Revitalization of Trakošćan Lake—Preliminary Analyses of the Sediment with the Possibility of Its Reuse in the Environment
Abstract
1. Introduction
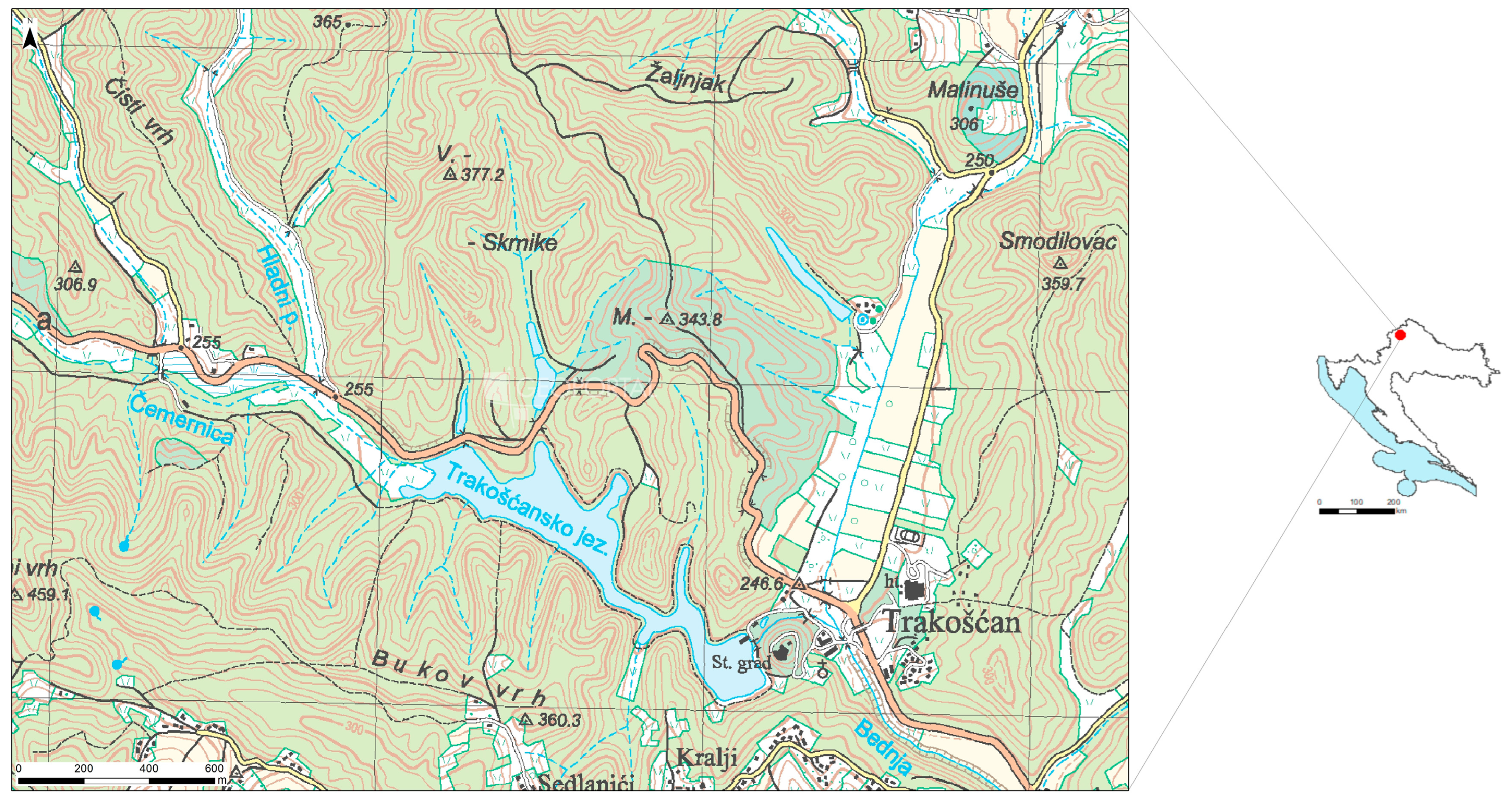
2. Study Area
2.1. Hydrology Characteristics
2.2. Geology of the Trakošćan Lake Catchment
3. Methods and Techniques of Analysis
3.1. Sampling and Laboratory Preparation of Lake Sediment
3.2. Laboratory Sediment Analyses
3.3. Statistical Analysis
4. Results
5. Discussion
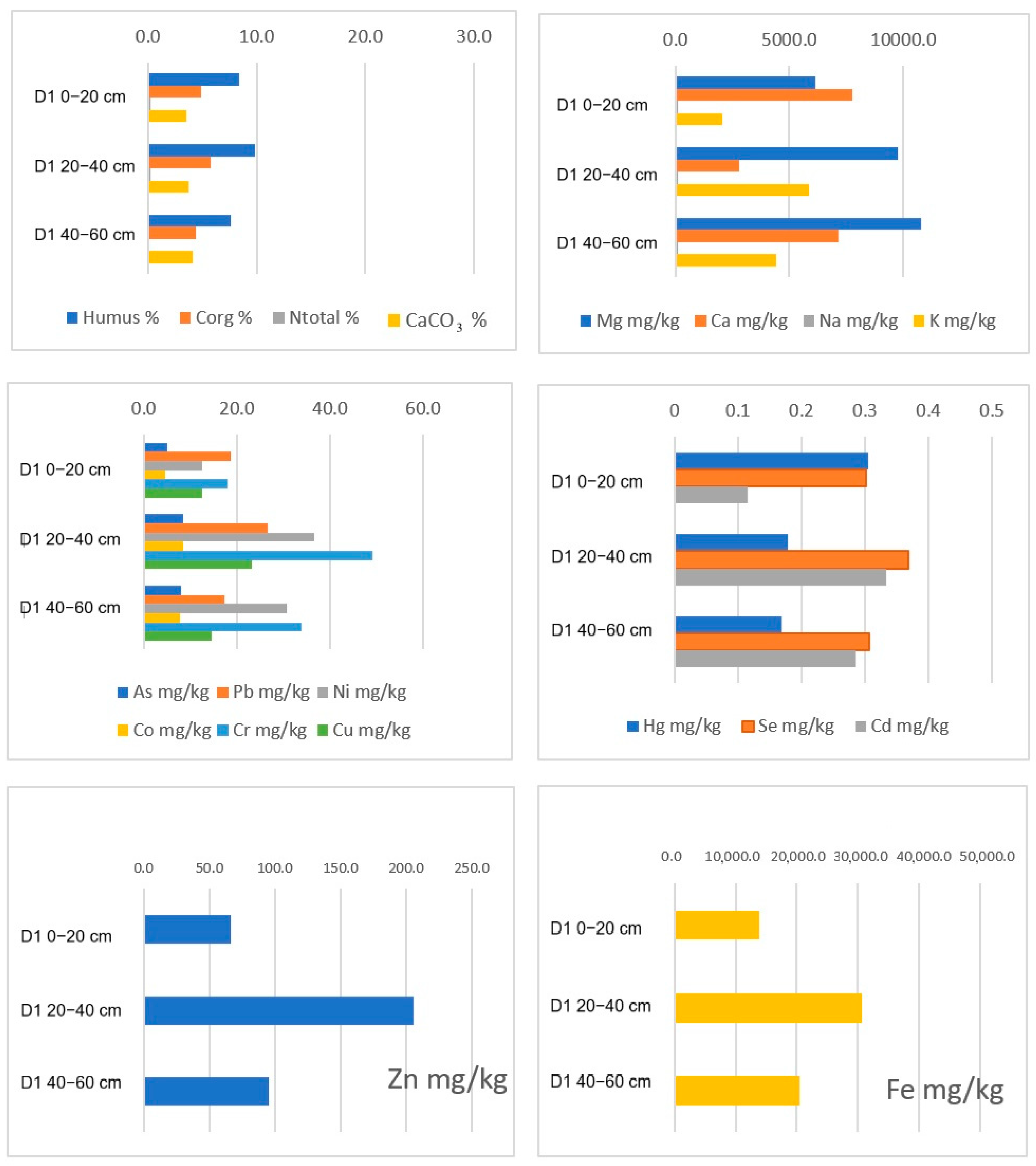
5.1. Distribution of Compounds and Elements Along the Depth of Sediments
5.2. Origin of Compounds and Elements
5.3. Sediment Quality
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pannard, A.; Souchu, P.; Chauvin, C.; Delabuis, M.; Gascuel-Odoux, C.; Jeppesen, E.; Gross, E.M. Why are there so many definitions of eutrophication? Ecol. Monogr. 2024, 94, e1616. [Google Scholar] [CrossRef]
- Vorosmarty, C.J.; Meybeck, M.; Fekete, B.; Sharma, K.; Green, P.; Syvitski, J.P.M. Anthropogenic sediment retention: Major global impact from registered river impoundments. Glob. Planet. Change 2003, 39, 169–190. [Google Scholar] [CrossRef]
- Verstraeten, G.; Bazzoffi, P.; Lajczak, A.; Rãdoane, M.; Rey, F.; Poesen, J.; de Vente, J.; Reservoir and Pond Sedimentation in Europe; Physical and Regional Geography Research Group; Katholieke Universiteit Leuven. Soil Erosion in Europe; Boardman, J., Poesen, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Oskoruš, D.; Leskovar, K.; Pavlić, K. Parametric methods for assessing the production of suspended sediment and its deposition in artificial lakes—An example of Lake Trakošćan, Croatia. In Proceedings of the EGU General Assembly 2023, Vienna, Austria, 24–28 April 2023; p. EGU23-13546. [Google Scholar] [CrossRef]
- Oskoruš, D.; Leskovar, K.; Pavlić, K.; Tošić, I. Assessing the long-term production of suspended sediment and the climate changes impact on its deposition in artificial lakes–a case study of Lake Trakošćan, Croatia. Climate 2023, 11, 167. [Google Scholar] [CrossRef]
