Heavy Metal Contamination and Bioaccumulation Patterns from a Ramsar Wetland Tributary, Northern Algeria: A Baseline Assessment
Abstract
1. Introduction
2. Materials and Methods
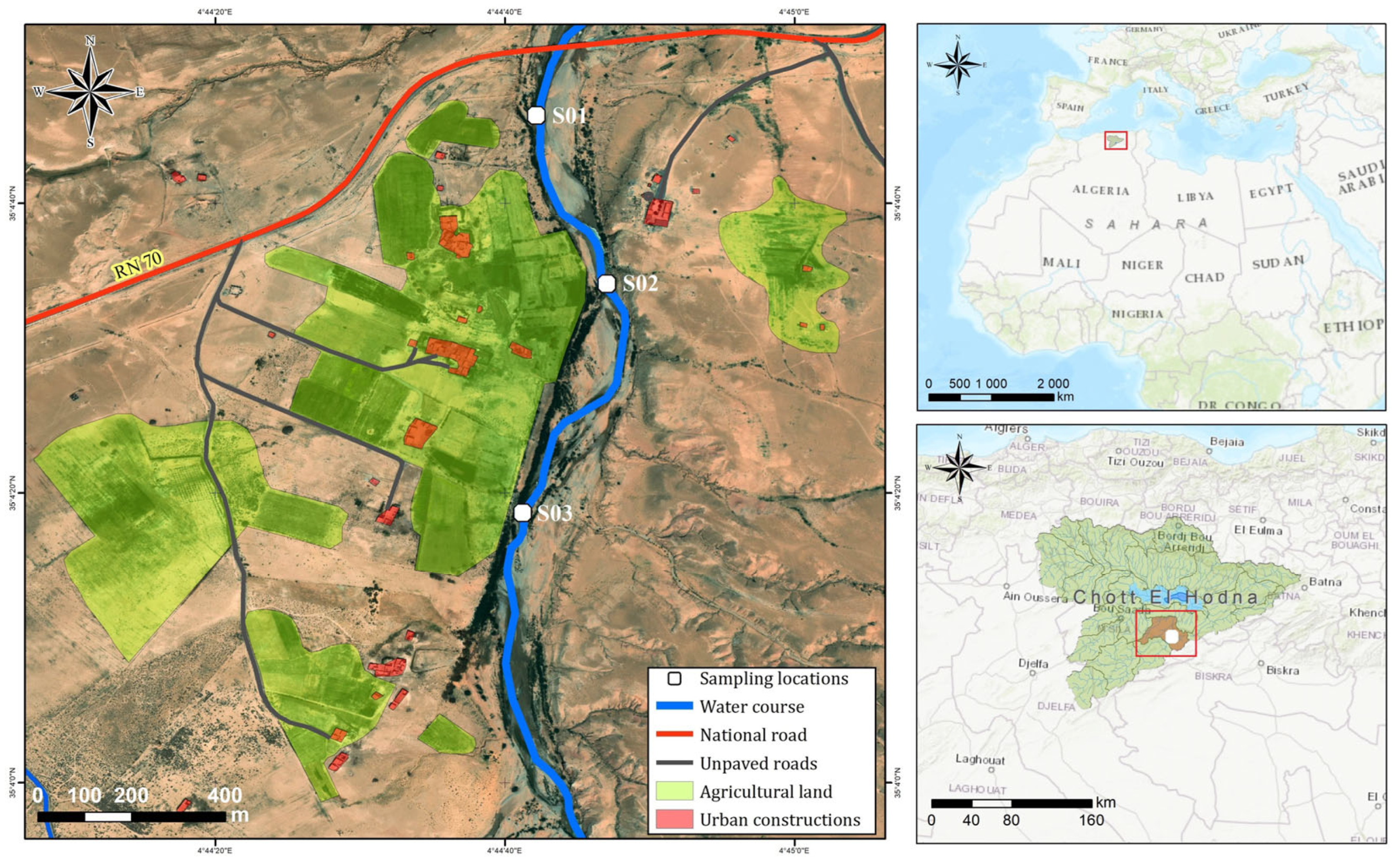
2.1. Study Area
2.2. Collection of Water, Fish, Algae, and Sediment Samples
2.3. Physical and Chemical Parameter Measurement
2.4. Digestion Methods for PTEs Concentration in Different Strata
2.5. Elemental Analysis, Quality Assurance and Quality Control
2.6. Bioaccumulation and Trophic Transfer Factors Measurement
2.7. Contamination and Ecological Risk Measurement
2.7.1. Geoaccumulation Index
2.7.2. Pollution Load Index and Contamination Factor
2.7.3. Ecological Risk Index
2.8. Health Risk Assessment
2.8.1. Estimated Daily Intake (EDI)
2.8.2. The Carcinogenic Risk (TR)
2.8.3. Non-Carcinogenic Health Risk
2.9. Statistical Analysis
3. Results and Discussion
3.1. River Water Physical and Chemical Parameters
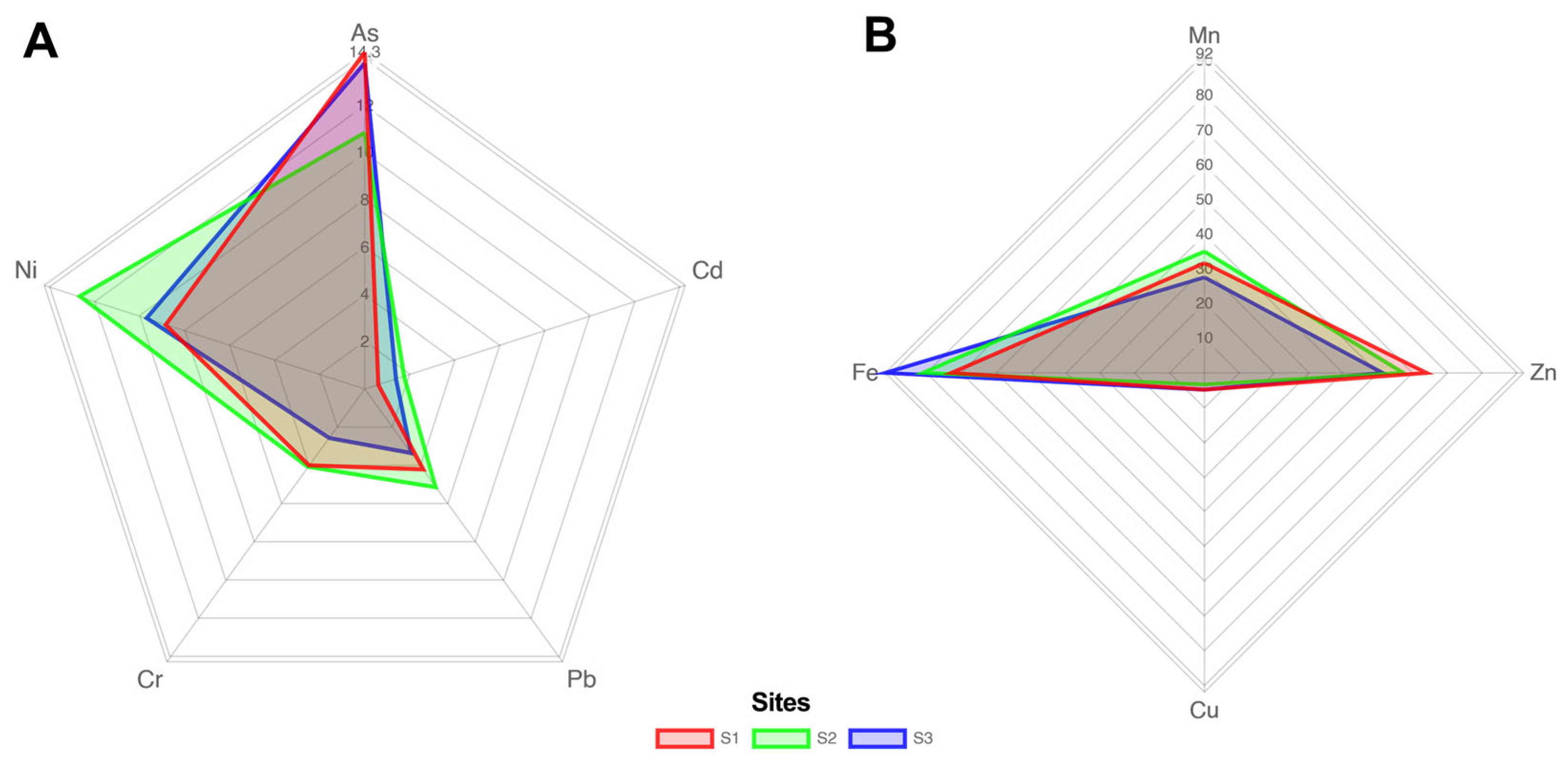
3.2. Element Concentration Variation and Distribution Patterns
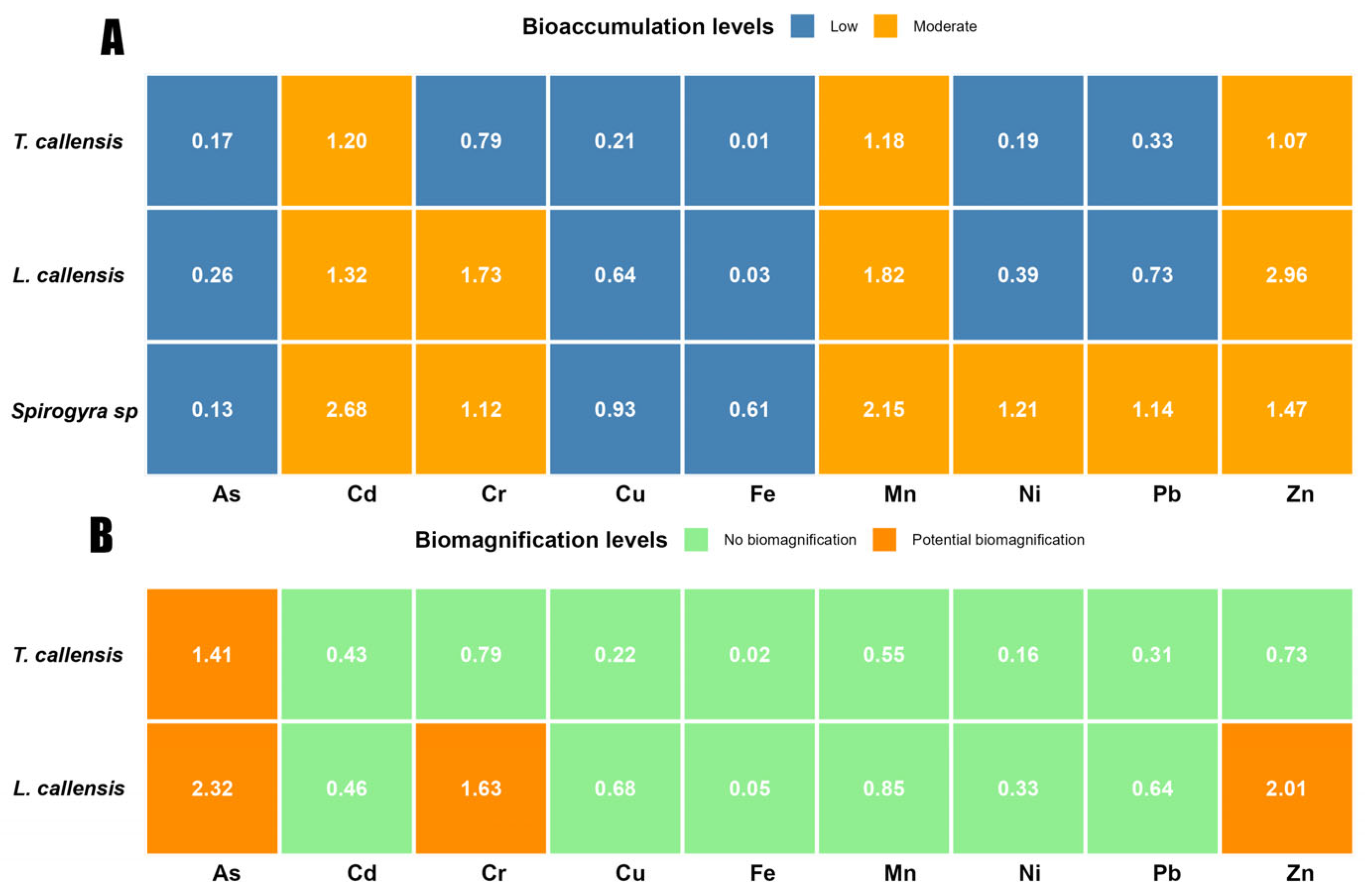
3.3. Bioaccumulation and Biomagnification of Elements
3.4. Ecological Impact and Health Concerns
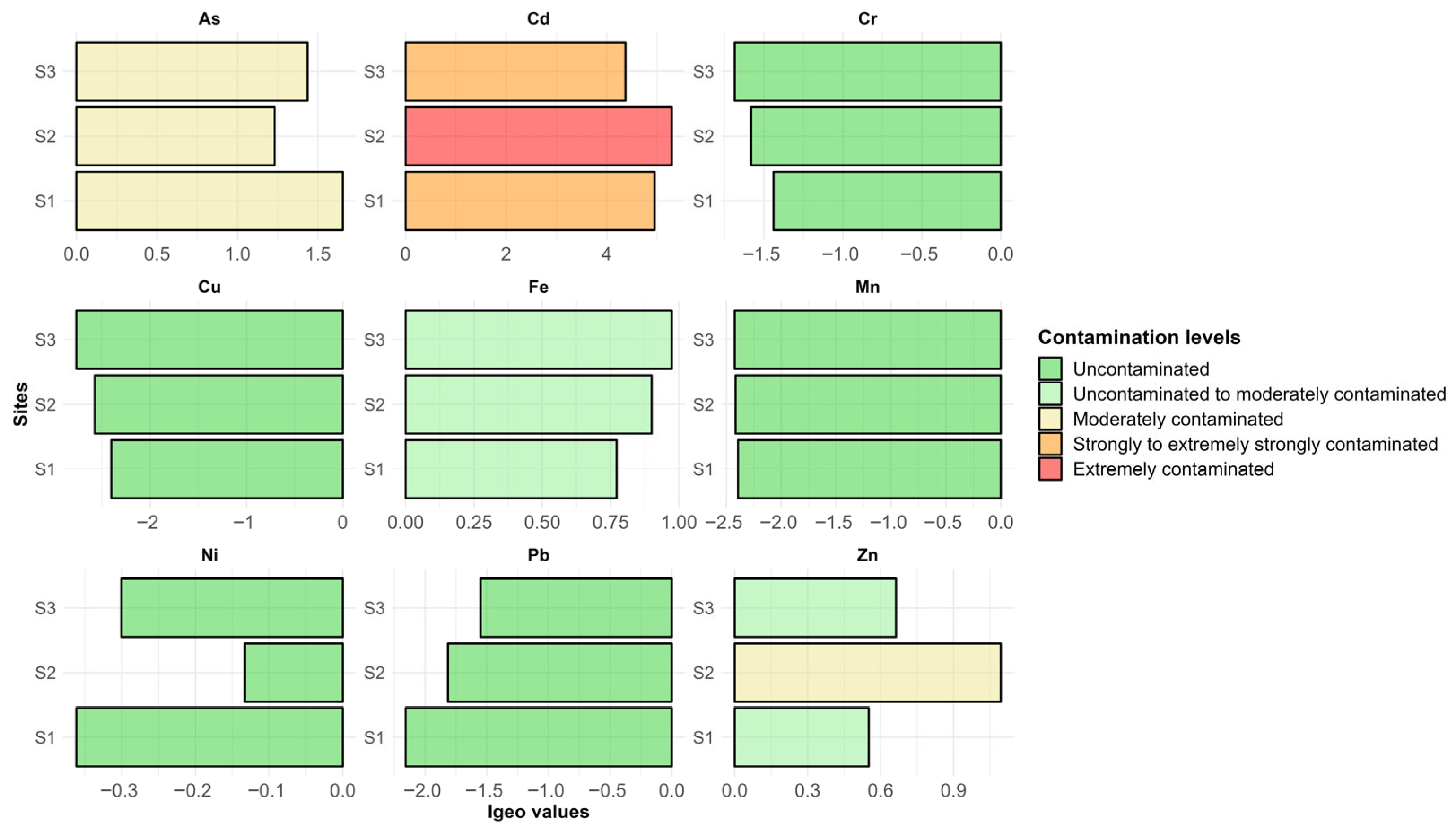
3.5. Sediment Contamination Level and Potential Environmental Risks
3.6. Health Risk Assessment
3.6.1. Dietary Exposure Assessment
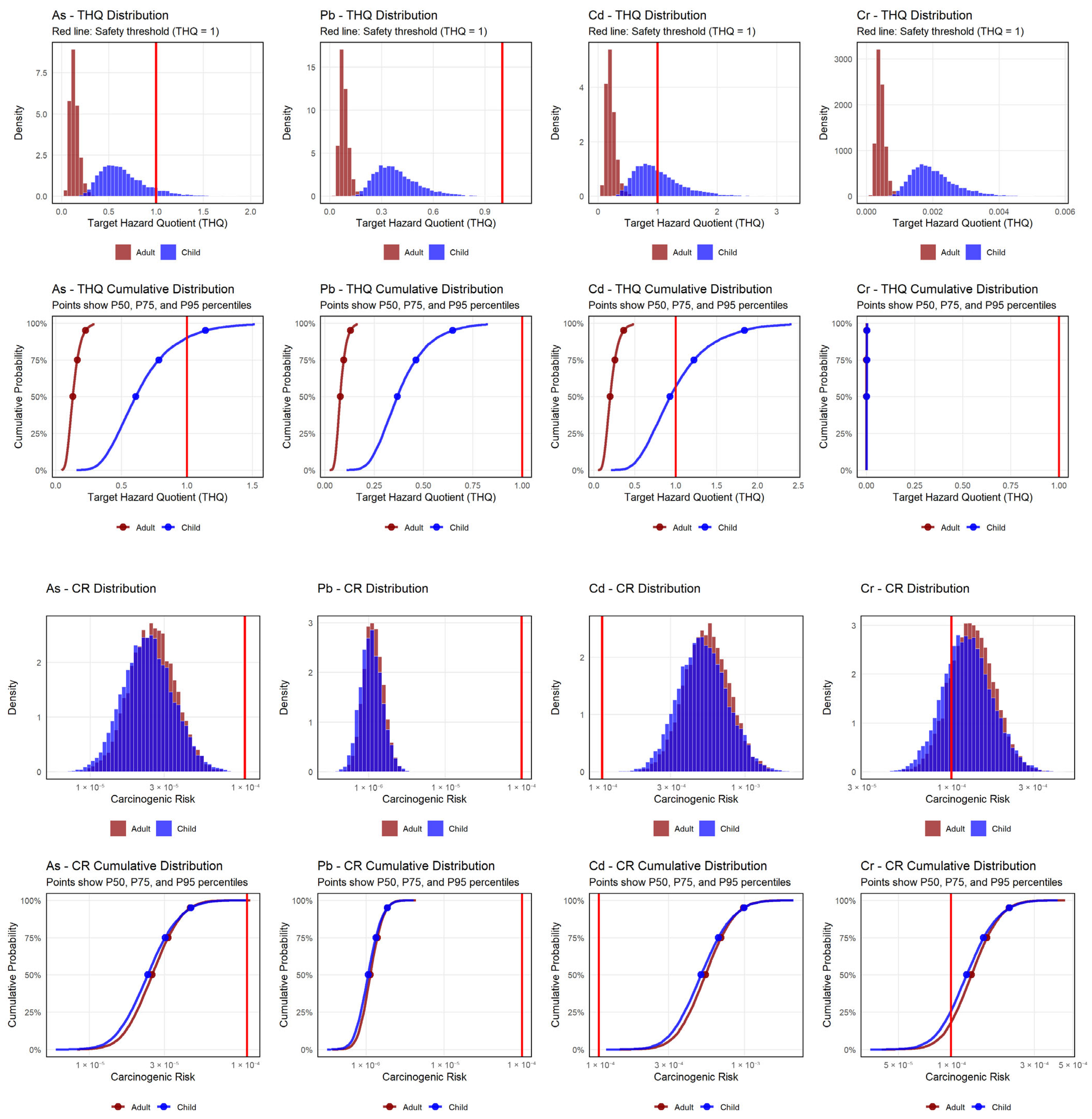
3.6.2. Target Hazard Quotient
3.6.3. Carcinogenic Risk
3.6.4. Monte Carlo Simulation for Health Risk Uncertainty Assessment
3.6.5. Study Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANAHR | Algerian National Agency of Hydraulic Resources |
| APHA | American Public Health Association |
| As | Arsenic |
| AT_car | Averaging Time for carcinogens |
| AT_nc | Averaging Time for non-carcinogens |
| BAF | Bioaccumulation Factor |
| BDL | Below Detection Limit |
| Bn | Background value of element in upper continental crust |
| BW | Body Weight |
| C | Concentration of heavy metals in fish muscle |
| Cd | Cadmium |
| CF | Contamination Factor |
