Glucose as a Metabolic Enhancer: Promoting Nonylphenol Detoxification by Chlorella pyrenoidosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Strain and Culture Conditions
2.2. Experimental Design of Exposure and Degradation
2.3. Analysis of Algal Growth, Morphologic Properties, and EPS
2.3.1. Algal Growth
2.3.2. Morphologic Properties
2.3.3. Extraction and Analysis of EPSs
2.4. Extraction and Detection of NP
2.4.1. Extraction of NP from Culture Medium, Adsorbed on Algal Cell Walls, and Absorbed Within Algal Cells
- (1)
- Extraction of NP from Culture Medium
- (2)
- Extraction of NP adsorbed on cell surfaces
- (3)
- Extraction of NP absorbed by Algal Cells
2.4.2. Detection of NP and Degradation Metabolites
2.5. Kinetic Equations for NP Degradation by C. pyrenoidosa
2.6. Statistical Analysis
3. Results and Discussion
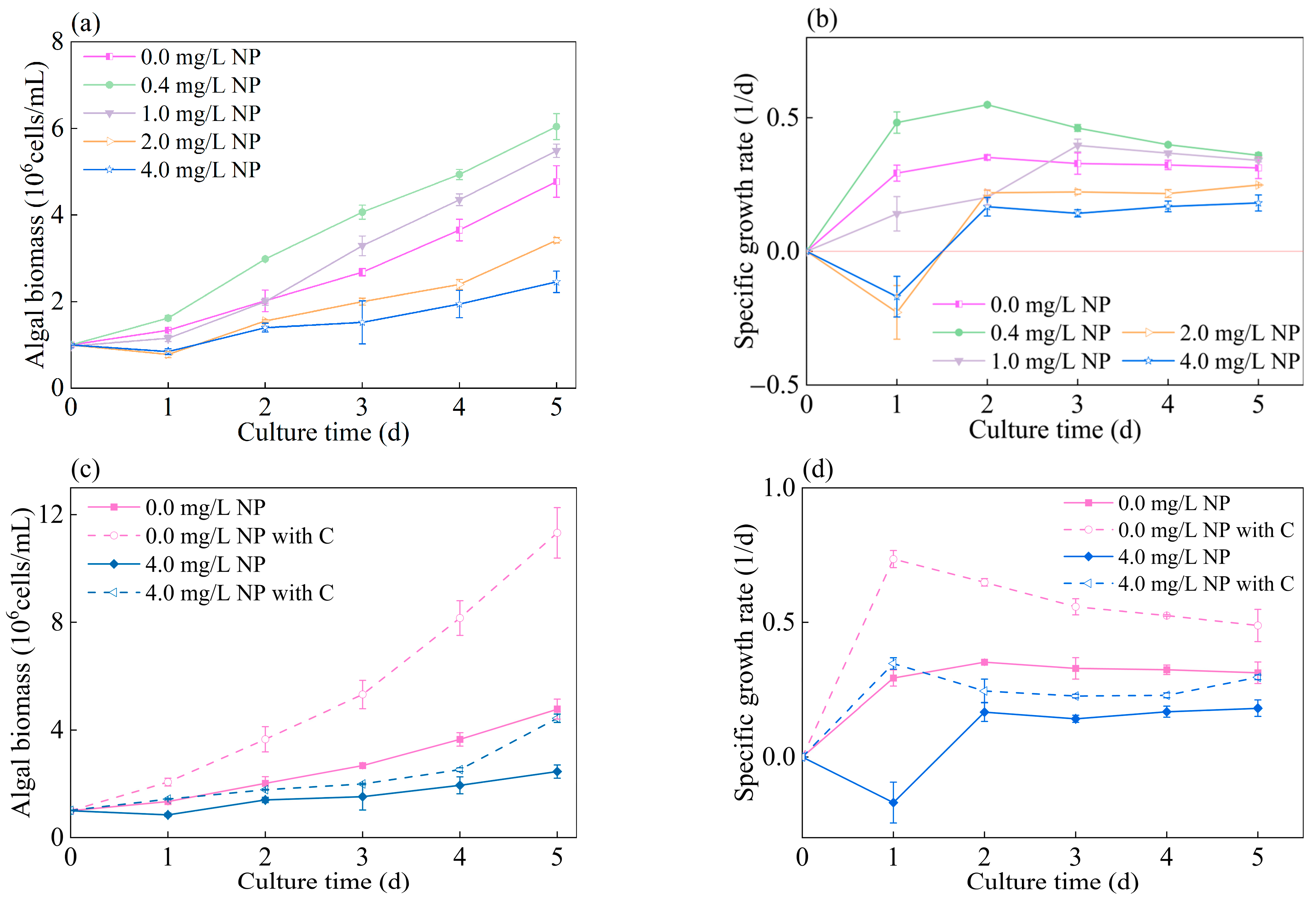
3.1. Detoxification Effect of Glucose on Growth of Algae Exposed to NP
3.2. Effect of Glucose on Structural Characterization of Algal Cells Exposed to NP
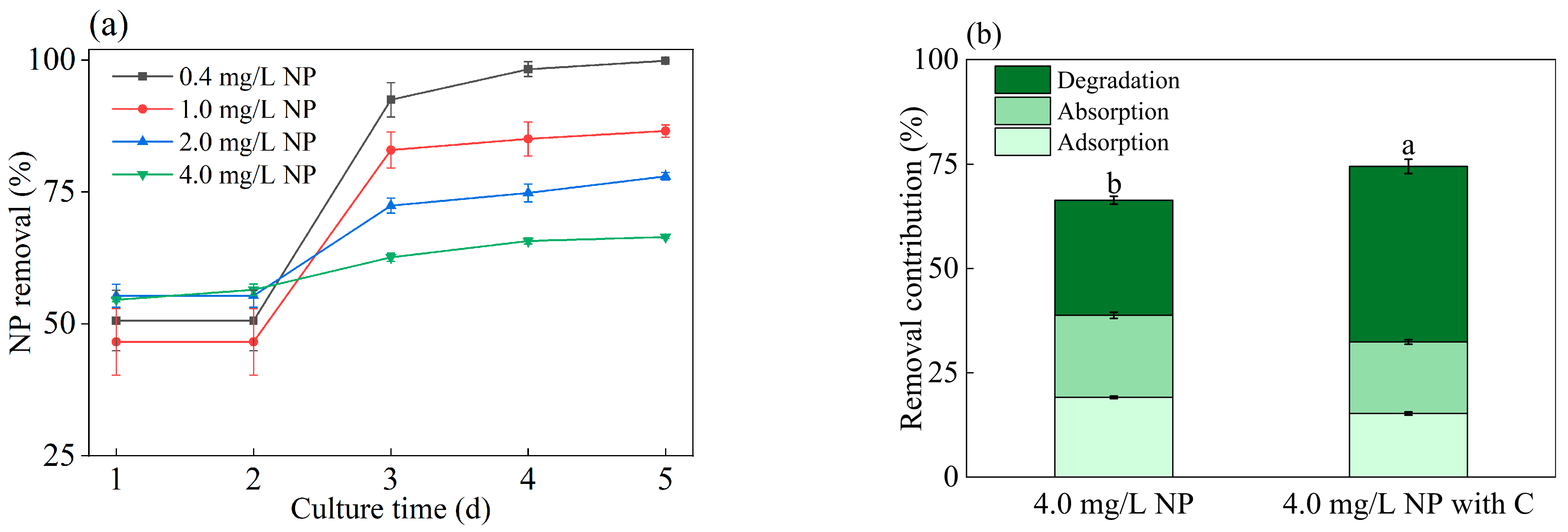
3.3. Glucose Improved NP-Removal Capacity of Algae
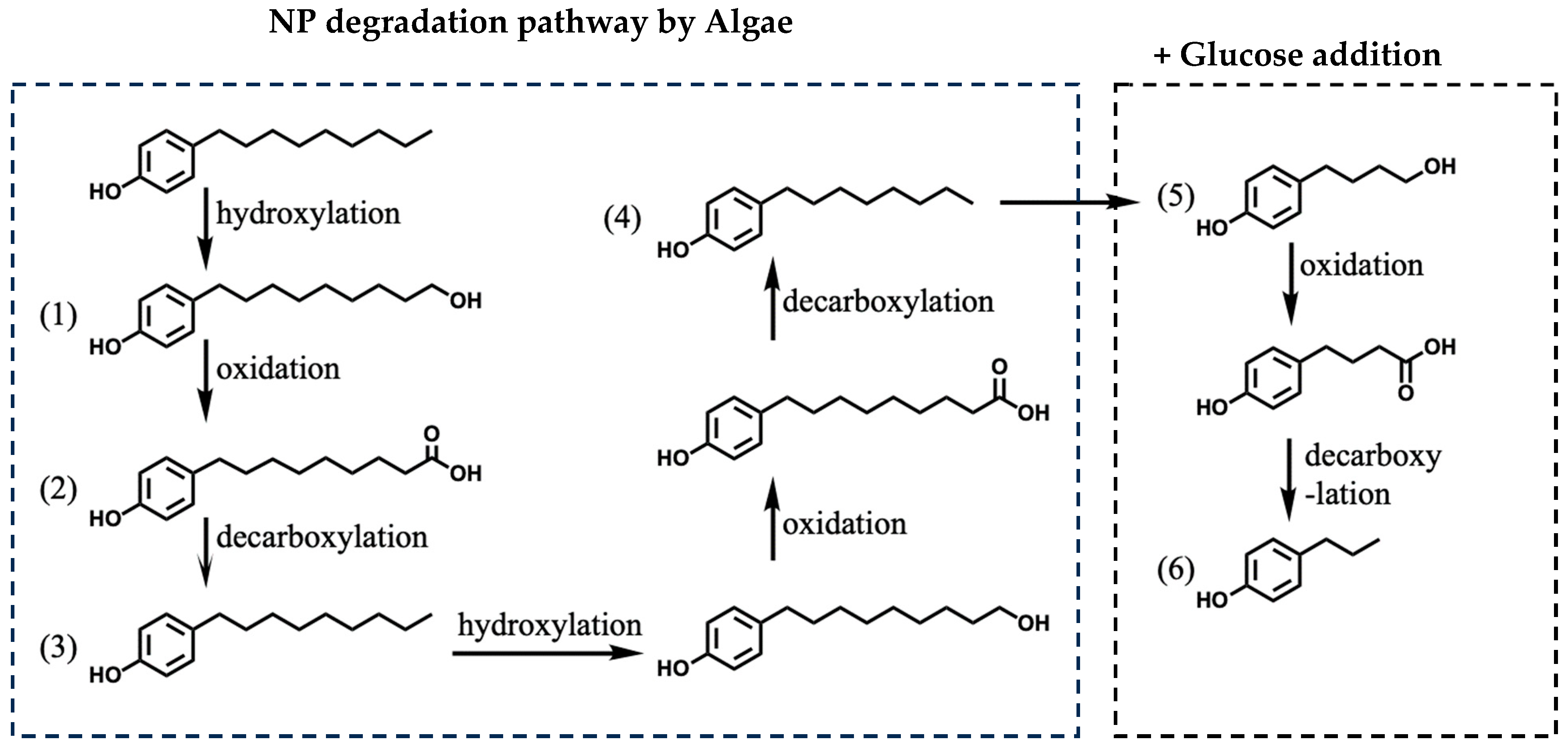
3.4. Effect of Glucose on NP-Degradation Pathway in Algae
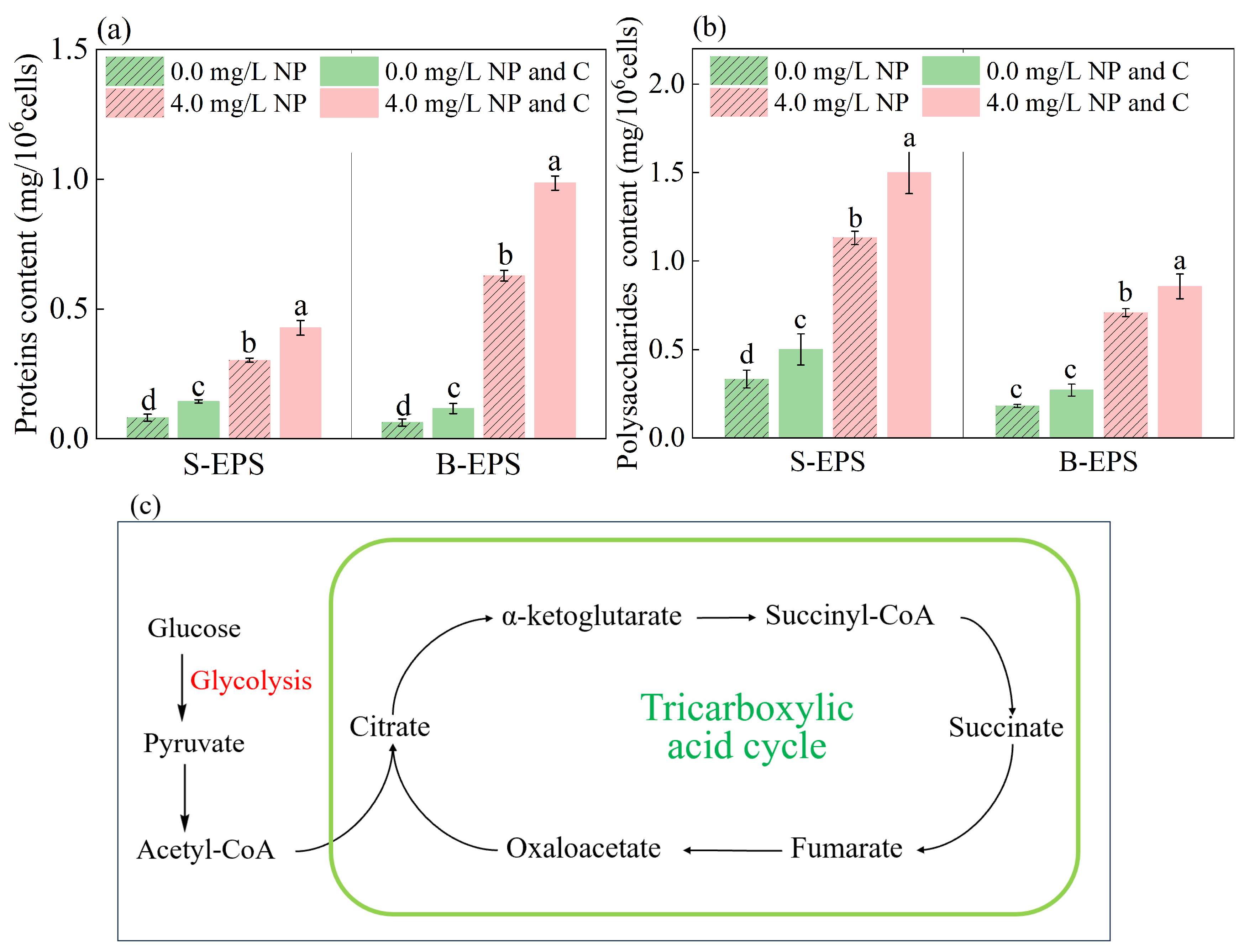
3.5. Glucose Stimulated EPS Secretion in Algae Exposed to NP
3.6. Practical Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhandari, G.; Bagheri, A.R.; Bhatt, P.; Bilal, M. Occurrence, Potential Ecological Risks, and Degradation of Endocrine Disrupter, Nonylphenol, from the Aqueous Environment. Chemosphere 2021, 275, 130013. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Zhao, Q.; Wang, L.; Ma, J.; Song, L.; Huang, D. Adsorption Behavior of Nonylphenol on Polystyrene Microplastics and Their Cytotoxicity in Human Caco-2 Cells. Water 2022, 14, 3288. [Google Scholar] [CrossRef]
- Rahmani, F.; Sajjadi, N.; Dehghani, M.H.; Zaeimdar, M. Modelling and Optimization of Nonylphenol Biosorption by Novel Low-Cost Magnetic Chlorella Vulgaris. Emerg. Contam. 2024, 10, 100310. [Google Scholar] [CrossRef]
- De La Parra-Guerra, A.; Olivero-Verbel, J. Toxicity of Nonylphenol and Nonylphenol Ethoxylate on Caenorhabditis Elegans. Ecotoxicol. Environ. Saf. 2020, 187, 109709. [Google Scholar] [CrossRef]
- Yang, W.; Gao, X.; Wu, Y.; Wan, L.; Tan, L.; Yuan, S.; Ding, H.; Zhang, W. The Combined Toxicity Influence of Microplastics and Nonylphenol on Microalgae Chlorella Pyrenoidosa. Ecotoxicol. Environ. Saf. 2020, 195, 110484. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kim, M.; Shim, W.J.; Yim, U.H.; Oh, J.-R.; Kwon, Y.-J. Seasonal Flux of Nonylphenol in Han River, Korea. Chemosphere 2004, 56, 1–6. [Google Scholar] [CrossRef]
