Plants Decrease Network Complexity and Increase Environmental Stability of Microbial Communities, Shifting the Dominant Environmental Controls from Carbon-Related Factors to pH in Newly Formed Wetlands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Description, Experimental Design and Soil Sampling
2.2. Soil Physicochemical Analysis
2.3. Soil Extracellular Enzyme Activities Analysis
2.4. Microbial DNA Extraction, PCR Amplification, Absolute Quantitative Sequencing and Data Processing
2.5. Data Analyses
3. Results
3.1. Microbial Composition and Structure in Areas with and Without Plants
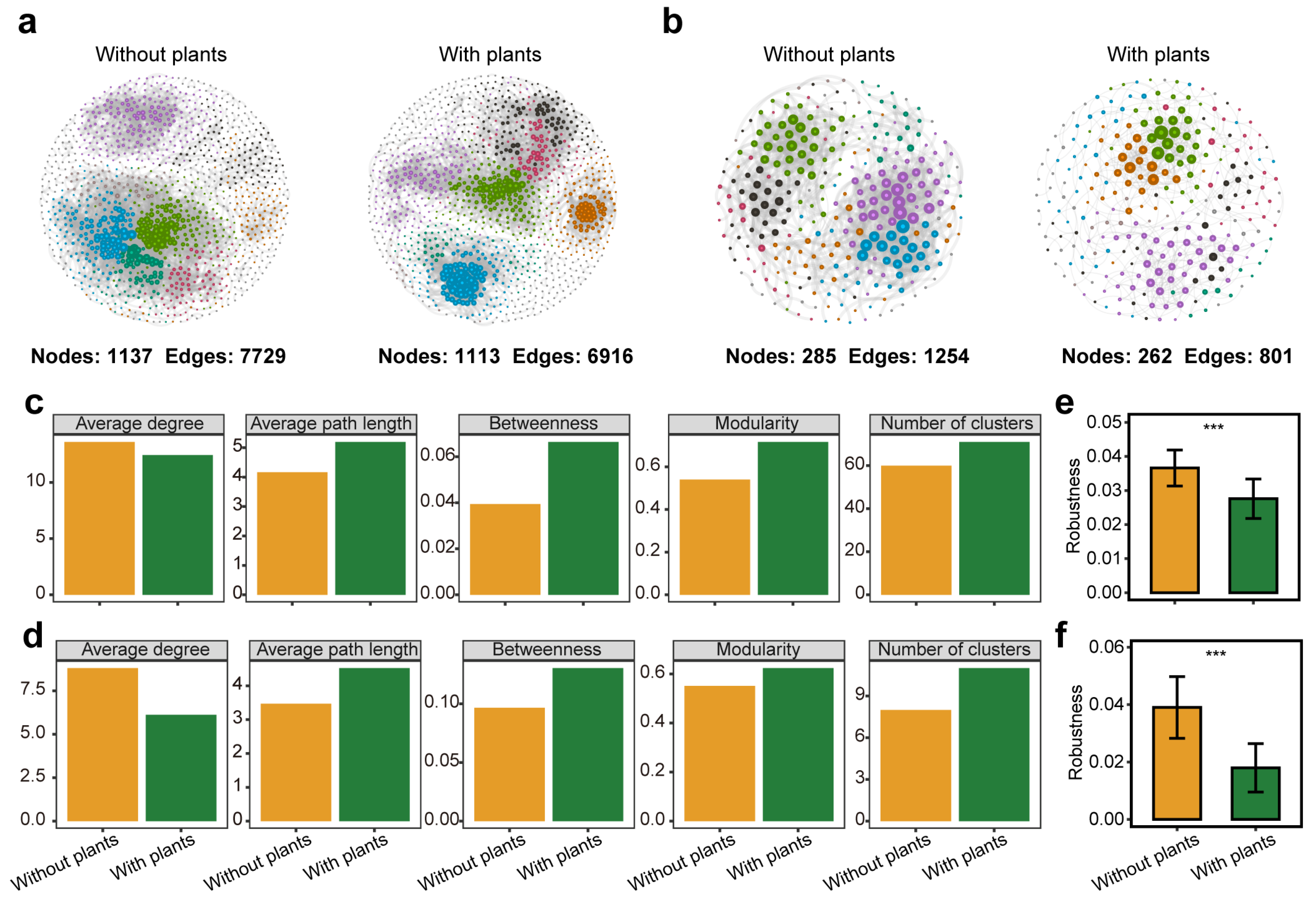
3.2. Co-Occurrence Network Structure and Stability
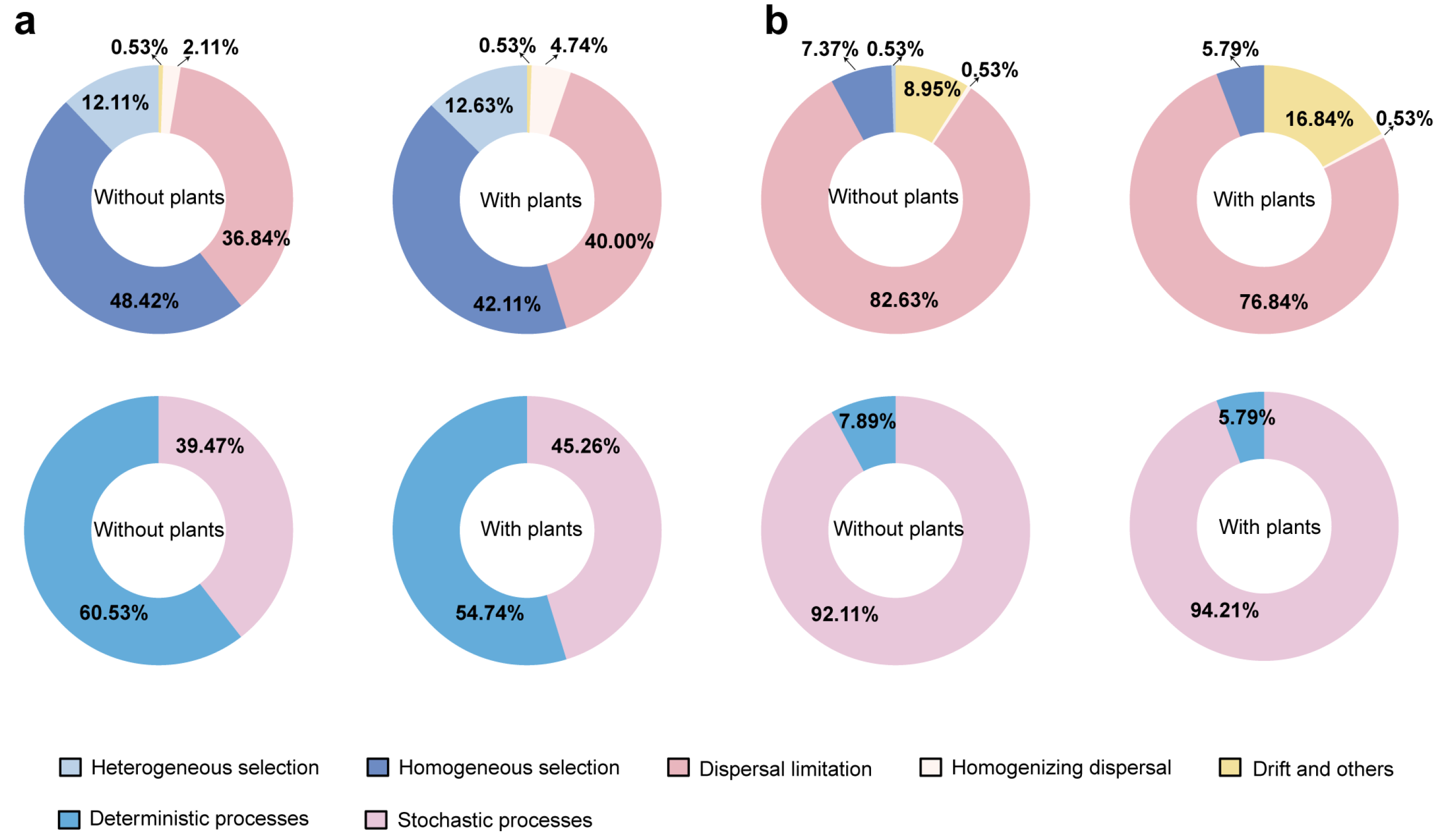
3.3. Microbial Community Assembly Mechanisms
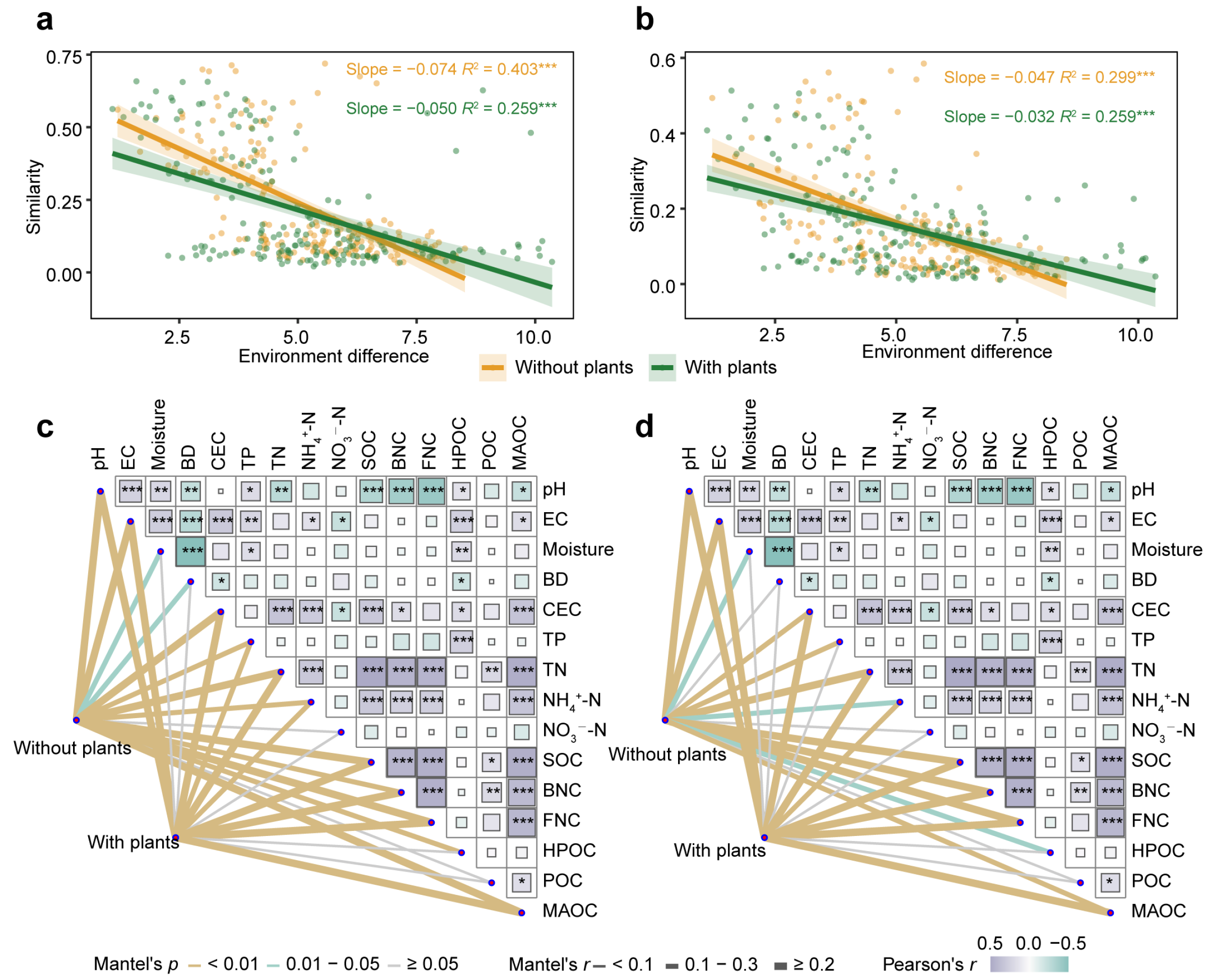
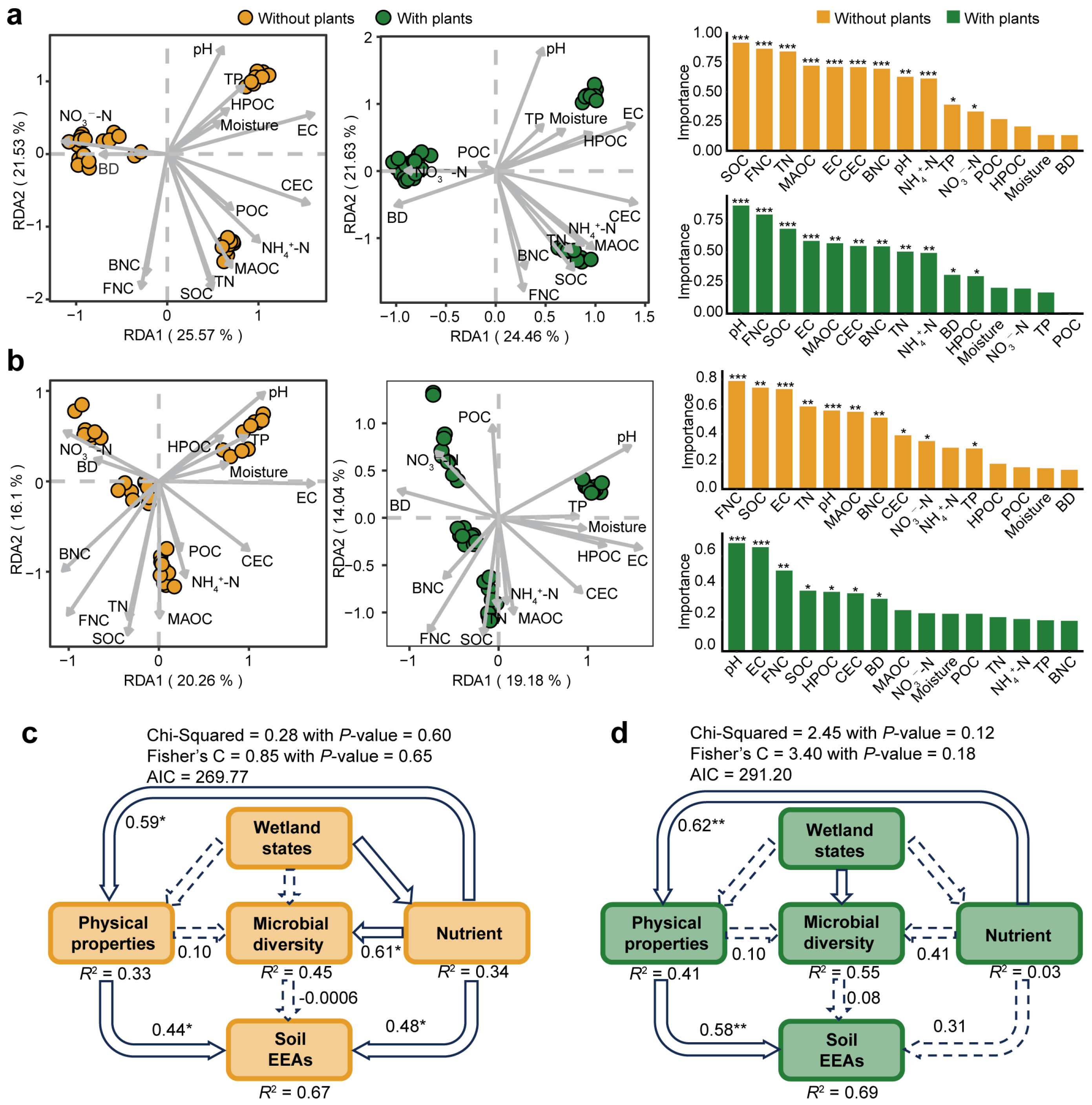
3.4. Environmental Factors Affecting Microbial Communities and Extracellular Enzyme
4. Discussion
4.1. Variations in Soil Microbial Community Characteristics Under the Presence of Plants
4.2. Ecological Processes Regulating Soil Microbial Communities
4.3. Environmental Factors Affecting Soil Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schuerch, M.; Spencer, T.; Temmerman, S.; Kirwan, M.L.; Wolff, C.; Lincke, D.; McOwen, C.J.; Pickering, M.D.; Reef, R.; Vafeidis, A.T.; et al. Future Response of Global Coastal Wetlands to Sea-Level Rise. Nature 2018, 561, 231–234. [Google Scholar] [CrossRef]
- Janse, J.H.; Van Dam, A.A.; Hes, E.M.A.; De Klein, J.J.M.; Finlayson, C.M.; Janssen, A.B.G.; Van Wijk, D.; Mooij, W.M.; Verhoeven, J.T.A. Towards a Global Model for Wetlands Ecosystem Services. Cur. Opin. Environ. Sustain. 2019, 36, 11–19. [Google Scholar] [CrossRef]
- Wang, F.; Sanders, C.J.; Santos, I.R.; Tang, J.; Schuerch, M.; Kirwan, M.L.; Kopp, R.E.; Zhu, K.; Li, X.; Yuan, J.; et al. Global Blue Carbon Accumulation in Tidal Wetlands Increases with Climate Change. Natl. Sci. Rev. 2021, 8, nwaa296. [Google Scholar] [CrossRef] [PubMed]
- Fluet-Chouinard, E.; Stocker, B.D.; Zhang, Z.; Malhotra, A.; Melton, J.R.; Poulter, B.; Kaplan, J.O.; Goldewijk, K.K.; Siebert, S.; Minayeva, T.; et al. Extensive Global Wetland Loss over the Past Three Centuries. Nature 2023, 614, 281–286. [Google Scholar] [CrossRef]
- Temmink, R.J.M.; Lamers, L.P.M.; Angelini, C.; Bouma, T.J.; Fritz, C.; Van De Koppel, J.; Lexmond, R.; Rietkerk, M.; Silliman, B.R.; Joosten, H.; et al. Recovering Wetland Biogeomorphic Feedbacks to Restore the World’s Biotic Carbon Hotspots. Science 2022, 376, eabn1479. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.J. The Extent and Drivers of Global Wetland Loss. Nature 2023, 614, 234–235. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Q.; Zhou, R.; Zhang, R.; Tian, D.; Gaffney, P.P.J.; Chen, W.; Gan, D.; Zhang, Z.; Niu, S.; et al. Elevating Water Table Reduces Net Ecosystem Carbon Losses from Global Drained Wetlands. Glob. Change Biol. 2024, 30, e17495. [Google Scholar] [CrossRef]
