Electrochemical Ammonia Oxidation in Water Treatment: A Comprehensive Review on Mechanisms, Catalysts, and Implementation Challenges
Abstract
1. Introduction
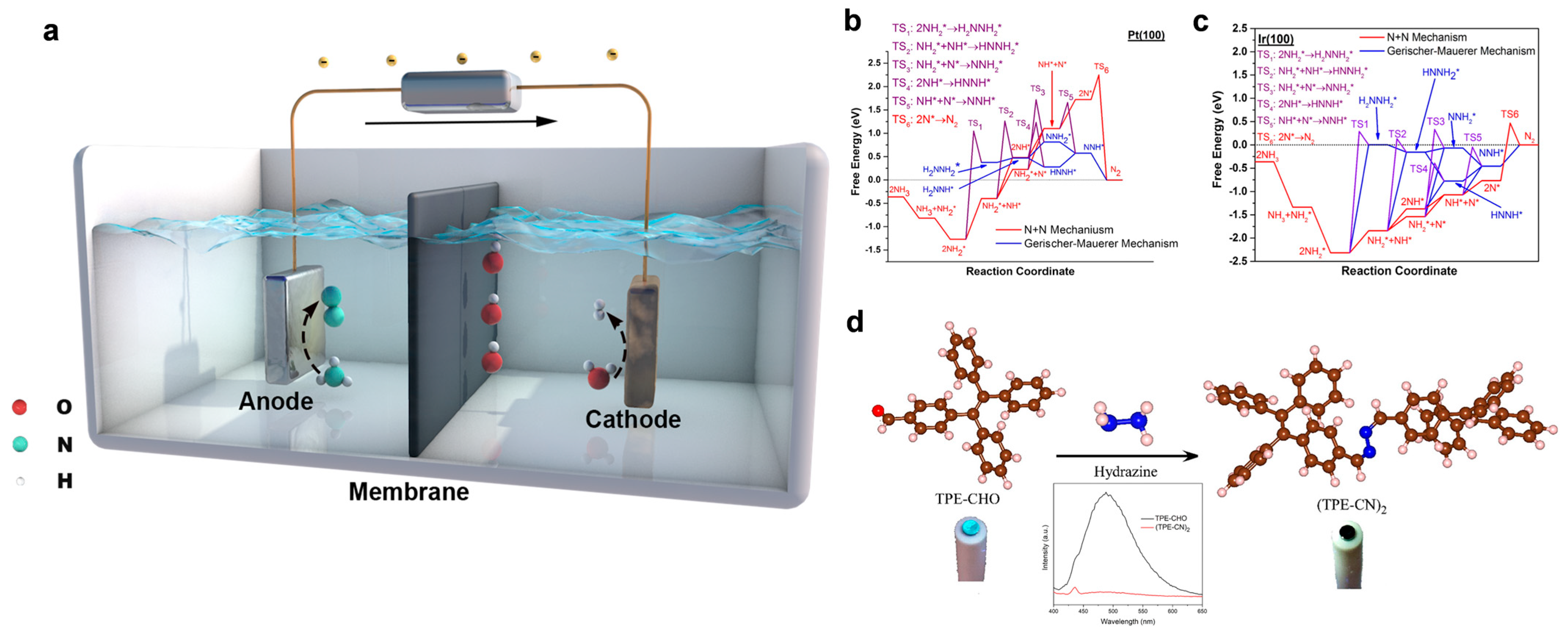
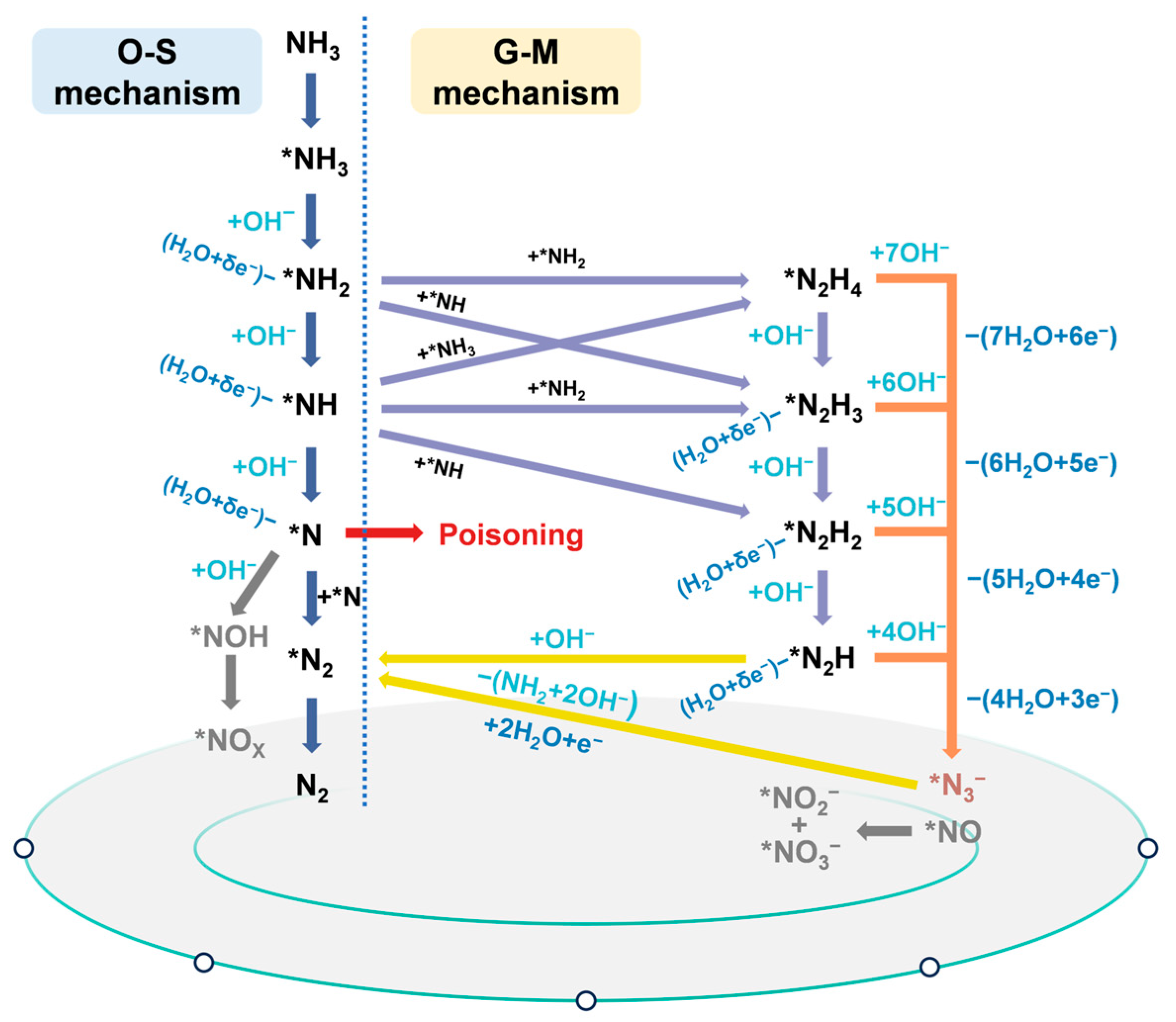
2. Direct eAOR
3. Indirect eAOR via RCS
3.1. Breakpoint Chlorination Oxidation Mechanism
3.2. Chlorine Radical Oxidation Mechanism
4. Fundamentals of the Design of Catalysts in eAOR
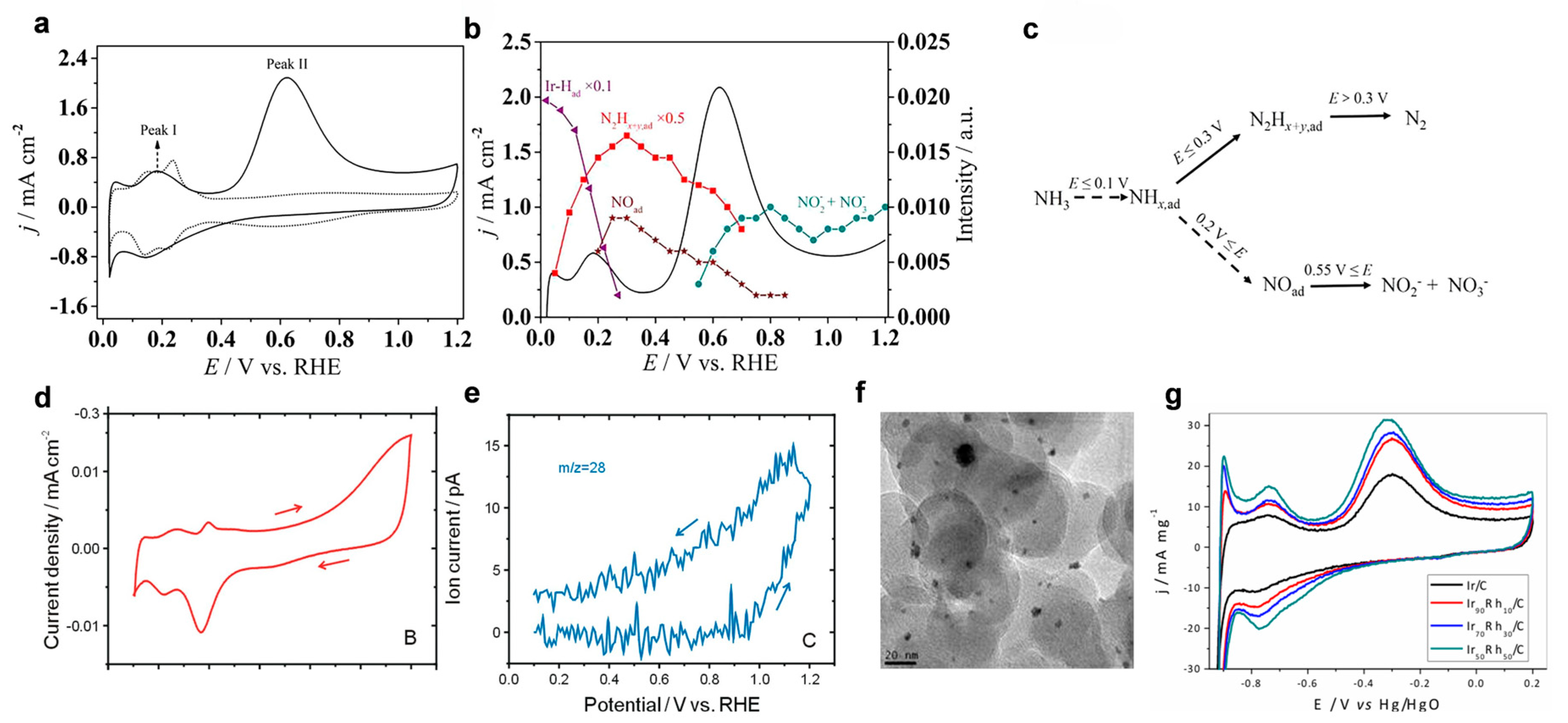
4.1. Noble Metal-Based Catalysts for Direct eAOR
4.2. Non-Noble Metal in Direct eAOR
4.3. Fundamentals of the Design of Catalysts in Indirect eAOR
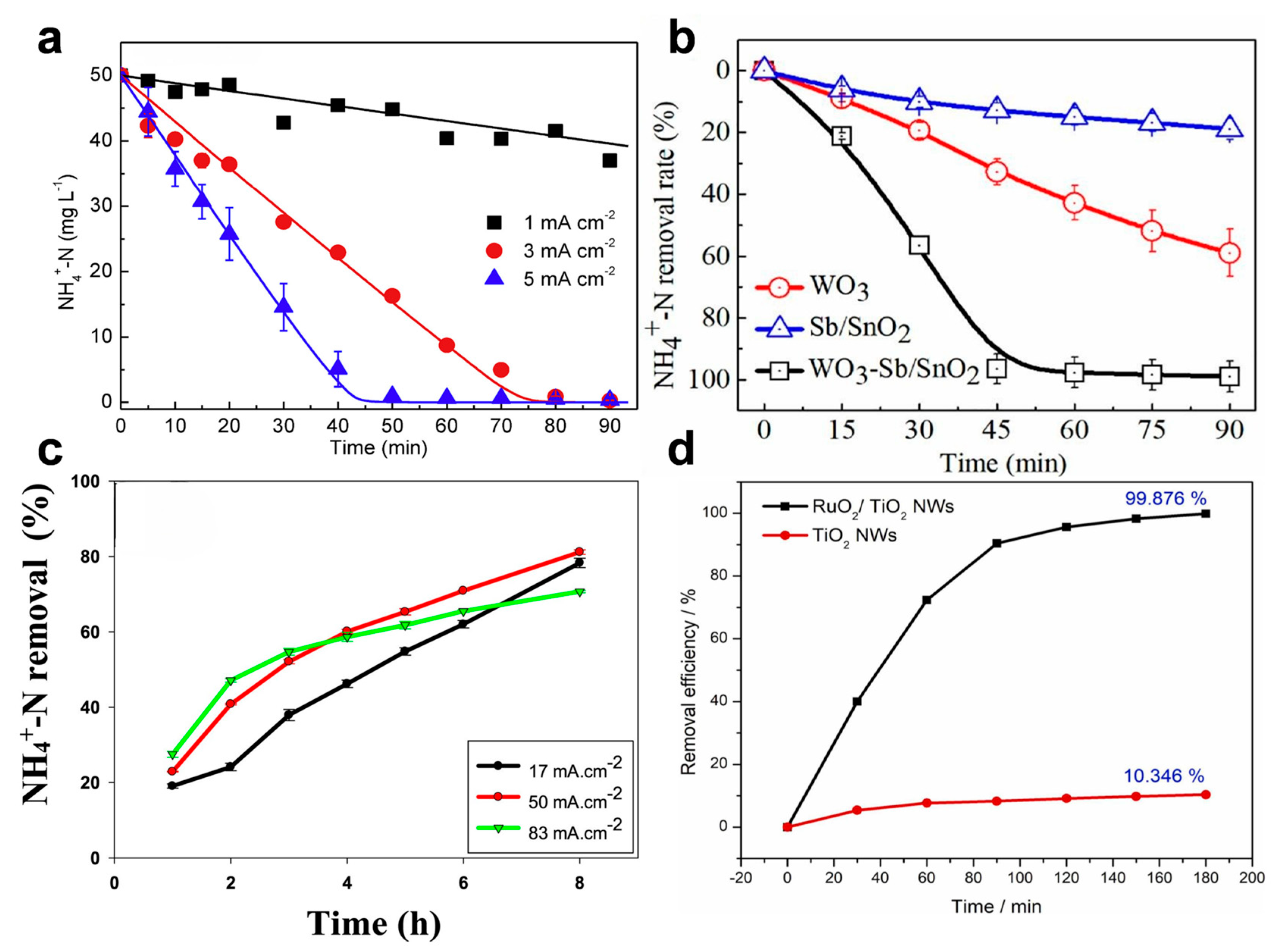
4.3.1. Noble Metal and Metal Oxide Anodes
4.3.2. Non-Metal and Diamond-Based Electrodes
4.3.3. Membrane Electrodes and Hybrid Materials
| Anode Material | Rate Constant or Removal Efficiency | Concentration of Ammonia | Concentration of Chlorine | Current Density/Potential | References |
|---|---|---|---|---|---|
| Ti/IrO2–RuO2 | 100%/25 min | 50 mg L−1 | 20 mM | 5 mA cm−2 | [79] |
| PbO2/Ti | 0.306 h−1 | 78.2 mg L−1 | 40 mM | 37.5 mA cm−2 | [36] |
| WO3-Sb/SnO2 | 3.516 h−1 | 30 mg L−1 | 50 mM | 2.0 V (VS Ag/AgCl) | [82] |
| 3D Co3O4 NWs | 4.836 h−1 | 56 mg L−1 | 50 mM | 1.8 V (VS Ag/AgCl) | [81] |
| Co3O4/MXene | 0.6059 h−1 | 200 mg L−1 | 50 mM | 20 mA cm−2 | [83] |
| Sn, Bi co-doped Co3O4 | 0.0055 min−1 | 100 mg L−1 | 600 mM | 10 mA cm−2 | [84] |
| Ti/BDD | >80%/480 min | 1006–1197 mg L−1 | 1880–2700 mg L−1 | 17–83 mA cm−2 | [85] |
| BDD | 100%/45 min | 8 mg L−1 | 26167 mg L−1 | 50 A m−2 | [86] |
| RuO2/TiO2 NW membrane | 99.876%/180 min | 200 mg L−1 | 2000 mg L−1 | 20 mA cm−2 | [80] |
| coal-based carbon membrane | 100%/120 min | 30 mg L−1 | 100 mM | 2.8 V | [88] |
| Ceramic membrane | 93.5%/60 min | 8.8 mg L−1 | 1781.9 mg L−1 | 25 mA cm−2 | [90] |
5. Key Environmental Factors in Practical Water Treatment
5.1. pH Dependence and Speciation Effects
5.2. Formation of Nitrogenous and Halogenated Byproducts
5.3. Background Matrix Effects and Coexisting Constituents
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, Z.; Huang, B.; Wang, Y.; Wang, K.; Tu, X.; Xie, P.; Fu, X. Ammonia as a renewable energy carrier from synthesis to utilization. Nat. Rev. Clean Technol. 2025, 1–16. [Google Scholar] [CrossRef]
- Edwards, T.M.; Puglis, H.J.; Kent, D.B.; Durán, J.L.; Bradshaw, L.M.; Farag, A.M. Ammonia and aquatic ecosystems—A review of global sources, biogeochemical cycling, and effects on fish. Sci. Total Environ. 2024, 907, 167911. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, V.; Cheng, L.; Hussain, A.; Ormeci, B. Nitrogen removal from wastewater: A comprehensive review of biological nitrogen removal processes, critical operation parameters and bioreactor design. J. Environ. Chem. Eng. 2022, 10, 107387. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Yan, M.; Zhao, T.; Liu, Y.; Zhu, T.; Ni, B.-J. Insights into N2O turnovers under polyethylene terephthalate microplastics stress in mainstream biological nitrogen removal process. Water Res. 2022, 224, 119037. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, S.; Li, H.; Liu, J.; Li, S.; Zhang, L. Treatment of ammonia-nitrogen wastewater by the ultrasonic strengthened break point chlorination method. J. Water Process Eng. 2022, 45, 102501. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, P.; Zhou, J.; Li, J.; Li, Z.; Wang, D. Formation of disinfection byproducts in an ammonia-polluted source water with UV/chlorine treatment followed by post-chlorination: A pilot-scale study. Environ. Technol. Innov. 2022, 26, 102266. [Google Scholar] [CrossRef]
