Impact of Polyethylene Terephthalate Microplastics on Aerobic Granular Sludge Structure and EPS Composition in Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactor Setup
2.2. Biomass Structure
2.3. Determination of EPSs
2.4. Statistical Analysis
3. Results and Discussion
3.1. Pollutant Removal Efficiency in GSBRs
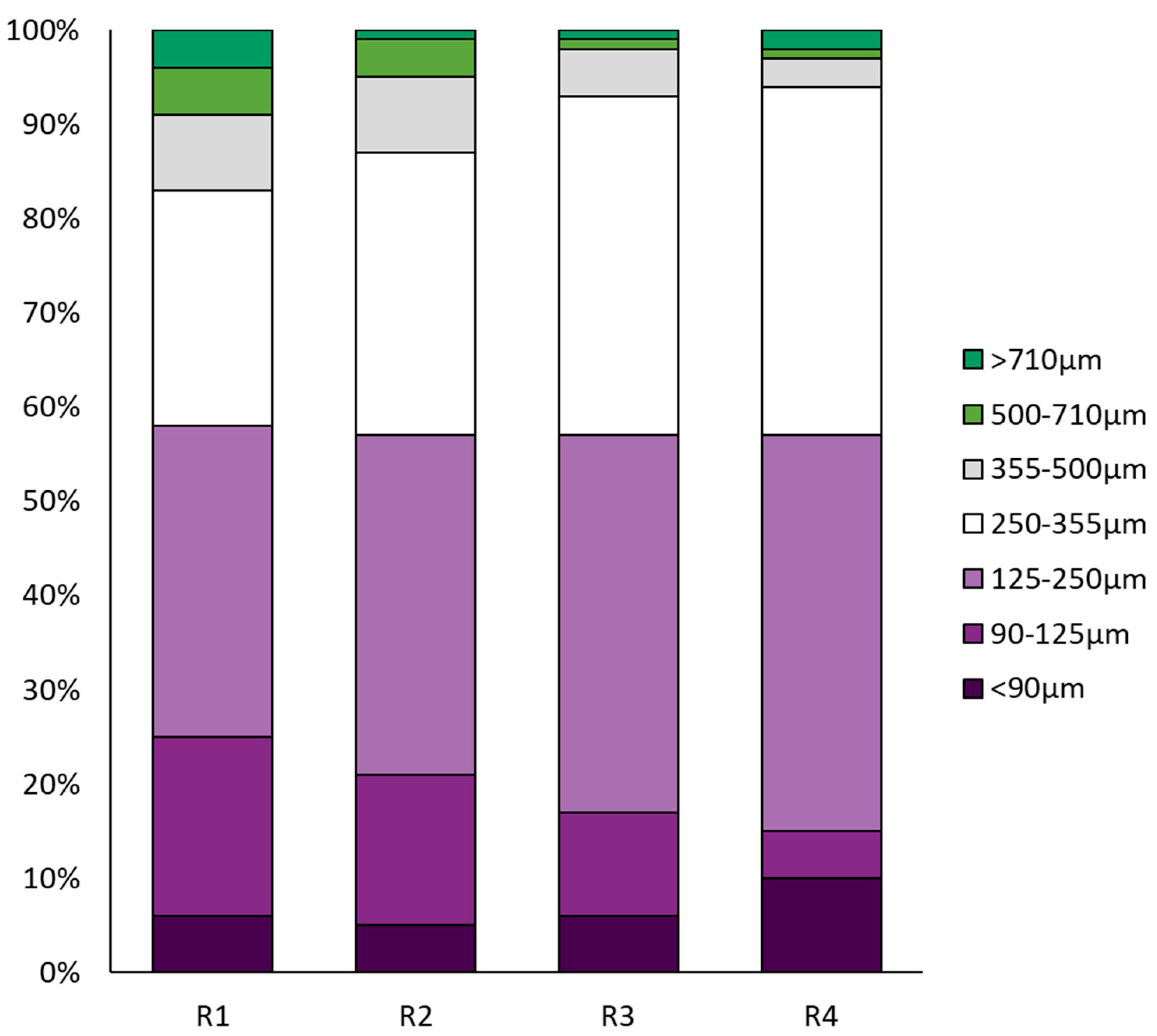
3.2. Biomass Characteristics of AGS
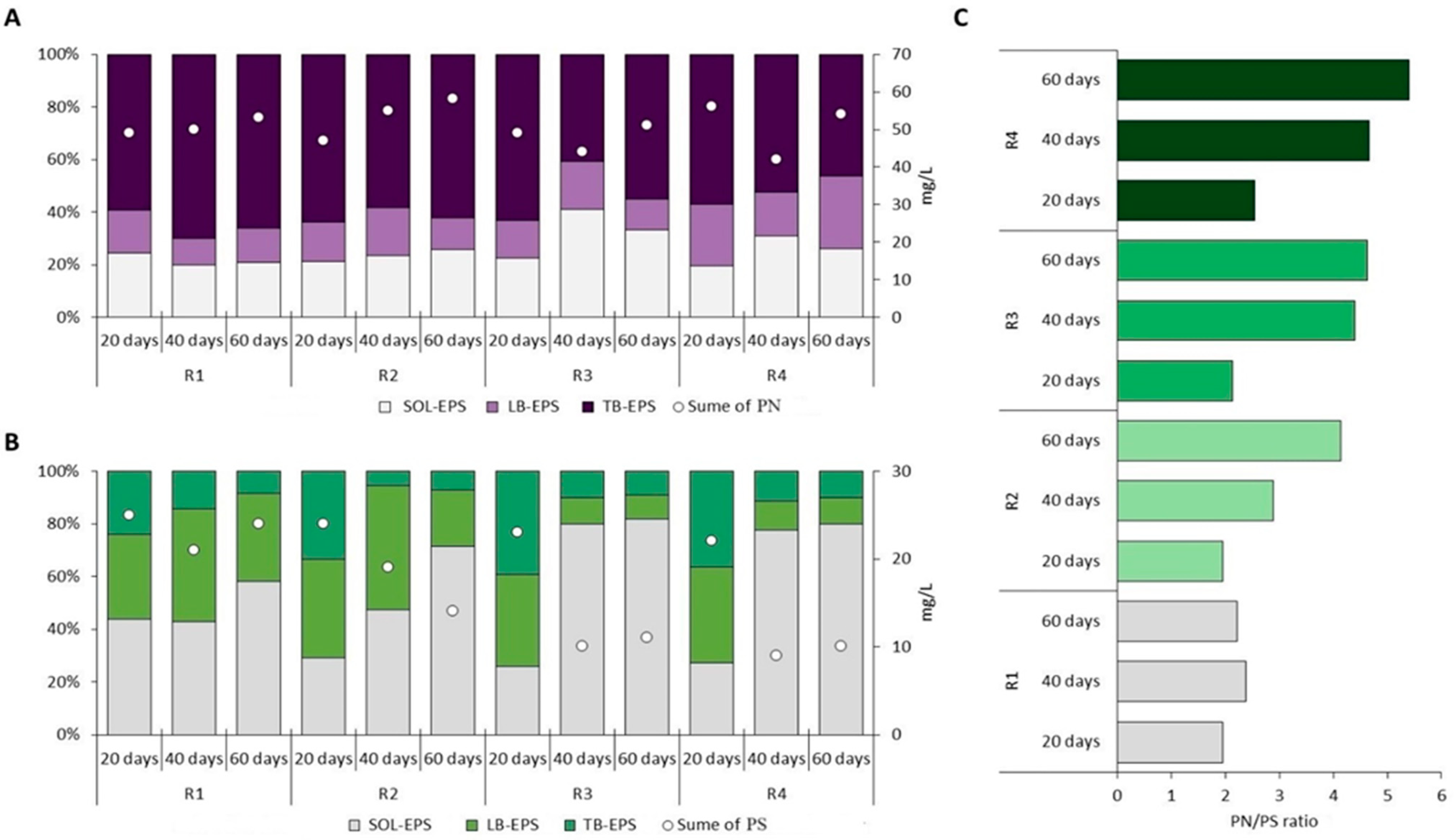
3.3. EPS Distribution in AGS
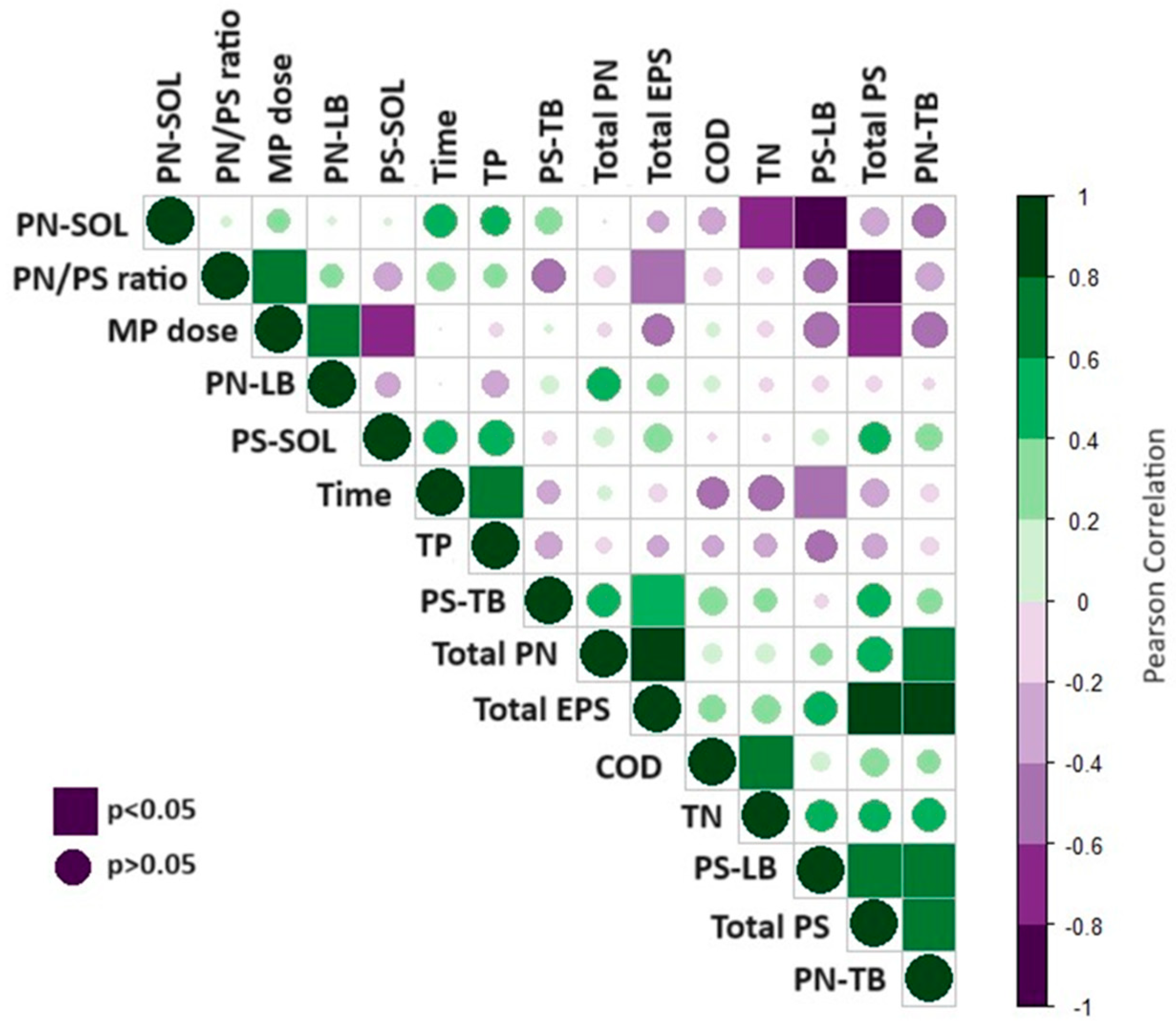
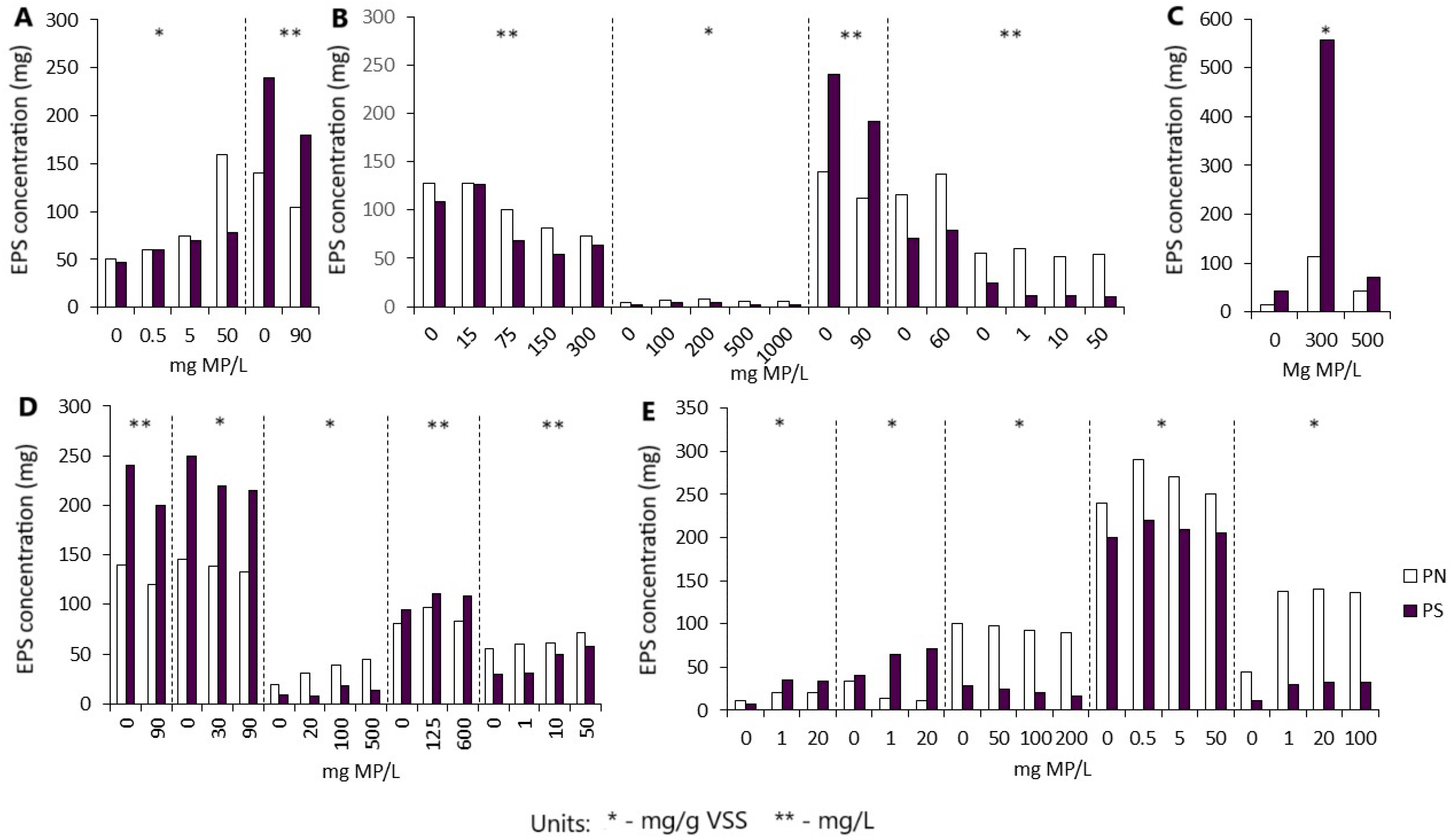
3.4. Comparison of Results with Existing Research
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jachimowicz, P.; Radzevičius, A.; Wojnarová, P.; Šadzevičius, R.; Horoszko, B.; Dapkienė, M.; Klik, B. Two Decades of Heavy Metal Fluctuations in Wastewater Sludge in Lithuania with Evolving Trends and Implications for Treatment Efficiency. J. Geochem. Explor. 2025, 269, 107642. [Google Scholar] [CrossRef]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Felis, E.; Bajkacz, S.; Jachimowicz, P.; Niestępski, S.; Konopka, I. Small-scale Wastewater Treatment Plants as a Source of the Dissemination of Antibiotic Resistance Genes in the Aquatic Environment. J. Hazard. Mater. 2020, 381, 121221. [Google Scholar] [CrossRef] [PubMed]
- Osinska, A.; Korzeniewska, E.; Harnisz, M.; Niestepski, S.; Jachimowicz, P. The Occurrence of Antibiotic-Resistant Bacteria, including Escherichia coli in Municipal Wastewater and River Water. E3S Web Conf. 2019, 100, 00061. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Grzyb, M.; Jachimowicz, P. Metatranscriptome Analysis of Bisphenol A-Exposed Aerobic Granular Sludge. Energies 2021, 14, 3263. [Google Scholar] [CrossRef]
- Jachimowicz, P.; Peng, R.; Hüffer, T.; Hofmann, T.; Cydzik-Kwiatkowska, A. Tire Materials Disturb Transformations of Nitrogen Compounds and Affect the Structure of Biomass in Aerobic Granular Sludge Reactors. J. Hazard. Mater. 2024, 465, 133223. [Google Scholar] [CrossRef] [PubMed]