- Yu, Y.; Zhai, H.; Hou, S.; Sun, H. Nonylphenol Ethoxylates and Their Metabolites in Sewage Treatment Plants and Rivers of Tianjin, China. Chemosphere 2009, 77, 1–7. [Google Scholar] [CrossRef]
- Sibali, L.L.; Okwonkwo, J.O.; McCrindle, R.I. Levels of Selected Alkylphenol Ethoxylates (APEs) in Water and Sediment Samples from the Jukskei River Catchment Area in Gauteng, South Africa. Water SA 2010, 36, 229–238. [Google Scholar]
- Rocha, M.J.; Silva, F.; Rocha, E. Annual Evaluation of 17 Oestrogenic Endocrine Disruptors and Hazard Indexes in the Douro River Estuary—The Atlantic Discharge of the Highest-Flow River of Southwestern Europe. Water 2022, 14, 2046. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Li, J.; Qin, N.; Wei, Q.; Li, Y.; Peng, Y.; Li, H. Assessment of Nonylphenol Exposure Based on Global Urinary Concentration Data and Its Risk Analysis. Environ. Res. 2024, 244, 117903. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ji, J.; Wu, Y.; Chen, S.; Xu, M.; Cao, X.; Liu, H.; Wang, Z.; Bi, H.; Guan, G.; et al. Nonylphenol and Its Derivatives: Environmental Distribution, Treatment Strategy, Management and Future Perspectives. Chemosphere 2024, 352, 141377. [Google Scholar] [CrossRef]
- Noorimotlagh, Z.; Mirzaee, S.A.; Martinez, S.S.; Rachoń, D.; Hoseinzadeh, M.; Jaafarzadeh, N. Environmental Exposure to Nonylphenol and Cancer Progression Risk–A Systematic Review. Environ. Res. 2020, 184, 109263. [Google Scholar] [CrossRef] [PubMed]
- Bodziach, K.; Staniszewska, M.; Nehring, I.; Ożarowska, A.; Zaniewicz, G.; Meissner, W. Endocrine Disrupting Bisphenol A, 4-Tert-Octylphenol and 4-Nonylphenol in Gonads of Long-Tailed Ducks Clangula Hyemalis Wintering in the Southern Baltic. Environ. Res. 2024, 243, 117772. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.; Watanabe, T. Distribution and Removal of Nonylphenol Ethoxylates and Nonylphenol from Textile Wastewater—A Comparison of a Cotton and a Synthetic Fiber Factory in Vietnam. Water 2017, 9, 386. [Google Scholar] [CrossRef]
- Lalonde, B.; Garron, C. Nonylphenol, Octylphenol, and Nonylphenol Ethoxylates Dissemination in the Canadian Freshwater Environment. Arch. Environ. Contam. Toxicol. 2021, 80, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-D.; Han, H.; Park, T.-J.; Park, J.-H.; Kim, Y.S. New Findings on the Occurrence, Removal, and Risk Assessment of Nonylphenol and Octylphenol in Industrial Wastewater Treatment Plants in Korea. J. Hazard. Mater. 2024, 461, 132615. [Google Scholar] [CrossRef] [PubMed]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.V.; Kumar, M.S. Detoxification of Water and Wastewater by Advanced Oxidation Processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Nabeel, F.; Iqbal, H.M.N.; Zhao, Y. Hazardous Contaminants in the Environment and Their Laccase-Assisted Degradation—A Review. J. Environ. Manag. 2019, 234, 253–264. [Google Scholar] [CrossRef]