| CFo | Conversion Factor |
| CM | Concentration in Medium |
| Cn | Concentration of element in sediment sample |
| Cr | Chromium |
| CSFo | Oral Carcinogenic Slope Factor |
| Cu | Copper |
| DO | Dissolved Oxygen |
| DV | Dual View |
| EC | Electrical Conductivity |
| ED | Exposure Duration |
| EDI EWI | Estimated Daily Intake Estimated Weekly Intake |
| EF | Exposure Frequency |
| Er | Potential Ecological Risk Factor |
| FAO | Food and Agriculture Organization |
| Fe | Iron |
| FIR | Fish Ingestion Rate |
| HI | Hazard Index |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectrometry |
| Igeo | The geoaccumulation Index |
| LoD | Limits of Detection |
| LoQ | Limits of Quantification |
| mCd | Modified Degree of Contamination |
| Mn | Manganese |
| Ni | Nickel |
| Pb | Lead |
| PEL | Probable effect level |
| PLI | Pollution Load Index |
| PTEs | Potentially toxic elements |
| RFD | Reference Dose |
| RI | Risk Index |
| Se | Selenium |
| PTWI | Provisional Tolerable Weekly Intake |
| TDS | Total Dissolved Solids |
| TEL | Threshold effect level |
| TH | Total Hardness |
| THQ | Target Hazard Quotient |
| TR | Carcinogenic Risk |
| Tr | Toxic Response Factor |
| TTF | Trophic Transfer Factor |
| USA | United States of America |
| USEPA | United States Environmental Protection Agency |
| WHO | World Health Organization |
| Zn | Zinc |
Appendix A
| Elements | Strata | Mean ± SD | Range | Local and International Guidelines | p Value |
|---|---|---|---|---|---|
| As | Spirogyra sp. (mg/kg) | 1.59 ± 0.63 | 0.86–2.00 | – | 0.01 * |
| L. callensis (mg/kg) | 3.28 ± 0.35 | 2.95–3.66 | 1.4 a | ||
| T. callensis (mg/kg) | 2.10 ± 0.69 | 1.51–2.86 | |||
| Sediment (mg/kg) | 8.19 ± 1.20 | 7.04–9.44 | 5.9 b, 17c | ||
| Water (µg/L) | 12.97 ± 1.85 | 10.85–14.26 | 10 d, 10 e | ||
| Cd | Spirogyra sp. (mg/kg) | 3.12 ± 0.85 | 2.14–3.67 | – | 0.03 * |
| L. callensis (mg/kg) | 1.36 ± 0.26 | 1.10–1.63 | 0.2 a | ||
| T. callensis (mg/kg) | 1.29 ± 0.33 | 1.06–1.67 | |||
| Sediment (mg/kg) | 4.54 ± 1.38 | 3.11–5.88 | 0.596 b, 3.53 c | ||