- Magnuszewski, A.; Moran, S.; Yu, G. Modelling lowland reservoir sedimentation conditions and the potential environmental consequences of dam removal: Wloclawek Reservoir, Vistula River, Poland. In Sediment Dynamics for a Changing Future, Proceedings of the ICCE Symposium Held at Warsaw University of Life Sciences—SGGW, Warsaw, Poland, 14–18 June 2010; IAHS Publ.: Oxford, UK, 2010; p. 337. [Google Scholar]
- Kelderman, P.; Yang, X.; Drossaert, W.M.E. Sediment pollution with respect to heavy metals and organic micropollutants in the city canals of Delft (The Netherlands)—Assessment of a data base of 188 sediment stations. In E-Water; Official Publication of the European Water Association (EWA): Hennef, Germany, 2005. [Google Scholar]
- Kelderman, P.; Drossaert, W.M.E.; Zhang, M.; Galione, L.S.; Okonkwo, L.C.; Clarisse, I.A. Pollution assessment of the canal sediments in the city of Delft (the Netherlands). Water Res. 2000, 34, 936–944. [Google Scholar] [CrossRef]
- Lawrence, P.; Atkinson, E. Deposition of fine sediments in irrigation canals. Irrig. Drain. Syst. 1998, 12, 371–385. [Google Scholar] [CrossRef]
- Maggi, I.; Maraga, F.; Ottone, C. Erosive rains related to in-channel sediment delivery in a small Alpine basin (North-Western Italy). In Proceedings of the 9th Conference of the European Network of Experimental and Representative Basins (ERB), Demänovská Dolina, Slovakia, 25–28 September 2002; pp. 91–99. [Google Scholar]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Savić, R.; Pantelić, S.; Belić, A.; Belić, S. Teški metali u sedimentima melioracionih kanala. Zb. Rad. Građevinskog Fak. 2005, 14, 260–265. [Google Scholar]
- Savić, R.; Josimov-Dunđerski, J.; Belić, A.; Ondrašek, G.; Letić, L.; Nikolić, V. Monitoring Kvalitata Vode i Sedimenta Manjih Vodotoka u Vojvodini—Primjer Vodotoka Tatarnica. 2025. Available online: http://doisrpska.nub.rs/index.php/agroznanje/article/viewFile/2293/2202 (accessed on 29 August 2025).