- Han, B.; Butterly, C.; Zhang, W.; He, J.-Z.; Chen, D. Adsorbent materials for ammonium and ammonia removal: A review. J. Clean. Prod. 2021, 283, 124611. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Tawalbeh, M. Electrochemical oxidation of ammonia on nickel oxide nanoparticles. Int. J. Hydrogen Energy 2020, 45, 10398–10408. [Google Scholar] [CrossRef]
- Zöllig, H.; Fritzsche, C.; Morgenroth, E.; Udert, K.M. Direct electrochemical oxidation of ammonia on graphite as a treatment option for stored source-separated urine. Water Res. 2015, 69, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Rahardjo, S.S.P.; Shih, Y.-J. Electrochemical characteristics of silver/nickel oxide (Ag/Ni) for direct ammonia oxidation and nitrogen selectivity in paired electrode system. Chem. Eng. J. 2023, 452, 139370. [Google Scholar] [CrossRef]
- Chauhan, R.; Srivastava, V.C. Mechanistic kinetic modeling of simultaneous electrochemical nitrate reduction and ammonium ion oxidation in wastewater. Chem. Eng. Sci. 2022, 247, 117025. [Google Scholar] [CrossRef]
- Yan, C.; Liu, L. Oxidation of gas phase ammonia via accelerated generation of radical species and synergy of photo electrochemical catalysis with persulfate activation by CuO-Co3O4 on cathode electrode. J. Hazard. Mater. 2020, 388, 121793. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Hsu, C.-H. Kinetics and highly selective N2 conversion of direct electrochemical ammonia oxidation in an undivided cell using NiCo oxide nanoparticle as the anode and metallic Cu/Ni foam as the cathode. Chem. Eng. J. 2021, 409, 128024. [Google Scholar] [CrossRef]
- Hansen, H.A.; Man, I.C.; Studt, F.; Abild-Pedersen, F.; Bligaard, T.; Rossmeisl, J. Electrochemical chlorine evolution at rutile oxide (110) surfaces. Phys. Chem. Chem. Phys. 2010, 12, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Candido, L.; Gomes, J.A.C.P. Evaluation of anode materials for the electro-oxidation of ammonia and ammonium ions. Mater. Chem. Phys. 2011, 129, 1146–1151. [Google Scholar] [CrossRef]
- Elnabawy, A.O.; Herron, J.A.; Karraker, S.; Mavrikakis, M. Structure sensitivity of ammonia electro-oxidation on transition metal surfaces: A first-principles study. J. Catal. 2021, 397, 137–147. [Google Scholar] [CrossRef]
- Siddharth, K.; Alam, P.; Hossain, M.D.; Xie, N.; Nambafu, G.S.; Rehman, F.; Lam, J.W.Y.; Chen, G.; Cheng, J.; Luo, Z.; et al. Hydrazine Detection during Ammonia Electro-oxidation Using an Aggregation-Induced Emission Dye. J. Am. Chem. Soc. 2021, 143, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Oswin, H.G.; Salomon, M. The Anodic Oxidation of Ammonia at Platinum Black Electrodes in Aqueous Koh Electrolyte. Can. J. Chem. 1963, 41, 1686–1694. [Google Scholar] [CrossRef]
- Gerischer, H.; Mauerer, A. Untersuchungen Zur anodischen Oxidation von Ammoniak an Platin-Elektroden. J. Electroanal. Chem. Interfacial Electrochem. 1970, 25, 421–433. [Google Scholar] [CrossRef]
- Katsounaros, I.; Chen, T.; Gewirth, A.A.; Markovic, N.M.; Koper, M.T.M. Evidence for Decoupled Electron and Proton Transfer in the Electrochemical Oxidation of Ammonia on Pt(100). J. Phys. Chem. Lett. 2016, 7, 387–392. [Google Scholar] [CrossRef]
- de Vooys, A.C.A.; Mrozek, M.F.; Koper, M.T.M.; van Santen, R.A.; van Veen, J.A.R.; Weaver, M.J. The nature of chemisorbates formed from ammonia on gold and palladium electrodes as discerned from surface-enhanced Raman spectroscopy. Electrochem. Commun. 2001, 3, 293–298. [Google Scholar] [CrossRef]
