already converge in their approaches and where important differences remain. The overall workflow of SOP collection and review is shown in Figure 1, which illustrates how national protocols were compiled into a common format, processed, and examined to provide the basis for subsequent integration.
Figure 1.
Workflow from SOP collection to harmonization synthesis.
2.2. Intercalibration (IC) Exercise
To assess the practical consistency of analytical performance across regional laboratories, a proficiency testing exercise was organized in 2023 within the framework of the QUASIMEME “Quality Assurance of Information for Marine Environmental Monitoring in Europe” (https://www.wepalquasimeme.nl/en/wepalquasimeme.htm, accessed on 25 October 2025) proficiency testing scheme, Round 2023.1. The exercise focused on sediment samples, specifically targeting trace metals and PAHs. Test materials were prepared and characterized by the scheme coordinator, Wageningen Evaluating Programs for Analytical Laboratories—WEPAL (Wageningen University, The Netherlands), and distributed to participating laboratories. Each laboratory analyzed the samples using its routine SOPs and submitted results through the QUASIMEME platform. Performance was evaluated against established criteria, interlaboratory statistics being calculated and issued by QUASIMEME. Assigned values and the dispersion used for assessment were derived with QUASIMEME’s Normal Distribution Approximation (NDA), a robust procedure consistent with ISO 13528 that estimates a consensus mean and robust standard deviation from all reported results without outlier removal; where dataset size or agreement is limited, values are flagged as ‘indicative’ rather than assigned. Participant results were summarized as z′-scores, which express how far a result is from the assigned value in units of the scheme’s proficiency dispersion (‘total error’); classification follows standard bands: |z′| ≤ 2 satisfactory, 2 < |z′| ≤ 3 questionable, |z′| > 3 unsatisfactory. Individual reports received by each participant provided the assigned value, its uncertainty, the dispersion, ranked and graphical z′-score overviews, allowing them to evaluate their own results in comparison with reference values.
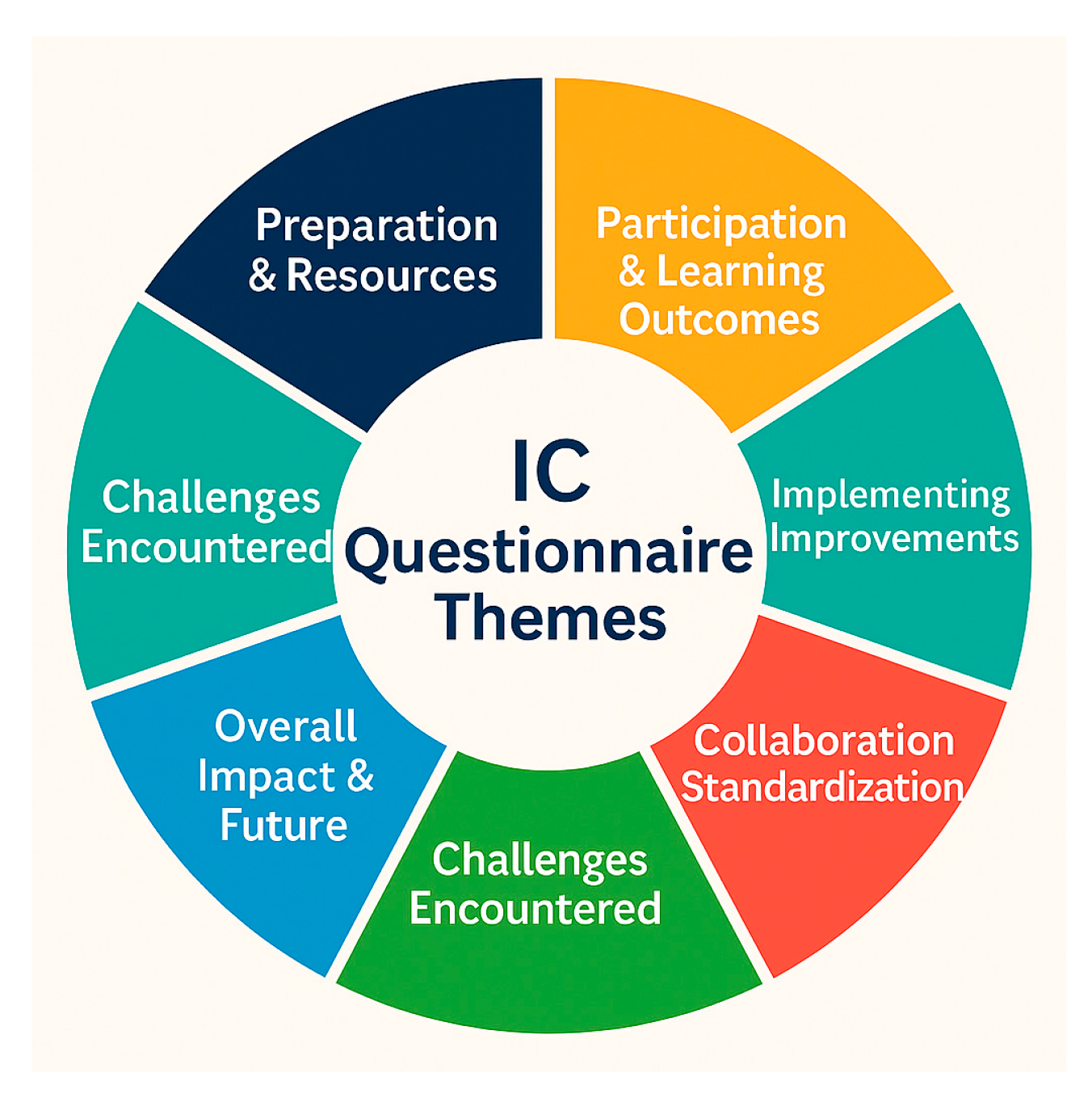
A post-intercalibration questionnaire (Appendix A.2) was circulated among project partners to capture qualitative feedback following analytical testing. This survey addressed all stages of the exercise, from preparation and resource adequacy to participation, learning outcomes, implementation of improvements, collaboration, and future perspectives. The main themes are illustrated in Figure 2, which summarizes the scope of the questionnaire. Laboratories reflected on the challenges they encountered, the parts of their SOPs that required adjustment, and the strengths and weaknesses that participation revealed in their analytical methods. They also described whether the exercise increased staff awareness of QA/QC requirements, and whether corrective measures were subsequently introduced, such as revised calibration procedures, improved blank corrections, expanded use of certified reference materials, or changes to the reporting of analytical limits and uncertainty.
Figure 2.
Themes of the IC questionnaire.
The responses were compiled into a feedback matrix that highlighted recurring challenges, capacity gaps, and examples of good practice. While detailed performance results remain confidential at the individual level, the aggregated outcomes provided an area-wide overview of strengths and weaknesses and informed recommendations for targeted coordination. Overall, the IC exercise served as both a diagnostic and benchmarking tool for assessing laboratory performance and SOP comparability. Beyond capacity validation, it also aimed to reinforce the importance of embedding regular proficiency testing into cross-border assessment programs, ensuring that QA/QC frameworks are applied consistently and that environmental evaluations are supported by robust and comparable data.
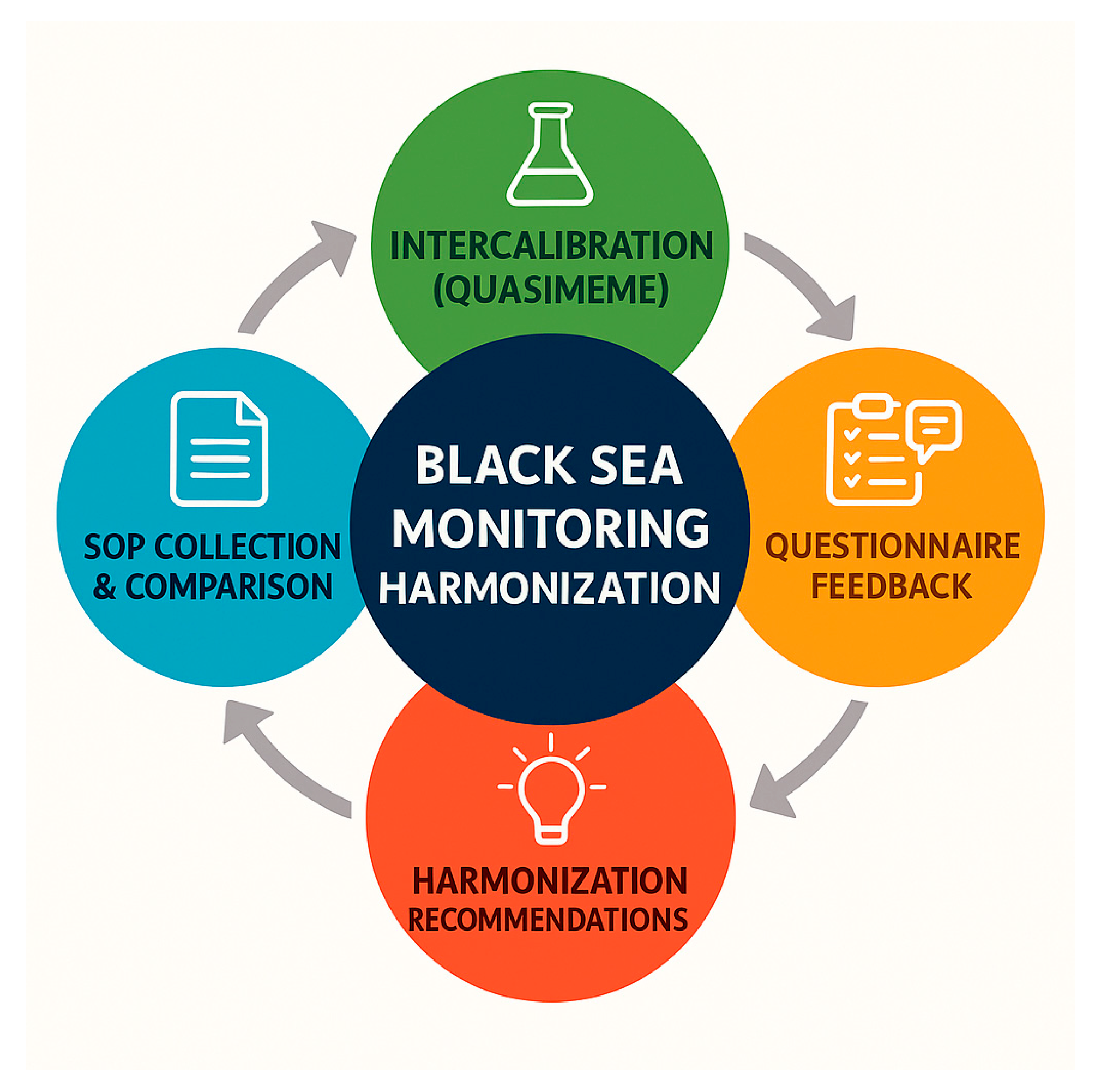
To illustrate the workflow of this study, a schematic diagram was developed summarizing the sequential steps: SOP collection and comparison, intercalibration through QUASIMEME, structured questionnaire feedback, and synthesis into recommendations (Figure 3).
Figure 3.
Process flow for harmonizing Black Sea contaminant monitoring. The four phases are (1) SOP collection and comparison, (2) intercalibration (QUASIMEME), (3) questionnaire feedback, and (4) formulation of recommendations. The circular structure reflects the iterative nature of continuous methodological improvement across regional laboratories.
3. Results
3.1. SOPs Analysis—Seawater Matrix
3.1.1. Heavy Metals (HMs) in Seawater
Across the Black Sea region, HM monitoring in seawater follows broadly comparable steps, yet several methodological differences remain that could affect the consistency of results. Most laboratories collect water samples from the surface layer using Niskin bottles or equivalent devices and immediately transfer them into acid-washed containers. A shared feature is the preservation of samples through acidification with ultrapure nitric acid to pH < 2, followed by storage at low temperature (4 °C) in the dark to minimize adsorption and transformation processes [11].
Divergences mainly occur in the fraction analyzed (dissolved vs. total) [12,13,14,15]. Most institutions analyze acidified unfiltered seawater and report total-recoverable metals—the dissolved fraction plus particle-bound metals solubilized by storage acidification (typically pH < 2 with HNO3). This differs from total metals, which require complete digestion of the whole sample (water + particulates) by a strong-acid protocol before analysis. Meanwhile, other laboratories apply 0.45 µm membrane filtration, ammonium pyrrolidine dithiocarbamate (APDC) chelation, followed by organic extraction of the metal–chelate complexes, and subsequent acidification with nitric acid, to specifically quantify the dissolved fraction. This difference is significant because dissolved and total concentrations are not specifically interchangeable when evaluating compliance with Environmental Quality Standards (EQS) [16]. Clear documentation of the fraction analyzed is therefore essential for interoperability of datasets across surveillance strategies.
Analytical determination relies primarily on inductively coupled plasma mass spectrometry (ICP-MS), which provides multi-element sensitivity at low detection limits. Flame or graphite furnace atomic absorption spectrometry (FAAS, GF-AAS) remains in use for some laboratories. Mercury determination is typically performed using dedicated techniques such as cold vapor AAS or direct mercury analyzers (DMA). These approaches are broadly harmonized, though method-specific variability (especially for volatile elements such as Hg) requires inter-method validation.
Quality assurance and quality control (QA/QC) practices show both convergence and gaps. Multi-point calibration curves are standard, and most laboratories employ procedural blanks, replicate analysis, and, where available, certified reference materials (CRMs). However, access to seawater CRMs for trace metals is rather limited [17], and reporting of measurement uncertainty and sensitivity thresholds is inconsistent. Recovery criteria for internal standards or spiked samples typically range between 80 and 120%, yet differences in reference scaling and blank correction procedures remain sources of variability.
In summary, HM determination in seawater is broadly harmonized with respect to sampling, conservation, and the use of modern analytical equipment. The main divergences concern fraction selection (total vs. dissolved), element-specific analytical techniques, and the extent of QA/QC documentation. These differences evince the need for the alignment of fraction reporting, wider use of CRMs, and consistent reporting of uncertainty, so that seawater datasets can be robustly integrated into chemical status analysis.