- He, Q.; Li, Z.; Daleo, P.; Lefcheck, J.S.; Thomsen, M.S.; Adams, J.B.; Bouma, T.J. Coastal Wetland Resilience through Local, Regional and Global Conservation. Nat. Rev. Biodivers. 2025, 1, 50–67. [Google Scholar] [CrossRef]
- Calderón, K.; Spor, A.; Breuil, M.-C.; Bru, D.; Bizouard, F.; Violle, C.; Barnard, R.L.; Philippot, L. Effectiveness of Ecological Rescue for Altered Soil Microbial Communities and Functions. ISME J. 2017, 11, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Averill, C.; Anthony, M.A.; Baldrian, P.; Finkbeiner, F.; van den Hoogen, J.; Kiers, T.; Kohout, P.; Hirt, E.; Smith, G.R.; Crowther, T.W. Defending Earth’s Terrestrial Microbiome. Nat. Microbiol. 2022, 7, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Candry, P.; Abrahamson, B.; Stahl, D.A.; Winkler, M.H. Microbially Mediated Climate Feedbacks from Wetland Ecosystems. Glob. Change Biol. 2023, 29, 5169–5183. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ma, Z. Ecological Restoration of Coastal Wetlands in China: Current Status and Suggestions. Biol. Conserv. 2024, 291, 110513. [Google Scholar] [CrossRef]
- Peay, K.G.; Baraloto, C.; Fine, P.V.A. Strong Coupling of Plant and Fungal Community Structure across Western Amazonian Rainforests. ISME J. 2013, 7, 1852–1861. [Google Scholar] [CrossRef]
- Rodriguez, P.A.; Rothballer, M.; Chowdhury, S.P.; Nussbaumer, T.; Gutjahr, C.; Falter-Braun, P. Systems Biology of Plant-Microbiome Interactions. Mol. Plant 2019, 12, 804–821. [Google Scholar] [CrossRef]
- Zhai, C.; Yang, Y.; Kong, L.; Wang, X.; Hou, J.; Zeng, Q.; Ge, A.; Yao, B.; Zhou, Z.; Feng, J.; et al. Nitrogen Deposition Decouples Grassland Plant Community from Soil Bacterial and Fungal Communities along a Precipitation Gradient. J. Ecol. 2025, 113, 1269–1280. [Google Scholar] [CrossRef]
- Martin, F.M.; Uroz, S.; Barker, D.G. Ancestral Alliances: Plant Mutualistic Symbioses with Fungi and Bacteria. Science 2017, 356, eaad4501. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going Back to the Roots: The Microbial Ecology of the Rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere Bacteriome Structure and Functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Y.; Janssens, I.A.; Deng, Y.; He, X.; Liu, L.; Yi, Y.; Xiao, N.; Wang, X.; Li, C.; et al. Divergent Rhizosphere and Non-rhizosphere Soil Microbial Structure and Function in Long-term Warmed Steppe Due to Altered Root Exudation. Glob. Change Biol. 2024, 30, e17111. [Google Scholar] [CrossRef]
- Dong, X.; Man, H.; Liu, C.; Wu, X.; Zhu, J.; Zheng, Z.; Ma, D.; Li, M.; Zang, S. Changes in Soil Bacterial Community along a Gradient of Permafrost Degradation in Northeast China. Catena 2023, 222, 106870. [Google Scholar] [CrossRef]