- Nafea, T.H.; Al-Maliki, A.J.; Al-Tameemi, I.M. Sources, Fate, Effects, and Analysis of Microplastic in Wastewater Treatment Plants: A Review. Environ. Eng. Res. 2024, 29, 1. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Milojevic, N.; Jachimowicz, P. The Fate of Microplastic in Sludge Management Systems. Sci. Total Environ. 2022, 848, 157466. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiu, C.; Bian, S.; Zheng, L.; Chen, Y.; Song, Y.; Fang, C. The Effects of Microplastics and Nanoplastics on Nitrogen Removal, Extracellular Polymeric Substances, and Microbial Community in Sequencing Batch Reactor. Bioresour. Technol. 2023, 379, 129001. [Google Scholar] [CrossRef]
- Winkler, M.K.H.; van Loosdrecht, M.C. Intensifying Existing Urban Wastewater. Science 2022, 375, 377–378. [Google Scholar] [CrossRef] [PubMed]
- de Kreuk, M.K.; Kishida, N.; van Loosdrecht, M.C. Aerobic Granular Sludge—State of the Art. Water Sci. Technol. 2007, 55, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ma, X.; Niu, X.; Li, Z.; Wang, Q.; Ma, Y.; Li, H. The Impacts of Biodegradable and Non-Biodegradable Microplastic on the Performance and Microbial Community Characterization of Aerobic Granular Sludge. Front. Microbiol. 2024, 15, 1389046. [Google Scholar] [CrossRef] [PubMed]
- Torresi, E.; Polesel, F.; Bester, K.; Christensson, M.; Smets, B.F.; Trapp, S. Diffusion and Sorption of Organic Micropollutants in Biofilms with Varying Thicknesses. Water Res. 2017, 123, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.; Quintelas, C.; Ferreira, E.C.; Mesquita, D.P. The Role of Extracellular Polymeric Substances in Micropollutant Removal. Front. Chem. Eng. 2022, 4, 778469. [Google Scholar] [CrossRef]
- Mahto, K.U.; Priyadarshanee, M.; Samantaray, D.P.; Das, S. Bacterial Biofilm and Extracellular Polymeric Substances in the Treatment of Environmental Pollutants: Beyond the Protective Role in Survivability. J. Clean. Prod. 2022, 379, 134759. [Google Scholar] [CrossRef]
- Zhao, W.; You, J.; Yin, S.; Yang, H.; He, S.; Feng, L.; Wei, L. Extracellular Polymeric Substances—Antibiotics Interaction in Activated Sludge: A Review. Environ. Sci. Ecotechnol. 2023, 13, 100212. [Google Scholar] [CrossRef]