3.1.2. Polycyclic Aromatic Hydrocarbons (PAHs) in Seawater
Measuring PAHs in seawater is generally built on established international guidelines (Appendix A.3), but practices across Black Sea laboratories display important procedural differences. Sampling is performed using acid-cleaned glass or high-quality polymer containers, typically collecting volumes of 2–5 L from the surface layer. Samples are protected from light and cooled to 4 °C immediately after collection. A common feature is the absence of filtration, ensuring that both dissolved and particle-bound fractions are retained, as PAHs readily adsorb to suspended matter.
Extraction procedures show the greatest variability. Solid-phase extraction (SPE) using polymeric or C18 cartridges is the predominant approach, but liquid–liquid extraction (LLE) with non-polar solvents (hexane, dichloromethane, or mixtures) is still practiced in some laboratories. More recently, solventless stir-bar sorptive extraction coupled with thermal desorption has also been introduced [18]. Differences arise in extraction volumes, solvent systems, and cleanup strategies. While most laboratories apply silica or alumina column cleanup to remove interferences, the sequence and composition of adsorbents vary, leading to potential discrepancies in recovery of higher molecular weight PAHs [19].
Analytical detection is relatively harmonized, with gas chromatography coupled to mass spectrometry (GC-MS or GC-MS/MS) being the dominant technique. Tandem MS operated in selected ion or multiple reaction monitoring (SIM/MRM) mode ensures greater sensitivity and selectivity, though some laboratories still rely on single quadrupole GC-MS. The lack of uniformity in instrumental configuration can affect comparability for trace-level compounds [20].
Quality control is based on the use of deuterated PAH surrogates, which are added prior to extraction to track recovery efficiency [21]. Most laboratories apply procedural blanks and replicate analyses, and recovery ranges between 70 and 120% are considered acceptable. Certified reference materials (CRMs) for PAHs in seawater are not fully available, with only limited coverage for selected compounds, and were not reported as being routinely used by Black Sea laboratories, meaning that interlaboratory comparability rests largely on spiking experiments and proficiency testing schemes [22]. Sensitivity thresholds are generally suitable for environmental assessment but vary depending on extraction efficiency and instrument sensitivity, underscoring the need for standardized reporting.
Overall, PAH analysis in seawater combines common elements: light-protected storage, non-filtered sampling, GC-MS detection, with some divergences in extraction and cleanup procedures. These differences highlight the importance of intercalibration exercises and harmonized QA/QC documentation to ensure that data from different laboratories can be reliably integrated for status assessments.
3.1.3. Organochlorine Pesticides (OCPs) and Polychlorinated Biphenyls (PCBs) in Seawater
Analysis of OCPs and PCBs in seawater across the Black Sea relies on established international protocols (Appendix A.3), yet procedural heterogeneity is more pronounced than for metals or PAHs. Sampling typically involves the collection of 2–5 L of seawater in pre-cleaned amber glass bottles to minimize photodegradation and sorption. Samples are kept at 4 °C and processed within 48–72 h to reduce losses of these hydrophobic and semi-volatile compounds. As with PAHs, filtration is generally not applied, ensuring that particulate-associated fractions are included [23,24].
Extraction strategies diverge considerably. Solid-phase extraction (SPE) is the most common method, using polymeric or C18 sorbents, but liquid–liquid extraction (LLE) with non-polar solvents (hexane, dichloromethane, or mixtures) is still practiced in several laboratories [24,25]. Cleanup procedures are applied in most cases to reduce co-extracted organic matter, using silica, alumina, or multilayer columns, though the sequence and selectivity of adsorbents differ. This variability can influence recovery rates, particularly for highly chlorinated PCBs [24].
Detection methods are less uniform than for PAHs. Gas chromatography coupled to tandem mass spectrometry (GC-MS/MS) is increasingly adopted for its high sensitivity and specificity. However, some laboratories continue to use single-quadrupole GC-MS or electron-capture detection (GC-ECD), particularly for halogenated targets. While GC-ECD offers excellent sensitivity for chlorinated compounds, it lacks the selectivity of mass spectrometric detection, increasing the risk of co-elution and false positives in complex seawater matrices [24].
Method control practices include the use of isotope-labelled surrogates (^13C-labelled standards) added before extraction, as well as blanks and replicate analyses [24]. Recovery acceptance ranges are like those applied for PAHs (typically 70–120%). Certified reference materials (CRMs) for OCPs and PCBs in seawater are not fully available, with only limited coverage for selected compounds, and were not reported as being routinely used by Black Sea laboratories. This limitation restricts external traceability and means that comparability relies largely on spiking experiments and interlaboratory proficiency testing. Detection limits differ depending on the analytical platform used, with GC-MS/MS generally providing lower and more reliable thresholds than GC-ECD.
In summary, OCP and PCB determination in seawater exhibits a shared foundation of amber-glass sampling, cold storage, and organic solvent extraction, but with differences in extraction strategy, cleanup, and detection technology, which can affect data comparability [26]. Strengthening consistency will require not only convergence in analytical methods but also the establishment of seawater reference materials and systematic benchmark assessment exercises to ensure robust assessments.
Overall, seawater monitoring of HMs and POPs in the Black Sea is broadly aligned with international reference procedures (Appendix A.3). These provide the common framework for sampling (acid-cleaned containers, amber glass for organics, immediate storage), preservation (acidification for metals, cold and dark storage), extraction or digestion, and instrumental analysis. QA/QC principles: use of blanks, surrogates, CRMs where available, and participation in proficiency testing schemes such as QUASIMEME are also widely recognized. Against this methodological backbone, the comparison reveals that sampling and storage are relatively well harmonized, but divergences remain in fraction determination (total vs. dissolved metals), extraction strategies for POPs (SPE, LLE, or sorptive techniques), and the choice of detection platforms (ICP-MS vs. AAS; GC-MS/MS vs. GC-ECD). Gaps are most evident in QA/QC traceability, where the absence of seawater CRMs for many POPs, combined with inconsistent reporting of analytical limits and measurement uncertainty, prevents full comparability. Overall, while the Black Sea laboratories operate within the scope of accepted international methods, greater alignment in fraction reporting, extraction protocols, and QA/QC documentation is needed to achieve coherent basin-wide assessments (Table 1).

Table 1.
Summary of SOPs for seawater analysis of HMs, PAHs, and OCPs/PCBs: common practices, major variations, and implications for comparability.
3.2. SOPs Analysis—Sediment Matrix
3.2.1. Heavy Metals (HMs) in Sediment
Sediment investigations for HMs in the Black Sea follow broadly comparable principles, but methodological variability still affects consistency of results. Sampling is commonly performed with grabs or box corers, targeting the surface layer as the most representative of recent deposition. Surface sediments (0–2 cm) are most often collected for HMs and POPs, as this layer represents the most recent deposition and best reflects present-day contamination inputs. Some protocols apply thicker layers, such as 0–5 cm or 0–10 cm, which integrate older deposits and may complicate comparisons between datasets [27,28,29]. After collection, sediments are transferred to acid-washed polyethylene, polypropylene, or Teflon containers to minimize contamination. Samples are then stored frozen (−20 °C) for long-term storage or refrigerated at 4 °C if processed shortly after collection.
A key divergence concerns the treatment of sediment grain size. International guidelines recommend the use of the <63 µm fine fraction, as this concentrates trace metals and reduces natural variability, thereby improving comparability [30,31]. This approach is widely applied across laboratories. However, many protocols in the Black Sea still analyze the whole sediment after removal of the coarse fraction (>2 mm) [31]. This bulk approach has the advantage of consistency with several ecotoxicological guidelines, such as the NOAA ERL/ERM or TEL/PEL sediment quality thresholds, which are expressed on a total-sediment basis [32,33,34]. Because grain size strongly influences metal concentrations, mixing fine-fraction and bulk-sediment datasets limits comparability and can obscure spatial or temporal patterns [35,36]. Standardization, therefore, requires not only methodological alignment but also clarity on whether datasets are intended for geochemical normalization or for direct comparison with ecotoxicological benchmarks.
Sediment digestion protocols vary across laboratories. Microwave-assisted acid digestion is most often applied, using concentrated nitric acid (HNO3) or aqua regia (HNO3 + HCl); in some cases, hot-plate digestion is still employed. Hydrogen peroxide (H2O2) may occasionally be added to oxidize organic matter prior to acid treatment. These procedures represent partial digestion, targeting the environmentally available fraction of metals that is most relevant for pollution assessment and consistent with international guidelines [29,37,38,39]. In parallel, some protocols apply total digestion, incorporating hydrofluoric acid (HF) to dissolve silicate minerals and achieve complete decomposition of the sediment matrix. Reported variants include HNO3 + HCl + HF (microwave), HNO3 + HF + HClO4 (hot plate), and aqua regia + HF (hot plate). These methods provide the total elemental content of sediments and are widely used in geochemical characterization [29,40]. The coexistence of partial and total digestion approaches leads to differences in reported concentrations, limiting dataset comparability and emphasizing the need for harmonized protocols in sediment assessment techniques [41].
For instrumental analysis, ICP-MS is the predominant method, offering multi-element sensitivity and low sensitivity thresholds. ICP-OES and GF-AAS are also used by other laboratories. Mercury is determined either by cold vapor AAS/ICP-MS after digestion or by a direct mercury analyzer (DMA). While each approach is valid, method-specific biases highlight the need for cross-validation and use of appropriate reference materials.
Performance verification is supported by multi-point calibration, blanks, replicate analysis, and the use of certified reference materials (CRMs) for sediments, which are available for several metals. However, the systematic application of CRMs and the reporting of detection limits and measurement uncertainty remain inconsistent across laboratories.
In summary, sediment methods for HMs in the Black Sea are broadly harmonized in sampling and instrumental techniques, yet important divergences remain in sampling strategy, grain-size fractionation, and digestion protocols. Most laboratories apply partial digestions aimed at the environmentally available fraction, while others use total digestion procedures that include hydrofluoric acid (HF) to determine the complete elemental content. This coexistence of approaches affects comparability across datasets. Greater convergence on fine-fraction analysis, harmonized digestion procedures, and systematic use of certified reference materials (CRMs) would enhance both the consistency and the policy relevance of sediment assessment results at the basin scale.
3.2.2. Polycyclic Aromatic Hydrocarbons (PAHs) in Sediment
The analysis of PAHs in sediments across the Black Sea follows broadly recognized protocols (Appendix A.3) but reveals important variations that could affect interoperability of datasets. Sediment samples are generally collected with grabs or box corers, stored in pre-cleaned glass jars, and kept frozen at −20 °C until processing to minimize losses of volatile compounds. PAHs are typically analyzed in the bulk sediment fraction < 2 mm, in line with international recommendations for organic contaminants [42,43].
Extraction is most often carried out using Soxhlet extraction with non-polar solvents such as hexane, dichloromethane, or mixtures, applied for several hours to ensure efficient recovery [44]. Some laboratories have adopted microwave-assisted extraction (MAE) or accelerated solvent extraction (ASE), both of which reduce solvent use and extraction time while maintaining good recovery [45]. Differences remain in the choice of solvent systems and extraction durations, which can affect the efficiency for high-molecular-weight PAHs. Cleanup procedures are widely used to remove co-extracted lipids and matrix interferences. The most common approach is silica or alumina column chromatography, while some laboratories employ multilayer silica/alumina systems for more complex matrices [46]. These differences in cleanup strategy may influence reproducibility, particularly for heavier and more strongly sorbed PAHs.
For detection, gas chromatography coupled with mass spectrometry (GC-MS) is the predominant technique. Tandem mass spectrometry (GC-MS/MS), operated in selected ion monitoring (SIM) or multiple reaction monitoring (MRM) modes, provides greater sensitivity and selectivity and is increasingly applied [47]. Single-quadrupole GC-MS is adequate but may suffer from co-elution interferences in complex sediment extracts [48].
Analytical validation practices include the use of deuterated PAH surrogates added before extraction to track recoveries, procedural blanks, and spiked samples [49]. CRMs for PAHs in sediments are only partially available and were not reported as being systematically applied by partner laboratories, which instead relied on surrogates, spiked samples, and procedural blanks. Acceptance criteria for surrogate recoveries typically range between 70 and 120%, but differences in surrogate sets and reporting of uncertainty still hinder full comparability.
In summary, PAH methods in sediments across the Black Sea are based on well-established international methodologies, with broadly harmonized sampling and storage practices and common reliance on solvent extraction followed by GC-MS analysis. The main divergences concern the choice of extraction technique, cleanup complexity, and access to advanced mass spectrometric platforms. Integration of surrogate standards, systematic use of sediment CRMs, and standardized reporting of analytical limits would substantially improve the reliability and comparability of PAH datasets in the region.
3.2.3. Organochlorine Pesticides (OCPs) and Polychlorinated Biphenyls (PCBs) in Sediment
Measurement of OCPs and PCBs in sediments across the Black Sea is generally based on international standards (Appendix A.3), but procedures diverge in ways that could affect data comparability. Sediment samples are typically collected with grabs or box corers, transferred into pre-cleaned glass containers to avoid contamination, and stored frozen (−20 °C) until analysis to preserve volatile and semi-volatile compounds. Like PAHs, most laboratories analyze the whole sediment fraction after sieving to 2 mm, as this aligns with many ecotoxicological guidelines that define thresholds on a total-sediment basis [50].
Extraction is most often performed by Soxhlet extraction with non-polar solvents such as hexane, dichloromethane, or their mixtures, typically over 6–8 h. Some laboratories have adopted microwave-assisted extraction (MAE) or accelerated solvent extraction (ASE), which provide shorter extraction times and reduced solvent consumption while maintaining good recoveries [51]. While both approaches achieve adequate recoveries, differences in solvent selection, extraction duration, and temperature can introduce variability, especially for higher chlorinated PCB congeners. Cleanup procedures are widely applied to eliminate lipids and interfering compounds. Silica and alumina chromatography is standard, though multilayer silica/alumina or Florisil columns are used in some protocols for more rigorous purification [52]. Variations in the cleanup sequence and adsorbent composition can influence the reproducibility of results, particularly for trace-level compounds.