- Kim, H.; Yang, W.; Lee, W.H.; Han, M.H.; Moon, J.; Jeon, C.; Kim, D.; Ji, S.G.; Chae, K.H.; Lee, K.-S.; et al. Operando Stability of Platinum Electrocatalysts in Ammonia Oxidation Reactions. ACS Catal. 2020, 10, 11674–11684. [Google Scholar] [CrossRef]
- Vidal-Iglesias, F.J.; Solla-Gullón, J.; Feliu, J.M.; Baltruschat, H.; Aldaz, A. DEMS study of ammonia oxidation on platinum basal planes. J. Electroanal. Chem. 2006, 588, 331–338. [Google Scholar] [CrossRef]
- Wasmus, S.; Vasini, E.J.; Krausa, M.; Mishima, H.T.; Vielstich, W. DEMS-cyclic voltammetry investigation of the electrochemistry of nitrogen compounds in 0.5 M potassium hydroxide. Electrochim. Acta 1994, 39, 23–31. [Google Scholar] [CrossRef]
- Gootzen, J.F.E.; Wonders, A.H.; Visscher, W.; van Santen, R.A.; van Veen, J.A.R. A DEMS and cyclic voltammetry study of NH3 oxidation on platinized platinum. Electrochim. Acta 1998, 43, 1851–1861. [Google Scholar] [CrossRef]
- Siddharth, K.; Chan, Y.; Wang, L.; Shao, M. Ammonia electro-oxidation reaction: Recent development in mechanistic understanding and electrocatalyst design. Curr. Opin. Electrochem. 2018, 9, 151–157. [Google Scholar] [CrossRef]
- Bunce, N.J.; Bejan, D. Mechanism of electrochemical oxidation of ammonia. Electrochim. Acta 2011, 56, 8085–8093. [Google Scholar] [CrossRef]
- He, J.; Zhang, C.; Yang, Y.; Kang, J.; Zhang, C.; He, D.; Ma, J. Chlorine-Mediated Ammonia and Organics Transformation during Electrochemical Ammonia Recovery from Human Urine. Environ. Sci. Technol. 2025, 59, 13096–13107. [Google Scholar] [CrossRef]
- Qiang, Z.; Adams, C.D. Determination of Monochloramine Formation Rate Constants with Stopped-Flow Spectrophotometry. Environ. Sci. Technol. 2004, 38, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chen, X.; Zhang, S.; Wu, D. Treatment of high chlorine-containing composting leachate biochemical effluent by Ti/RuO2-IrO2 anodic electrochemical oxidation: Optimization and evolution of pollutants. J. Environ. Chem. Eng. 2023, 11, 109674. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, N.; Feng, C.; Chen, F.; Wang, H.; Kuang, P.; Feng, Z.; Liu, T.; Gao, Y.; Hu, W. Treatment of organic wastewater containing nitrogen and chlorine by combinatorial electrochemical system: Taking biologically treated landfill leachate treatment as an example. Chem. Eng. J. 2019, 364, 349–360. [Google Scholar] [CrossRef]
- Liu, Z.; Tao, Y.; Zhang, Z.; He, J.; Yang, K.; Ma, J. Active chlorine mediated ammonia oxidation in an electrified SnO2–Sb filter: Reactivity, mechanisms and response to matrix effects. Sep. Purif. Technol. 2023, 312, 123369. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Bai, J.; Li, L.; Chen, S.; Zhou, T.; Wang, J.; Xia, L.; Xu, Q.; Zhou, B. Extremely Efficient Decomposition of Ammonia N to N2 Using ClO• from Reactions of HO• and HOCl Generated in Situ on a Novel Bifacial Photoelectroanode. Environ. Sci. Technol. 2019, 53, 6945–6953. [Google Scholar] [CrossRef]
- Yan, Z.; Dai, Z.; Zheng, W.; Lei, Z.; Qiu, J.; Kuang, W.; Huang, W.; Feng, C. Facile ammonium oxidation to nitrogen gas in acid wastewater by in situ photogenerated chlorine radicals. Water Res. 2021, 205, 117678. [Google Scholar] [CrossRef]
- Lu, S.; Li, X.; Liao, Y.; Zhang, Z.; Luo, H.; Zhang, G. Boosting generation of reactive oxygen and chlorine species on TNT photoanode and Ni/graphite fiber cathode towards efficient oxidation of ammonia wastewater. Chemosphere 2023, 313, 137363. [Google Scholar] [CrossRef]