- Wang, C.; Wei, W.; Zhang, Y.T.; Dai, X.; Ni, B.J. Different sizes of polystyrene microplastics induced distinct microbial responses of anaerobic granular sludge. Water Res. 2022, 220, 118607. [Google Scholar] [CrossRef]
- Melo, A.; Costa, J.; Quintelas, C.; Ferreira, E.C.; Mesquita, D.P. Effect of Ibuprofen on Extracellular Polymeric Substances (EPS) Production and Composition, and Assessment of Microbial Structure by Quantitative Image Analysis. J. Environ. Manag. 2021, 293, 112852. [Google Scholar] [CrossRef]
- Okoffo, E.D.; Tscharke, B.J.; Thomas, K.V. Predicted Growth in Plastics Entering Biosolids and Agricultural Lands Exceeds Efforts to Control Source Emissions. ACS ES&T Water 2023, 3, 2238–2246. [Google Scholar] [CrossRef]
- Van Do, M.; Le, T.X.T.; Vu, N.D.; Dang, T.T. Distribution and Occurrence of Microplastics in Wastewater Treatment Plants. Environ. Technol. Innov. 2022, 26, 102286. [Google Scholar] [CrossRef]
- Jachimowicz, P.; Mądzielewska, W.; Cydzik-Kwiatkowska, A. Microplastics in Granular Sequencing Batch Reactors: Effects on Pollutant Removal Dynamics and the Microbial Community. J. Hazard. Mater. 2024, 476, 135061. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.A.; Russo, C.; Araujo, O.Q. Optimization of a Sequencing Batch Reactor for Biological Nitrogen Removal. Water Res. 2000, 34, 2809–2817. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water And Wastewater, 22nd ed.; American Public Health Association, Standard Methods: Washington, DC, USA, 2012; ISBN 978-087553-013-0. [Google Scholar]
- Cydzik-Kwiatkowska, A.; Białowiec, A.; Wojnowska-Baryła, I.; Smoczyński, L. Characteristic of Granulated Activated Sludge Fed with Glycerin Fraction from Biodiesel Production. Arch. Environ. Prot. 2009, 35, 41–52. [Google Scholar]
- Rusanowska, P.; Cydzik-Kwiatkowska, A.; Wojnowska-Baryła, I. Microbial Origin of Excreted DNA in Particular Fractions of Extracellular Polymers (EPS) in Aerobic Granules. Water Air Soil Pollut. 2019, 230, 203. [Google Scholar] [CrossRef]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of Extracellular Polymers from Activated Sludge Using a Cation Exchange Resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wei, W.; Sun, J.; Xu, Q.; Ni, B.J. Long-Term Effects of Polyvinyl Chloride Microplastics on Anaerobic Granular Sludge for Recovering Methane from Wastewater. Environ. Sci. Technol. 2020, 54, 9662–9671. [Google Scholar] [CrossRef]