- Leko-Kos, M. Nastanak i Pokretljivost Sedimenta Onečišćenog Teškim Metalima u Melioracijskim Kanalima Ravničarskih Područja. Ph.D. Thesis, Sveučilište Josipa Jurja Strossmayera u Osijeku, Građevinski i arhitektonski fakultet Osijek, Osijek, Croatia, 2019. [Google Scholar]
- Hrvatske Vode. Jezero Trakošćan Zapunjeno Vodom. Available online: https://www.voda.hr/hr/novost/jezero-trakoscan-zapunjeno-vodom (accessed on 8 September 2025).
- Zakon o Gospodarenju Otpadom, NN 84/2021, 142/2023. Available online: https://www.zakon.hr/z/2848/zakon-o-gospodarenju-otpadom (accessed on 8 September 2025).
- Javna Ustanova “Priroda Varaždinske Županije”. Trakošćan. Available online: https://priroda-vz.hr/podrucja/trakoscan/ (accessed on 10 September 2025).
- Lemière, B.; Gebert, J.; Wijdeveld, A. The CE white paper. In Proceedings of the SedNet WG Circular Economy WGCE7, Delft, The Netherlands, 5 July 2022. [Google Scholar]
- Ministarstvo Poljoprivrede. Pravilnik o Zaštiti Poljoprivrednog Zemljišta od Onečišćenja NN 71/2019. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2019_07_71_1507.html (accessed on 8 September 2025).
- Tanocki, Z.; Crljenko, I. Jezera Hrvatske; Školska knjiga: Zagreb, Hrvatska, 2011. [Google Scholar]
- Geokon-Zagreb, d.d. Geotechnical Study of a Survey of the Thickness and Composition of Sediment and Water Quality in Lake Trakošćan; Geokon-Zagreb d.d.: Zagreb, Croatia, 2019. [Google Scholar]
- Kerovec, M. Studija Utvrđivanja Stanja Vode u Jezeru Trakošćan sa Programom Mjera za Njegovu Sanaciju i Revitalizaciju; Studeni: Zagreb, Croatia, 2007. [Google Scholar]