- Saltonstall, K.; Van Breugel, M.; Navia, W.; Castillo, H.; Hall, J.S. Soil Microbial Communities in Dry and Moist Tropical Forests Exhibit Distinct Shifts in Community Composition but Not Diversity with Succession. Microbiol. Spectr. 2025, 13, e01931-24. [Google Scholar] [CrossRef]
- Ge, M.; Gao, J.; McClellan, S.A.; Hou, A.; Yu, J.; Yang, J.; Song, T.; Guan, B. Changes of Bacterial Communities in Restored Phragmites australis Wetlands Indicate the Improvement of Soil in the Yellow River Delta. Land Degrad. Dev. 2023, 34, 1897–1909. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-Bacterial Diversity and Microbiome Complexity Predict Ecosystem Functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Mendes, L.W.; Kuramae, E.E.; Navarrete, A.A.; Van Veen, J.A.; Tsai, S.M. Taxonomical and Functional Microbial Community Selection in Soybean Rhizosphere. ISME J. 2014, 8, 1577–1587. [Google Scholar] [CrossRef]
- Wang, D.; Deng, S.; Yang, H.; Li, N.; Feng, Q.; Liu, J.; Yin, H. The Microbial Network Exhibits Higher Complexity in the Rhizosphere than in Bulk Soils along Elevational Gradients in the Alpine Forests. Appl. Soil Ecol. 2025, 213, 106264. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, K.; Wang, Z.; Liu, D.; Li, T.; Hou, H.; Zhang, Z.; Chen, D.; Zhang, S.; Yu, A.; et al. Soil Microbial Subcommunity Assembly Mechanisms Are Highly Variable and Intimately Linked to Their Ecological and Functional Traits. Mol. Ecol. 2024, 33, e17302. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Lin, X.; Konopka, A.E.; Fredrickson, J.K. Stochastic and Deterministic Assembly Processes in Subsurface Microbial Communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Riddley, M.; Hepp, S.; Hardeep, F.; Nayak, A.; Liu, M.; Xing, X.; Zhang, H.; Liao, J. Differential Roles of Deterministic and Stochastic Processes in Structuring Soil Bacterial Ecotypes across Terrestrial Ecosystems. Nat. Commun. 2025, 16, 2337. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, K.; Krause, S.M.B.; Li, S.; Wang, X.; Zhang, Z.; Shen, M.; Yang, Q.; Lian, J.; Wang, X.; et al. Changes in Assembly Processes of Soil Microbial Communities during Secondary Succession in Two Subtropical Forests. Soil Biol. Biochem. 2021, 154, 108144. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, H.; Yang, Y.; Deng, Y.; Ju, F. Fungi as a Critical Component of Lake Microbiota in Response to Cyanobacterial Harmful Algal Blooms. Environ. Sci. Technol. 2025, 59, 11167–11180. [Google Scholar] [CrossRef]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Using Soil Bacterial Communities to Predict Physico-Chemical Variables and Soil Quality. Microbiome 2020, 8, 79. [Google Scholar] [CrossRef]