- Jachimowicz, P.; Jo, Y.J.; Cydzik-Kwiatkowska, A. Polyethylene Microplastics Increase Extracellular Polymeric Substances Production in Aerobic Granular Sludge. Sci. Total Environ. 2022, 851, 158208. [Google Scholar] [CrossRef]
- Wei, F.; Xu, C.; Chen, C.; Wang, Y.; Lan, Y.; Long, L.; Yang, G. Distribution of Microplastics in the Sludge of Wastewater Treatment Plants in Chengdu, China. Chemosphere 2022, 287, 132357. [Google Scholar] [CrossRef]
- Laspidou, C.S.; Rittmann, B.E. A Unified Theory for Extracellular Polymeric Substances, Soluble Microbial Products, and Active and Inert Biomass. Water Res. 2002, 36, 2711–2720. [Google Scholar] [CrossRef]
- Deng, S.; Wang, L.; Su, H. Role and Influence of Extracellular Polymeric Substances on the Preparation of Aerobic Granular Sludge. J. Environ. Manag. 2016, 173, 49–54. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wei, W.; Huang, Q.S.; Wang, C.; Wang, Y.; Ni, B.J. Insights into the Microbial Response of Anaerobic Granular Sludge during Long-Term Exposure to Polyethylene Terephthalate Microplastics. Water Res. 2020, 179, 115898. [Google Scholar] [CrossRef]
- McSwain, B.S.; Irvine, R.L.; Hausner, M.; Wilderer, P. Composition and Distribution of Extracellular Polymeric Substances in Aerobic Flocs and Granular Sludge. Appl. Environ. Microbiol. 2005, 71, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Peng, D.C.; Hou, Y.P.; Li, H.J.; Pei, L.Y.; Yu, L.F. The Important Implications of Particulate Substrate in Determining the Physicochemical Characteristics of Extracellular Polymeric Substances (EPS) in Activated Sludge. Water Res. 2014, 129, 133–142. [Google Scholar] [CrossRef]
- Campo, R.; Corsino, S.F.; Torregrossa, M.; Di Bella, G. The Role of Extracellular Polymeric Substances on Aerobic Granulation with Stepwise Increase of Salinity. Sep. Purif. 2018, 195, 12–20. [Google Scholar] [CrossRef]