- Al-Khiat, S.H.; Bukhari, N.A.; Ameen, F.; Abdel-Raouf, N. Comparison of the Microalgae Phormidium Tenue and Chlorella Vulgaris as Biosorbents of Cd and Zn from Aqueous Environments. Environ. Res. 2023, 235, 116675. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-L.; Nagarajan, D.; Chen, J.-H.; Yen Chen, C.; Wu, Y.-J.; Whang, L.-M.; Lee, D.-J.; Chang, J.-S. Microalgae-Bacteria Consortia for the Treatment of Raw Dairy Manure Wastewater Using a Novel Two-Stage Process: Process Optimization and Bacterial Community Analysis. Chem. Eng. J. 2023, 473, 145388. [Google Scholar] [CrossRef]
- Ma, Y.; Lin, S.; Guo, T.; Guo, C.; Li, Y.; Hou, Y.; Gao, Y.; Dong, R.; Liu, S. Exploring the Influence of Sulfadiazine-Induced Stress on Antibiotic Removal and Transformation Pathway Using Microalgae Chlorella sp. Environ. Res. 2024, 256, 119225. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, H.; He, N.; Sun, D.; Duan, S. Biosorption and Biodegradation of the Environmental Hormone Nonylphenol By Four Marine Microalgae. Sci. Rep. 2019, 9, 5277. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gao, X.; Wu, Y.; Wan, L.; Lu, C.; Huang, J.; Chen, H.; Yang, Y.; Ding, H.; Zhang, W. Chemical- and Species-Specific Toxicity of Nonylphenol and Octylphenol to Microalgae Chlorella Pyrenoidosa and Scenedesmus Obliquus. Environ. Toxicol. Pharmacol. 2021, 81, 103517. [Google Scholar] [CrossRef] [PubMed]
- López-Pacheco, I.Y.; Salinas-Salazar, C.; Silva-Núñez, A.; Rodas-Zuluaga, L.I.; Donoso-Quezada, J.; Ayala-Mar, S.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldívar, R. Removal and Biotransformation of 4-Nonylphenol by Arthrospira Maxima and Chlorella Vulgaris Consortium. Environ. Res. 2019, 179, 108848. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, A.; Fu, W.; Song, D. Growth Performance, Antioxidant Response, Biodegradation and Transcriptome Analysis of Chlorella Pyrenoidosa after Nonylphenol Exposure. Sci. Total Environ. 2022, 806, 150507. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Sun, X.; Zhong, Y.; Sun, K.; Liu, W.; Duan, S. Removal and Biodegradation of Nonylphenol by Four Freshwater Microalgae. Int. J. Environ. Res. Public. Health 2016, 13, 1239. [Google Scholar] [CrossRef]
