Material Conversion, Microbial Community Composition, and Metabolic Functional Succession During Algal Sludge Composting
Abstract
1. Introduction
2. Materials and Methods
2.1. Composting Process and Sample Collection
2.2. Physicochemical Analysis
2.3. DNA Extraction, Sequencing, and Sequence Analysis
2.4. Statistical Analyses
3. Results and Discussion
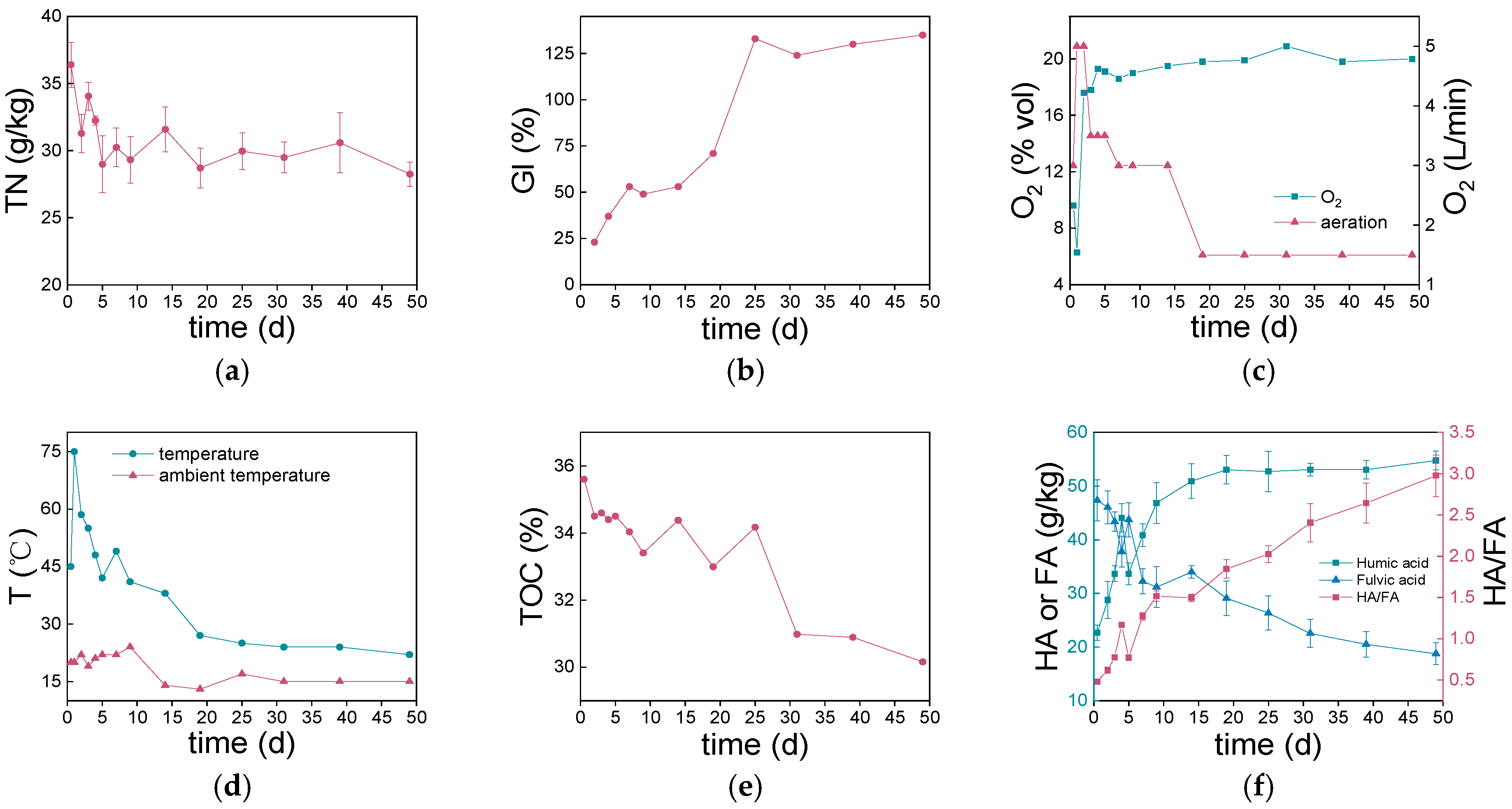
3.1. Variations of Physicochemical Properties
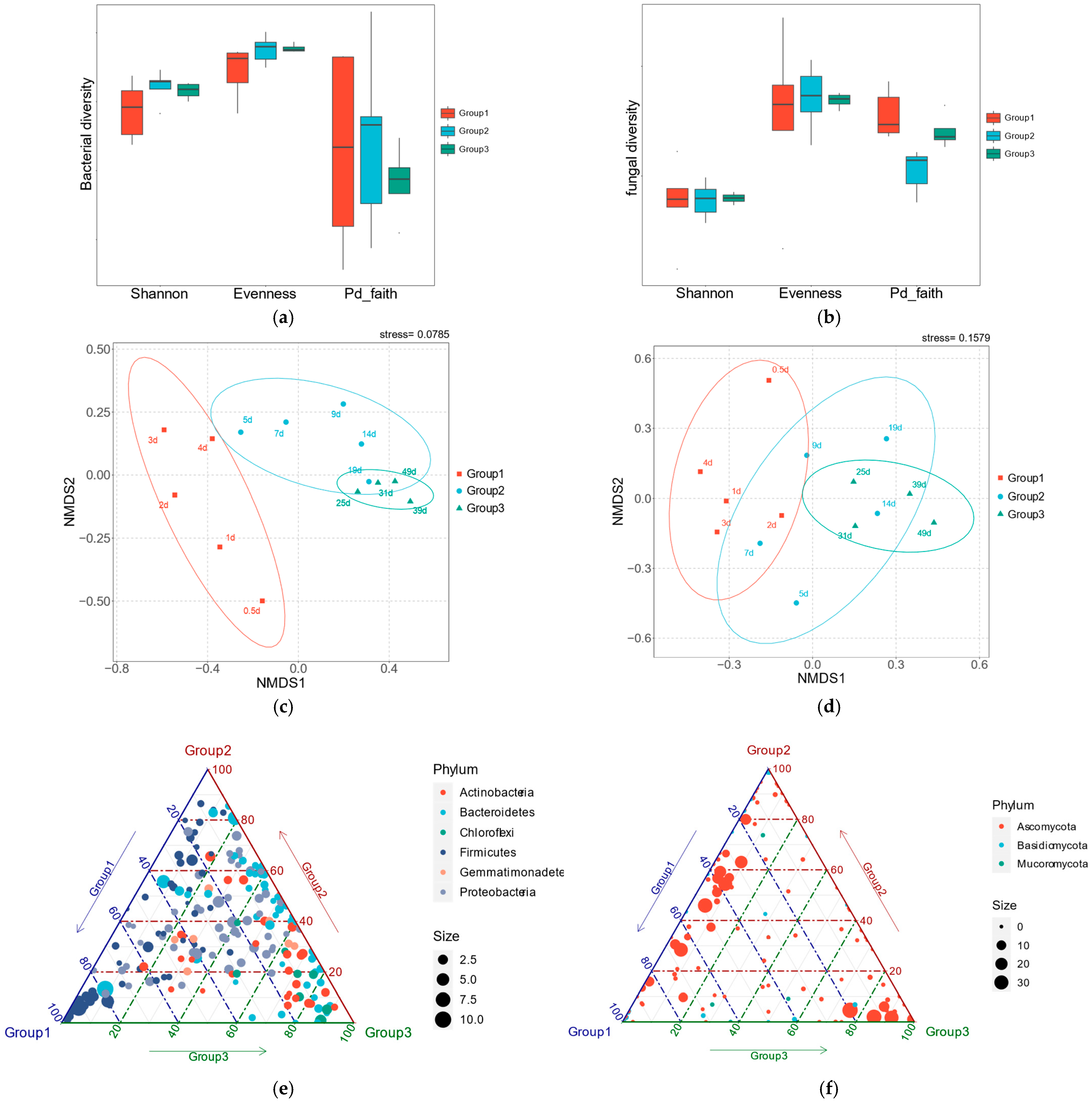
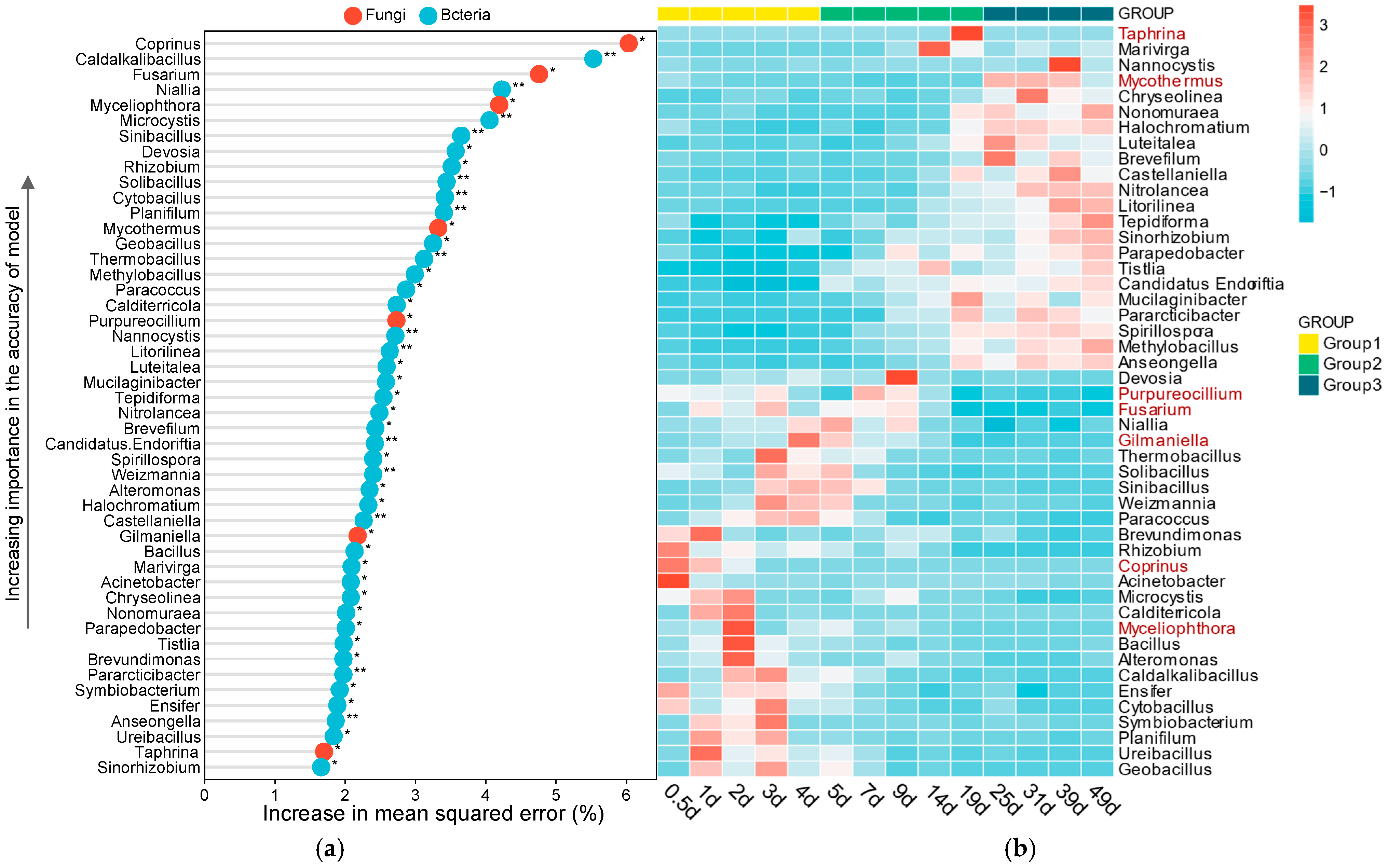
3.2. Microbial Succession During Composting
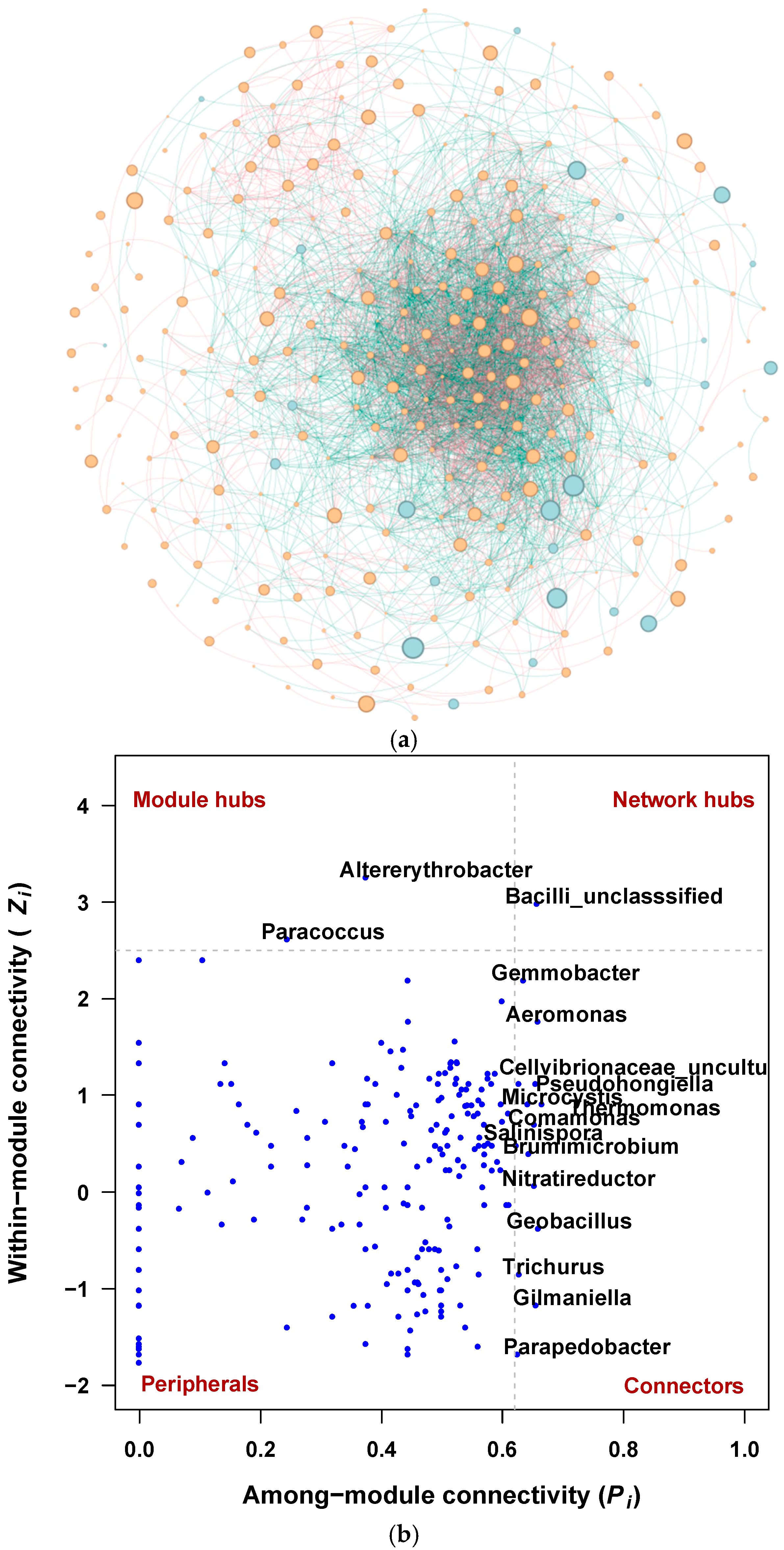