- Zhang, D.; Li, W.; Hou, C.; Shen, J.; Jiang, X.; Sun, X.; Liu, X. Aerobic Granulation Accelerated by Biochar for the Treatment of Refractory Wastewater. J. Chem. Eng. 2017, 314, 88–97. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, S.; Zhou, N.; Chen, Y.; Su, H. Granulation Enhancement and Microbial Community Shift of Tylosin-Tolerant Aerobic Granular Sludge on the Treatment of Tylosin Wastewater. Bioresour. Technol. 2020, 318, 124041. [Google Scholar] [CrossRef]
- González-Menéndez, C.; Sol, D.; Laca, A.; Laca, A.; Díaz, M. Interrelation between Extracellular Polymer Substances (EPSs) and MPs in an MBR. J. Environ. Chem. Eng. 2024, 12, 112021. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Liu, J. Composition Analysis of Fractions of Extracellular Polymeric Substances from an Activated Sludge Culture and Identification of Dominant Forces Affecting Microbial Aggregation. Sci. Rep. 2016, 6, 28391. [Google Scholar] [CrossRef]
- Li, X.Y.; Yang, S.F. Influence of Loosely Bound Extracellular Polymeric Substances (EPS) on the Flocculation, Sedimentation and Dewaterability of Activated Sludge. Water Res. 2007, 41, 1022–1030. [Google Scholar] [CrossRef]
- Wang, W.; Zang, Y.; Wang, C.; Wang, K.; Li, R. Effects of Four Kinds of Oxide Nanoparticles on Proteins in Extracellular Polymeric Substances of Sludge. BioMed Res. Int. 2020, 2020, 1754134. [Google Scholar] [CrossRef]
- Bassin, J.P.; Pronk, M.; Kraan, R.; Kleerebezem, R.; Van Loosdrecht, M.C. Ammonium Adsorption in Aerobic Granular Sludge, Activated Sludge and Anammox Granules. Water Res. 2011, 45, 5257–5265. [Google Scholar] [CrossRef] [PubMed]
- Zafar, R.; Arshad, Z.; Choi, N.E.; Li, X.; Hur, J. Unravelling the Complex Adsorption Behavior of Extracellular Polymeric Substances onto Pristine and UV-Aged Microplastics Using Two-Dimensional Correlation Spectroscopy. J. Chem. Eng. 2023, 438, 129528. [Google Scholar] [CrossRef]
- Burzio, C.; Ekholm, J.; Modin, O.; Falås, P.; Svahn, O.; Persson, F.; Wilén, B.M. Removal of Organic Micropollutants from Municipal Wastewater by Aerobic Granular Sludge and Conventional Activated Sludge. J. Hazard. Mater. 2022, 438, 129528. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Wang, Y.; Wang, J.; Fang, F.; Yan, P.; Liu, Z. Effect of Different Forms and Components of EPS on Sludge Aggregation During Granulation Process of Aerobic Granular Sludge. Chemosphere 2022, 303, 135116. [Google Scholar] [CrossRef]