For detection, gas chromatography coupled to mass spectrometry (GC-MS or GC-MS/MS) is increasingly adopted, offering selectivity and sensitivity. However, some laboratories continue to employ electron capture detection (GC-ECD), which is highly sensitive to halogenated compounds but more prone to co-elution interferences in complex sediment extracts [53]. As a result, comparability between datasets can be limited when different detection technologies are applied. As documented in the literature [24], GC–ECD is widely used for OCPs and ortho-PCBs but is prone to co-elution-driven misidentification in complex sediment extracts (e.g., p, p′-DDD with cis-nonachlor; p, p′-DDE with dieldrin or PCB-85). GC–MS/MS provides higher selectivity and greater sensitivity, with reported instrumental limits of detection around ~0.1 pg for key OCPs versus ~0.5–1 pg typical for ECD, thereby reducing false positives through confirmatory ions and production transitions.
Accuracy and precision checks include the use of isotope-labelled surrogates (^13C-labelled OCPs/PCBs) added prior to extraction, procedural blanks, and spiked recoveries [54]. Surrogate recovery ranges between 70 and 120% and is generally considered acceptable. Nonetheless, CRMs for OCPs and PCBs in sediments are not consistently used, which reduces traceability. Reporting method quantification boundaries and measurement uncertainty is also inconsistent across laboratories.
In summary, sediment analysis of OCPs and PCBs in the Black Sea is based on shared methodological foundations: glass storage, solvent extraction, and GC-based analysis, but key divergences persist in extraction conditions, cleanup strategies, and the choice of detection platform. These differences highlight the need for wider adoption of harmonized QA/QC practices, systematic proficiency testing, and greater access to sediment CRMs to ensure comparability and reliability of surveillance results across the basin.
Overall, the determination of HMs and POPs in sediments across the Black Sea builds on internationally recognized reference methods (Appendix A.3). These protocols recommend standardized sampling of the surface layer, storage in inert containers under cold conditions, and subsequent preparation of either fine fractions (<63 µm) for metals or bulk sediment (<2 mm) for organics, depending on the assessment objective. Acid digestion with HNO3 or aqua regia is the most widely applied procedure for HMs, while Soxhlet extraction with non-polar solvents remains the classical approach for POPs, with accelerated solvent extraction (ASE) and microwave-assisted extraction (MAE) increasingly adopted. Analytical determination generally relies on ICP-MS for metals and GC-MS/MS for organics, supported by blanks, surrogates, and CRMs where available.
Against this common methodological backbone, several divergences persist. Differences in the thickness of sediment layers collected (0–2 cm versus 0–5 cm or 0–10 cm) influence the temporal representativeness of results. Variability in fractionation (fine vs. bulk), digestion protocols (partial versus total with hydrofluoric acid, HF), and extraction techniques (Soxhlet, ASE, MAE) contributes to systematic differences in measured concentrations. Additional variability arises from solvent choice, cleanup procedures, and detection platforms (GC-MS/MS vs. GC-ECD). While QA/QC practices such as reference scaling, blanks, and surrogate recoveries are widely applied, inconsistent use of CRMs and limited reporting of measurement uncertainty reduce data traceability. Thus, sediment assessment in the Black Sea is consistent with international reference methods in principle, but further consistency in sampling, fraction preparation, digestion/extraction procedures, and QA/QC implementation is required to ensure comparability and policy relevance of status assessments (Table 2).

Table 2.
Summary of SOPs for sediment analysis of HMs, PAHs, and OCPs/PCBs: common practices, major variations, and implications for comparability.
3.3. SOPs Analysis—Biota Matrix
3.3.1. Heavy Metals (HMs) in Biota
Biota evaluation for HMs in the Black Sea is generally aligned with international recommendations (Appendix A.3), but methodological differences among laboratories could influence interoperability of datasets. The most widely monitored organisms are bivalves such as Mytilus galloprovincialis, used for their sedentary lifestyle and accumulation potential, and fish (mainly dorsal muscle of commercial species). The choice of organism and tissue is largely harmonized, but differences persist, with some protocols analyzing whole soft tissue of mussels while others restrict analysis to particular organs [55]. These distinctions are important, as concentration levels vary substantially with tissue type.
Sample preservation is relatively uniform: organisms are depurated, dissected under clean conditions, and stored frozen until analysis. Homogenization of tissue prior to digestion is standard, yet protocols differ in whether results are reported on a wet-weight or dry-weight basis. Most laboratories follow the regulatory requirement to express results on a wet-weight basis, particularly for fish intended for human consumption, while others prefer dry-weight reporting for comparability with historical datasets. This divergence complicates direct comparison unless conversion factors are provided.
Digestion is typically performed with concentrated nitric acid, either by microwave-assisted procedures or hot-plate mineralization [56]. Hydrogen peroxide may be added to enhance oxidation of organic matter, though this is not uniformly applied. Mercury is analyzed using cold vapor AAS or DMA, while other elements are quantified mainly by ICP-MS, with GF-AAS or ICP-OES used as alternatives [57].
Reliability framework practices include the use of certified reference materials (CRMs) such as mussel tissue or fish homogenates, procedural blanks, and replicate analyses [58]. Participation in intercalibration schemes is also reported by several laboratories. Nevertheless, inconsistencies remain in the systematic reporting of sensitivity thresholds, measurement uncertainty, and CRM recovery. In summary, HMs analysis in biota is broadly harmonized in terms of species selection, tissue preservation, and analytical techniques. However, differences in tissue choice, wet- vs. dry-weight reporting, and the completeness of QA/QC documentation continue to limit comparability across datasets. Alignment with international reference methods, systematic use of CRMs, and explicit weight-basis conversion are essential steps toward coherent assessments.
3.3.2. Polycyclic Aromatic Hydrocarbons (PAHs) in Biota
The determination of PAHs in biota across the Black Sea builds on established reference methods (Appendix A.3), but practice still varies across laboratories. Bivalves (Mytilus galloprovincialis) are the most frequently used sentinel organisms, owing to their filtering capacity and ability to accumulate hydrophobic compounds, while fish muscle is also analyzed to address both environmental status and food-safety concerns.
Sample collection and conservation are relatively consistent: organisms are wrapped in pre-cleaned aluminum foil or placed in glass containers, avoiding contact with plastics, and stored at −20 °C until analysis. Homogenization of soft tissue is standard, though variability remains in whether whole soft tissue or specific organs (e.g., muscle, hepatopancreas) are targeted. These choices affect reported concentrations, since hydrophobic PAHs often accumulate preferentially in lipid-rich tissues [59].
Extraction is predominantly performed using Soxhlet extraction with non-polar solvents (hexane, dichloromethane, or mixtures). Microwave-assisted extraction (MAE) with solvent mixtures (hexane/acetone, hexane/dichloromethane/methanol) and accelerated solvent extraction (ASE) are also applied in several protocols. [51]. Although recoveries were broadly similar across methods, differences in solvent systems, extraction duration, and temperature conditions contribute to interlaboratory variability. Cleanup steps are routinely applied to remove co-extracted lipids. Silica or alumina column chromatography is the most common approach, and some laboratories also employ Florisil for additional purification. The choice of cleanup method can influence recovery, especially for higher molecular weight PAHs [60]. Detection relies primarily on GC-MS or GC-MS/MS, operated in SIM or MRM modes for sensitivity and selectivity. Tandem MS is increasingly used, but single-quadrupole GC-MS remains common in some institutions [61]. Differences in instrumentation and detection settings may therefore affect comparability for low-level compounds.
Analytical validation practices include the use of deuterated PAH surrogates added before extraction, procedural blanks, and recovery checks [45]. CRMs for PAHs in biota are not fully available, with only a limited number covering selected compounds and tissues and were not reported as being routinely used by partner laboratories. Recovery acceptance ranges (typically 70–120%) are applied, but the choice of surrogate sets and the extent of uncertainty reporting vary across laboratories. In summary, PAH evaluation in biota is based on harmonized international guidelines and shows broad convergence in species choice, preservation, and reliance on GC-MS detection. However, divergence persists in tissue selection, extraction, and cleanup methods, and QA/QC practices. Greater standardization of surrogate use, systematic interlaboratory comparison, and the development of suitable biota CRMs would significantly improve the comparability of PAH datasets in the region.
3.3.3. Organochlorine Pesticides (OCPs) and Polychlorinated Biphenyls (PCBs) in Biota
Analysis of OCPs and PCBs in biota is an established component of contaminant surveillance in the Black Sea, guided by reference protocols (Appendix A.3). Mussels (Mytilus galloprovincialis) and fish muscle are the most analyzed matrices, providing information on both environmental exposure and potential human health risks. However, methodological differences persist that reduce the consistency of results. Sample conservation practices are generally harmonized: organisms are wrapped in pre-combusted aluminum foil or stored in solvent-rinsed glass containers and frozen at −20 °C. Prior to analysis, tissues are homogenized, though laboratories differ in whether whole soft tissue or specific muscle is selected. Such choices strongly influence measured concentrations, since OCPs and PCBs accumulate in lipid-rich tissues [24].
Extraction typically employs Soxhlet with non-polar solvents (hexane, dichloromethane, or mixtures), with extraction durations of 6–8 h being common. In addition, microwave-assisted extraction (MAE) and accelerated solvent extraction (ASE) with mixed solvent systems (hexane/acetone and hexane/dichloromethane/methanol) are also applied in some laboratories, improving efficiency. These pressurized techniques offer reduced solvent use and shorter processing times, while maintaining acceptable recoveries [51]. However, variability in solvent composition, extraction duration, and applied temperature may influence results, particularly for higher-chlorinated congeners. Cleanup is a crucial step due to the high lipid content of biological samples. Silica or alumina columns are widely used, while multilayer or Florisil columns are applied in some protocols for more effective lipid removal [24]. These differences influence reproducibility, particularly for trace-level congeners. Detection is most often performed by GC-MS/MS, providing sensitivity and selectivity across multiple compounds. Some laboratories still employ GC-ECD, which is highly sensitive to halogenated compounds but prone to co-elution with interfering substances [24,62]. As a result, comparability across datasets may be limited when different detection platforms are used.
Method control measures include the use of isotope-labelled surrogates (^13C-labelled OCPs and PCBs), procedural blanks, and recovery checks [26]. Recovery acceptance ranges of 70–120% are typically applied. CRMs for OCPs and PCBs in biota are not fully available and were not reported as being routinely used by Black Sea laboratories, limiting external traceability. Reporting of detection limits and uncertainty also remains inconsistent. In summary, OCP and PCB determination in biota across the Black Sea is broadly harmonized with international practices, especially in terms of sample preservation, solvent extraction, and GC-based analysis. Divergences in tissue selection, extraction and cleanup strategies, and detection platforms remain limiting factors to comparability. Progress toward standardization will require systematic use of isotope-labelled surrogates, wider access to CRMs, and routine cross-laboratory evaluation to ensure that results can be reliably integrated into status assessments.
Overall, biota assessment for HMs and organic contaminants in the Black Sea broadly follows internationally recognized reference procedures (Appendix A.3). These frameworks guide organism selection (mussels, fish), conservation (frozen storage, avoidance of plastics), solvent extraction, and instrumental detection, with performance verification measures such as blanks, surrogates, and CRMs. Across the region, practices are relatively well aligned in terms of species choice and preservation, but divergences remain that affect data comparability. Differences in tissue selection (whole soft tissue vs. muscle) and wet- vs. dry-weight reporting complicate direct comparisons. For PAHs, OCPs, and PCBs, laboratories diverge in extraction solvents, lipid cleanup strategies, and detection platforms (GC-MS/MS vs. GC-ECD). For POPs in biota, Soxhlet, MAE, and ASE are all in use. These approaches yield acceptable recoveries but differ in efficiency and solvent requirements, introducing variability that hampers the alignment of results across laboratories. CRMs seem more readily available for trace metals than for organics, where their scarcity represents a structural gap. Overall, while the methodological backbone is shared across Black Sea laboratories, further alignment is needed in tissue selection, reporting basis, cleanup procedures, and QA/QC traceability to ensure basin-wide comparability of contaminant levels in marine organisms (Table 3).

Table 3.
Summary of SOPs for biota analysis of HMs, PAHs, and OCPs/PCBs: common practices, major variations, and implications for comparability.
3.4. Intercalibration Exercise
Intercalibration (IC) exercises are an established cornerstone of quality assurance in marine environmental surveillance. They provide laboratories with a neutral framework to evaluate analytical comparability under international standards. Within BRIDGE-BS, IC was carried out through participation in the QUASIMEME scheme, a long-running European proficiency testing program designed specifically for contaminants in marine matrices. QUASIMEME has been instrumental in building trust in large-scale environmental data since the early 1990s [8], echoing the earlier calls for harmonized QA practice in marine chemistry [7] and complementing more recent basin-level initiatives such as HarmoNIA in the Adriatic–Ionian Seas [9].
For the Black Sea partners, participation in QUASIMEME ensured that analytical determinations of HMs and POPs were benchmarked against certified reference values under conditions consistent with regional monitoring obligations. Importantly, the BRIDGE-BS IC exercise did not aim to assess or disclose individual laboratory performance, which remains confidential, but to highlight common methodological and QA/QC challenges across the region. To capture these practical challenges, a detailed questionnaire was circulated among participants following the QUASIMEME round. The questionnaire focused on crucial points that could influence cross-study alignment, including reference scaling, blank correction, CRMs, digestion and extraction protocols, instrument maintenance, reporting of sensitivity thresholds and uncertainty, and interpretation of SOPs. Detailed content of the questionnaire is provided in Appendix A.2.
Feedback revealed that instrument tuning practices were not always aligned with the expected concentration of intercalibration samples. In some cases, standards were prepared outside the relevant range, leading to potential bias. Blank contamination was also identified as a recurring concern, with several laboratories acknowledging that elevated values in trace-level determinations could be linked to insufficient blank correction. The use of CRMs showed clear variability: while some institutions applied matrix-matched CRMs systematically, others lacked appropriate reference materials or did not integrate correction factors into routine workflows. Digestion and extraction protocols also differed, with variations in temperature, acid volume, and digestion time noted as influencing outcomes. Instrument maintenance and troubleshooting emerged as another significant theme, with reports of instrument drift and unresolved maintenance issues underscoring the importance of routine checks and preventive servicing. Reporting practices were inconsistent across laboratories, as not all institutions calculated or communicated measurement uncertainty, and limits of detection (LOD) and quantification (LOQ) were sometimes reported in non-standard ways or omitted altogether. Finally, ambiguities in SOP language were noted: even when nominally identical procedures were applied, different interpretations led to small but significant divergences in implementation, affecting comparability across institutions. These themes are summarized in Table 4, which compiles the feedback obtained through the questionnaire.