| Water (µg/L) | 1.26 ± 0.58 | 0.63–1.77 | 10 d, 5 e | ||
| Cr | Spirogyra sp. (mg/kg) | 4.02 ± 2.04 | 2.66–6.38 | – | 0.03 * |
| L. callensis (mg/kg) | 5.98 ± 1.21 | 5.24–7.38 | 2.3 a | ||
| T. callensis (mg/kg) | 2.69 ± 0.32 | 2.38–3.03 | |||
| Sediment (mg/kg) | 17.74 ± 1.52 | 16.32–19.36 | 37.3 b, 90 c | ||
| Water (µg/L) | 3.55 ± 0.87 | 2.55–4.09 | 50 d, 100 e | ||
| Cu | Spirogyra sp. (mg/kg) | 3.85 ± 0.17 | 3.67–4.02 | – | 0.01 * |
| L. callensis (mg/kg) | 2.61 ± 0.38 | 2.24–3.01 | 73.3 a | ||
| T. callensis (mg/kg) | 0.85 ± 0.17 | 0.67–1.02 | |||
| Sediment (mg/kg) | 6.30 ± 0.79 | 5.52–7.10 | 35.7 b, 197 c | ||
| Water (µg/L) | 4.31 ± 0.88 | 3.29–4.90 | 50 d, 9 e | ||
| Fe | Spirogyra sp. (mg/kg) | 49.41 ± 2.43 | 47.22–52.03 | – | 0.01 * |
| L. callensis (mg/kg) | 2.47 ± 0.67 | 1.88–3.20 | 425.5 a | ||
| T. callensis (mg/kg) | 1.02 ± 0.17 | 0.86–1.20 | |||
| Sediment (mg/kg) | 96.92 ± 6.78 | 89.67–103.11 | NS b, NS c | ||
| Water (µg/L) | 82.04 ± 9.53 | 73.00–92.00 | 1000 d, NS e | ||
| Mn | Spirogyra sp. (mg/kg) | 67.40 ± 9.06 | 57.72–75.70 | – | 0.01 * |
| L. callensis (mg/kg) | 55.88 ± 8.37 | 49.62–65.39 | 500 a | ||
| T. callensis (mg/kg) | 36.73 ± 2.68 | 33.70–38.79 | |||
| Sediment (mg/kg) | 169.20 ± 1.87 | 167.70–171.30 | NS b, NS c | ||
| Water (µg/L) | 31.38 ± 3.70 | 27.59–35.00 | 300 d, NS e | ||
| Ni | Spirogyra sp. (mg/kg) | 12.33 ± 2.34 | 10.33–14.91 | – | 0.01 * |
| L. callensis (mg/kg) | 3.91 ± 0.92 | 3.02–4.87 | 67 a | ||
| T. callensis (mg/kg) | 1.89 ± 0.28 | 1.57–2.11 | |||
| Sediment (mg/kg) | 25.02 ± 2.08 | 23.35–27.36 | 18 b, 36 c | ||
| Water (µg/L) | 10.42 ± 2.01 | 8.87–12.70 | NS d, 52 e | ||
| Pb | Spirogyra sp. (mg/kg) | 4.63 ± 0.53 | 4.06–5.11 | – | 0.01 * |
| L. callensis (mg/kg) | 2.94 ± 0.55 | 2.40–3.50 | 0.3 a | ||
| T. callensis (mg/kg) | 1.42 ± 0.60 | 0.74–1.86 | |||
| Sediment (mg/kg) | 8.49 ± 1.76 | 6.72–10.24 | 35 b, 91.3 c | ||
| Water (µg/L) | 4.21 ± 0.89 | 3.32–5.11 | 50 d, 10 e | ||
| Se | Spirogyra sp. (mg/kg) | BDL | BDL | – | – |