- Cheng, Q.; Jiang, Y.; Jin, Z.; Hui, C.; Xu, L.; Zhou, Q.; Zhao, Y.; Du, L.; Jiang, H. Enhanced Excretion of Extracellular Polymeric Substances Associated with Nonylphenol Tolerance in Dictyosphaerium sp. J. Hazard. Mater. 2020, 395, 122644. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.; Zhai, J.; Wei, H. Effect of Nitrogen Limitation on Biochemical Composition and Photosynthetic Performance for Fed-Batch Mixotrophic Cultivation of Microalga Spirulina Platensis. Bioresour. Technol. 2018, 263, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-Q.; Kurade, M.B.; Kim, J.R.; Roh, H.-S.; Jeon, B.-H. Ciprofloxacin Toxicity and Its Co-Metabolic Removal by a Freshwater Microalga Chlamydomonas Mexicana. J. Hazard. Mater. 2017, 323, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.N.P.; Ngo, H.H.; Guo, W.; Nguyen, K.H.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Liu, Y.; Ding, A.; Bui, X.T. Micropollutants Cometabolism of Microalgae for Wastewater Remediation: Effect of Carbon Sources to Cometabolism and Degradation Products. Water Res. 2020, 183, 115974. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Ma, H.; Sun, M.; Yin, X.; Feng, Q.; Song, H.; Gai, H. Characterization of Cometabolic Degradation of P-Cresol with Phenol as Growth Substrate by Chlorella Vulgaris. Bioresour. Technol. 2019, 281, 296–302. [Google Scholar] [CrossRef]
- Cheng, Q.; Du, L.; Xu, L.; Zhao, Y.; Ma, J.; Lin, H. Toxicity Alleviation and Metabolism Enhancement of Nonylphenol in Green Algae Dictyosphaerium sp. by NaHCO3. Sci. Total Environ. 2022, 848, 157698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, R.; Li, H.; Du, Q.; Lu, J.; Huang, Y.; Yan, Z.; Chen, J. Mechanism Analysis for the Process-Dependent Driven Mode of NaHCO3 in Algal Antibiotic Removal: Efficiency, Degradation Pathway and Metabolic Response. J. Hazard. Mater. 2020, 394, 122531. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic Cultures of Microalgae: Metabolism and Potential Products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, Y.; Wang, X. Heterotrophic Cultivation of Chlorella Pyrenoidosa Using Sucrose as the Sole Carbon Source by Co-Culture with Rhodotorula Glutinis. Bioresour. Technol. 2016, 220, 615–620. [Google Scholar] [CrossRef]
- Liu, N.; Guo, B.; Cao, Y.; Wang, H.; Yang, S.; Huo, H.; Kong, W.; Zhang, A.; Niu, S. Effects of Organic Carbon Sources on the Biomass and Lipid Production by the Novel Microalga Micractinium Reisseri FM1 under Batch and Fed-Batch Cultivation. South Afr. J. Bot. 2021, 139, 329–337. [Google Scholar] [CrossRef]
- Mao, X.; Wang, X.; Ge, M.; Chen, F.; Liu, B. New Insights into Xanthophylls and Lipidomic Profile Changes Induced by Glucose Supplementation in the Marine Diatom Nitzschia Laevis. Mar. Drugs 2022, 20, 456. [Google Scholar] [CrossRef] [PubMed]