- Aničić, B.; Juriša, M. Osnovna Geološka Karta SFRJ 1:100 000—Tumač List Rogatec L 33 68; Geološki Zavod: Ljubljana, Slovenia, 1985. [Google Scholar]
- Avanić, R.; Pavelić, D.; Pecskay, Z.; Miknić, M.; Tibljaš, D.; Wacha, L. Tidal deposits in the Early Miocene Central Parathethys: The Vučji Jarek and Čemernica members of the Macelj formation (NW Croatia). Geol. Croat. 2021, 74, 41–56. [Google Scholar] [CrossRef]
- Halamić, J.; Miko, S. Geokemijski atlas Republike Hrvatske; Hrvatski Geološki Institut: Zagreb, Hrvatska, 2009. [Google Scholar]
- Statistica; Software Version 7; StatSoft: Tulsa, OK, USA, 2005.
- Ćosić, T.; Čoga, L.; Pavlović, I.; Patek, M.; Slunjski, S. Materijali za Vježbe iz Ishrane Bilja; Zavod za ishranu bilja, Agronomski fakultet Sveučilišta u Zagrebu: Zagreb, Hrvatska, 2007. [Google Scholar]



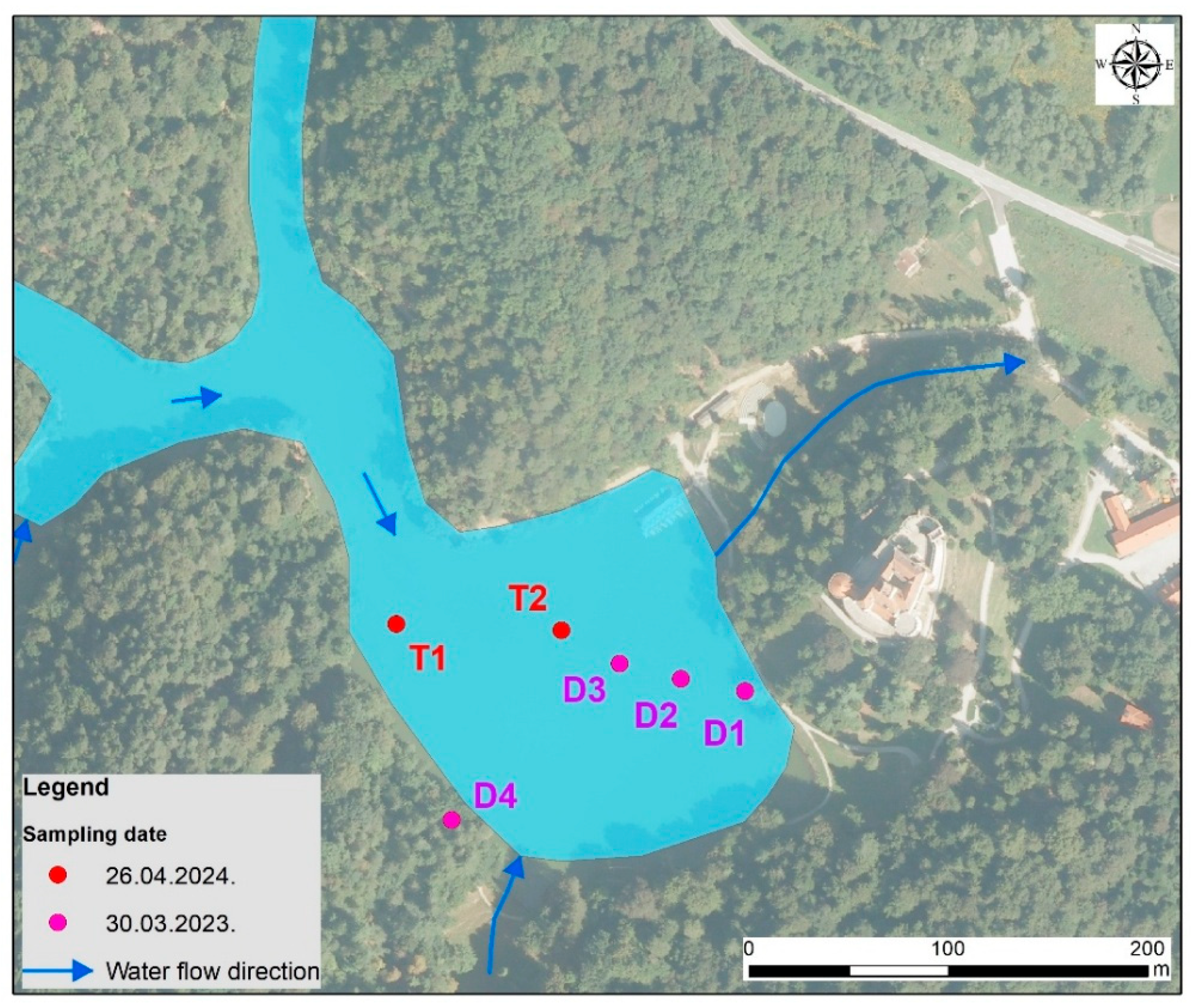


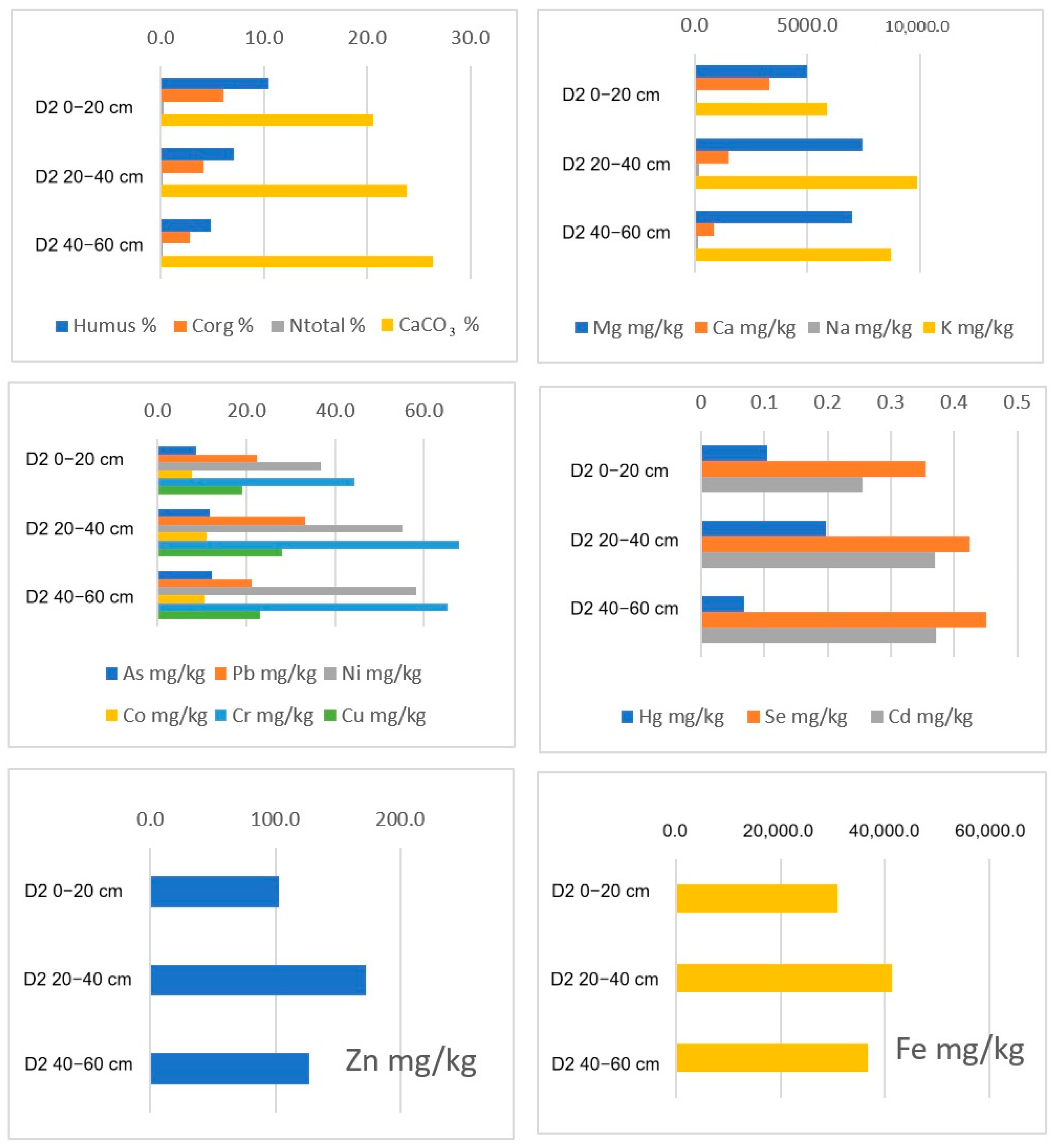
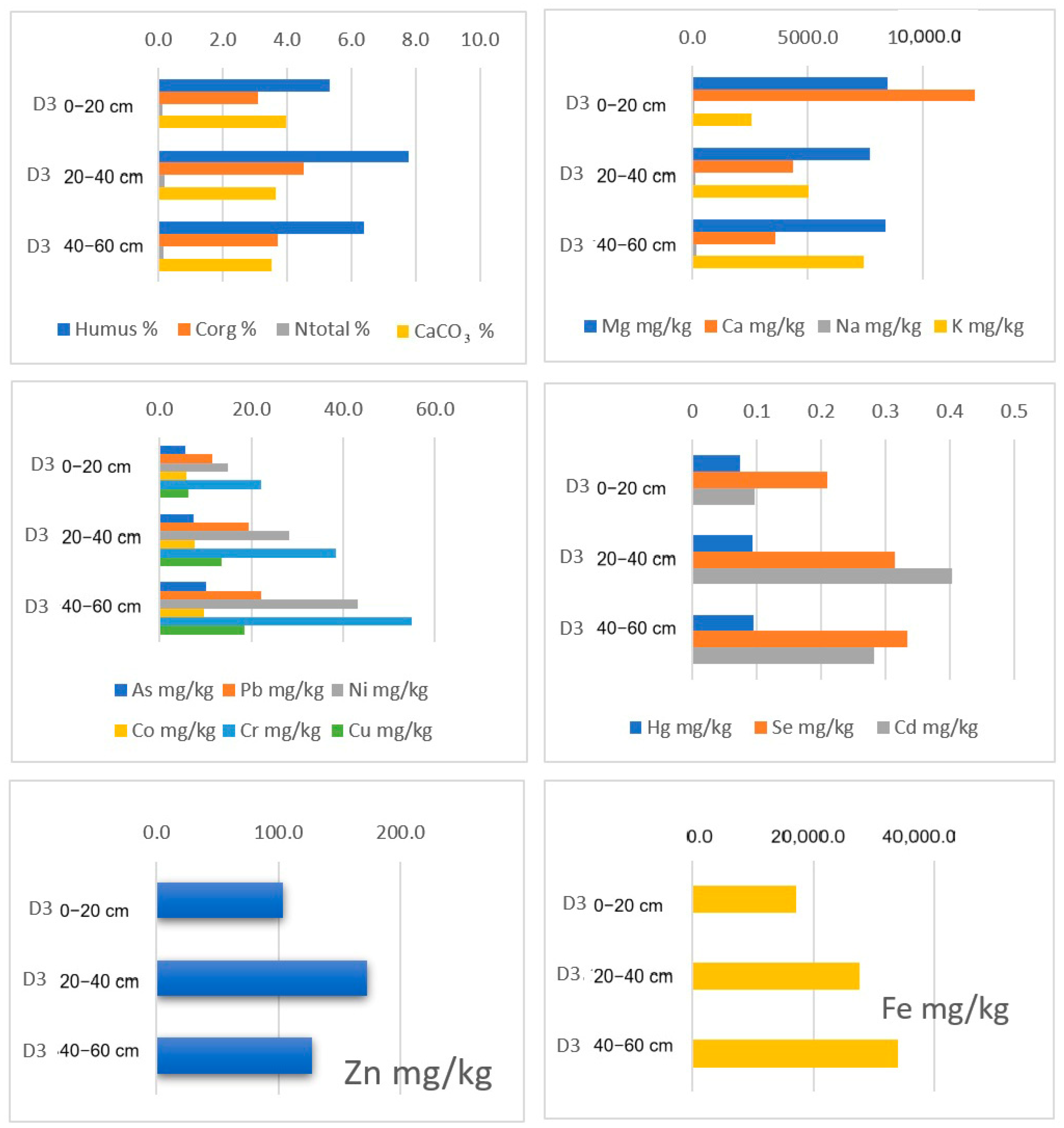

| TRAKOŠĆAN LAKE | pH (KCl) | pH (Water) | Humus % | Corg % | Total N % | P2O5 mg/100 g | K2O mg/100g | CaCO3 % |
|---|---|---|---|---|---|---|---|---|
| T1 26.04.24. surface | 5.10 | 5.35 | 13.67 | 7.93 | 0.23 | 3.05 | 8.36 | 0.34 |
| T2 26.04.24. bottom on 4 m | 4.29 | 5.07 | 2.85 | 1.65 | 0.09 | 30.48 | 11.70 | 0.33 |
| D1 0–20 30.03.23. | 6.46 | 6.67 | 8.43 | 4.89 | 0.22 | 45.56 | 12.60 | 3.51 |
| D1 20–40 | 6.41 | 6.58 | 9.87 | 5.72 | 0.24 | 16.96 | 23.02 | 3.75 |
| D1 40–60 | 6.38 | 6.56 | 7.63 | 4.43 | 0.15 | 22.30 | 13.46 | 4.08 |
| D2 0–20 | 6.62 | 7.00 | 10.44 | 6.06 | 0.25 | 11.83 | 20.61 | 2.72 |
| D2 20–40 | 6.13 | 6.50 | 7.06 | 4.09 | 0.17 | 11.73 | 23.88 | 1.47 |
| D2 40–60 | 6.14 | 6.40 | 4.80 | 2.78 | 0.13 | 15.97 | 26.34 | 1.14 |