Table 4.
Summary of feedback from the BRIDGE-BS intercalibration questionnaire, with identified challenges and examples of corrective measures reported by participating laboratories.
Beyond documenting technical shortcomings, the IC exercise also provided a structured opportunity for participating laboratories to reflect on their own practices and to identify strengths and weaknesses. Many reported that elements of their protocols required clarification or adjustment to achieve more reproducible results. The exercise increased staff awareness of QA/QC requirements and emphasized the importance of systematic documentation. For several laboratories, participation itself functioned as a training exercise, reinforcing good practices and drawing attention to the procedural steps that most strongly influence comparability.
Follow-up actions were also identified. In some cases, reference scaling protocols were revised to better align with environmentally relevant concentration ranges, blank correction procedures were modified to minimize contamination, or digestion/extraction steps were adjusted to improve recovery and reproducibility. The use of CRMs was expanded, either through the adoption of additional reference sets or through more consistent application of existing ones. Reporting practices were strengthened as well, with several institutions committing to systematic documentation of LOD/LOQ values and measurement uncertainty. These changes underline the dual role of proficiency testing: not only benchmarking laboratory performance against reference values but also catalyzing corrective measures that progressively improve comparability across the region.
Taken together, the combination of QUASIMEME participation and structured feedback illustrates the dual value of IC: providing an independent benchmark for analytical performance, while at the same time creating a platform for laboratories to exchange experiences and implement improvements. The issues raised: calibration consistency, CRM availability, digestion protocol clarity, maintenance routines, standardized reporting, and unambiguous SOPs, represent actionable priorities for future harmonization. By embedding these lessons into the refinement of regional SOPs and ensuring continuous participation in benchmark assessment, the Black Sea community can strengthen the comparability and reliability of pollutant assessment, aligning with both EU Directives (WFD, MSFD) and the Bucharest Convention’s Integrated Monitoring and Assessment Program (BSIMAP).
4. Discussion
4.1. Regional Synthesis of SOP Harmonization and Its Implications
The comparative review of SOPs for seawater, sediment, and biota highlights a broadly shared methodological backbone rooted in international reference procedures (Appendix A.3) but also reveals various divergences that could affect interoperability of datasets. Sampling and conservation steps are relatively well harmonized: seawater is collected with Niskin bottles and acidified to pH < 2 with ultrapure nitric acid (for HMs), sediments are obtained by grabs or box corers and stored frozen, while biota samples are homogenized and frozen to preserve integrity. These common practices provide a consistent basis for cross-border monitoring. Although field procedures appear largely aligned, the credibility of analytical data ultimately depends on how consistently these sampling protocols are applied in practice. Sampling procedures exert a critical influence on the credibility and comparability of contaminant data. General marine sampling principles emphasize maintaining sample integrity and minimizing disturbance by reproducing in-situ conditions as closely as possible and by preventing contamination, chemical alteration, or particle resuspension during collection and handling [63]. Inconsistent field practices, such as variable coring depth, heterogeneous sediment sub-sampling, non-cleaned or inappropriate containers, or delayed preservation, can introduce contamination or analyte loss and reduce data reliability [64]. The representativeness of samples may also be compromised by uneven spatial coverage or insufficient replication [65]. Scientifically sound practice therefore requires clean sampling tools, controlled sampling depth, use of field blanks and duplicates to assess variability, and immediate stabilization of samples through cooling or chemical preservation [66]. Small deviations from these practices are acceptable only within documented quality-control limits that ensure reproducibility [67]. Observed deficiencies in regional datasets, including incomplete documentation of field procedures, absence of field QA/QC checks, and inconsistent handling conditions, evince the need for stricter adherence to standardized sampling principles to preserve the integrity and traceability of environmental monitoring data.
Divergences become pronounced in subsequent steps and are concentrated in a few high-impact areas. For HMs, main differences include the treatment of seawater fractions (unfiltered total acid-leachable vs. filtered dissolved), the sediment fraction analyzed (<63 μm vs. bulk < 2 mm), digestion techniques, and the tissue and reporting basis for biota (whole tissue vs. muscle; wet vs. dry weight). These methodological choices strongly influence measured concentrations and determine comparability with Environmental Quality Standards (EQS), sediment guidelines (ERL/ERM), or food-safety thresholds.
Multiple studies show that the filtration-based phase definition governs metal partitioning: relative to total, dissolved values are lower because filtration removes particulates; Fe and Mn are largely particulate, Cu is mostly dissolved (particulate ≈10% of total), and Ni/Zn are intermediate. Within the <0.45 µm dissolved pool, a non-negligible colloidal share is typical (Cu ≈ 11%, Zn ≈ 5%, Ni ≈ 6%, Co/Mn < 5%); therefore, phase definitions must be stated for comparability [68]. Recent research confirms element-specific gaps that vary by site and water type: Fe and Mn are typically much lower in the dissolved fraction than total, Co is largely dissolved (≈90–100%), Ni (≈25–35%) and Zn (≈60–70%) are intermediate, and Cu is moderately partitioned (≈40–50%) [69].
Grain-size normalization can further shift metal levels: in sediments with >40% sand, concentrations in the <63 µm fraction were up to ~7× higher than in bulk (<2 mm), with smaller differences in fine-sediment [31]. Likewise, in Yellow River sediments, 78–82% of total metal load resided in particles < 16 µm, with enrichment factors ~1.18 (Cu), 1.16 (Zn), 1.15 (Pb) for clay–fine silt relative to coarser classes [70].
Digestion chemistry influences reported metal concentrations [71,72]. “Total” digests that include HF dissolve silicates lattices and aluminosilicate coatings; partial digests (nitric or aqua-regia type) do not. Elements weakly associated with silicates or hosted in carbonates/Fe–Mn oxides (Cu, Pb, Zn, partly As) often agree within ~0–10% between partial and HF digests, whereas silicate-affinity metals (Ni, Cr, Co, partly Cd) are commonly ~5–25% lower in partial digests. Nitric-acid-only extractions can under-recover further in refractory matrices [71].
For organic contaminants (PAHs, OCPs, PCBs), variability is most evident in extraction and cleanup. While solvent extraction is a shared approach, techniques differ (solid-phase extraction, liquid–liquid extraction, Soxhlet, microwave- or accelerated-solvent extraction), as do cleanup strategies (silica/alumina vs. multilayer columns). Literature shows that method choice governs recovery and selectivity. For PAHs, reported recoveries span roughly 70–120% across common workflows: pressurized solvent extraction typically yields ~75–110% with relative standard deviations ~3–20%; solid-phase or ultrasonic extraction often gives ~82–121% in water and sediments; dispersive liquid–liquid microextraction achieves ~80% at sub-ng/L; supercritical-fluid extraction shortens extraction time with high efficiency [45]. For OCPs/PCBs, several extraction routes (agitation solid–liquid, pressurized liquid, Soxhlet) can deliver comparable recoveries when parameters are optimized; extending agitation from 1 h to 16 h changed median recovery only marginally (≈88% → ≈ 89%). Reported method-related uncertainties of ~20–50% highlight the need to report key parameters (solvent system, time/temperature, cleanup, surrogates/CRMs) and set acceptance ranges for surrogate recoveries [72].
Detection techniques also vary: ICP-MS and GC-MS/MS provide sensitive and selective measurements, but GF-AAS and GC-ECD also remain in use in some laboratories. For POPs detection, gas chromatography with tandem mass spectrometry provides higher specificity than single-quadrupole mass spectrometry or non-mass-selective detectors by confirming identity with multiple ions, reducing false positives in complex extracts [45]. Across routine metal determinations, ICP-MS and ICP-OES offer higher throughput and multi-element capability than GF-AAS. A cadmium study shows GF-AAS and ICP-MS agree at lower concentrations, while at higher levels ICP-MS aligns with ICP-OES, while GF-AAS tends to read lower. ICP-MS generally yields the best overall accuracy but at higher instrument and argon cost; ICP-OES is robust for mid–high concentrations with careful spectral line selection to avoid interferences; GF-AAS remains suitable for targeted low-level single-element work under tight control [57].
Quality assurance is recognized as essential, and most laboratories apply calibration, blanks, and recovery checks. However, systematic use of certified reference materials (CRMs) is uneven: CRMs are available for metals in sediments and biota but remain limited for PAHs, OCPs, and PCBs in seawater and biota. Reporting of sensitivity thresholds, uncertainty, and the fraction analyzed is inconsistent, and calibration ranges, blank corrections, and digestion/extraction conditions still differ among laboratories. Participation in external proficiency testing (e.g., QUASIMEME) is an asset, but results confirm that variability persists in key QA/QC steps.
As summarized in Table 5, these methodological divergences could determine whether results are comparable across institutions and suitable for use in basin-wide evaluations. Similar patterns have been documented in other regional seas. The HarmoNIA project in the Adriatic–Ionian region found that heterogeneity in sediment fraction, tissue selection, and QA documentation undermined comparability despite a shared regulatory framework [9]. The Adriatic–Ionian heavy metals case study further demonstrated that incomplete metadata, inconsistent grain-size selection, and divergent matrix definitions contributed to large variability in datasets [10]. Regionally, comparability is impacted by heterogeneous practices: sediments analyzed on mixed grain-size bases (<63 µm, <500 µm, <2 mm, or unsieved) and inconsistent core layers (0–2 cm vs. 0–10 cm); waters mixing dissolved (<0.45 µm) with total/particulate without clear phase labels; biota differing in tissue basis (fish fillet vs. whole mussel vs. mussel muscle) and weight basis (wet vs. dry) without conversions; uneven QA reporting (LOQ/LOD not tied to EQS, inconsistent CRM use, limited intercalibration evidence) and metadata gaps (sample depth, layer thickness, pre-treatments, method, instrument, station depth). After QC, most sediment entries were “good,” with notable fractions “probably bad” or <LOQ, evidencing large cross-dataset variability driven by these inconsistencies [9,10].

Table 5.
Summary of harmonization status across seawater, sediment, and biota matrices for HMs and organic contaminants (PAHs, OCPs, PCBs).
Our findings for the Black Sea, carried out under BRIDGE-BS, confirm that alignment efforts should be pragmatic and targeted, focusing on a limited set of high-leverage procedures rather than attempting complete convergence of all analytical details. Priority actions should include:
- Standardizing fraction selection across matrices and explicitly reporting it in datasets.
- Promoting convergence toward advanced extraction and detection methods while phasing out less selective techniques.
- Expanding access to CRMs for organic contaminants and enforcing systematic reporting of uncertainty and detection limits.
- Embedding regular intercalibration exercises (e.g., QUASIMEME) to validate analytical performance and strengthen confidence.
Such targeted consistency maximizes improvements in cross-border data usability while minimizing disruption to established workflows. Embedding these practices into BSIMAP and linking them with EU and Bucharest Convention reporting would align the Black Sea with best practices in other European seas and enhance the policy relevance of monitoring results.
4.2. Role of Intercalibration and QA/QC
The QUASIMEME round highlighted six recurring comparability gaps: calibration-range mismatches, insufficient blank correction, uneven access/use of certified reference materials (CRMs), digestion/extraction variability, instrument drift/maintenance, and inconsistent reporting of LOD/LOQ and uncertainty. These were observed across matrices and platforms, indicating procedural rather than instrument-specific causes. Participating laboratories reported corrective steps already implemented, including revised reference-scaling and calibration policies, stricter blank protocols, expanded CRM use, clarified SOP steps, and routine proficiency-testing participation to verify performance and improve long-term comparability.
Across matrices, most reviewed SOPs are anchored in ISO/CEN frameworks or equivalent international references, typically the ISO 5667 sampling series and ISO/IEC 17025 QA/QC, complemented by OSPAR/HELCOM guidance and UNEP/IAEA reference methods. Several SOPs apply EPA or IAEA/UNEP methods with lab-specific, ISO/IEC 17025–style validation; we treat these as partial alignments. Only a small subset relies on legacy national methods without explicit ISO/CEN anchoring and is flagged for priority harmonization. The underlying standards cited by all SOPs are compiled in Appendix A.3.
The intercalibration exercise performed within this study, using the QUASIMEME proficiency testing scheme, showed that Black Sea laboratories generally achieved satisfactory performance, but also revealed recurring weaknesses in reference scaling selection, recovery correction, and the systematic reporting of analytical limits and measurement uncertainty. These findings mirror lessons from earlier European QA initiatives, including ICES (International Council for the Exploration of the Sea) proficiency tests, OSPARCOM (Oslo and Paris Commissions) monitoring programs, and BCR (Community Bureau of Reference, later integrated into the European Commission’s Joint Research Centre—JRC) guidelines, which demonstrated that even when laboratories nominally apply the same methods, results can diverge significantly without rigorous QA/QC oversight [7,8].
Regular participation in interlaboratory comparisons is therefore not only a tool for performance evaluation but also a mechanism for continuous training, benchmarking, and mutual learning. Proficiency testing historically served to identify methodological weaknesses, promote corrective actions, and ensure alignment with evolving regulatory standards. Embedding such exercises within the Black Sea Integrated Monitoring and Assessment Program (BSIMAP) would consolidate regional capacity, enhance institutional trust, and ensure that pollutant data are defensible in both scientific and policy contexts [6].
This study also highlighted that QA/QC measures, such as systematic use of certified reference materials (CRMs), regular calibration verification, and transparent reporting of detection limits and uncertainty, should be considered essential components of a surveillance system, not optional add-ons. The availability of IAEA-MESL (Marine Environmental Studies Laboratory, Monaco) CRMs for trace metals and POPs in seawater, sediment, and biota provides a global reference framework that should be systematically integrated into Black Sea monitoring workflows [73].
Finally, the QUASIMEME framework itself has proven the most effective mechanism for QA/QC standardization in Europe, institutionalizing z-score evaluation, systematic error identification, and corrective feedback loops [74]. Its systematic adoption in the Black Sea region would align assessment techniques with long-established practices in the North-East Atlantic (OSPAR) and the Baltic (HELCOM), thereby strengthening the scientific robustness and policy credibility of large-scale assessments.