| L. callensis (mg/kg) | BDL | BDL | NS | ||
| T. callensis (mg/kg) | BDL | BDL | NS | ||
| Sediment (mg/kg) | BDL | BDL | NS | ||
| Water (µg/L) | BDL | BDL | NS d, 50 e | ||
| Zn | Spirogyra sp. (mg/kg) | 84.40 ± 5.80 | 78.50–90.10 | – | 0.01 * |
| L. callensis (mg/kg) | 168.83 ± 15.40 | 152.20–182.60 | 99.4 a | ||
| T. callensis (mg/kg) | 61.40 ± 7.29 | 54.60–69.10 | |||
| Sediment (mg/kg) | 184.07 ± 38.05 | 156.10–227.40 | 123 b, 315 c | ||
| Water (µg/L) | 57.66 ± 6.38 | 51.41–64.18 | 500 d, 118 e |
References
- Yadav, D.; Rangabhashiyam, S.; Verma, P.; Singh, P.; Devi, P.; Kumar, P.; Hussain, C.M.; Gaurav, G.K.; Kumar, K.S. Environmental and Health Impacts of Contaminants of Emerging Concerns: Recent Treatment Challenges and Approaches. Chemosphere 2021, 272, 129492. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.K. River Pollution and Perturbation: Perspectives and Processes. In Riverine Ecology, 1st ed.; Springer: Cham, Switzerland, 2021; Volume 2, pp. 443–530. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Ren, B.; Luo, J.; Yuan, J.; Ding, X.; Bian, H.; Yao, X. Trends and Health Risks of Dissolved Heavy Metal Pollution in Global River and Lake Water from 1970 to 2017. Rev. Environ. Contam. Toxicol. 2020, 251, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Yadav, P.; Pal, A.K.; Mishra, V. Water Pollutants: Origin and Status. In Sensors in Water Pollutants Monitoring: Role of Material; Pooja, D., Kumar, P., Singh, P., Patil, S., Eds.; Springer: Singapore, 2020; pp. 5–20. [Google Scholar] [CrossRef]
- Williams-Subiza, E.A.; Epele, L.B. Drivers of Biodiversity Loss in Freshwater Environments: A Bibliometric Analysis of the Recent Literature. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 2469–2480. [Google Scholar] [CrossRef]
- Palmer, M.; Ruhi, A. Linkages between Flow Regime, Biota, and Ecosystem Processes: Implications for River Restoration. Science 2019, 365, eaaw2087. [Google Scholar] [CrossRef]