| D3 0–20 | 6.99 | 7.17 | 5.33 | 3.09 | 0.12 | 13.16 | 10.84 | 3.97 |
| D3 20–40 | 6.71 | 7.00 | 7.78 | 4.51 | 0.17 | 13.40 | 15.08 | 3.64 |
| D3 40–60 | 6.57 | 6.71 | 6.39 | 3.71 | 0.14 | 16.49 | 20.06 | 3.50 |
| D4 0–20 (near forest) | 4.88 | 5.62 | 4.82 | 2.80 | 0.14 | 2.69 | 27.76 | 0.38 |
| Mean value without D4 | 6.16 | 6.46 | 7.66 | 4.44 | 0.17 | 18.27 | 19.43 | 2.59 |
| Median without D4 | 6.41 | 6.58 | 7.63 | 4.43 | 0.17 | 15.97 | 15.08 | 3.50 |
| min. value | 4.29 | 5.07 | 2.85 | 1.65 | 0.09 | 3.05 | 8.36 | 0.33 |
| max. value | 6.99 | 7.17 | 13.67 | 7.93 | 0.25 | 45.56 | 26.34 | 4.08 |
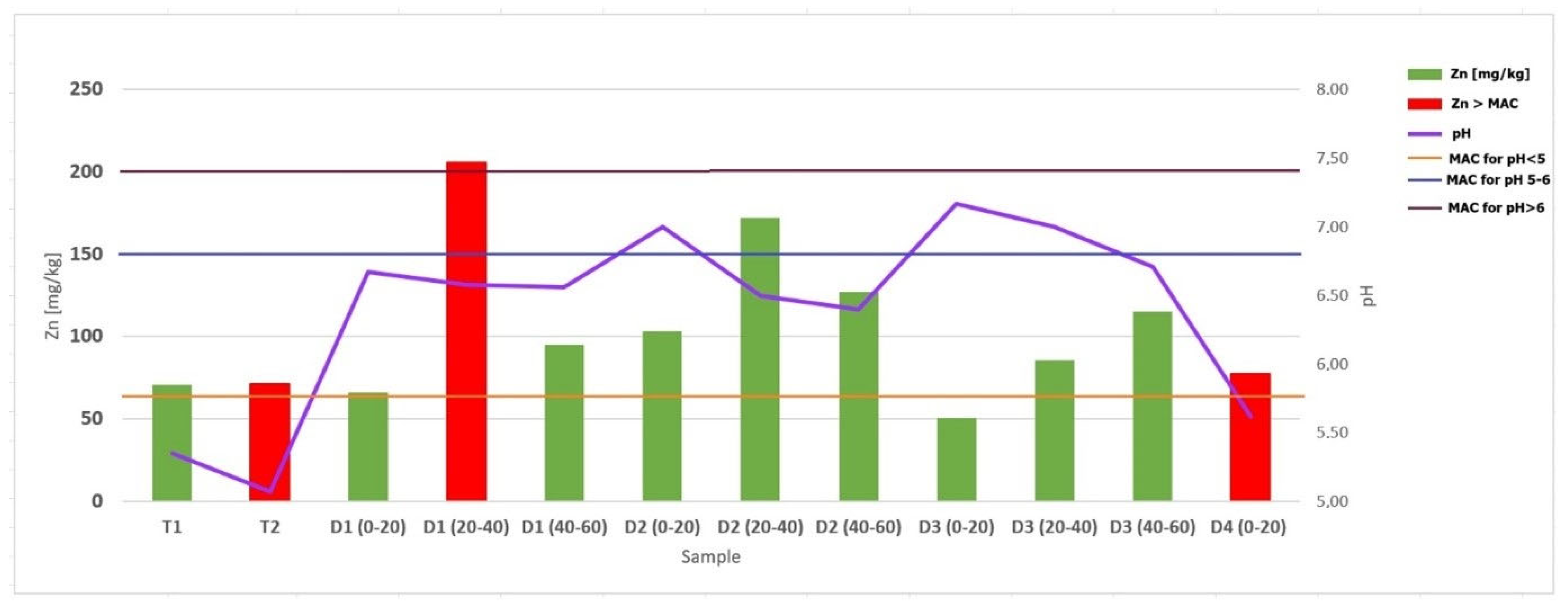
| TRAKOŠĆAN LAKE | Cu [mg/kg] | Cr [mg/kg] | Zn [mg/kg] | Mn [mg/kg] | Fe [mg/kg] | Mg [mg/kg] | Ca [mg/kg] | Na [mg/kg] | K [mg/kg] | Ag [mg/kg] | In [mg/kg] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 26.04.24. surface | 15.023 | 31.174 | 70.892 | 236.620 | 21,910.80 | 3708.92 | 846.948 | 110.61 | 3056.338 | <DL | <DL | |
| T2 26.04.24. bottom on 4 m | 10.728 | 28.738 | 71.408 | 194.175 | 21,286.41 | 3466.02 | 238.35 | 62.913 | 1980.583 | <DL | <DL | |
| D1 0–20 30.03.23. | 12.500 | 17.963 | 66.389 | 322.685 | 13,898.15 | 6157.41 | 7768.519 | 86.065 | 2069.444 | <DL | <DL | |
| D1 20–40 | 23.171 | 49.073 | 205.756 | 411.220 | 30,575.61 | 9780.49 | 2809.756 | 118.537 | 5853.659 | <DL | <DL | |
| D1 40–60 | 14.597 | 33.934 | 94.834 | 309.479 | 20,412.32 | 10,758.29 | 7137.441 | 89.1 | 4436.019 | <DL | <DL | |
| D2 0–20 | 18.986 | 44.378 | 103.134 | 417.512 | 30,967.74 | 5000.00 | 3321.659 | 112.903 | 5852.535 | <DL | <DL | |
| D2 20–40 | 27.959 | 67.959 | 172.194 | 477.551 | 41,377.55 | 7448.98 | 1484.694 | 218.367 | 9852.041 | <DL | <DL | |