- Zheng, W.; Zhu, L.; Liang, S.; Ye, J.; Yang, X.; Lei, Z.; Yan, Z.; Li, Y.; Wei, C.; Feng, C. Discovering the Importance of ClO• in a Coupled Electrochemical System for the Simultaneous Removal of Carbon and Nitrogen from Secondary Coking Wastewater Effluent. Environ. Sci. Technol. 2020, 54, 9015–9024. [Google Scholar] [CrossRef]
- Li, F.; Peng, X.; Liu, Y.; Mei, J.; Sun, L.; Shen, C.; Ma, C.; Huang, M.; Wang, Z.; Sand, W. A chloride-radical-mediated electrochemical filtration system for rapid and effective transformation of ammonia to nitrogen. Chemosphere 2019, 229, 383–391. [Google Scholar] [CrossRef]
- Chen, X.; Dai, T.; Yin, M.-Y.; Xia, X.-Y.; Xing, Q.-J.; Tian, L.; Zou, J.-P. Enhanced anodic mass transfer enables interfacial Cl• for efficient ammonia oxidation. Chin. Chem. Lett. 2025, 111445. [Google Scholar] [CrossRef]
- Yuan, K.-X.; Wu, Q.; Hu, K.; Liu, Y.-L.; Wang, W.; Feng, H.; Liu, Y.; Bao, X.; Ma, J. Harnessing Electrochemistry Synergy in Reverse Osmosis: Modulating Ammonium Localized Oxidation and Restricted Transport. Environ. Sci. Technol. 2025, 59, 4188–4198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Wang, R.; Hu, Q.; Zhang, Y.; Wang, Z.; Zhou, J.; Qu, G.; Wang, T.; Jia, H.; et al. Underlying mechanisms of promoted formation of haloacetic acids disinfection byproducts after indometacin degradation by non-thermal discharge plasma. Water Res. 2022, 220, 118701. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Pillai, H.S.; Lattimer, J.; Mohd Adli, N.; Karakalos, S.; Chen, M.; Guo, L.; Xu, H.; Yang, J.; et al. Ternary PtIrNi Catalysts for Efficient Electrochemical Ammonia Oxidation. ACS Catal. 2020, 10, 3945–3957. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Huang, Y.-H.; Huang, C.P. Electrocatalytic ammonia oxidation over a nickel foam electrode: Role of Ni(OH)2(s)-NiOOH(s) nanocatalysts. Electrochim. Acta 2018, 263, 261–271. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Huang, Y.-H.; Huang, C.P. In-situ electrochemical formation of nickel oxyhydroxide (NiOOH) on metallic nickel foam electrode for the direct oxidation of ammonia in aqueous solution. Electrochim. Acta 2018, 281, 410–419. [Google Scholar] [CrossRef]
- Jiang, X.; Ying, D.; Liu, X.; Liu, M.; Zhou, S.; Guo, C.; Zhao, G.; Wang, Y.; Jia, J. Identification of the role of Cu site in Ni-Cu hydroxide for robust and high selective electrochemical ammonia oxidation to nitrite. Electrochim. Acta 2020, 345, 136157. [Google Scholar] [CrossRef]
- Xu, W.; Du, D.; Lan, R.; Humphreys, J.; Miller, D.N.; Walker, M.; Wu, Z.; Irvine, J.T.S.; Tao, S. Electrodeposited NiCu bimetal on carbon paper as stable non-noble anode for efficient electrooxidation of ammonia. Appl. Catal. B Environ. 2018, 237, 1101–1109. [Google Scholar] [CrossRef]
- He, S.; Chen, Y.; Wang, M.; Nuomin, H.; Novello, P.; Li, X.; Zhu, S.; Liu, J. Metal nitride nanosheets enable highly efficient electrochemical oxidation of ammonia. Nano Energy 2021, 80, 105528. [Google Scholar] [CrossRef]
- He, S.; Somayaji, V.; Wang, M.; Lee, S.-H.; Geng, Z.; Zhu, S.; Novello, P.; Varanasi, C.V.; Liu, J. High entropy spinel oxide for efficient electrochemical oxidation of ammonia. Nano Res. 2022, 15, 4785–4791. [Google Scholar] [CrossRef]
- Li, L.; Xu, L.; Wang, H.; Wei, H.; Tang, C.; Li, G.; Dou, Y.; Liu, H.K.; Dou, S.X. Electrocatalytic nitrogen cycle: Mechanism, materials, and momentum. Energy Environ. Sci. 2024, 17, 9027–9050. [Google Scholar] [CrossRef]