- Bagheri, S.; Gholamhosseini, A.; Banaee, M. Investigation of Different Nutritional Effects of Dietary Chromium in Fish: A Literature Review. Biol. Trace Elem. Res. 2023, 201, 2546–2554. [Google Scholar] [CrossRef]
- Arab, L.H.; Boutaleb, A.; Berdous, D. Environmental assessment of heavy metal pollution in the polymetallic district of Kef Oum Teboul (El Kala, Northeast Algeria). Environ. Earth Sci. 2021, 80, 277. [Google Scholar] [CrossRef]
- Vinci, G.; Maddaloni, L.; Mancini, L.; Prencipe, S.A.; Ruggeri, M.; Tiradritti, M. The Health of the Water Planet: Challenges and Opportunities in the Mediterranean Area. An Overview. Earth 2021, 2, 894–919. [Google Scholar] [CrossRef]
- MedWet Secretariat. Stratégie Nationale de Gestion Écosystémique des Zones Humides d’Algérie. République Algérienne Démocratique et Populaire, Ministère de l’Agriculture, du Développement Rural et de la Pêche, Direction Générale des Forêts; 2017. Available online: https://medwet.org/wp-content/uploads/2017/04/Strategie-Nationale-des-Zones-Humides_Algerie.pdf (accessed on 25 June 2025).
- Talbi, H.; Kachi, S. Evaluation of Heavy Metal Contamination in Sediments of the Seybouse River, Guelma–Annaba, Algeria. J. Water Land Dev. 2019, 40, 81–86. [Google Scholar] [CrossRef]
- Napoletano, P.; Guezgouz, N.; Di Iorio, E.; Colombo, C.; Guerriero, G.; De Marco, A. Anthropic Impact on Soil Heavy Metal Contamination in Riparian Ecosystems of Northern Algeria. Chemosphere 2022, 313, 137522. [Google Scholar] [CrossRef]
- Adesiyan, I.M.; Bisi-Johnson, M.; Aladesanmi, O.T.; Okoh, A.I.; Ogunfowokan, A.O. Concentrations and human health risk of heavy metals in rivers in Southwest Nigeria. J. Health Pollut. 2018, 8, 180907. [Google Scholar] [CrossRef]
- Dehbi, M.; Kanjal, M.I.; Tahraoui, H.; Zamouche, M.; Amrane, A.; Assadi, A.A.; Hadadi, A.; Mouni, L. Analysis of Heavy Metal Contamination in Macroalgae from Surface Waters in Djelfa, Algeria. Water 2023, 15, 974. [Google Scholar] [CrossRef]