4.3. Metadata and Data Management
This study confirms that coordination must extend beyond laboratory procedures to encompass metadata and data management. Without systematic documentation of sampling depth, fraction analyzed, sensitivity thresholds, uncertainty, and QA/QC history, datasets cannot be reliably used for sea-wide assessments. The Adriatic–Ionian Sea case study demonstrated that metadata gaps can be as significant a barrier to comparability as methodological heterogeneity itself [10], a conclusion that is directly relevant to the Black Sea context.
The adoption of FAIR principles (Findable, Accessible, Interoperable, Reusable) has already improved marine data management in European infrastructures such as EMODnet Chemistry and SeaDataNet [75,76,77]. These systems ensure interoperability through controlled vocabularies, standardized formats, and quality-flagging protocols. Integrating these principles into the Black Sea Integrated Monitoring and Assessment Program (BSIMAP) would ensure that sub-basin datasets are not only consistent at the point of collection but also compatible with EU-wide and global repositories, enhancing their scientific and policy value.
Metadata completeness is equally critical for transparency and accountability. Without explicit information on the analytical method applied, the detection limits used, or whether biota concentrations are expressed on a wet- or dry-weight basis, it is impossible to integrate results across institutions or to judge compliance with environmental quality standards. Thus, the unification of SOPs must proceed in parallel with harmonization of reporting formats and metadata structures. The policy backbone for this approach is provided by European Commission Decision 2017/848 laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardised methods for monitoring and assessment, which explicitly calls for regional cooperation in cases where thresholds and methods remain undefined.
4.4. Lessons from European Harmonization Frameworks
The trajectory of QA/QC in European marine evaluation strategies highlights the long-term value of coordination. The London Declaration on the Protection of the North Sea (1987) first called for coordinated methods and reliability framework in marine monitoring. This was followed by the ICES Cooperative Research Report No. 175 (1991), and later the OSPAR CEMP Guidelines for Contaminants in Sediment and Biota (OSPAR Agreement 2014-04), which institutionalized cross-laboratory evaluation exercises and the systematic use of reference materials (Appendix A.3). The QUASIMEME programme further demonstrated the effectiveness of structured, iterative learning schemes, showing how regular proficiency testing can both identify analytical weaknesses and drive convergence across laboratories [8].
Our study, undertaken within the BRIDGE-BS framework, extends this legacy into the Black Sea. Results show that analytical capacity is largely in place, but institutionalizing harmonized SOPs, metadata standards, and QA/QC routines at sea-wide level remains the next pivotal step. By doing so, the Black Sea region can leverage decades of European experience and avoid repeating pitfalls already encountered in the North Sea, North Atlantic, and Mediterranean monitoring frameworks. Complementary models are already available: the HELCOM Monitoring Manual (2018) and UNEP/MAP IMAP Guidelines (2016) both provide structured protocols for contaminant methods, including matrix prioritization and common indicators, which can be adapted to the Black Sea context (Appendix A.3).
A recent assessment under EMODnet Chemistry evaluated laboratory practices for contaminants across European seas [78]. The survey confirmed that most laboratories show high technical proficiency, with qualified staff, well-maintained instruments, systematic quality checks, and use of CRMs. Nevertheless, methodological heterogeneity remains evident. Differences were noted in sampling practices, particularly for biota, in the digestion and analytical methods and in the calculation of detection and quantification limits. While analytical methods for hydrocarbons, pesticides, PCBs, and metals are broadly consistent, important divergences persist in the details of their application and in the detection platforms employed. The study also highlighted that QA/QC metadata are often incomplete, limiting full comparability and reusability of datasets [78].
Comparable issues have been highlighted in the Eastern Mediterranean, where Lipizer et al. (2022) [79] analyzed large datasets of trace metals and PAHs in sediments. That study showed that local concentration ranges and background levels can serve as effective quality control tools to distinguish natural variability from anthropogenic enrichment. However, it also emphasized that heterogeneity in digestion and extraction protocols, coupled with limited metadata on sediment characteristics, hampers comparability across laboratories and datasets. Very high values observed in known contamination hot spots demonstrated the need to separate true signals from analytical inconsistencies.
Similar challenges have also been documented in the Adriatic–Ionian Seas, where Molina Jack et al. (2020) [10] highlighted that heavy metal datasets remain fragmented and heterogeneous, with differences in sampling, analytical methods, and metadata reporting limiting their integration. The study stressed that long-term datasets and interoperable infrastructures such as EMODnet Chemistry and SeaDataNet are essential to ensure FAIR data principles and reliable trend analysis. These observations reinforce our findings for the Black Sea, where alignment must address not only laboratory protocols but also the quality, interoperability, and accessibility of metadata to support coherent sea-wide assessments.
These findings parallel our observations for the Black Sea, where observation methods are broadly aligned with international standards, but divergences in sampling, fractionation, digestion and extraction methods, instrumental approaches, and QA/QC documentation continue to constrain data comparability and integration. This historical perspective also highlights the importance of continuity. Harmonization is not a one-off exercise but an ongoing process that requires regular updates to SOPs, continuous intercalibration, and adaptation to emerging pollutants and evolving regulatory thresholds. Embedding these practices into the institutional culture of the Black Sea laboratories and agencies will be essential to ensure long-term comparability, credibility, and policy relevance of environmental data.
4.5. Toward a Regional Harmonization Strategy
The findings of this study suggest that effective coordination in the Black Sea requires a two-tiered approach.
First, technical alignment should target the methodological steps with the greatest impact on consistency of results, like sediment size class, tissue basis in biota, reference scaling practices, and uncertainty reporting. By addressing these high-leverage areas, laboratories can ensure that results are comparable and defensible in large scale assessments, even if minor procedural differences remain. Best practices are already available through international frameworks, such as ISO 5667-3:2018 for water sampling (2018), ICES TIMES methods for validated contaminant protocols (2020), and OSPAR CEMP normalization rules (2014) (Appendix A.3).
Second, systemic improvements are required in QA/QC documentation, metadata completeness, and alignment of data infrastructures with FAIR principles (Findable, Accessible, Interoperable, Reusable). This tier ensures that data are not only comparable at the point of production but also reusable across time and borders, integrated into European infrastructures such as EMODnet Chemistry and SeaDataNet, and suitable for use in global assessments [76,77].
By addressing both tiers, Black Sea monitoring will be able to deliver data that is nationally relevant and regionally coherent, specifically supporting the implementation of BSIMAP, the Bucharest Convention, and EU directives. In doing so, institutions can ensure that pollutant measurements contribute to robust Good Environmental Status (GES) assessments, inform evidence-based policy, and sustain public trust in environmental reporting.
The outcomes of the proficiency testing exercise provide a vital complement to the SOPs review undertaken in the framework of BRIDGE-BS, as they reveal how methodological differences translate into practical challenges during routine laboratory work. The feedback collected through the accompanying questionnaire (Table 4; Appendix A.2) highlighted recurring issues, such as reference scaling alignment, CRM availability, digestion protocol variability, and inconsistent reporting of uncertainty, that could affect comparability. These observations confirm earlier European QA experiences [7,8] and reinforce the need to embed intercalibration results into the refinement of SOPs. Regular participation in proficiency testing, coupled with structured feedback loops, must become a cornerstone of sea-wide consistency under BSIMAP and related regional frameworks.
Beyond the alignment of analytical procedures, the unification of assessment methodologies represents the next essential step for the Black Sea region. Achieving coherence in the application of indicators, thresholds, and criteria is essential to ensure that monitoring data are consistently interpreted and can be applied reliably in the evaluation of ecological status. Figure 4 presents this conceptual framework: analytical procedures (sampling, digestion/extraction, detection, QA/QC) form the technical foundation, assessment methodologies (indicators, thresholds, criteria) provide the evaluative layer, and QA/QC together with intercalibration (IC) act as the central bridge linking the two. This integration ensures that laboratory-generated data are both comparable and policy-relevant, supporting evaluations under BSIMAP, the Bucharest Convention, and EU directives. While the present study has focused primarily on analytical consistency, advancing toward a shared evaluative framework remains a priority for future efforts.
Figure 4.
Conceptual framework for harmonization of pollutant monitoring in the Black Sea.
Beyond the alignment of SOPs and intercalibration, future coordination efforts in the Black Sea must also encompass the assessment dimension. Bellas et al. (2020) [80] highlighted that assessment strategies under WFD and MSFD still follow different conceptual frameworks: risk-based versus ecosystem-based and argued for complementing chemical measurements with effect-based tools such as biomarkers and ecotoxicological assays. Similarly, the ICON project [81] demonstrated that combining chemical analyses with biological effect indicators (e.g., PAH metabolites, oxidative stress markers, genotoxicity, fish disease) within a unified framework and applying ICES/OSPAR assessment criteria (BAC/EAC) provides more comprehensive insights into contaminant impacts than chemical data alone. Both perspectives reinforce that the Black Sea should gradually move toward an integrated chemical–biological evaluation framework, coupling harmonized laboratory protocols with effect-based assessments, to ensure robust evaluations of ecological status and full compatibility with European marine policy requirements.
4.6. Recommendations for Harmonization
Basin-wide comparability is governed primarily by a small set of harmonized methodological design choices covering sampling, fraction definition, preparation, and detection principles, rather than using identical instrumentation. Each monitoring program should therefore adopt clear, stable fraction policies and apply them consistently over time. In seawater, select a single fraction per station: dissolved or total, according to assessment purpose and maintain it across years. In sediments, target recent deposits and state both the sampling thickness and the grain-size policy; where a fine fraction is used to enhance trend sensitivity, declare it explicitly. In biota, fix species and tissue a priori and report concentrations on a consistent weight basis suitable for compliance assessment, adding conversion information only when required.
Sample preparation and analysis must be purpose-driven and transparent. The digestion or extraction approach should be tailored to the monitoring objective and explicitly stated with the method description. For programmes focused on routine surveillance and temporal trends, preparation should prioritize reproducibility and matrix comparability; where the objective is quantitative accounting of contaminant stocks and fluxes, preparation should be more exhaustive to maximize recovery and control speciation, and this rationale should be documented within the analytical workflow.
Detection should rely on methods that provide confirmatory selectivity, so that co-elution and misidentification are controlled by specific identifiers and predefined criteria. Screening approaches may be applied for preliminary triage, but they are presumptive and must be followed by confirmation that demonstrates identity using independent identifiers and meets stated acceptance thresholds. The method description should state the confirmation criteria and summarize key performance characteristics (e.g., selectivity, LOD/LOQ, recovery, and precision) to make the evidential basis explicit.
Quality assurance must be systematic and transparent. Laboratories should participate in external proficiency testing at defined intervals and document outcomes, corrective actions, and performance trends. Participation frequency and scope should match monitoring goals and regulatory obligations, while acknowledging resource constraints that may limit regular interlaboratory exercises; where necessary, interim checks (e.g., shared reference materials, blind duplicates, or data audits) should bridge gaps between full rounds.
Routine QA/QC elements: recoveries, blanks, duplicates, detection and quantification limits, and stated uncertainty, should be reported in a standardized format so that results are interpretable beyond the originating laboratory. Intercalibration should function as a recurring feedback mechanism that reveals bias, aligns acceptance bands for accuracy and precision, and triggers SOP updates and targeted training.
Interoperability depends on complete metadata. Every dataset should carry SOP-level description of the sampling context (device, layer, fraction, tissue or grain size), preparation and cleanup, detection principle, analytical limits and uncertainty, QC flags, and any normalizers applied, aligned to shared regional vocabularies. Implementing these principles will reduce method-induced variance, increase the reliability of status classification, and enable regional aggregation for MSFD and BSIMAP reporting, while allowing laboratories to select instruments and methods that meet agreed performance criteria.
Harmonization in the Black Sea is progressing as a continuous process. Priorities are to broaden the framework to matrices and substance groups with limited evidence, especially emerging contaminants, and to strengthen designs that capture spatial and temporal structure where relevant (e.g., vertical and seasonal variability) using the same principles of explicit fraction policies, purpose-aligned preparation, and confirmatory detection.
In parallel, metadata and uncertainty reporting should be embedded as standard practice, so datasets are FAIR by design and readily reusable for regional assessments and trend analyses. Finally, intercalibration and external proficiency testing should be maintained as routine feedback mechanisms, with participation planned pragmatically considering resource constraints and complemented by interim checks when full rounds are not feasible.
5. Conclusions
Comparative review across Black Sea SOPs and practices indicates that basin-wide comparability is governed primarily by a core set of methodological design choices, rather than by uniformity in instrumentation or analytical platforms. The principal drivers are: (i) fraction definition in seawater; (ii) surface-layer thickness and grain-size policy in sediments; (iii) species/tissue selection and reporting basis in biota; and (iv) preparation aligned to assessment purpose with confirmatory detection, underpinned by explicit QA/QC and complete metadata. Variation at these points influences trend detection and may change outcomes of environmental status evaluations.
Interlaboratory evidence from proficiency testing and follow-up questionnaires highlights practical factors that amplify these differences, including calibration-range selection, blank correction, access to and use of certified reference materials, protocol interpretation, instrument maintenance, and incomplete reporting of detection limits and uncertainty. These issues are tractable but require systematic attention beyond the analytical step itself.
Accordingly, harmonization has two complementary layers. First, matrix-specific levers should be fixed and declared: stable fraction policies in water, recent-deposit sampling with stated thickness and granulometry in sediments, and predefined species/tissue with a consistent weight basis in biota, while preparation and detection remain purpose-matched and supported by confirmation. Second, measurement quality should be made visible through routine external proficiency testing, agreed acceptance bands for accuracy and precision, documented corrective actions, and interoperable metadata.
Embedding these rules within BSIMAP will reduce method-induced variance, improve the reliability of status classification, and enable cross-border aggregation for MSFD and the Bucharest Convention. Parallel progress on assessment coherence: agreement on indicators, thresholds, and evaluation criteria will ensure that technically comparable data translate into consistent judgments of environmental status.