- Agharezaei, P.; Ghuman, K.K. Designing Trimetallic Single-Doped Alloy Catalysts for Sustainable Ammonia Production: The Role of Dopants in Active Site Engineering. ACS Catal. 2025, 15, 7853–7866. [Google Scholar] [CrossRef]
- de Vooys, A.C.A.; Koper, M.T.M.; van Santen, R.A.; van Veen, J.A.R. The role of adsorbates in the electrochemical oxidation of ammonia on noble and transition metal electrodes. J. Electroanal. Chem. 2001, 506, 127–137. [Google Scholar] [CrossRef]
- Wei, R.-L.; Liu, Y.; Chen, Z.; Jia, W.-S.; Yang, Y.-Y.; Cai, W.-B. Ammonia oxidation on iridium electrode in alkaline media: An in situ ATR-SEIRAS study. J. Electroanal. Chem. 2021, 896, 115254. [Google Scholar] [CrossRef]
- Zhu, C.; Lan, B.; Wei, R.-L.; Wang, C.-N.; Yang, Y.-Y. Potential-Dependent Selectivity of Ethanol Complete Oxidation on Rh Electrode in Alkaline Media: A Synergistic Study of Electrochemical ATR-SEIRAS and IRAS. ACS Catal. 2019, 9, 4046–4053. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Ren, J.; Li, Q.-X.; Zhou, Z.-Y.; Sun, S.-G.; Cai, W.-B. Electrocatalysis of Ethanol on a Pd Electrode in Alkaline Media: An in Situ Attenuated Total Reflection Surface-Enhanced Infrared Absorption Spectroscopy Study. ACS Catal. 2014, 4, 798–803. [Google Scholar] [CrossRef]
- Silva, J.C.M.; Assumpção, M.H.M.T.; Hammer, P.; Neto, A.O.; Spinacé, E.V.; Baranova, E.A. Iridium−Rhodium Nanoparticles for Ammonia Oxidation: Electrochemical and Fuel Cell Studies. ChemElectroChem 2017, 4, 1101–1107. [Google Scholar] [CrossRef]
- Peng, W.; Xiao, L.; Huang, B.; Zhuang, L.; Lu, J. Inhibition Effect of Surface Oxygenated Species on Ammonia Oxidation Reaction. J. Phys. Chem. C 2011, 115, 23050–23056. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Wu, Z.-L.; Huang, Y.-H.; Huang, C.-P. Electrochemical nitrate reduction as affected by the crystal morphology and facet of copper nanoparticles supported on nickel foam electrodes (Cu/Ni). Chem. Eng. J. 2020, 383, 123157. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Kapałka, A.; Cally, A.; Neodo, S.; Comninellis, C.; Wächter, M.; Udert, K.M. Electrochemical behavior of ammonia at Ni/Ni(OH)2 electrode. Electrochem. Commun. 2010, 12, 18–21. [Google Scholar] [CrossRef]
- Wang, M.; Liu, M.; Zou, H.; Liu, G. Efficient Removal of Ni-Edta Complexes Utilizing 3d Ni-Rm Electro-Fenton System. Sep. Purif. Technol. 2025, 369, 133067. [Google Scholar] [CrossRef]
- Yao, K.; Cheng, Y.F. Investigation of the electrocatalytic activity of nickel for ammonia oxidation. Mater. Chem. Phys. 2008, 108, 247–250. [Google Scholar] [CrossRef]
- Yao, K.; Cheng, Y.F. Electrodeposited Ni–Pt binary alloys as electrocatalysts for oxidation of ammonia. J. Power Sources 2007, 173, 96–101. [Google Scholar] [CrossRef]
- Despić, A.R.; Dražić, D.M.; Rakin, P.M. Kinetics of electrochemical oxidation of ammonia in alkaline solution. Electrochim. Acta 1966, 11, 997–1005. [Google Scholar] [CrossRef]
- Medford, A.J.; Vojvodic, A.; Hummelshøj, J.S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J.K. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef]
- Xu, M.; Wei, M. Layered Double Hydroxide-Based Catalysts: Recent Advances in Preparation, Structure, and Applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Xie, J.; Gao, L.; Cao, S.; Liu, W.; Lei, F.; Hao, P.; Xia, X.; Tang, B. Copper-incorporated hierarchical wire-on-sheet α-Ni(OH)2 nanoarrays as robust trifunctional catalysts for synergistic hydrogen generation and urea oxidation. J. Mater. Chem. A 2019, 7, 13577–13584. [Google Scholar] [CrossRef]