- Lohmann, U.; Sausen, R.; Bengtsson, L.; Cubasch, U.; Perlwitz, J.; Roeckner, E. The Köppen Climate Classification as a Diagnostic Tool for General Circulation Models. Clim. Res. 1993, 3, 177–193. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 6th ed.; American Public Health Association: Washington, DC, USA, 1926. [Google Scholar]
- Arnot, J.A.; Gobas, F.A. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Kim, S.-K. Trophic Transfer of Organochlorine Pesticides through Food-Chain in Coastal Marine Ecosystem. Environ. Eng. Res. 2020, 25, 43–51. [Google Scholar] [CrossRef]
- Salhi, S.; Chaibi, R.; Badache, H.; Hamidouche, M.; Laouar, R. Seasonal Variation in the Diet and the Morphometric Parameters of the Genus Pseudophoxinus sp. (Cyprinidae) in Eastern Algeria. Biosyst. Divers. 2021, 29, 326–333. [Google Scholar] [CrossRef]
- Müller, G. Index of Geoaccumulation in Sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Geochemical Evolution of the Continental Crust. Rev. Geophys. 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Reimann, C.; De Caritat, P. Chemical Elements in the Environment: Factsheets for the Geochemist and Environmental Scientist; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 398. [Google Scholar]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the Assessment of Heavy-Metal Levels in Estuaries and the Formation of a Pollution Index. Helgol. Mar. Res. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Hoang, H.-G.; Lin, C.; Chiang, C.F.; Bui, X.T.; Lukkhasorn, W.; Bui, T.P.T.; Tran, H.T.; Vo, T.-D.H.; Le, V.G.; Nghiem, L.D. The Individual and Synergistic Indexes for Assessments of Heavy Metal Contamination in Global Rivers and Risk: A Review. Curr. Pollut. Rep. 2021, 7, 247–262. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.; Zheng, X.; Wang, Z.; Cheng, X. Nutritional Value and Bioaccumulation of Heavy Metals in Nine Commercial Fish Species from Dachen Fishing Ground, East China Sea. Sci. Rep. 2022, 12, 6927. [Google Scholar] [CrossRef]
- FAO. Algeria–Fishery and Aquaculture Country Profile; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; Available online: https://www.fao.org/fishery/facp/DZA (accessed on 9 July 2025).