- Přibyl, P.; Cepák, V. Screening for Heterotrophy in Microalgae of Various Taxonomic Positions and Potential of Mixotrophy for Production of High-Value Compounds. J. Appl. Phycol. 2019, 31, 1555–1564. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, F.; Wang, Z.; Zhou, R.; Tang, Y.; Li, Y. The Potential Growth and Lipid Accumulation in Coccomyxa Subellipsoidea Triggered by Glucose Combining with Sodium Acetate. World J. Microbiol. Biotechnol. 2019, 35, 110. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, D.; Zhang, Y.; Chen, F. Glucose Triggers Cell Structure Changes and Regulates Astaxanthin Biosynthesis in Chromochloris Zofingiensis. Algal Res. 2019, 39, 101455. [Google Scholar] [CrossRef]
- Joun, J.; Sirohi, R.; Sim, S.J. The Effects of Acetate and Glucose on Carbon Fixation and Carbon Utilization in Mixotrophy of Haematococcus Pluvialis. Bioresour. Technol. 2023, 367, 128218. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhao, F.; Feng, J.; Liu, Q.; Nan, F.; Liu, X. Transcriptomic Analysis Reveals the Mechanism on the Response of Chlorococcum sp. GD to Glucose Concentration in Mixotrophic Cultivation. Bioresour. Technol. 2019, 288, 121568. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Yang, S.; Guo, B.; Wang, H.; Huo, H.; Zhang, A.; Niu, S. Growth Behavior, Glucose Consumption and Phenol Removal Efficiency of Chlorella Vulgaris under the Synergistic Effects of Glucose and Phenol. Ecotoxicol. Environ. Saf. 2019, 186, 109762. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, J.; Wang, Z.; Torres, O.L.; Guo, R.; Chen, J. Exogenous Organic Carbon as an Artificial Enhancement Method to Assist the Algal Antibiotic Treatment System. J. Clean. Prod. 2018, 194, 624–634. [Google Scholar] [CrossRef]
- Han, C.; Ren, J.; Wang, B.; Wang, Z.; Yin, H.; Ke, F.; Xu, D.; Zhang, L.; Si, X.; Shen, Q. Ignored Effects of Phosphite (P+III) on the Growth Responses of Three Typical Algae Species. Environ. Pollut. 2022, 294, 118672. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, Y.; Wu, F.; Pan, X.; Li, W.; Han, J. Transcriptomics Revealed the Key Molecular Mechanisms of Ofloxacin-Induced Hormesis in Chlorella Pyrenoidosa at Environmentally Relevant Concentration. Environ. Pollut. 2024, 361, 124887. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Y.; Liang, C.; Peng, L.; Zhou, Y. Nitrogen Removal by Algal-Bacterial Consortium during Mainstream Wastewater Treatment: Transformation Mechanisms and Potential N2O Mitigation. Water Res. 2023, 235, 119890. [Google Scholar] [CrossRef]
- Shang, Z.; Li, M.; Ma, Y.; Si, B.; Wei, Z.; Zhang, X. Deciphering Metabolomic Insights into Benzoic Acid-Mediated Nutrient Enrichment in Chlorella Pyrenoidosa. Food Biosci. 2024, 59, 104157. [Google Scholar] [CrossRef]