| D2 40–60 | 23.000 | 65.286 | 127.143 | 488.095 | 36,752.38 | 6976.19 | 858.095 | 158.571 | 8714.286 | <DL | <DL | |
| D3 0–20 | 6.308 | 22.205 | 50.872 | 293.333 | 17,061.54 | 8435.90 | 12,225.641 | 93.59 | 2564.103 | <DL | <DL | |
| D3 20–40 | 13.493 | 38.373 | 85.598 | 376.077 | 27,645.93 | 7703.35 | 4344.498 | 100.957 | 5019.139 | <DL | <DL | |
| D3 40–60 | 18.593 | 55.025 | 115.075 | 411.558 | 33,984.92 | 8366.83 | 3582.915 | 153.769 | 7432.161 | <DL | <DL | |
| D4 0–20 (near forest) | 10.567 | 36.598 | 77.474 | 446.907 | 21,546.39 | 5051.55 | 476.289 | 121.031 | 3726.804 | <DL | <DL | |
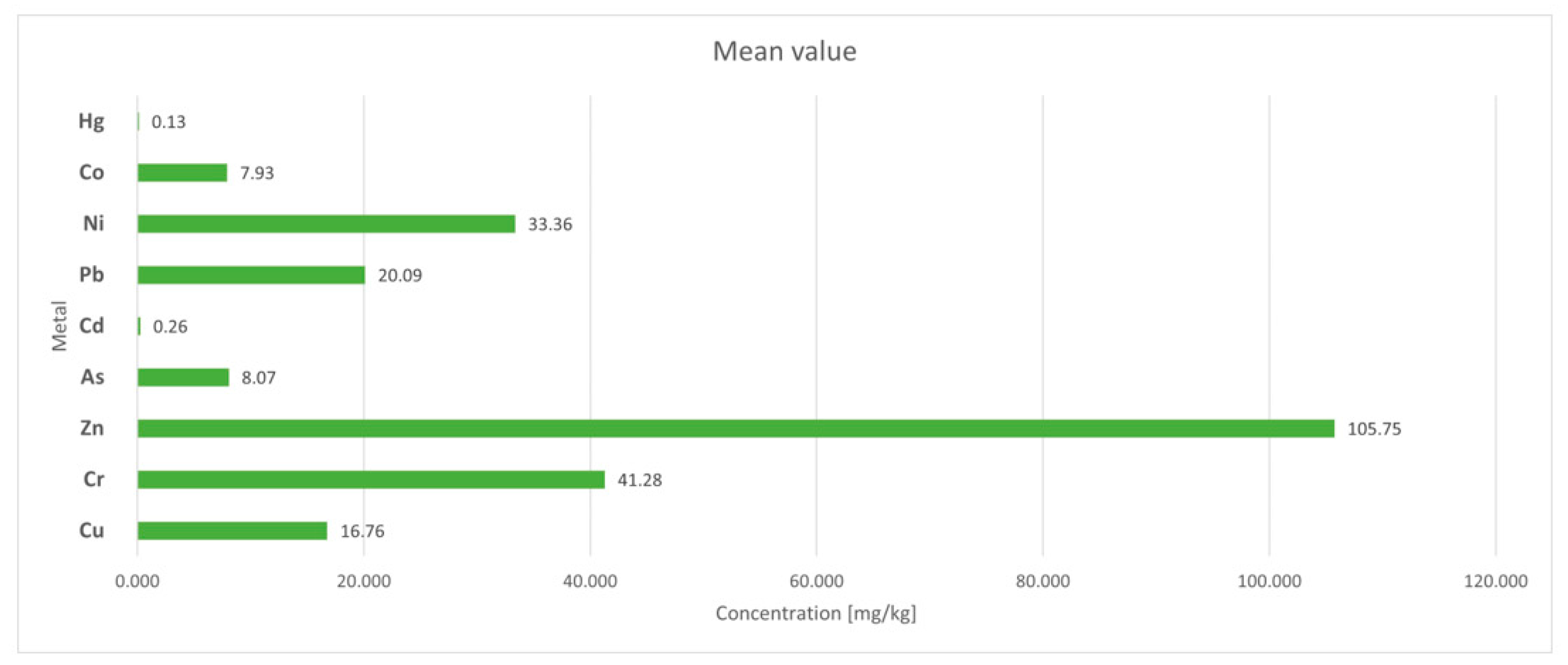
| Mean value without D4 | 16.760 | 41.283 | 105.754 | 358.028 | 26,897.58 | 7072.94 | 4056.229 | 118.671 | 5166.392 | <DL | <DL | |
| Median without D4 | 15.02 | 38.37 | 94.83 | 376.08 | 27,645.93 | 7448.98 | 3321.66 | 110.61 | 5019.14 | <DL | <DL | |
| min. value | 6.31 | 17.96 | 50.87 | 194.18 | 13,898.15 | 3466.02 | 238.35 | 62.91 | 1980.58 | <DL | <DL | |
| max. value | 27.96 | 67.96 | 205.76 | 488.10 | 41,377.55 | 10,758.29 | 12,225.64 | 218.37 | 9852.04 | <DL | <DL | |
| TRAKOŠĆAN LAKE | Au [mg/kg] | Pd [mg/kg] | Pt [mg/kg] | As [mg/kg] | Se [mg/kg] | Cd [mg/kg] | Pb [mg/kg] | Ni [mg/kg] | Co [mg/kg] | Hg [mg/kg] | ||
| T1 26.04.24. surface | 8.122 | 4.601 | <DL | 8.12 | 0.361 | 0.206 | 18.015 | 25.070 | 6.551 | 0.078 | ||
| T2 26.04.24. bottom on 4 m | 7.184 | 5.485 | <DL | 3.548 | 0.345 | 0.137 | 10.428 | 25.801 | 7.385 | 0.017 | ||
| D1 0–20 30.03.23. | 5.093 | 4.444 | <DL | 5.082 | 0.301 | 0.115 | 18.765 | 12.532 | 4.519 | 0.305 | ||