- U.S. Environmental Protection Agency (USEPA). Exposure and Human Health Reassessment of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and Related Compounds. Draft Final; Exposure Assessment and Risk Characterization Group; National Center for Environmental Assessment-Washington Office; Office of Research and Development, EPA/600/P-00/00/A-c; U.S. EPA: Washington, DC, USA, 2000.
- World Health Organization. STEPS Survey for Noncommunicable Disease Risk Factors, Algeria 2016–2017; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps (accessed on 9 July 2025).
- UNICEF. Multiple Indicator Cluster Survey (MICS) 2018, Algeria Final Report; United Nations Children’s Fund: New York, NY, USA, 2019; Available online: https://mics.unicef.org/surveys (accessed on 9 July 2025).
- U.S. Environmental Protection Agency. Risk Based Concentration Table; U.S. EPA: Washington, DC, USA, 2009.
- Dinno, A.; Dinno, M.A. Package ‘dunn.test’. CRAN Repos. 2017, 10, 1–7. [Google Scholar]
- Buijs, P.; Toader, C. Proposed System of Surface Water Quality Standards for Moldova; Technical Report; OECD: Paris, France, 2007. [Google Scholar]
- Agence Nationale des Ressources Hydrauliques. Qualité des Eaux Superficielles Dans les Bassins du Kebir-Rhumel, de la Seybouse et de la Medjerda-Mellegue, Algérie (2004–2007); Agence Nationale des Ressources Hydrauliques: El Mouradia, Algérie, 2009. [Google Scholar]
- Ordóñez, J.I.; Cortés, S.; Maluenda, P.; Soto, I. Biosorption of Heavy Metals with Algae: Critical Review of Its Application in Real Effluents. Sustainability 2023, 15, 552. [Google Scholar] [CrossRef]
- Benabdelkader, A.; Taleb, A.; Probst, J.-L.; Belaidi, N.; Probst, A. Anthropogenic contribution and influencing factors on metal features in fluvial sediments from a semi-arid Mediterranean river basin (Tafna River, Algeria): A multi-indices approach. Sci. Total Environ. 2018, 626, 899–914. [Google Scholar] [CrossRef]
- Alloway, B.J. Trace Metals and Metalloids in Soils and Their Bioavailability. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 3–9. [Google Scholar]
- Jeong, H.; Ra, K. Source Apportionment and Health Risk Assessment for Potentially Toxic Elements in Size-Fractionated Road Dust in Busan Metropolitan City, Korea. Environ. Monit. Assess. 2022, 194, 350. [Google Scholar] [CrossRef] [PubMed]
- De Paiva Magalhães, D.; da Costa Marques, M.R.; Baptista, D.F.; Buss, D.F. Metal bioavailability and toxicity in freshwaters. Environ. Chem. Lett. 2015, 13, 69–87. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Huang, X.; Zhang, L.; Yang, C.; Li, E.; Wang, Z. Heavy Metal Distribution and Bioaccumulation Combined with Ecological and Human Health Risk Evaluation in a Typical Urban Plateau Lake, Southwest China. Front. Environ. Sci. 2022, 10, 814678. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy Metal–Induced Stress in Eukaryotic Algae—Mechanisms of Heavy Metal Toxicity and Tolerance with Particular Emphasis on Oxidative Stress in Exposed Cells and the Role of Antioxidant Response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef]
- Cervantes-Ramírez, L.T.; Ramírez-López, M.; Mussali-Galante, P.; Ortiz-Hernández, M.L.; Sánchez-Salinas, E.; Tovar-Sánchez, E. Heavy metal biomagnification and genotoxic damage in two trophic levels exposed to mine tailings: A network theory approach. Rev. Chil. Hist. Nat. 2018, 91, 6. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Kumari, B.; Kumar, V.; Sinha, A.K.; Ahsan, J.; Ghosh, A.K.; Wang, H.; DeBoeck, G. Toxicology of Arsenic in Fish and Aquatic Systems. Environ. Chem. Lett. 2016, 15, 43–64. [Google Scholar] [CrossRef]
- Food Additives and Contaminants. Joint FAO/WHO Food Standards Programme, ALINORM; Food and Agricultural Organization: Rome, Italy, 2001; Volume 1, pp. 1–289. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives; World Health Organization. Safety Evaluation of Certain Food Additives and Contaminants. In Proceedings of the Seventy-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Geneva, Switzerland, 8–17 June 2010; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, H.; Hwang, U.K.; Kang, J.C.; Kang, Y.J.; Kim, K.I.; Kim, J.H. Toxic Effects of Lead Exposure on Bioaccumulation, Oxidative Stress, Neurotoxicity, and Immune Responses in Fish: A Review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef]