Beyond the present focus on heavy metals and priority organics, basin-wide harmonization should advance further for microplastics and emerging contaminants. Extending the same principles means applying standardized SOPs, shared QA/QC checks, intercalibration, and explicit uncertainty and metadata to less-studied matrices and contaminant classes where validated methods and datasets are limited, so outputs remain transparent, comparable, and reproducible. Future projects should widen participation across countries, formalize data-exchange formats, and pilot aligned protocols with provider-run proficiency testing. Institutionalizing these practices will make datasets FAIR by design and reusable for regional synthesis and evaluation. Taken together, these steps move Black Sea monitoring from nominal method alignment to a fully harmonized, performance-based framework that supports consistent, policy-relevant assessments.
Author Contributions
Conceptualization, A.O., V.C., Y.O., H.A., E.A., L.B. (Levent Bat), N.M., A.B., N.Ç.B., N.E.K. and L.B. (Laura Boicenco); methodology, A.O., V.C., Y.O., H.A., E.A., L.B. (Levent Bat), N.M., A.B., N.Ç.B., N.E.K. and L.B. (Laura Boicenco); validation, A.O., V.C., Y.O., H.A., E.A., L.B. (Levent Bat), N.M., A.B., N.Ç.B., N.E.K. and L.B. (Laura Boicenco); formal analysis, A.O., V.C., Y.O., H.A., E.A., L.B. (Levent Bat), N.M., A.B., N.Ç.B., N.E.K. and L.B. (Laura Boicenco); investigation, A.O., V.C., Y.O., H.A., E.A., L.B. (Levent Bat), N.M., A.B., N.Ç.B., N.E.K. and L.B. (Laura Boicenco); resources, A.O., V.C., Y.O., H.A., E.A., L.B. (Levent Bat), N.M., A.B., N.Ç.B., N.E.K. and L.B. (Laura Boicenco); data curation, A.O., V.C., Y.O., H.A., E.A., L.B. (Levent Bat), N.M., A.B., N.Ç.B., N.E.K. and L.B. (Laura Boicenco); writing—original draft preparation, A.O., V.C., Y.O., H.A., E.A., L.B. (Levent Bat), N.M., A.B., N.Ç.B., N.E.K. and L.B. (Laura Boicenco); writing—review and editing, A.O., V.C., Y.O., H.A., E.A., L.B. (Levent Bat), N.M., A.B., N.Ç.B., N.E.K. and L.B. (Laura Boicenco); visualization, A.O., V.C., Y.O., H.A., E.A., L.B. (Levent Bat), N.M., A.B., N.Ç.B., N.E.K. and L.B. (Laura Boicenco); supervision, A.O. and L.B. (Laura Boicenco); project administration, L.B. (Laura Boicenco); funding acquisition, L.B. (Laura Boicenco) All authors have read and agreed to the published version of the manuscript.
Funding
This research and APC were supported by the BRIDGE-BS Project from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement No. 101000240.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank all BRIDGE-BS partner institutions for providing Standard Operating Procedures (SOPs) and intercalibration feedback that formed the basis of this study. The authors also acknowledge the QUASIMEME (Quality Assurance of Information for Marine Environmental Monitoring in Europe) proficiency testing scheme for providing the framework used in the intercalibration exercise. The authors acknowledge the assistance of OpenAI’s ChatGPT-5 Plus (August 2025 release), which was used to generate schematic figures. These figures were subsequently reviewed and edited by the authors.
Conflicts of Interest
Author Nino Machitadze was employed by Research Center GAMMA Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAS | Atomic Absorption Spectrometry |
| APDC | Ammonium Pyrrolidine Dithiocarbamate |
| ASE | Accelerated Solvent Extraction |
| BAC/EAC | Background Assessment Criteria/Environmental Assessment Criteria |
| BCR | Community Bureau of Reference |
| BSIMAP | Black Sea Integrated Monitoring and Assessment Program |
| CEN | European Committee for Standardization |
| CRMs | Certified Reference Materials |
| CV-AAS | Cold Vapor Atomic Absorption Spectrometry |
| DMA | Direct Mercury Analyzer |
| EQS | Environmental Quality Standards |
| ERL/ERM | Effects Range Low/Effects Range Median |
| EU | European Union |
| FAAS | Flame Atomic Absorption Spectrometry |
| FAIR | Findable, Accessible, Interoperable, Reusable |
| GC-ECD | Gas Chromatography with Electron Capture Detector |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| GC-MS/MS | Gas Chromatography–Tandem Mass Spectrometry |
| GES | Good Environmental Status |
| GF-AAS | Graphite Furnace Atomic Absorption Spectrometry |
| HELCOM | Helsinki Commission (Baltic Marine Environment Protection Commission) |
| HF | Hydrofluoric Acid |
| HMs | Heavy Metals |
| IAEA-MESL | International Atomic Energy Agency—Marine Environmental Studies Laboratory |
| IC | Intercalibration |
| ICES | International Council for the Exploration of the Sea |
| ICP-MS | Inductively Coupled Plasma—Mass Spectrometry |
| ICP-OES | Inductively Coupled Plasma—Optical Emission Spectrometry |
| IMAP | Integrated Monitoring and Assessment Programme (Mediterranean, UNEP/MAP) |
| ISO | International Organization for Standardization |
| JRC | Joint Research Centre (European Commission) |
| LOD/LOQ | Limit of Detection/Limit of Quantification |
| LLE | Liquid–Liquid Extraction |
| MAE | Microwave-Assisted Extraction |
| MSFD | Marine Strategy Framework Directive |
| NOAA | National Oceanic and Atmospheric Administration |
| OCPs | Organochlorine Pesticides |
| OSPAR | Oslo and Paris Commissions |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PCBs | Polychlorinated Biphenyls |
| QA/QC | Quality Assurance/Quality Control |
| QUASIMEME | Quality Assurance of Information for Marine Environmental Monitoring in Europe |
| SIM/MRM | Selected Ion Monitoring/Multiple Reaction Monitoring |
| SOPs | Standard Operating Procedures |
| UNEP | United Nations Environment Programme |
| UNEP/MAP | United Nations Environment Programme/Mediterranean Action Plan |
| WEPAL | Wageningen Evaluating Programs for Analytical Laboratories |
| WFD | Water Framework Directive |
Appendix A
Appendix A.1. Standard Operating Procedure (SOP) Template (For HMs and POPs in Seawater, Sediment, and Biota)
Note: partners were provided with the following standardized template to document SOPs. The same structure applies across matrices. Fields are to be completed with institution-specific information.
SOP Template
1. Title of SOP
(Specify determinant group)
2. Purpose
(State objective and intended application of the method)
3. Principle and Operation
(Describe the underlying principle of analysis: digestion/extraction, instrumental method)
4. Materials and Equipment
Reagents and solvents
Certified reference materials (if available)
Instruments and accessories
5. Sampling and Storage
Sampling gear and procedure
Container material and preparation
Preservation steps
Storage and transport conditions
6. Sample Preparation
Pre-treatment steps (e.g., filtration, sieving, homogenization, tissue selection)
Digestion or extraction procedure
Cleanup steps (if applicable)
7. Analytical Method
Instrument type
Measurement parameters/settings
Detection mode
8. Calibration and Standards
Calibration strategy
Reference/standard solutions used
Frequency of calibration
9. QA/QC Measures
Blanks (field, procedural)
Replicates/duplicates
Spikes and recovery tests
CRMs (if applied)
LOD/LOQ and uncertainty reporting
10. Data Reporting
Units
Weight basis (wet or dry, biota)
Metadata to be included (sampling date, site, conditions, etc.)
Appendix A.2. Intercalibration (IC) Questionnaire
Note: This questionnaire is reproduced from BRIDGE-BS Deliverable D1.4, with only minor formatting adjustments for clarity and journal style.
Section 1. General Information
Institution name and country
Laboratory code (if applicable)
Contact person and email
Matrices analyzed (water, sediment, biota)
Contaminants targeted (heavy metals, PAHs, PCBs, OCPs, others)
Section 2. Preparation and Resources
Was sufficient information provided in advance to prepare for the IC exercise?
Were laboratory resources (staff, equipment, consumables) adequate to complete the exercise?
Did you experience difficulties in sample storage, preparation, or handling?
Section 3. Participation and Learning Outcomes
What challenges did your laboratory encounter during analysis?
Which parts of your SOPs required adjustments to complete the exercise?
Did participation highlight strengths or weaknesses in your laboratory methods?
Did the exercise increase staff awareness of QA/QC requirements?
Section 4. Implementing Improvements
Were corrective actions identified as a result of participation?
Did you adopt new calibration practices or revise blank correction methods?
Was the use of Certified Reference Materials (CRMs) improved or expanded?
Did you change reporting procedures for LOD/LOQ or uncertainty?
Section 5. Collaboration and Standardization
Did the IC exercise promote dialogue with other Black Sea laboratories?
Were differences in SOP interpretation clarified through the exercise?
Do you consider intercalibration useful for harmonization of monitoring practices at
basin level?
What are your recommendations for future IC rounds?
Section 6. Overall Impact and Future
How would you rate the overall usefulness of the IC exercise for your institution?
Do you plan to maintain regular participation in QUASIMEME or other proficiency
testing schemes?
Which areas require most support for achieving harmonized monitoring (calibration,
CRMs, digestion/extraction, maintenance, reporting)?
Additional comments.
Appendix A.3
References from SOPs (HMs and POPs)
These references were explicitly declared by partner laboratories in their Standard Operating Procedures (SOPs) collected under BRIDGE-BS. They represent the operational basis for monitoring in the Black Sea region.
Agilent Technologies. ICP-MS Operator’s Manual (Agilent 7700X); Internal Standard and Tune Solutions. Agilent Technologies: Santa Clara, CA, USA.
Allen, H.E.; Minear, R.A. (1982). Examination of Water for Pollution Control: Physical, Chemical and Radiological Examination. Pergamon Press: Oxford, UK.
APHA/AWWA/WPCF. (1980). Standard Methods for the Examination of Water and Wastewater, 15th ed.; American Public Health Association: Washington, DC, USA.
Bruland, K.W.; Coale, K.H.; March, L. (1985). Analysis of seawater for dissolved cadmium, copper, and lead: An intercomparison of voltammetric and atomic absorption methods. Mar. Chem. 17, 285–300.
Canlı, O.; Çetintürk, K.; Öktem Olgun, E.E. (2020). Determination of 117 endocrine disruptors in water using SBSE TD–GC-MS/MS under the European Water Framework Directive. Anal. Bioanal. Chem. 412, 5169–5178.
De Mora, S.; Tolosa, I.; Fowler, S.W.; Villeneuve, J.P.; Cassi, R.; Cattini, C. (2010). Distribution of petroleum hydrocarbons and organochlorinated contaminants in marine biota and coastal sediments from the ROPME Sea Area during 2005. Mar. Pollut. Bull. 60, 2323–2349.
DSTU ISO 9000. (2015). Quality Management Systems. Basic Provisions and Glossary of Terms. State Standard of Ukraine.
DSTU ISO/IEC 17025. (2019). General Requirements for the Competence of Testing and Calibration Laboratories. State Standard of Ukraine.
EPA. (1986). Method 3510C: Separatory Funnel Liquid–Liquid Extraction. U.S. Environmental Protection Agency: Washington, DC, USA.
EPA. (1995). Test Methods for Evaluating Solid Waste, Method 3545 (Pressurized Fluid Extraction). SW-846, 3rd ed., Update III. U.S. Environmental Protection Agency: Washington, DC, USA.
EPA. (2003). Sample Preparation Procedure for Spectrochemical Determination of Total Recoverable Elements in Biological Tissues. U.S. Environmental Protection Agency: Washington, DC, USA.
EPA. (2008). Determination of Trace Elements in Waters and Wastes by ICP-MS. U.S. Environmental Protection Agency: Washington, DC, USA.
EPA. Method 3052: Microwave-Assisted Acid Digestion of Siliceous and Organically Based Matrices. U.S. Environmental Protection Agency: Washington, DC, USA.
EPA. Method 6020B: Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) for Trace Metals in Environmental Samples. U.S. Environmental Protection Agency: Washington, DC, USA.
Grasshoff, K.; Kremling, K.; Ehrhardt, M. (Eds.) (1999). Methods of Seawater Analysis, 3rd ed.; Wiley-VCH: Weinheim, Germany.
Grimmer, G.; Böhnke, H. (1975). Polycyclic aromatic hydrocarbon profile analysis of high-protein foods, oils, and fats by gas chromatography. J. Assoc. Off. Anal. Chem. 58, 725–733.
IAEA-MEL. (1999). Training Manual: Measurement of Heavy Metals in Environmental Samples. International Atomic Energy Agency, Marine Environmental Laboratory: Monaco.
IAEA-MEL/MESL. (1995). Training Manual on the Measurement of Organochlorine Compounds and Petroleum Hydrocarbons in Environmental Samples. International Atomic Energy Agency: Monaco.
IAEA-MEL/MESL. (2011). MEDPOL—Trace Organic Contaminants Training Course. Monaco, 5–16 December 2011.
ISO. (2001). ISO 9000: Quality Management Systems—Principles and Vocabulary. International Organization for Standardization: Geneva, Switzerland.
ISO. (2003). ISO 5667-2: Water Quality—Sampling—Part 2: Guidance on Sampling Techniques. International Organization for Standardization: Geneva, Switzerland.
ISO. (2004). ISO 5667-19: Water Quality—Sampling—Part 19: Guidance on Sampling in Marine Sediments. International Organization for Standardization: Geneva, Switzerland.
ISO. (2005). ISO 5667-9: Water Quality—Sampling—Part 9: Guidance on Sampling from Marine Waters. International Organization for Standardization: Geneva, Switzerland.
ISO. (2005). ISO 17025: General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland.
ISO. (2022). ISO 5667-3: Water Quality—Sampling—Part 3: Preservation and Handling of Water Samples. International Organization for Standardization: Geneva, Switzerland.
ISO. ISO 11466: Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia. International Organization for Standardization: Geneva, Switzerland.
ISO. ISO 12914: Microbeam Analysis—Calibration of Energy Dispersive X-ray Spectrometers. International Organization for Standardization: Geneva, Switzerland.
Karacık, B.; Okay, O.; Henkelmann, B.; Pfister, G.; Schramm, K.-W. (2013). Water concentrations of PAH, PCB, and OCP by using semipermeable membrane devices and sediments. Mar. Pollut. Bull. 70, 258–265.
Milestone. Microwave Digestion Application Reports. Milestone Srl: Sorisole, Italy.