| D1 20–40 | 11.951 | 6.098 | <DL | 8.379 | 0.369 | 0.334 | 26.679 | 36.498 | 8.529 | 0.179 | ||
| D1 40–60 | 6.588 | 4.976 | <DL | 8.103 | 0.306 | 0.285 | 17.333 | 30.763 | 7.831 | 0.169 | ||
| D2 0–20 | 7.742 | 4.654 | <DL | 8.588 | 0.354 | 0.256 | 22.241 | 36.696 | 7.601 | 0.104 | ||
| D2 20–40 | 10.561 | 6.531 | <DL | 11.815 | 0.425 | 0.369 | 33.328 | 55.204 | 10.997 | 0.198 | ||
| D2 40–60 | 9.524 | 5.714 | <DL | 12.132 | 0.451 | 0.371 | 21.216 | 58.333 | 10.607 | 0.068 | ||
| D3 0–20 | 1.846 | 2.923 | <DL | 5.532 | 0.210 | 0.097 | 11.473 | 14.790 | 5.91 | 0.074 | ||
| D3 20–40 | 5.407 | 3.684 | <DL | 7.367 | 0.314 | 0.403 | 19.374 | 28.182 | 7.638 | 0.093 | ||
| D3 40–60 | 7.839 | 2.864 | <DL | 10.134 | 0.334 | 0.282 | 22.127 | 43.075 | 9.63 | 0.095 | ||
| D4 0–20 (near forest) | 0.825 | 1.340 | <DL | 7.637 | 0.245 | 0.144 | 18.604 | 29.881 | 8.286 | 0.006 | ||
| Mean value without D4 | 7.442 | 4.725 | <DL | 8.073 | 0.343 | 0.260 | 20.089 | 33.359 | 7.927 | 0.125 | ||
| Median without D4 | 7.74 | 4.65 | - | 8.12 | 0.35 | 0.28 | 19.37 | 30.76 | 7.64 | 0.10 | ||
| min. value | 1.85 | 2.86 | 0.00 | 3.55 | 0.21 | 0.10 | 10.43 | 12.53 | 4.52 | 0.02 | ||
| max. value | 11.95 | 6.53 | 0.00 | 12.13 | 0.45 | 0.40 | 33.33 | 58.33 | 11.00 | 0.31 | ||
| Element | Sediment Concentration [mg/kg] d.w. | Geochem. Atlas Range [mg/kg] [%] | Median [mg/kg] [%] |
|---|---|---|---|
| As | 8.073 | 1.8–59 mg/kg | 8.4 mg/kg |
| Ca | 4056.229 | 0.07–26.79% | 0.52% |
| Cd | 0.260 | 0.2–9.4 mg/kg | 0.2 mg/kg |
| Co | 7.927 | 3–36 mg/kg | 11 mg/kg |
| Cr | 41.283 | 28–524 mg/kg | 74 mg/kg |
| Cu | 16.760 | 3–248 mg/kg | 19 mg/kg |
| Fe | 26,897.58 | 0.6–6.94% | 2.96% |