- Liao, B.Q.; Allen, D.G.; Droppo, I.G.; Leppard, G.G.; Liss, S.N. Surface Properties of Sludge and Their Role in Bioflocculation and Settleability. Water Res. 2001, 35, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.; Wang, J. Interaction of Nanoplastics with Extracellular Polymeric Substances (EPS) in the Aquatic Environment: A Special Reference to Eco-Corona Formation and Associated Impacts. Water Res. 2021, 201, 117319. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular Polymeric Substances (EPS) of Microbial Aggregates in Biological Wastewater Treatment Systems: A Review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Dai, H.-H.; Gao, J.-F.; Wang, Z.-Q.; Zhao, Y.-F.; Zhang, D. Behavior of Nitrogen, Phosphorus and Antibiotic Resistance Genes under Polyvinyl Chloride Microplastics Pressures in an Aerobic Granular Sludge System. J. Clean. Prod. 2020, 256, 120402. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, B.; Liu, Y.; Feng, X.; Shi, W. Revealing the Influencing Mechanisms of Polystyrene Microplastics (MPs) on the Performance and Stability of the Algal-Bacterial Granular Sludge. Bioresour. Technol. 2022, 354, 127202. [Google Scholar] [CrossRef]
- Tang, M.; Zhou, S.; Huang, J.; Sun, L.; Lu, H. Stress Responses of Sulfate-Reducing Bacteria Sludge upon Exposure to Polyethylene Microplastics. Water Res. 2022, 220, 118646. [Google Scholar] [CrossRef]
- Gan, Y.; Gong, B.; Huang, X.; Fang, F.; Peng, T.; Liu, Z. Response of Aerobic Granular Sludge under Acute Inhibition by Polystyrene Microplastics: Activity, Aggregation Performance, and Microbial Analysis. Environ. Pollut. 2024, 349, 123923. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, Q.; He, D.; Yang, F.; Ma, X.; Wang, Y.; Wang, D. Exposure of Polyethylene Microplastics Affects Sulfur Migration and Transformation in Anaerobic System. J. Hazard. Mater. 2024, 472, 134520. [Google Scholar] [CrossRef]