- Lee, J.W.; Jo, A.H.; Lee, D.C.; Choi, C.Y.; Kang, J.C.; Kim, J.H. Review of Cadmium Toxicity Effects on Fish: Oxidative Stress and Immune Responses. Environ. Res. 2023, 236, 116600. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kar, I.; Patra, A.K. Cadmium induced bioaccumulation, histopathology, gene regulation in fish and its amelioration–a review. J. Trace Elem. Med. Biol. 2023, 79, 127202. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; et al. Update of the Risk Assessment of Inorganic Arsenic in Food. EFSA J. 2024, 22, e8488. [Google Scholar] [CrossRef]
- Smith, S.L.; MacDonald, D.D.; Keenleyside, K.A.; Ingersoll, C.G.; Field, L.J. A Preliminary Evaluation of Sediment Quality Assessment Values for Freshwater Ecosystems. J. Great Lakes Res. 1996, 22, 624–638. [Google Scholar] [CrossRef]
- Cobbinah, R.T.; Boadi, N.O.; Saah, S.A.; Agorku, E.S.; Badu, M.; Kortei, N.K. Cancer risk from heavy metal contamination in fish and implications for public health. Sci. Rep. 2025, 15, 24162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Noman, A.; Zhang, C.; Al-Bukhaiti, W.Q.; Abed, S.M. Effect of fish-heavy metals contamination on the generation of reactive oxygen species and its implications on human health: A review. Front. Mar. Sci. 2024, 11, 1500870. [Google Scholar] [CrossRef]


| Parameters | Mean Concentration (SD) | Local Guidelines | International Guidelines |
|---|---|---|---|
| pH | 8.15 (0.44) | 6.5–8.5 | 6.5–9 |
| Electrical Conductivity (µs/cm) | 5971 (465.18) | 2800 | 2500 |
| Dissolved Oxygen (mg/L) | 12.73(3.04) | 3–5 | 4–7 |
| Total Hardness (mg/L) | 2149 (501.26) | 500 | – |
| Total Dissolved Solids (mg/L) | 2770.67 (455.13) | 1600 | 1300 |
| Sulfate SO42− (mg/L) | 486.73 (38.67) | 200–300 | 300 |
| Phosphate PO43− (mg /L) | 0.08 (0.05) | 0.01–0.1 | 0.1 |
| Nitrate NO3− (mg/L) | 21.10 (3.47) | 10–20 | 9.1 |
| Nitrite NO2− (mg/L) | 0.31 (0.47) | 0.01–0.1 | 0.02 |
| Ammonium NH4+ (mg/L) | 0.95 (0.96) | 0.01–0.1 | 0.4–0.8 |
| Sodium Na+2 (mg/L) | 24.93 (7.09) | 100–200 | – |
| Iron Fe2+ (mg/L) | 0.06 (0.04) | 0.3 | – |
| Chloride Cl− (mg/L) | 907.28 (25.83) | 150–300 | 300 |
| Rivers | Trophic Levels | Elements | Current Study Concentrations mg/kg | Reference Concentrations mg/kg | References |
|---|---|---|---|---|---|
| El Mellah River | Spirogyra sp. | Zn | 84.40 | 40.83 | [14] |
| Cu | 3.85 | 32.66 | |||
| Fe | 49.41 | 1514.17 | |||
| Pb | 4.63 | 19.98 | |||
| Tafna River | Sediment | Cu | 6.30 | 17.51 | [35] |
| Fe | 96.92 | 17.63 | |||
| As | 8.19 | 5.13 | |||
| Cd | 4.54 | 0.19 | |||
| Pb | 8.49 | 43.37 |
| EWI (mg/kg) | THQ | TTHQ | CR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Trace Elements | PTWI (mg/kg) | Ad | Ch | Ad | Ch | Ad | Ch | Ad | Ch |
| As | 0.015 | 0.0003± 0.0001 | 0.0013 ± 0.0004 | 0.137 ± 0.042 | 0.640 ± 0.194 | 2.65 × 10−5 ± 8.01 × 10−6 | 2.47 × 10−5 ± 7.47 × 10−6 | ||
| Pb | 0.025 | 0.0023 ± 0.0010 | 0.0109 ± 0.0049 | 0.084 ± 0.037 | 0.390 ± 0.175 | 1.22 × 10−6 ± 5.45 × 10−7 | 1.14 × 10−6 ± 5.09 × 10−7 | ||
| Cd | 0.007 | 0.0014 ± 0.0003 | 0.0066 ± 0.0014 | 0.203 ± 0.041 | 0.946 ± 0.194 | 0.555 ± 0.128 | 2.592 ± 0.596 | 5.48 × 10−4 ± 1.12 × 10−4 | 5.11 × 10−4 ± 1.05 × 10−4 |
| Cr | 0.015 | 0.0046 ± 0.0021 | 0.0216 ± 0.0098 | 0.000 ± 0.000 | 0.002 ± 0.001 | 1.42 × 10−4 ± 6.45 × 10−5 | 1.33 × 10−4 ± 6.02 × 10−5 | ||
| Ni | 0.035 | 0.0031 ± 0.0014 | 0.0145 ± 0.0063 | 0.022 ± 0.010 | 0.104 ± 0.045 | - | - | ||
| Mn | 0.2 | 0.0496 ± 0.0127 | 0.2312 ± 0.0593 | 0.051 ± 0.013 | 0.236 ± 0.060 | - | - | ||
| Zn | 7 | 0.1232 ± 0.0640 | 0.5748 ± 0.2987 | 0.059 ± 0.030 | 0.274 ± 0.142 | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salhi, S.; Mellal, M.K.; Chelli, A.; Khelifa, R. Heavy Metal Contamination and Bioaccumulation Patterns from a Ramsar Wetland Tributary, Northern Algeria: A Baseline Assessment. Water 2025, 17, 2975. https://doi.org/10.3390/w17202975
Salhi S, Mellal MK, Chelli A, Khelifa R. Heavy Metal Contamination and Bioaccumulation Patterns from a Ramsar Wetland Tributary, Northern Algeria: A Baseline Assessment. Water. 2025; 17(20):2975. https://doi.org/10.3390/w17202975
Chicago/Turabian StyleSalhi, Selma, Mohammed Khalil Mellal, Abdelmadjid Chelli, and Rassim Khelifa. 2025. "Heavy Metal Contamination and Bioaccumulation Patterns from a Ramsar Wetland Tributary, Northern Algeria: A Baseline Assessment" Water 17, no. 20: 2975. https://doi.org/10.3390/w17202975
APA StyleSalhi, S., Mellal, M. K., Chelli, A., & Khelifa, R. (2025). Heavy Metal Contamination and Bioaccumulation Patterns from a Ramsar Wetland Tributary, Northern Algeria: A Baseline Assessment. Water, 17(20), 2975. https://doi.org/10.3390/w17202975