- Qu, F.; Liang, H.; He, J.; Ma, J.; Wang, Z.; Yu, H.; Li, G. Characterization of Dissolved Extracellular Organic Matter (dEOM) and Bound Extracellular Organic Matter (bEOM) of Microcystis Aeruginosa and Their Impacts on UF Membrane Fouling. Water Res. 2012, 46, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, F.; Liu, N.; Ge, F.; Xiao, H.; Yang, Y. Role of Extracellular Polymeric Substances from Chlorella Vulgaris in the Removal of Ammonium and Orthophosphate under the Stress of Cadmium. Bioresour. Technol. 2015, 190, 299–306. [Google Scholar] [CrossRef]
- Morris, D.L. Quantitative Determination of Carbohydrates With Dreywood’s Anthrone Reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Zhou, Q.; Jin, Z.; Jiang, Y.; Xu, L.; Jiang, H.; Zhao, Y. Bioaccumulation, Growth Performance, and Transcriptomic Response of Dictyosphaerium sp. after Exposure to Nonylphenol. Sci. Total Environ. 2019, 687, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, Y.; Zhang, Y.; Cheng, Q.; Li, X.; Zhao, C.; Zhang, D. Different Pathways for 4-n-Nonylphenol Biodegradation by Two Aspergillus Strains Derived from Estuary Sediment: Evidence from Metabolites Determination and Key-Gene Identification. J. Hazard. Mater. 2018, 359, 203–212. [Google Scholar] [CrossRef]
- Park, J.; Yamashita, N.; Wu, G.; Tanaka, H. Removal of Pharmaceuticals and Personal Care Products by Ammonia Oxidizing Bacteria Acclimated in a Membrane Bioreactor: Contributions of Cometabolism and Endogenous Respiration. Sci. Total Environ. 2017, 605–606, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.N.P.; Ngo, H.H.; Guo, W.; Liu, Y.; Woong Chang, S.; Nguyen, D.D.; Zhang, X.; Liang, H.; Xue, S. Selective Carbon Sources and Salinities Enhance Enzymes and Extracellular Polymeric Substances Extrusion of Chlorella sp. for Potential Co-Metabolism. Bioresour. Technol. 2020, 303, 122877. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Show, P.L.; Ngo, H.H.; Ho, S.-H. Algae-Mediated Antibiotic Wastewater Treatment: A Critical Review. Environ. Sci. Ecotechnol. 2022, 9, 100145. [Google Scholar] [CrossRef]
- Qian, Z.; Na, L.; Bao-Long, W.; Tao, Z.; Peng-Fei, M.; Wei-Xiao, Z.; Sraboni, N.Z.; Zheng, M.; Ying-Qi, Z.; Liu, Y. Capabilities and Mechanisms of Microalgae on Nutrients and Florfenicol Removing from Marine Aquaculture Wastewater. J. Environ. Manag. 2022, 320, 115673. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Zabczynski, S.; Göbel, A.; Hoffmann, B.; Löffler, D.; McArdell, C.S.; Ternes, T.A.; Thomsen, A.; Siegrist, H. Biological Degradation of Pharmaceuticals in Municipal Wastewater Treatment: Proposing a Classification Scheme. Water Res. 2006, 40, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of Microalgae Based Technology for the Removal of Antibiotics from Wastewater: A Review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Chen, P.; Addy, M.; Zhang, R.; Deng, X.; Ma, Y.; Cheng, Y.; Hussain, F.; Chen, C.; Liu, Y.; et al. Carbon-Dependent Alleviation of Ammonia Toxicity for Algae Cultivation and Associated Mechanisms Exploration. Bioresour. Technol. 2018, 249, 99–107. [Google Scholar] [CrossRef] [PubMed]