- De Nijs, E.A.; Hicks, L.C.; Leizeaga, A.; Tietema, A.; Rousk, J. Soil Microbial Moisture Dependences and Responses to Drying–Rewetting: The Legacy of 18 Years Drought. Glob. Change Biol. 2019, 25, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, X.; Liu, J.; Zhang, Z.; Zhang, W.; Qi, Y.; Li, W.; Chen, Y. Changes of Soil Bacterial Community, Network Structure, and Carbon, Nitrogen and Sulfur Functional Genes under Different Land Use Types. Catena 2023, 231, 107385. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, J.; Bourque, C.P.-A.; Xiang, Q.; Zhang, J.; Yang, X.; Zhu, J.; Ma, J. Soil Depth Affects Bacterial, but Not Fungal Community Structure and Assembly in Robinia pseudoacacia Plantations. Eur. J. Soil Biol. 2025, 126, 103747. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Zhang, C.; Wang, R.; Wang, H.; Zheng, P. Vegetation Succession Enhances Microbial Diversity, Network Complexity, and Stability in Coastal Wetlands, Underscoring the Pivotal Role of Soil Salinity and Key Microbial Species. J. Environ. Manag. 2025, 388, 125997. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Song, M.; Du, H.; Jiang, S.; Zeng, F.; Chen, H.; Song, T. Assembly Processes and Networks of Soil Microbial Communities along Karst Forest Succession. Catena 2025, 248, 108574. [Google Scholar] [CrossRef]
- Liu, J.; Jia, X.; Yan, W.; Zhong, Y.; Shangguan, Z. Changes in Soil Microbial Community Structure during Long-term Secondary Succession. Land Degrad. Dev. 2020, 31, 1151–1166. [Google Scholar] [CrossRef]
- Barberán, A.; McGuire, K.L.; Wolf, J.A.; Jones, F.A.; Wright, S.J.; Turner, B.L.; Essene, A.; Hubbell, S.P.; Faircloth, B.C.; Fierer, N. Relating Belowground Microbial Composition to the Taxonomic, Phylogenetic, and Functional Trait Distributions of Trees in a Tropical Forest. Ecol. Lett. 2015, 18, 1397–1405. [Google Scholar] [CrossRef]
- Yang, W.; Cai, A.; Wang, J.; Luo, Y.; Cheng, X.; An, S. Exotic Spartina Alterniflora Loisel. Invasion Significantly Shifts Soil Bacterial Communities with the Successional Gradient of Saltmarsh in Eastern China. Plant Soil 2020, 449, 97–115. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Zhang, W. Landscape Pattern Changes and Its Drivers Inferred from Salt Marsh Plant Variations in the Coastal Wetlands of the Liao River Estuary, China. Ecol. Indic. 2022, 145, 109719. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, X.; Xu, X.; Zou, Z.; Chen, B.; Qin, Y.; Zhang, X.; Dong, J.; Liu, D.; Pan, L.; et al. Rebound in China’s Coastal Wetlands Following Conservation and Restoration. Nat. Sustain. 2021, 4, 1076–1083. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Gao, H.; Wang, F.; Shang, B.; Zhang, M.; Fu, T. Spatial Pattern of Critical Wetland Patches and Its Influencing Factors in a Coastal Area, North China. J. Environ. Manag. 2025, 373, 123741. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhao, Y.; Zhang, X.; Wang, Y.; Cao, Q.; Liu, J. Microbial Necromass Carbon Contributed to Soil Organic Carbon Accumulation and Stabilization in the Newly Formed Inland Wetlands. Environ. Res. 2025, 264, 120397. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, G.; Li, C.; Zhao, Y.; Dong, J.; Wang, Z.; Lu, G.; Chen, Z.; Dong, Z.; Liu, K.; et al. Natural Succession and Artificial Management Have Different Effects on Soil Microbial Ecological Patterns in Wetland Resulting from Land-Use Change. Appl. Soil Ecol. 2025, 205, 105783. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing Soil Organic Matter into Particulate and Mineral-associated Forms to Address Global Change in the 21st Century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Liu, F.; Qin, S.; Fang, K.; Chen, L.; Peng, Y.; Smith, P.; Yang, Y. Divergent Changes in Particulate and Mineral-Associated Organic Carbon upon Permafrost Thaw. Nat. Commun. 2022, 13, 5073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Amelung, W. Gas Chromatographic Determination of Muramic Acid, Glucosamine, Mannosamine, and Galactosamine in Soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Joergensen, R.G. Amino Sugars as Specific Indices for Fungal and Bacterial Residues in Soil. Biol. Fertil. Soils 2018, 54, 559–568. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of Soil Enzyme Activity at Global Scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.; Hu, B.; Li, F.; Wei, D.; Wang, Z.; Huang, L.; Bao, W. Climate and Soil Properties Shape Latitudinal Patterns of Soil Extracellular Enzyme Activity and Stoichiometry: Evidence from Southwest China. Appl. Soil Ecol. 2024, 197, 105319. [Google Scholar] [CrossRef]
- Smets, W.; Leff, J.W.; Bradford, M.A.; McCulley, R.L.; Lebeer, S.; Fierer, N. A Method for Simultaneous Measurement of Soil Bacterial Abundances and Community Composition via 16S rRNA Gene Sequencing. Soil Biol. Biochem. 2016, 96, 145–151. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-Species Living Tree Project (LTP)” Taxonomic Frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Kõljalg, U.; Larsson, K.; Abarenkov, K.; Nilsson, R.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; et al. UNITE: A Database Providing Web-based Methods for the Molecular Identification of Ectomycorrhizal Fungi. New Phytol. 2005, 166, 1063–1068. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise Structural Equation Modelling in r for Ecology, Evolution, and Systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Ning, D.; Yuan, M.; Wu, L.; Zhang, Y.; Guo, X.; Zhou, X.; Yang, Y.; Arkin, A.P.; Firestone, M.K.; Zhou, J. A Quantitative Framework Reveals Ecological Drivers of Grassland Microbial Community Assembly in Response to Warming. Nat. Commun. 2020, 11, 4717. [Google Scholar] [CrossRef]
- Wen, T.; Xie, P.; Yang, S.; Niu, G.; Liu, X.; Ding, Z.; Xue, C.; Liu, Y.; Shen, Q.; Yuan, J. ggClusterNet: An R Package for Microbiome Network Analysis and Modularity-based Multiple Network Layouts. iMeta 2022, 1, e32. [Google Scholar] [CrossRef]