- Hong, X.; Niu, B.; Sun, H.; Zhou, X. Insight into Response Characteristics and Inhibition Mechanisms of Anammox Granular Sludge to Polyethylene Terephthalate Microplastics Exposure. Bioresour. Technol. 2023, 385, 129355. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wei, W.; Wang, C.; Ni, B.J. Understanding and Mitigating the Distinctive Stresses Induced by Diverse Microplastics on Anaerobic Hydrogen-Producing Granular Sludge. J. Hazard. Mater. 2022, 440, 129771. [Google Scholar] [CrossRef]
- Yi, K.; Huang, J.; Li, X.; Li, S.; Pang, H.; Liu, Z.; Shu, W. Long-Term Impacts of Polyethylene Terephthalate (PET) Microplastics in Membrane Bioreactor. J. Environ. Manag. 2022, 323, 116234. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Liu, Y.; Zhou, Z.; Hu, W.; Lin, L.; Wu, Z. Effects of Microplastics Accumulation on Performance of Membrane Bioreactor for Wastewater Treatment. Chemosphere 2022, 287, 131968. [Google Scholar] [CrossRef]
- Ren, Z.; Guo, H.; Jin, H.; Wang, Y.; Zhang, G.; Zhou, J.; Wang, T. P, N, and C-related Functional Genes in SBR System Promoted Antibiotics Resistance Gene Transmission under Polystyrene Microplastics Stress. Water Res. 2023, 235, 119884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, S.; Wu, L.; Guo, Y.; Shi, W.; Lens, P.N. Micro (nano) Plastic Size and Concentration Co-Differentiate the Treatment Performance and Toxicity Mechanism in Aerobic Granular Sludge Systems. Chem. Eng. J. 2023, 457, 141212. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wei, W.; Wang, C.; Ni, B.J. Microbial and Physicochemical Responses of Anaerobic Hydrogen-Producing Granular Sludge to Polyethylene Micro (nano) Plastics. Water Res. 2022, 221, 118745. [Google Scholar] [CrossRef]
- Gong, X.; Ge, Z.; Ma, Z.; Li, Y.; Huang, D.; Zhang, J. Effect of Different Size Microplastic Particles on the Construction of Algal-Bacterial Biofilms and Microbial Communities. J. Environ. Manag. 2023, 343, 118246. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, Y.; Liu, L.; Wang, L.; Tang, J. Interaction between Microplastic Biofilm Formation and Antibiotics: Effect of Microplastic Biofilm and Its Driving Mechanisms on Antibiotic Resistance Gene. J. Hazard. Mater. 