- MtibaÃ, R.; Ezzanad, A.; Aranda, E.; Pozo, C.; Ghariani, B.; Moraga, J.; Nasri, M. Biodegradation and Toxicity Reduction of Nonylphenol, 4-Tert-Octylphenol and 2,4-Dichlorophenol by the Ascomycetous Fungus Thielavia Sp HJ22: Identification of Fungal Metabolites and Proposal of a Putative Pathway. Sci. Total Environ. 2020, 708, 135129. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular Polymeric Substances (EPS) of Microbial Aggregates in Biological Wastewater Treatment Systems: A Review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhao, F.; Feng, J.; Liu, Q.; Nan, F.; Xie, S. Extraction of Extracellular Polymeric Substances (EPS) from a Newly Isolated Self-Flocculating Microalga Neocystis Mucosa SX with Different Methods. Algal Res. 2019, 40, 101479. [Google Scholar] [CrossRef]
- Guo, X.; Yuan, D.; Li, Q.; Jiang, J.; Chen, F.; Zhang, H. Spectroscopic Techniques for Quantitative Characterization of Cu (II) and Hg (II) Complexation by Dissolved Organic Matter from Lake Sediment in Arid and Semi-Arid Region. Ecotoxicol. Environ. Saf. 2012, 85, 144–150. [Google Scholar] [CrossRef] [PubMed]
- You, G.; Hou, J.; Xu, Y.; Wang, C.; Wang, P.; Miao, L.; Ao, Y.; Li, Y.; Lv, B. Effects of CeO2 Nanoparticles on Production and Physicochemical Characteristics of Extracellular Polymeric Substances in Biofilms in Sequencing Batch Biofilm Reactor. Bioresour. Technol. 2015, 194, 91–98. [Google Scholar] [CrossRef]
- Xin, K.; Chen, X.; Zhang, Z.; Zhang, Z.; Pang, H.; Yang, J.; Jiang, H.; Lu, J. Trace Antibiotics Increase the Risk of Antibiotic Resistance Genes Transmission by Regulating the Biofilm Extracellular Polymeric Substances and Microbial Community in the Sewer. J. Hazard. Mater. 2022, 432, 128634. [Google Scholar] [CrossRef]
- Xu, L.-Z.-J.; Wu, J.; Xia, W.-J.; Jin, L.-Y.; Zhao, Y.-H.; Fan, N.-S.; Huang, B.-C.; Jin, R.-C. Adaption and Restoration of Anammox Biomass to Cd(II) Stress: Performance, Extracellular Polymeric Substance and Microbial Community. Bioresour. Technol. 2019, 290, 121766. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wu, J.; Du, W.; Nafees, M.; Yin, Y.; Ji, R.; Banwart, S.A.; Guo, H. Response of Soil Bacterial Communities to Sulfadiazine Present in Manure: Protection and Adaptation Mechanisms of Extracellular Polymeric Substances. J. Hazard. Mater. 2021, 408, 124887. [Google Scholar] [CrossRef] [PubMed]