- Hu, M.; Sardans, J.; Sun, D.; Yan, R.; Wu, H.; Ni, R.; Peñuelas, J. Microbial Diversity and Keystone Species Drive Soil Nutrient Cycling and Multifunctionality Following Mangrove Restoration. Environ. Res. 2024, 251, 118715. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming Effects: Interactions between Living and Dead Organic Matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Shi, L.; Marshall, M.R.; Kuzyakov, Y.; Blagodatskaya, E.; Zang, H. Strong Priming of Soil Organic Matter Induced by Frequent Input of Labile Carbon. Soil Biol. Biochem. 2021, 152, 108069. [Google Scholar] [CrossRef]
- Shi, J.; Yang, L.; Liao, Y.; Li, J.; Jiao, S.; Shangguan, Z.; Deng, L. Soil Labile Organic Carbon Fractions Mediate Microbial Community Assembly Processes during Long-term Vegetation Succession in a Semiarid Region. iMeta 2023, 2, e142. [Google Scholar] [CrossRef]
- Schmidt, R.; Mitchell, J.; Scow, K. Cover Cropping and No-till Increase Diversity and Symbiotroph:Saprotroph Ratios of Soil Fungal Communities. Soil Biol. Biochem. 2019, 129, 99–109. [Google Scholar] [CrossRef]
- Winterfeldt, S.; Cruz-Paredes, C.; Rousk, J.; Leizeaga, A. Microbial Resistance and Resilience to Drought across a European Climate Gradient. Soil Biol. Biochem. 2024, 199, 109574. [Google Scholar] [CrossRef]
- Jing, C.; Xu, Z.; Zou, P.; Tang, Q.; Li, Y.; You, X.; Zhang, C. Coastal Halophytes Alter Properties and Microbial Community Structure of the Saline Soils in the Yellow River Delta, China. Appl. Soil Ecol. 2019, 134, 1–7. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liu, C.; Wang, Q.; Yao, M.; Rui, J.; Zhang, S.; Li, X. Soil Bacterial Community Structure in Chinese Wetlands. Geoderma 2019, 337, 290–299. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Cappelli, S.L.; Shrestha, R.; Gerin, S.; Lohila, A.K.; Heinonsalo, J.; Nelson, D.B.; Kahmen, A.; Duan, P.; Sebag, D.; et al. Plant Diversity Drives Positive Microbial Associations in the Rhizosphere Enhancing Carbon Use Efficiency in Agricultural Soils. Nat. Commun. 2024, 15, 8065. [Google Scholar] [CrossRef]
- Zhou, T.; Liang, G.; Reich, P.B.; Delgado-Baquerizo, M.; Wang, C.; Zhou, Z. Promoting Effect of Plant Diversity on Soil Microbial Functionality Is Amplified over Time. One Earth 2024, 7, 2139–2148. [Google Scholar] [CrossRef]
- Zhu, L.; Luan, L.; Chen, Y.; Wang, X.; Zhou, S.; Zou, W.; Han, X.; Duan, Y.; Zhu, B.; Li, Y.; et al. Community Assembly of Organisms Regulates Soil Microbial Functional Potential through Dual Mechanisms. Glob. Change Biol. 2024, 30, e17160. [Google Scholar] [CrossRef]
- Romdhane, S.; Spor, A.; Aubert, J.; Bru, D.; Breuil, M.-C.; Hallin, S.; Mounier, A.; Ouadah, S.; Tsiknia, M.; Philippot, L. Unraveling Negative Biotic Interactions Determining Soil Microbial Community Assembly and Functioning. ISME J. 2022, 16, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Lyu, Y.-M.; Liu, Y.; Wang, Y.-Q.; Xiong, M.-M.; Tang, Y.; Li, X.-Y.; Sun, H.; Xu, J.-L. Differential Spatial Responses and Assembly Mechanisms of Soil Microbial Communities across Region-Scale Taiga Ecosystems. J. Environ. Manag. 2024, 370, 122653. [Google Scholar] [CrossRef]