| Hg | 0.125 | 0.005–4.535 mg/kg | 0.05 mg/kg |
| K | 5166.392 | 0.33–3.28% | 1.6% |
| Mg | 7072.94 | 0.23–7.52% | 0.67% |
| Mn | 358.028 | 131–5619 mg/kg | 551 mg/kg |
| Na | 118.671 | 0.11–3.21% | 0.79% |
| Ni | 33.359 | 12–427 mg/kg | 33 mg/kg |
| Pb | 20.089 | 14–217 mg/kg | 27 mg/kg |
| Zn | 105.754 | 28–477 mg/kg | 73 mg/kg |
| Element mg/kg | pH Value | ||
|---|---|---|---|
| <5 | 5–6 | >6 | |
| Cd | 1 | 1.5 | 2 |
| Cr | 40 | 80 | 120 |
| Cu | 60 | 90 | 120 |
| Hg | 0.5 | 1 | 1.5 |
| Ni | 30 | 50 | 75 |
| Pb | 50 | 100 | 150 |
| Zn | 60 | 150 | 200 |
| As | 15 | 25 | 30 |
| Co | 30 | 50 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavrtnik, S.; Oskoruš, D.; Kapelj, S.; Loborec, J. Revitalization of Trakošćan Lake—Preliminary Analyses of the Sediment with the Possibility of Its Reuse in the Environment. Water 2025, 17, 3055. https://doi.org/10.3390/w17213055
Zavrtnik S, Oskoruš D, Kapelj S, Loborec J. Revitalization of Trakošćan Lake—Preliminary Analyses of the Sediment with the Possibility of Its Reuse in the Environment. Water. 2025; 17(21):3055. https://doi.org/10.3390/w17213055
Chicago/Turabian StyleZavrtnik, Saša, Dijana Oskoruš, Sanja Kapelj, and Jelena Loborec. 2025. "Revitalization of Trakošćan Lake—Preliminary Analyses of the Sediment with the Possibility of Its Reuse in the Environment" Water 17, no. 21: 3055. https://doi.org/10.3390/w17213055
APA StyleZavrtnik, S., Oskoruš, D., Kapelj, S., & Loborec, J. (2025). Revitalization of Trakošćan Lake—Preliminary Analyses of the Sediment with the Possibility of Its Reuse in the Environment. Water, 17(21), 3055. https://doi.org/10.3390/w17213055