- Wei, C.; Sun, Y.; Scherer, G.G.; Fisher, A.C.; Sherburne, M.; Ager, J.W.; Xu, Z.J. Surface Composition Dependent Ligand Effect in Tuning the Activity of Nickel–Copper Bimetallic Electrocatalysts toward Hydrogen Evolution in Alkaline. J. Am. Chem. Soc. 2020, 142, 7765–7775. [Google Scholar] [CrossRef]
- Sonia Theres, G.; Velayutham, G.; Santhana Krishnan, P.; Shanthi, K. Synergistic impact of Ni–Cu hybrid oxides deposited on ordered mesoporous carbon scaffolds as non-noble catalyst for methanol oxidation. J. Mater. Sci. 2019, 54, 1502–1519. [Google Scholar] [CrossRef]
- Jabłońska, M.; Beale, A.M.; Nocuń, M.; Palkovits, R. Ag-Cu based catalysts for the selective ammonia oxidation into nitrogen and water vapour. Appl. Catal. B Environ. 2018, 232, 275–287. [Google Scholar] [CrossRef]
- Yang, A.; Wang, J.; Su, K.; Lei, W.; Qiu, X.; Tang, Y. Modulating Hydroxyl-Rich Interfaces on Nickel–Copper Double Hydroxide Nanotyres to Pre-activate Alkaline Ammonia Oxidation Reactivity. Chem.—A Eur. J. 2021, 27, 4869–4875. [Google Scholar] [CrossRef]
- Xu, W.; Lan, R.; Du, D.; Humphreys, J.; Walker, M.; Wu, Z.; Wang, H.; Tao, S. Directly growing hierarchical nickel-copper hydroxide nanowires on carbon fibre cloth for efficient electrooxidation of ammonia. Appl. Catal. B Environ. 2017, 218, 470–479. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Chen, T.-C.; Juang, Y.; Hua, L.-C.; Huang, C. High catalytic performance of CuCo/nickel foam electrode for ammonia electrooxidation. Electrochem. Commun. 2020, 121, 106875. [Google Scholar] [CrossRef]
- Wang, R.; Liu, H.; Zhang, K.; Zhang, G.; Lan, H.; Qu, J. Ni(II)/Ni(III) redox couple endows Ni foam-supported Ni2P with excellent capability for direct ammonia oxidation. Chem. Eng. J. 2021, 404, 126795. [Google Scholar] [CrossRef]
- Schiffer, Z.J.; Lazouski, N.; Corbin, N.; Manthiram, K. Nature of the First Electron Transfer in Electrochemical Ammonia Activation in a Nonaqueous Medium. J. Phys. Chem. C 2019, 123, 9713–9720. [Google Scholar] [CrossRef]
- Gieshoff, T.; Kehl, A.; Schollmeyer, D.; Moeller, K.D.; Waldvogel, S.R. Insights into the Mechanism of Anodic N–N Bond Formation by Dehydrogenative Coupling. J. Am. Chem. Soc. 2017, 139, 12317–12324. [Google Scholar] [CrossRef]
- Xiao, S.; Qu, J.; Zhao, X.; Liu, H.; Wan, D. Electrochemical process combined with UV light irradiation for synergistic degradation of ammonia in chloride-containing solutions. Water Res. 2009, 43, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Y. Ammonia removal in electrochemical oxidation: Mechanism and pseudo-kinetics. J. Hazard. Mater. 2009, 161, 1010–1016. [Google Scholar] [CrossRef]
- Kapałka, A.; Katsaounis, A.; Michels, N.-L.; Leonidova, A.; Souentie, S.; Comninellis, C.; Udert, K.M. Ammonia oxidation to nitrogen mediated by electrogenerated active chlorine on Ti/PtOx-IrO2. Electrochem. Commun. 2010, 12, 1203–1205. [Google Scholar] [CrossRef]
- Jung, Y.J.; Baek, K.W.; Oh, B.S.; Kang, J.-W. An investigation of the formation of chlorate and perchlorate during electrolysis using Pt/Ti electrodes: The effects of pH and reactive oxygen species and the results of kinetic studies. Water Res. 2010, 44, 5345–5355. [Google Scholar] [CrossRef]