- Sun, S.; Li, S.; Avera, B.N.; Strahm, B.D.; Badgley, B.D. Soil Bacterial and Fungal Communities Show Distinct Recovery Patterns during Forest Ecosystem Restoration. Appl. Environ. Microbiol. 2017, 83, e00966-17. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.; Wang, B.; Xue, Z.; Wang, Y.; An, S.; Chang, S.X. Deciphering Factors Driving Soil Microbial Life-history Strategies in Restored Grasslands. iMeta 2023, 2, e66. [Google Scholar] [CrossRef]
- Rodrigues, J.L.M.; Pellizari, V.H.; Mueller, R.; Baek, K.; Jesus, E.D.C.; Paula, F.S.; Mirza, B.; Hamaoui, G.S.; Tsai, S.M.; Feigl, B.; et al. Conversion of the Amazon Rainforest to Agriculture Results in Biotic Homogenization of Soil Bacterial Communities. Proc. Natl. Acad. Sci. USA 2013, 110, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Chu, H.; Zhang, B.; Wei, X.; Chen, W.; Wei, G. Linking Soil Fungi to Bacterial Community Assembly in Arid Ecosystems. iMeta 2022, 1, e2. [Google Scholar] [CrossRef]
- Chaffron, S.; Delage, E.; Budinich, M.; Vintache, D.; Henry, N.; Nef, C.; Ardyna, M.; Zayed, A.A.; Junger, P.C.; Galand, P.E.; et al. Environmental Vulnerability of the Global Ocean Epipelagic Plankton Community Interactome. Sci. Adv. 2021, 7, eabg1921. [Google Scholar] [CrossRef]
- Bertolet, B.L.; Rodriguez, L.C.; Murúa, J.M.; Favela, A.; Allison, S.D. The Impact of Microbial Interactions on Ecosystem Function Intensifies Under Stress. Ecol. Lett. 2024, 27, e14528. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, J.; Chen, X.; Meng, Z.; Xu, R.; Duoji, D.; Zhang, J.; He, J.; Wang, Z.; Chen, J.; et al. Soil Microbial Network Complexity Predicts Ecosystem Function along Elevation Gradients on the Tibetan Plateau. Soil Biol. Biochem. 2022, 172, 108766. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere Size and Shape: Temporal Dynamics and Spatial Stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Zhang, C.; Lei, S.; Wu, H.; Liao, L.; Wang, X.; Zhang, L.; Liu, G.; Wang, G.; Fang, L.; Song, Z. Simplified Microbial Network Reduced Microbial Structure Stability and Soil Functionality in Alpine Grassland along a Natural Aridity Gradient. Soil Biol. Biochem. 2024, 191, 109366. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The Interplay between Microbial Communities and Soil Properties. Nat. Rev. Microbiol. 2023, 22, 226–239. [Google Scholar] [CrossRef]
- Crocker, K.; Lee, K.K.; Chakraverti-Wuerthwein, M.; Li, Z.; Tikhonov, M.; Mani, M.; Gowda, K.; Kuehn, S. Environmentally Dependent Interactions Shape Patterns in Gene Content across Natural Microbiomes. Nat. Microbiol. 2024, 9, 2022–2037. [Google Scholar] [CrossRef]