QUASIMEME. Proficiency Testing for Marine Environmental Monitoring. Wageningen University: Wageningen, The Netherlands.
UNEP. (1984). Determination of Total Cd, Zn, Pb, and Cu in Selected Marine Organisms by Flameless AAS. Reference Methods for Marine Pollution Studies No. 11 (Rev. 1). United Nations Environment Programme: Nairobi, Kenya.
UNEP. (1985). GESAMP: Cadmium, Lead, and Tin in the Marine Environment. UNEP Regional Seas Reports and Studies No. 56. United Nations Environment Programme: Nairobi, Kenya.
UNEP/FAO/IOC/IAEA. (1993). Guidelines for Monitoring Chemical Contaminants in the Sea Using Marine Organisms. Reference Methods for Marine Pollution Studies No. 6. United Nations Environment Programme: Nairobi, Kenya.
UNEP/IOC/IAEA. (1995). Manual for the Geochemical Analysis of Marine Sediments and Suspended Particulate Matter. Reference Methods for Marine Pollution Studies No. 63. United Nations Environment Programme: Nairobi, Kenya.
Policy and Methodological Regional Frameworks
These references were not cited in partner SOPs but provide overarching international frameworks, guidelines, and QA/QC infrastructures (e.g., ISO, OSPAR, HELCOM, UNEP/MAP, IAEA-MESL, JRC) that shape contaminant monitoring practices. They are included here to give context and highlight alignment opportunities for Black Sea harmonization.
International Conference on the Protection of the North Sea. (1987). London Declaration on the Protection of the North Sea. London, UK, 24–25 November 1987.
ICES. (1991). Report of the ICES Advisory Committee on Marine Pollution, 1991. ICES Cooperative Research Report No. 175, International Council for the Exploration of the Sea: Copenhagen, Denmark.
OSPAR Commission. (2014). OSPAR CEMP Guidelines: Coordinated Environmental Monitoring Programme (CEMP) Guidelines for Contaminants in Sediment and Biota. OSPAR Agreement 2014-04. Available online: https://www.ospar.org (accessed on 20 August 2025).
HELCOM. (2018). HELCOM Monitoring Manual: Contaminants. Helsinki Commission (Baltic Marine Environment Protection Commission): Helsinki, Finland. Available online: https://helcom.fi/action-areas/monitoring-and-assessment/monitoring-manual/ (accessed on 20 August 2025).
UNEP/MAP. (2016). Integrated Monitoring and Assessment Programme (IMAP) of the Mediterranean Sea and Coast and Related Assessment Criteria. UNEP(DEPI)/MED IG.22/28: Athens, Greece.
WEPAL/QUASIMEME. (2025). WEPAL-QUASIMEME Proficiency Testing Scheme for Marine Environmental Monitoring. Wageningen University: Wageningen, The Netherlands. Available online: https://www.wepal.nl (accessed on 20 August 2025).
IAEA-MESL. (2024). Catalogue of Certified Reference Materials (CRMs) for Marine Environmental Studies. International Atomic Energy Agency, Marine Environmental Studies Laboratory: Monaco. Available online: https://analytical-reference-materials.iaea.org (accessed on 20 August 2025).
International Organization for Standardization (ISO). (2018). ISO 5667-3:2018 Water Quality—Sampling—Part 3: Preservation and Handling of Water Samples. ISO: Geneva, Switzerland.
ICES. (2020). ICES Techniques in Marine Environmental Sciences (TIMES) series. International Council for the Exploration of the Sea: Copenhagen, Denmark. Available online: https://ices-library.figshare.com (accessed on 20 August 2025).
EMODnet Chemistry. (2025). Quality Control Steps and Data Aggregation for Contaminants Datasets. Available online: https://emodnet-chemistry.eu (accessed on 20 August 2025).
SeaDataNet. (2025). Common Vocabularies and Data Standards for Marine Data Management. Available online: https://www.seadatanet.org (accessed on 20 August 2025).
European Commission—JRC. (2001). BCR Information—The Community Bureau of Reference Programme (1973–2000). JRC: Geel, Belgium.
Joint Research Centre—Reference Materials Unit. (2018). Certified Reference Materials—Catalogue and Production Guidelines. JRC: Geel, Belgium.
References
- Oros, A.; Coatu, V.; Secrieru, D.; Tiganus, D.; Vasiliu, D.; Atabay, H.; Beken, C.; Tolun, L.; Moncheva, S.; Bat, L. Results of the Assessment of the Western Black Sea Contamination Status in the Frame of the MISIS Joint Cruise. Cercet. Mar. 2016, 46, 61–81. [Google Scholar]
- Krutov, A. (Ed.) State of the Environment of the Black Sea (2009–2014/5); Black Sea Commission: Istanbul, Turkey, 2019. [Google Scholar]
- Editura CD Press. ANEMONE Deliverable 2.3 “Black Sea State of Environment Based on ANEMONE Joint Cruise”; Lazar, L., Ed.; Editura CD Press: Bucharest, Romania, 2021. [Google Scholar]
- Editura CD Press. ANEMONE Deliverable 2.2 “Anthropogenic Pressures and Impacts on the Black Sea Coastal Ecosystem”; Lazar, L., Ed.; Editura CD Press: Bucharest Constanța, Romania, 2021; ISBN 978-606-528-529-3. [Google Scholar]
- Editura CD Press. ANEMONE Deliverable 2.1, 2021. “Impact of the Rivers on the Black Sea Ecosystem”; Lazar, L., Ed.; Editura CD Press: Bucharest Constanța, Romania, 2021; ISBN 978-606-528-528-6. [Google Scholar]
- Editura CD Press. ANEMONE Deliverable 1.3 “Black Sea Monitoring and Assessment Guideline”; Todorova, V., Ed.; Editura CD Press: Bucharest Constanța, Romania, 2021; ISBN 978-606-528-527-9. [Google Scholar]
- Topping, G. The Role and Application of Quality Assurance in Marine Environmental Protection. Mar. Pollut. Bull. 1992, 25, 61–66. [Google Scholar] [CrossRef]
- Quevauviller, P. The QUASIMEME Project from a European Union Viewpoint. Mar. Pollut. Bull. 1997, 35, 140–145. [Google Scholar] [CrossRef]
- Berto, D.; Formalewicz, M.; Giorgi, G.; Manfra, L.; Lipizer, M.; Molina Jack Adrianna Aravantinou, M.-E.; Bajt, O.; Cara, M.; Castelli, A.; Cermelj, B.; et al. HarmoNIA Methodological Proposals. Part I: Harmonizing Monitoring of Marine Contaminants and Sharing Data Quality Control Procedures Harmonization and Networking for Contaminant Assessment in the Ionian and Adriatic Seas; Istituto Nazionale di Oceanografia e di Geofisica Sperimentale: Venice, Italy, 2020. [Google Scholar] [CrossRef]
- Molina Jack, M.E.; Bakiu, R.; Castelli, A.; Čermelj, B.; Fafanđel, M.; Georgopoulou, C.; Giorgi, G.; Iona, A.; Ivankovic, D.; Kralj, M.; et al. Heavy Metals in the Adriatic-Ionian Seas: A Case Study to Illustrate the Challenges in Data Management When Dealing with Regional Datasets. Front. Mar. Sci. 2020, 7, 571365. [Google Scholar] [CrossRef]
- Batley, G.E.; Gardner, D. Sampling and Storage of Natural Waters for Trace Metal Analysis. Water Res. 1977, 11, 745–756. [Google Scholar] [CrossRef]
- Horowitz, A.J.; Lum, K.R.; Garbarino, J.R.; Hall, G.E.M.; Lemieux, C.; Demas, C.R. The Effect of Membrane Filtration on Dissolved Trace Element Concentrations. Water Air Soil Pollut. 1996, 90, 281–294. [Google Scholar] [CrossRef]
- Cuong, D.T.; Karuppiah, S.; Obbard, J.P. Distribution of Heavy Metals in the Dissolved and Suspended Phase of the Sea-Surface Microlayer, Seawater Column and in Sediments of Singapore’s Coastal Environment. Environ. Monit. Assess. 2008, 138, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, C.; Zhang, G.; Guo, Y.; Song, Y.; Thangaraj, S.; Zhang, X.; Sun, J. Dissolved and Particulate Heavy Metal Pollution Status in Seawater and Sedimentary Heavy Metals of the Bohai Bay. Mar. Environ. Res. 2023, 191, 106158. [Google Scholar] [CrossRef]
- Satybaldiyev, B.; Ismailov, B.; Nurpeisov, N.; Kenges, K.; Snow, D.D.; Malakar, A.; Uralbekov, B. Evaluation of Dissolved and Acid-Leachable Trace Element Concentrations in Relation to Practical Water Quality Standards in the Syr Darya, Aral Sea Basin, South Kazakhstan. Chemosphere 2023, 313, 137465. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. Off. J. Eur. Union 2013, L 226, 1–17. [Google Scholar]
- Ebeling, A.; Zimmermann, T.; Klein, O.; Irrgeher, J.; Pröfrock, D. Analysis of Seventeen Certified Water Reference Materials for Trace and Technology-Critical Elements. Geostand. Geoanalytical Res. 2022, 46, 351–378. [Google Scholar] [CrossRef]
- Manousi, N.; Zachariadis, G.A. Recent Advances in the Extraction of Polycyclic Aromatic Hydrocarbons from Environmental Samples. Molecules 2020, 25, 2182. [Google Scholar] [CrossRef] [PubMed]
- Manoli, E.; Samara, C. Polycyclic Aromatic Hydrocarbons in Natural Waters: Sources, Occurrence and Analysis. TrAC Trends Anal. Chem. 1999, 18, 417–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, L.; He, S.; Tao, H.; Xie, W.; Zhang, X.; Ren, X.; Jiang, T.; Li, L.; Zhu, Z. Contemporary Research Progress on the Detection of Polycyclic Aromatic Hydrocarbons. Int. J. Environ. Res. Public Health 2022, 19, 2790. [Google Scholar] [CrossRef]
- Soursou, V.; Campo, J.; Picó, Y. Revisiting the Analytical Determination of PAHs in Environmental Samples: An Update on Recent Advances. Trends Environ. Anal. Chem. 2023, 37, e00195. [Google Scholar] [CrossRef]
- Dosis, I.; Ricci, M.; Emteborg, H.; Emons, H. A Journey towards Whole Water Certified Reference Materials for Organic Substances: Measuring Polycyclic Aromatic Hydrocarbons as Required by the European Union Water Framework Directive. Anal. Bioanal. Chem. 2021, 413, 2283–2293. [Google Scholar] [CrossRef] [PubMed]
- Karacık, B.; Okay, O.S.; Henkelmann, B.; Pfister, G.; Schramm, K.-W. Water Concentrations of PAH, PCB and OCP by Using Semipermeable Membrane Devices and Sediments. Mar. Pollut. Bull. 2013, 70, 258–265. [Google Scholar] [CrossRef]
- Muir, D.; Sverko, E. Analytical Methods for PCBs and Organochlorine Pesticides in Environmental Monitoring and Surveillance: A Critical Appraisal. Anal. Bioanal. Chem. 2006, 386, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Helaleh, M.I.H.; Al-Rashdan, A.; Ibtisam, A. Simultaneous Analysis of Organochlorinated Pesticides (OCPs) and Polychlorinated Biphenyls (PCBs) from Marine Samples Using Automated Pressurized Liquid Extraction (PLE) and Power Prep™ Clean-Up. Talanta 2012, 94, 44–49. [Google Scholar] [CrossRef]
- Ohoro, C.R.; Wepener, V. Review of Scientific Literature on Available Methods of Assessing Organochlorine Pesticides in the Environment. Heliyon 2023, 9, e22142. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, J.; Wang, M.; Zhao, J. Towards More Accurate Risk Assessment of Sediment Trace Metals: Integrating Sedimentary Background Determination and Probabilistic Evaluation in Chaohu Lake, China. Water 2025, 17, 1383. [Google Scholar] [CrossRef]
- Bao, Q.; Liu, C.; Friese, K.; Dadi, T.; Yu, J.; Fan, C.; Shen, Q. Understanding the Heavy Metal Pollution Pattern in Sediments of a Typical Small- and Medium-Sized Reservoir in China. Int. J. Environ. Res. Public Health 2022, 20, 708. [Google Scholar] [CrossRef]
- UNEP/IOC/IAEA. Manual for the Geochemical Analysis of Marine Sediments and Suspended Particulate Matter; Reference Methods for Marine Pollution Studies No. 63; United Nations Environment Programme: Nairobi, Kenya, 1995. [Google Scholar]
- Szava-Kovats, R.C. Grain-Size Normalization as a Tool to Assess Contamination in Marine Sediments: Is the <63μm Fraction Fine Enough? Mar. Pollut. Bull. 2008, 56, 629–632. [Google Scholar] [CrossRef]
- Vdović, N.; Lučić, M.; Mikac, N.; Bačić, N. Partitioning of Metal Contaminants between Bulk and Fine-Grained Fraction in Freshwater Sediments: A Critical Appraisal. Minerals 2021, 11, 603. [Google Scholar] [CrossRef]
- Buchman, M.F. Screening Quick Reference Tables (SQuiRTs); National Oceanic and Atmospheric Administration (NOAA): Seattle, WA, USA, 2008.