- Zhang, C.; He, D.; Ma, J.; Waite, T.D. Active chlorine mediated ammonia oxidation revisited: Reaction mechanism, kinetic modelling and implications. Water Res. 2018, 145, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chen, R.; Wen, Y.; Li, Y.; Zhan, W.; Ma, F.; Jiang, X.; He, W.; Ni, H. Trace amount of RuO2 loaded on TiO2 nanowires for efficient electrocatalytic degradation of ammonia nitrogen in wastewater. J. Alloys Compd. 2022, 928, 167058. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, W.; Bai, J.; Li, J.; Wang, J.; Zhou, T.; Guan, X.; Zhou, B. Highly efficient removal of total nitrogen and dissolved organic compound in waste reverse osmosis concentrate mediated by chlorine radical on 3D Co3O4 nanowires anode. J. Hazard. Mater. 2022, 424, 127662. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Bai, J.; Li, J.; Li, L.; Zhou, T.; Chen, S.; Wang, J.; Rahim, M.; Guan, X.; et al. Efficient degradation of N-containing organic wastewater via chlorine oxide radical generated by a photoelectrochemical system. Chem. Eng. J. 2020, 392, 123695. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Mao, X.; Zou, H.; Liu, G. Enhanced electrochlorination for efficient ammonia oxidation facilitated by accelerating electron cycling on Co2+/Co3+. J. Environ. Chem. Eng. 2025, 13, 115415. [Google Scholar] [CrossRef]
- Wu, K.; Cao, J.; Zhang, R.; Pei, Y.; Peng, T.; Chen, G. Micro-doping tin-bismuth on modification of Co3O4 electrocatalyst and degradation of ammonia nitrogen. Environ. Res. 2025, 275, 121366. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.L. Post treatment of ICEAS-biologically landfill leachate using electrochemical oxidation with Ti/BDD and Ti/RuO2 anodes. Environ. Technol. Innov. 2020, 20, 101099. [Google Scholar] [CrossRef]
- Díaz, V.; Ibáñez, R.; Gómez, P.; Urtiaga, A.M.; Ortiz, I. Kinetics of electro-oxidation of ammonia-N, nitrites and COD from a recirculating aquaculture saline water system using BDD anodes. Water Res. 2011, 45, 125–134. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, H.; Ma, Z.; Li, N.; Li, G.; Zhang, T.; Lu, P.; Gong, X. Biochar sacrificial anode assisted water electrolysis for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 36482–36492. [Google Scholar] [CrossRef]
- Pan, Z.; Xu, J.; Zhou, X.; Xu, R.; Yu, H.; Hong, J.; Zhao, S.; Fan, X.; Song, C.; Wang, T. Efficient removal of ammonia from aqueous solution using coal-based carbon membrane via electrochemical oxidation in the present of chloride ion. J. Environ. Chem. Eng. 2024, 12, 114335. [Google Scholar] [CrossRef]
- Chi, M.; Luo, B.; Zhang, Q.; Jiang, H.; Chen, C.; Wang, S.; Min, D. Lignin-based monolithic carbon electrode decorating with RuO2 nanospheres for high-performance chlorine evolution reaction. Ind. Crops Prod. 2021, 159, 113088. [Google Scholar] [CrossRef]
- Li, Y.; Yi, Q.; Wang, D.; Wu, Z.; Wang, Z. Efficient treatment of landfill leachate using an electrochemical ceramic membrane filtration system: Chlorine-mediated oxidation. Chem. Eng. J. 2022, 450, 138102. [Google Scholar] [CrossRef]
- Devkota, L.M.; Williams, D.S.; Matta, J.H.; Albertson, O.E.; Grasso, D.; Fox, P. Variation of oxidation–reduction potential along the breakpoint curves in low-ammonia effluents. Water Environ. Res. 2000, 72, 610–617. [Google Scholar] [CrossRef]
- Guo, K.; Wu, Z.; Chen, C.; Fang, J. UV/Chlorine Process: An Efficient Advanced Oxidation Process with Multiple Radicals and Functions in Water Treatment. Acc. Chem. Res. 2022, 55, 286–297. [Google Scholar] [CrossRef]
- Bagastyo, A.Y.; Radjenovic, J.; Mu, Y.; Rozendal, R.A.; Batstone, D.J.; Rabaey, K. Electrochemical oxidation of reverse osmosis concentrate on mixed metal oxide (MMO) titanium coated electrodes. Water Res. 2011, 45, 4951–4959. [Google Scholar] [CrossRef]