- Dao, G.-H.; Wu, G.-X.; Wang, X.-X.; Zhang, T.-Y.; Zhan, X.-M.; Hu, H.-Y. Enhanced Microalgae Growth through Stimulated Secretion of Indole Acetic Acid by Symbiotic Bacteria. Algal Res. 2018, 33, 345–351. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.; Hill, R.T. Microalgal and Bacterial Auxin Biosynthesis: Implications for Algal Biotechnology. Curr. Opin. Biotechnol. 2022, 73, 300–307. [Google Scholar] [CrossRef]
- Shasaltaneh, M.D.; Moosavi-Nejad, Z.; Gharavi, S.; Fooladi, J. Cane Molasses as a Source of Precursors in the Bioproduction of Tryptophan by Bacillus Subtilis. Iran. J. Microbiol. 2013, 5, 285–292. [Google Scholar]
- Cao, X.; Wang, X.; Chen, H.; Li, H.; Tariq, M.; Wang, C.; Zhou, Y.; Liu, Y. Neurotoxicity of Nonylphenol Exposure on Caenorhabditis Elegans Induced by Reactive Oxidative Species and Disturbance Synthesis of Serotonin. Environ. Pollut. 2019, 244, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Luo, X.; Lv, Y.; Wu, L.; Hu, Z.; Huang, W.; Zhan, S.; Shen, X.; Hui, C.; Yu, D.; et al. Essential Oils Ameliorate the Intestinal Damages Induced by Nonylphenol Exposure by Modulating Tryptophan Metabolism and Activating Aryl Hydrocarbon Receptor via Gut Microbiota Regulation. Chemosphere 2024, 362, 142571. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.-P.; Han, B.; Yu, X. Coupling of Abiotic Stresses and Phytohormones for the Production of Lipids and High-Value by-Products by Microalgae: A Review. Bioresour. Technol. 2019, 274, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, B.; Gupta, B.; Thakur, I.S. Biosorption of Cr (VI) from Aqueous Solution by Extracellular Polymeric Substances (EPS) Produced by Parapedobacter sp. ISTM3 Strain Isolated from Mawsmai Cave, Meghalaya, India. Environ. Res. 2020, 191, 110064. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Zhao, L.; Li, Y.; Xing, G.; Chen, D.; Yang, Y. Glucose as a Metabolic Enhancer: Promoting Nonylphenol Detoxification by Chlorella pyrenoidosa. Water 2025, 17, 244. https://doi.org/10.3390/w17020244
Yuan J, Zhao L, Li Y, Xing G, Chen D, Yang Y. Glucose as a Metabolic Enhancer: Promoting Nonylphenol Detoxification by Chlorella pyrenoidosa. Water. 2025; 17(2):244. https://doi.org/10.3390/w17020244
Chicago/Turabian StyleYuan, Jinrui, Lin Zhao, Yanting Li, Guodong Xing, Danning Chen, and Yongkui Yang. 2025. "Glucose as a Metabolic Enhancer: Promoting Nonylphenol Detoxification by Chlorella pyrenoidosa" Water 17, no. 2: 244. https://doi.org/10.3390/w17020244
APA StyleYuan, J., Zhao, L., Li, Y., Xing, G., Chen, D., & Yang, Y. (2025). Glucose as a Metabolic Enhancer: Promoting Nonylphenol Detoxification by Chlorella pyrenoidosa. Water, 17(2), 244. https://doi.org/10.3390/w17020244