2023, 459, 132099. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, T.G.; Sivasankar, V.; Prabhakaran, M.; Omine, K. Microplastics–Pollutants’ Interactions, Mechanisms, and Potential Toxicity. In Organic Pollutants: Toxicity and Solutions; Omine, K., Ed.; Springer: Cham, Switzerland, 2022; pp. 551–582. [Google Scholar]
- Panthi, G.; Bajagain, R.; Chaudhary, D.K.; Kim, P.G.; Kwon, J.H.; Hong, Y. The Release, Degradation, and Distribution of PVC Microplastic-Originated Phthalate and Non-Phthalate Plasticizers in Sediments. J. Hazard. Mater. 2024, 470, 134167. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, P.; Schwarz, C.; Zhang, B.; Huo, L.; Shi, B.; Alvarez, P.J. Phthalate Esters Released from Plastics Promote Biofilm Formation and Chlorine Resistance. Environ. Sci. Technol. 2022, 56, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
| R1 | R2 | R3 | R4 | |
|---|---|---|---|---|
| COD | 85.5 ± 4.7% | 84.4% ± 5.3% | 85.1% ± 7.4% | 86.4% ± 7.5% |
| TN | 98.0% ± 3.9% | 94.3% ± 3.1% | 95.0% ± 3.4% | 96.9% ± 4.4% |
| TP | 64.4% ± 4.2% | 58.5% ± 5.4% | 64.6% ± 4.4% | 60.03% ± 6.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jachimowicz, P.; Cydzik-Kwiatkowska, A. Impact of Polyethylene Terephthalate Microplastics on Aerobic Granular Sludge Structure and EPS Composition in Wastewater Treatment. Water 2025, 17, 270. https://doi.org/10.3390/w17020270
Jachimowicz P, Cydzik-Kwiatkowska A. Impact of Polyethylene Terephthalate Microplastics on Aerobic Granular Sludge Structure and EPS Composition in Wastewater Treatment. Water. 2025; 17(2):270. https://doi.org/10.3390/w17020270
Chicago/Turabian StyleJachimowicz, Piotr, and Agnieszka Cydzik-Kwiatkowska. 2025. "Impact of Polyethylene Terephthalate Microplastics on Aerobic Granular Sludge Structure and EPS Composition in Wastewater Treatment" Water 17, no. 2: 270. https://doi.org/10.3390/w17020270
APA StyleJachimowicz, P., & Cydzik-Kwiatkowska, A. (2025). Impact of Polyethylene Terephthalate Microplastics on Aerobic Granular Sludge Structure and EPS Composition in Wastewater Treatment. Water, 17(2), 270. https://doi.org/10.3390/w17020270