- Kim, H.; Chung, M.W.; Choi, C.H. NOx-induced deactivation of Pt electrocatalysis towards the ammonia oxidation reaction. Electrochem. Commun. 2018, 94, 31–35. [Google Scholar] [CrossRef]
- Lu, S.; Shang, C.; Sun, B.; Xiang, Y. Dominant Dissolved Oxygen-Independent Pathway to Form Hydroxyl Radicals and the Generation of Reactive Chlorine and Nitrogen Species in Breakpoint Chlorination. Environ. Sci. Technol. 2023, 57, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, M.; Su, P.; Lv, Q.; Zeng, G.; An, L.; Cao, J.; Zhou, Y.; Snyder, S.A.; Ma, J.; et al. Refinement of kinetic model and understanding the role of dichloride radical (Cl2•−) in radical transformation in the UV/NH2Cl process. Water Res. 2024, 254, 121440. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, I.M.; Mitch, W.A. Enhanced Nitrogenous Disinfection ByProduct Formation near the Breakpoint: Implications for Nitrification Control. Environ. Sci. Technol. 2007, 41, 7039–7046. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Y.; Chen, Y.; Wang, W.-L.; Chen, Y.-L.; Wu, Q.-Y. The Impact of Dissolved Organic Matter in Natural Receiving Systems on the Formation Potential and Toxicity of Disinfection By-products: Insights from Origins, Chemical Properties, and Transformations. Curr. Pollut. Rep. 2025, 11, 29. [Google Scholar] [CrossRef]


| Anode Material | Electrolyte | Condition (mV s−1) | Onset Potential | Peak Potential | Peak Current Density | References |
|---|---|---|---|---|---|---|
| Pt | 0.05 mol L−1 KOH + 0.1 mol L−1 NH3 | 50 | 0.4 V vs. RHE | 0.7 V vs. RHE | – | [22] |
| Pt/SiO2-CNT-COOH | 1.0 mol L−1 KOH + 0.1 mol L−1 NH3 | 5 | 0.484 V vs. RHE | – | 77.3 A g−1 | [41] |
| PtIrNi1/SiO2-CNT-COOH | 1.0 mol L−1 KOH + 0.1 mol L−1 NH3 | 5 | 0.399 V vs. RHE | – | 124 A g−1 | [41] |
| NiO-TiO2 | 100 mmol L−1 NaNO3 + 200 mmol L−11 NH4+ | 100 | 0.5 V vs. Hg/HgO | ~0.9 V | – | [8] |
| NiCo oxyhydroxide/ Ni foam | 0.1 mol L−1 Na2SO4 + 50 mmol L−1 NH3 | 10 | – | – | – | [13] |
| Ni(OH)2/NiOOH | 0.1 mol L−1 Na2SO4 + 0.003 mol L−1 NH3 | 100 | 0.65 V vs. Hg/HgO | 0.9 V | – | [42] |
| Ni/NiOOH | 0.01 mol L−1 Na2SO4 + 50 ppm NH3 | 10 | 0.6 V vs. Hg/HgO | 1.0 V | – | [43] |
| Ni0.8Cu0.2LHs | 0.5 mol L−1 NaOH + 55 mmol L−1 NH4Cl | 25 | 0.4 V vs. Ag/AgCl | ~0.8 V | – | [44] |
| NiCu/CP electrode | 1 mol L−1 NaOH + 55 mmol L−1 NH4Cl | 25 | 0.47 V vs. Ag/AgCl | ~0.7 V | – | [45] |
| Ni0.8Cu0.2 hydroxide/ oxyhydroxide | 0.1 mol L−1 KOH + 1 mmol L−1 NH4+ | 10 | 1.4 V vs. RHE | – | – | [44] |
| NiCo2N | NH3 saturated 0.1 mol L−1 KPF6 | – | 0.55 V vs. NHE | – | – | [46] |
| (Mn,Fe,Co,Ni,Cu)3O4 oxides | NH3 saturated 0.1 mol L−1 KPF6 | – | 0.7 V vs. NHE | – | – | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Ma, F. Electrochemical Ammonia Oxidation in Water Treatment: A Comprehensive Review on Mechanisms, Catalysts, and Implementation Challenges. Water 2025, 17, 3106. https://doi.org/10.3390/w17213106
Shen X, Ma F. Electrochemical Ammonia Oxidation in Water Treatment: A Comprehensive Review on Mechanisms, Catalysts, and Implementation Challenges. Water. 2025; 17(21):3106. https://doi.org/10.3390/w17213106
Chicago/Turabian StyleShen, Xuanxu, and Fang Ma. 2025. "Electrochemical Ammonia Oxidation in Water Treatment: A Comprehensive Review on Mechanisms, Catalysts, and Implementation Challenges" Water 17, no. 21: 3106. https://doi.org/10.3390/w17213106
APA StyleShen, X., & Ma, F. (2025). Electrochemical Ammonia Oxidation in Water Treatment: A Comprehensive Review on Mechanisms, Catalysts, and Implementation Challenges. Water, 17(21), 3106. https://doi.org/10.3390/w17213106