- Liao, J.; Cui, X.; Feng, H.; Yan, S. Environmental Background Values and Ecological Risk Assessment of Heavy Metals in Watershed Sediments: A Comparison of Assessment Methods. Water 2021, 14, 51. [Google Scholar] [CrossRef]
- Elobaid, E.A.; Yigiterhan, O.; Al-Ansari, E.M.A.S.; Chen, Z.; Mohieldeen, Y.E.; Abdalla, R. Ecological Risk Assessment of Heavy Metals in the Marine Sediments Associated with the Petroleum Hydrocarbon Industry in the Central Arabian Gulf. J. Hazard. Mater. Adv. 2025, 19, 100749. [Google Scholar] [CrossRef]
- Özşeker, K.; Erüz, C.; Terzi, Y. Evaluation of Toxic Metals in Different Grain Size Fractions of Sediments of the Southeastern Black Sea. Mar. Pollut. Bull. 2022, 182, 113959. [Google Scholar] [CrossRef]
- Imsilp, K.; Sirinupong, P.; Yeesin, P.; Thong-asa, W.; Tanhan, P. Potential Risks and Spatial Variation of Heavy Metals in Water and Surface Sediment of Pattani Bay, Thailand. Toxics 2025, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Bettinelli, M.; Beone, G.M.; Spezia, S.; Baffi, C. Determination of Heavy Metals in Soils and Sediments by Microwave-Assisted Digestion and Inductively Coupled Plasma Optical Emission Spectrometry Analysis. Anal. Chim. Acta 2000, 424, 289–296. [Google Scholar] [CrossRef]
- Tuzen, M.; Sari, H.; Soylak, M. Microwave and Wet Digestion Procedures for Atomic Absorption Spectrometric Determination of Trace Metals Contents of Sediment Samples. Anal. Lett. 2004, 37, 1925–1936. [Google Scholar] [CrossRef]
- Idera, F.; Omotola, O.; Paul, U.J.; Adedayo, A. Evaluation of the Effectiveness of Different Acid Digestion on Sediments. IOSR J. Appl. Chem. 2014, 7, 39–47. [Google Scholar] [CrossRef]
- Gallello, G.; Ramacciotti, M.; Puchol, O.G.; Lezzerini, M.; McClure, S.B.; Pastor, A. Total vs. Partial Acid Digestion Methods for Trace Element Analysis in Archaeological Sediments. Minerals 2022, 12, 685. [Google Scholar] [CrossRef]
- Nnodum, C.F.; Yusuf, K.A.; Wusu, A.D. A Comparison of Two Digestion Methods and Heavy Metals Determination in Sediments. In Organic and Natural Product Synthesis; De Gruyter: Boston, MA, USA, 2022; pp. 51–70. [Google Scholar]
- Mitra, S.; Dickhut, R.M.; Kuehl, S.A.; Kimbrough, K.L. Polycyclic Aromatic Hydrocarbon (PAH) Source, Sediment Deposition Patterns, and Particle Geochemistry as Factors Influencing PAH Distribution Coefficients in Sediments of the Elizabeth River, VA, USA. Mar. Chem. 1999, 66, 113–127. [Google Scholar] [CrossRef]
- McGrath, J.A.; Joshua, N.; Bess, A.S.; Parkerton, T.F. Review of Polycyclic Aromatic Hydrocarbons (PAHs) Sediment Quality Guidelines for the Protection of Benthic Life. Integr. Environ. Assess. Manag. 2019, 15, 505–518. [Google Scholar] [CrossRef]
- Bayer, V.J.; Hümmler, A.; Brinkmann, N.; Achten, C. Varying Extractability of Petrogenic and Pyrogenic Polycyclic Aromatic Hydrocarbons (PAH) in Urban Soils: Evaluation of Sample Preparation and Extraction of 71 PAH and Alkylated PAH. Environ. Pollut. 2025, 366, 125411. [Google Scholar] [CrossRef]
- Mogashane, T.M.; Mokoena, L.; Tshilongo, J. A Review on Recent Developments in the Extraction and Identification of Polycyclic Aromatic Hydrocarbons from Environmental Samples. Water 2024, 16, 2520. [Google Scholar] [CrossRef]
- Drábová, L.; Dvořáková, D.; Urbancová, K.; Gramblička, T.; Hajšlová, J.; Pulkrabová, J. Critical Assessment of Clean-Up Techniques Employed in Simultaneous Analysis of Persistent Organic Pollutants and Polycyclic Aromatic Hydrocarbons in Fatty Samples. Toxics 2022, 10, 12. [Google Scholar] [CrossRef]
- Sørensen, L.; Meier, S.; Mjøs, S.A. Application of Gas Chromatography/Tandem Mass Spectrometry to Determine a Wide Range of Petrogenic Alkylated Polycyclic Aromatic Hydrocarbons in Biotic Samples. Rapid Commun. Mass Spectrom. 2016, 30, 2052–2058. [Google Scholar] [CrossRef]
- Ahad, J.M.E.; Macdonald, R.W.; Parrott, J.L.; Yang, Z.; Zhang, Y.; Siddique, T.; Kuznetsova, A.; Rauert, C.; Galarneau, E.; Studabaker, W.B.; et al. Polycyclic Aromatic Compounds (PACs) in the Canadian Environment: A Review of Sampling Techniques, Strategies and Instrumentation. Environ. Pollut. 2020, 266, 114988. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-D.; Chen, C.-F.; Chen, C.-W. Determination of Polycyclic Aromatic Hydrocarbons in Industrial Harbor Sediments by GC-MS. Int. J. Environ. Res. Public Health 2012, 9, 2175–2188. [Google Scholar] [CrossRef]
- Opel, O.; Palm, W.-U.; Steffen, D.; Ruck, W.K.L. Inside-Sediment Partitioning of PAH, PCB and Organochlorine Compounds and Inferences on Sampling and Normalization Methods. Environ. Pollut. 2011, 159, 924–931. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Wang, Y.; Wang, T.; Li, X.; Ding, L.; Jiang, G. Evaluation of Soxhlet Extraction, Accelerated Solvent Extraction and Microwave-Assisted Extraction for the Determination of Polychlorinated Biphenyls and Polybrominated Diphenyl Ethers in Soil and Fish Samples. Anal. Chim. Acta 2010, 663, 43–48. [Google Scholar] [CrossRef]
- Pan, B.; Liu, W.X.; Shi, Z.; Cao, J.; Shen, W.R.; Qing, B.P.; Sun, R.; Tao, S. Sample Purification for Analysis of Organochlorine Pesticides in Sediment and Fish Muscle. J. Environ. Sci. Health Part B 2004, 39, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Catarro, G.; Pelixo, R.; Feijó, M.; Rosado, T.; Socorro, S.; Araújo, A.R.T.S.; Gallardo, E. Analytical Approaches Using GC-MS for the Detection of Pollutants in Wastewater Towards Environmental and Human Health Benefits: A Comprehensive Review. Chemosensors 2025, 13, 253. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Yang, Z.-Y.; Zeng, E.Y. Measurements of Coeluting Unlabeled and 13C-Labeled Polychlorinated Biphenyl Congeners with Partially Overlapping Fragment Profiles Using a Tandem Mass Spectrometry. J. Chromatogr. A 2010, 1217, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Saion, E.; Halimah, M.K.; Yap, C.K. Utilizing Mollusk Soft Tissue and Shells as Biomarkers for Monitoring Heavy Metal Pollution in Mangrove Forests. MethodsX 2023, 11, 102281. [Google Scholar] [CrossRef]
- Mohammed, E.; Mohammed, T.; Mohammed, A. Optimization of an Acid Digestion Procedure for the Determination of Hg, As, Sb, Pb and Cd in Fish Muscle Tissue. MethodsX 2017, 4, 513–523. [Google Scholar] [CrossRef]
- Chen, K.; Mou, P.; Zhu, A.; Chen, P.; Chen, J.; Gao, G.; Wang, X.; Feng, X.; Yu, C. A Comparative Study of Different Methods for the Determination of Cadmium in Various Tissues of Ramie (Boehmeria nivea L.). Environ. Monit. Assess. 2023, 195, 1009. [Google Scholar] [CrossRef]
- Wippermann, D.; Zonderman, A.; Zimmermann, T.; Pröfrock, D. Determination of Technology-Critical Elements in Seafood Reference Materials by Inductively Coupled Plasma-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2024, 416, 2797–2807. [Google Scholar] [CrossRef]
- Meador, J.P.; Stein, J.E.; Reichert, W.L.; Varanasi, U. Bioaccumulation of Polycyclic Aromatic Hydrocarbons by Marine Organisms. Rev. Environ. Contam. Toxicol. 1995, 143, 79–165. [Google Scholar]
- Fuoco, R.; Giannarelli, S.; Onor, M.; Ceccarini, A.; Carli, V. Optimized Cleanup Methods of Organic Extracts for the Determination of Organic Pollutants in Biological Samples. Microchem. J. 2005, 79, 69–76. [Google Scholar] [CrossRef]
- Santos, F.J.; Galceran, M.T. Modern Developments in Gas Chromatography–Mass Spectrometry-Based Environmental Analysis. J. Chromatogr. A 2003, 1000, 125–151. [Google Scholar] [CrossRef]
- van Leeuwen, S.P.J.; de Boer, J. Advances in the Gas Chromatographic Determination of Persistent Organic Pollutants in the Aquatic Environment. J. Chromatogr. A 2008, 1186, 161–182. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Guo, J.; Yu, Z.; Lin, Y.; Wang, Y. Advances and Development in Sampling Techniques for Marine Water Resources: A Comprehensive Review. Front. Mar. Sci. 2024, 11, 1365019. [Google Scholar] [CrossRef]
- He, S.; Peng, Y.; Jin, Y.; Wan, B.; Liu, G. Review and Analysis of Key Techniques in Marine Sediment Sampling. Chin. J. Mech. Eng. 2020, 33, 66. [Google Scholar] [CrossRef]
- Krumgalz, B.S.; Fainshtein, G.; Sahler, M.; Gorfunkel, L. ‘Field Error’ Related to Marine Sediment Contamination Studies. Mar. Pollut. Bull. 1989, 20, 64–69. [Google Scholar] [CrossRef]
- Tuit, C.; Wait, A. A Review of Marine Sediment Sampling Methods. Environ. Forensics 2020, 21, 291–309. [Google Scholar] [CrossRef]
- Reichelt-Brushett, A. Collecting, Measuring, and Understanding Contaminant Concentrations in the Marine Environment. In Marine Pollution—Monitoring, Management and Mitigation; Reichelt-Brushett, A., Ed.; Springer Textbooks in Earth Sciences, Geography and Environment; Springer: Cham, Switzerland, 2023; pp. 23–51. [Google Scholar]
- Sigg, L.; Xue, H.; Kistler, D.; Sshönenberger, R. Size Fractionation (Dissolved, Colloidal and Particulate) of Trace Metals in the Thur River, Switzerland. Aquat. Geochem. 2000, 6, 413–434. [Google Scholar] [CrossRef]
- Tirez, K.; Dehaspe, J.; Van Stappen, J.; Joris, I.; Vanhoof, C.; Voorspoels, S.; Annys, J. An Inorganic “Non-Targeted” Characterization Platform for Comprehensive Monitoring of Elements in Surface Water. Environ. Sci. Eur. 2025, 37, 73. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, X.; Jian, H.; Chen, H.; Yu, Z. Characterization of the Particle Size Fraction Associated with Heavy Metals in Suspended Sediments of the Yellow River. Int. J. Environ. Res. Public Health 2015, 12, 6725–6744. [Google Scholar] [CrossRef]
- Peña-Icart, M.; Villanueva Tagle, M.E.; Alonso-Hernández, C.; Rodríguez Hernández, J.; Behar, M.; Pomares Alfonso, M.S. Comparative Study of Digestion Methods EPA 3050B (HNO3–H2O2–HCl) and ISO 11466.3 (Aqua Regia) for Cu, Ni and Pb Contamination Assessment in Marine Sediments. Mar. Environ. Res. 2011, 72, 60–66. [Google Scholar] [CrossRef]
- Mülow-Stollin, U.; Lehnik-Habrink, P.; Kluge, S.; Bremser, W.; Piechotta, C. Efficiency Evaluation of Extraction Methods for Analysis of OCPs and PCBs in Soils of Varying TOC. J. Environ. Prot. 2017, 08, 693–713. [Google Scholar] [CrossRef]
- Tolosa, I.; Cassi, R.; Huertas, D. A New Marine Sediment Certified Reference Material (CRM) for the Determination of Persistent Organic Contaminants: IAEA-459. Environ. Sci. Pollut. Res. 2019, 26, 7347–7355. [Google Scholar] [CrossRef]
- De Albano, F.M.; ten Caten, C.S. Proficiency Tests for Laboratories: A Systematic Review. Accredit. Qual. Assur. 2014, 19, 245–257. [Google Scholar] [CrossRef]
- Tanhua, T.; Pouliquen, S.; Hausman, J.; O’Brien, K.; Bricher, P.; de Bruin, T.; Buck, J.J.H.; Burger, E.F.; Carval, T.; Casey, K.S.; et al. Ocean FAIR Data Services. Front. Mar. Sci. 2019, 6, 440. [Google Scholar] [CrossRef]
- Giorgetti, A.; Lipizer, M.; Molina Jack, M.E.; Holdsworth, N.; Jensen, H.M.; Buga, L.; Sarbu, G.; Iona, A.; Gatti, J.; Larsen, M.; et al. Aggregated and Validated Datasets for the European Seas: The Contribution of EMODnet Chemistry. Front. Mar. Sci. 2020, 7, 583657. [Google Scholar] [CrossRef]
- Schaap, D.M.A.; Lowry, R.K. SeaDataNet—Pan-European Infrastructure for Marine and Ocean Data Management: Unified Access to Distributed Data Sets. Int. J. Digit. Earth 2010, 3, 50–69. [Google Scholar] [CrossRef]
- French, M.A.; Lipizer, M. Improving Data Reliability to Support Marine Pollution Assessment According to MSFD Descriptor 8 in the European Seas: The Contribution of EMODnet Chemistry. Front. Mar. Sci. 2023, 10, 1275097. [Google Scholar] [CrossRef]
- Lipizer, M.; Berto, D.; Cermelj, B.; Fafandjel, M.; Formalewicz, M.; Hatzianestis, I.; Ilijanić, N.; Kaberi, H.; Kralj, M.; Matijevic, S.; et al. Trace Metals and Polycyclic Aromatic Hydrocarbons in the Eastern Mediterranean Sediments: Concentration Ranges as a Tool for Quality Control of Large Data Collections. Mar. Pollut. Bull. 2022, 185, 114181. [Google Scholar] [CrossRef] [PubMed]
- Bellas, J.; Hylland, K.; Burgeot, T. Editorial: New Challenges in Marine Pollution Monitoring. Front. Mar. Sci. 2020, 6, 820. [Google Scholar] [CrossRef]
- Hylland, K.; Burgeot, T.; Martínez-Gómez, C.; Lang, T.; Robinson, C.D.; Svavarsson, J.; Thain, J.E.; Vethaak, A.D.; Gubbins, M.J. How Can We Quantify Impacts of Contaminants in Marine Ecosystems? The ICON Project. Mar. Environ. Res. 2017, 124, 2–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).