- Wang, G.; Van Der Putten, W.H.; Klironomos, J.; Zhang, F.; Zhang, J. Steering Plant-Soil Feedback for Sustainable Agriculture. Science 2025, 389, eads2506. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a pH Gradient in an Arable Soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Feng, K.; Deng, Y. Intertwined Relationship Between Soil pH and Microbes in Biogeography. Glob. Change Biol. 2025, 31, e70208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jia, J.; Zhao, Q.; Wang, W.; Wang, D.; Bai, J. Seasonality and Assembly of Soil Microbial Communities in Coastal Salt Marshes Invaded by a Perennial Grass. J. Environ. Manag. 2023, 331, 117247. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Y.; He, L.; Fan, B.; Liu, K.; Ding, M.; Hu, Y.; Jing, X.; Zhu, B.; Wang, S.; et al. Responses of Subsoil Organic Carbon to Climate Warming and Cooling Is Determined by Microbial Community Rather Than Its Molecular Composition. Ecol. Lett. 2025, 28, e70162. [Google Scholar] [CrossRef]
- Deng, Z.; Dong, F.; Dai, Q.; Jiang, R.; Du, W.; Zhao, Y.; Liu, M.; Chen, Z. Karst Wetland Litter Alters Soil Microbial Community Structure and Metabolic Activity. Plant Soil 2025, 1–18. [Google Scholar] [CrossRef]
- Wang, G.; Gao, Q.; Yang, Y.; Hobbie, S.E.; Reich, P.B.; Zhou, J. Soil Enzymes as Indicators of Soil Function: A Step toward Greater Realism in Microbial Ecological Modeling. Glob. Change Biol. 2022, 28, 1935–1950. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Dong, J.; Liu, X.; Li, C.; Zhao, Y.; Wang, Y.; Liu, J. Plants Decrease Network Complexity and Increase Environmental Stability of Microbial Communities, Shifting the Dominant Environmental Controls from Carbon-Related Factors to pH in Newly Formed Wetlands. Water 2025, 17, 3054. https://doi.org/10.3390/w17213054
Wang Y, Dong J, Liu X, Li C, Zhao Y, Wang Y, Liu J. Plants Decrease Network Complexity and Increase Environmental Stability of Microbial Communities, Shifting the Dominant Environmental Controls from Carbon-Related Factors to pH in Newly Formed Wetlands. Water. 2025; 17(21):3054. https://doi.org/10.3390/w17213054
Chicago/Turabian StyleWang, Yijing, Junyu Dong, Xiaoke Liu, Changchao Li, Yongkang Zhao, Yan Wang, and Jian Liu. 2025. "Plants Decrease Network Complexity and Increase Environmental Stability of Microbial Communities, Shifting the Dominant Environmental Controls from Carbon-Related Factors to pH in Newly Formed Wetlands" Water 17, no. 21: 3054. https://doi.org/10.3390/w17213054
APA StyleWang, Y., Dong, J., Liu, X., Li, C., Zhao, Y., Wang, Y., & Liu, J. (2025). Plants Decrease Network Complexity and Increase Environmental Stability of Microbial Communities, Shifting the Dominant Environmental Controls from Carbon-Related Factors to pH in Newly Formed Wetlands. Water, 17(21), 3054. https://doi.org/10.3390/w17213054