3.3. Interactions Between Bacterial and Fungal Communities
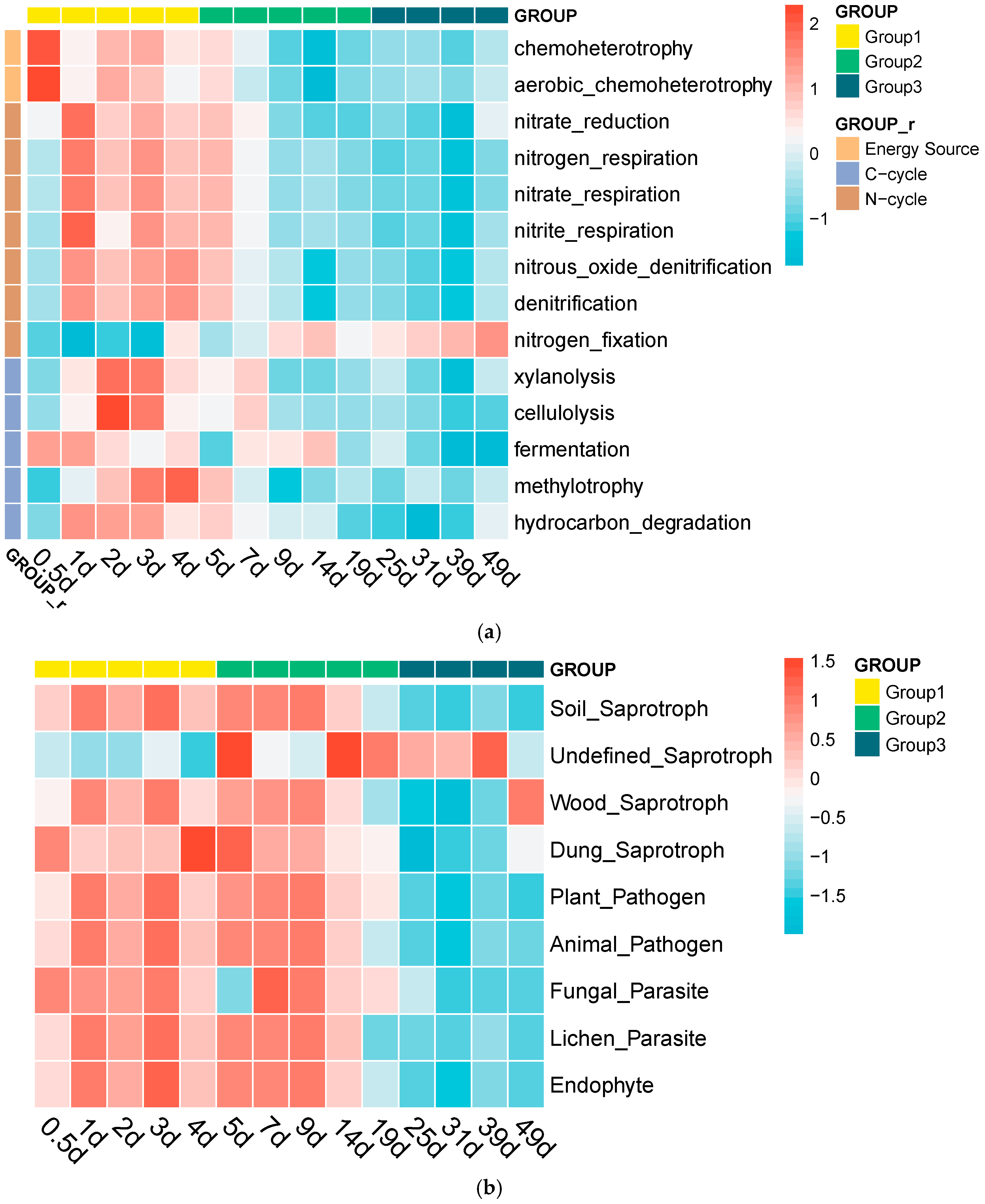
3.4. Microbial Metabolic Functions During Composting
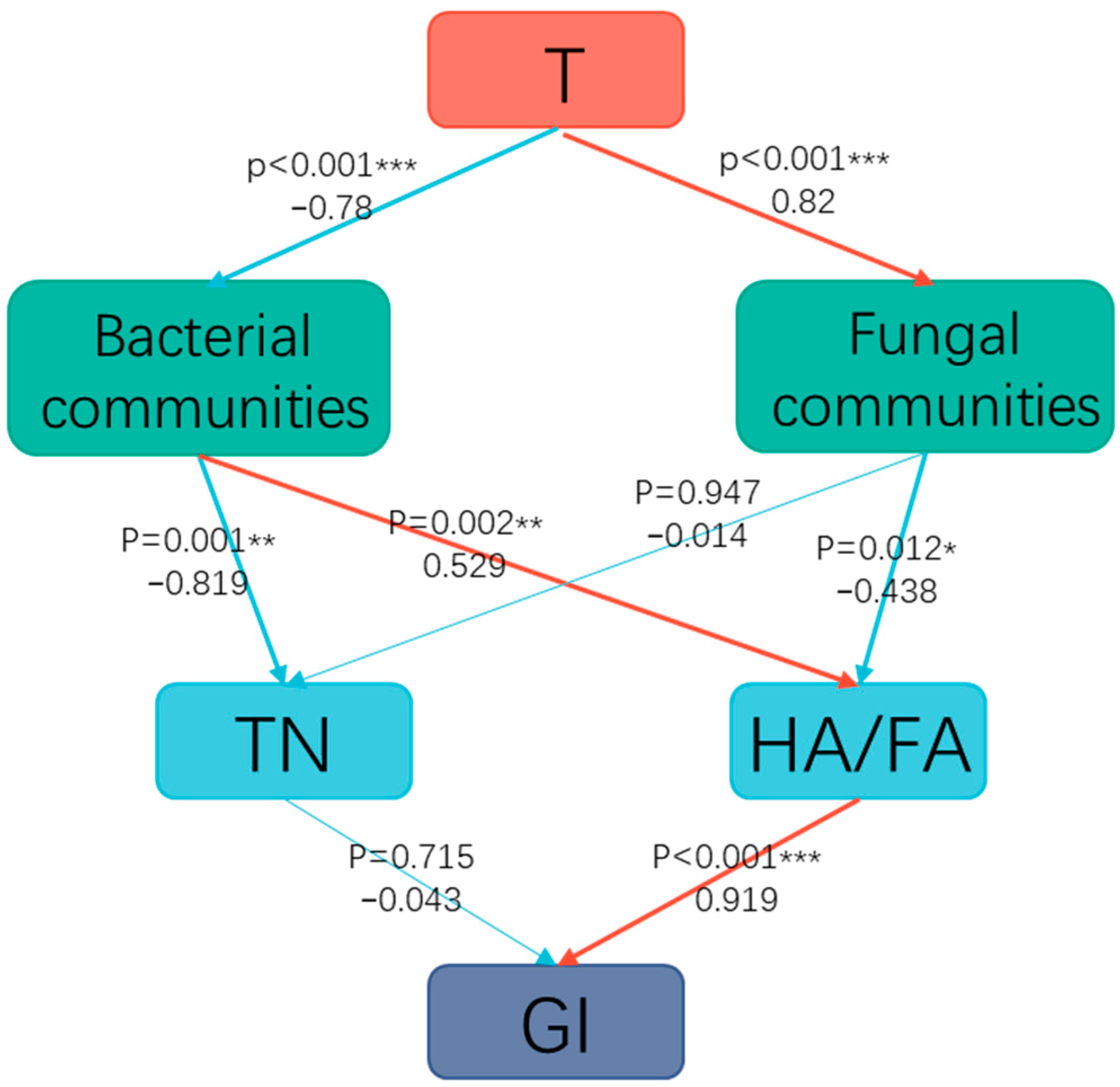
3.5. Relationship Between Physicochemical Characteristics and Microorganisms During Composting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Wang, X.; Li, S.; Bai, Z.; Ma, L. Advanced Composting Technologies Promotes Environmental Benefits and Eco-Efficiency: A Life Cycle Assessment. Bioresour. Technol. 2022, 346, 126576. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Sarsaiya, S.; Wang, Q.; Wang, M.; Chen, H.; Ren, X.; Kumar, S.; Zhang, Z. Mitigation of Global Warming Potential for Cleaner Composting. In Biosynthetic Technology and Environmental Challenges; Varjani, S.J., Parameswaran, B., Kumar, S., Khare, S.K., Eds.; Springer: Singapore, 2018; pp. 271–305. ISBN 978-981-10-7434-9. [Google Scholar]
- Huhe, J.C.; Wu, Y.; Cheng, Y. Bacterial and Fungal Communities and Contribution of Physicochemical Factors during Cattle Farm Waste Composting. MicrobiologyOpen 2017, 6, e00518. [Google Scholar] [CrossRef]
- Wang, X.; Wan, J.; Jiang, G.; Yang, T.; Banerjee, S.; Wei, Z.; Mei, X.; Friman, V.-P.; Xu, Y.; Shen, Q. Compositional and Functional Succession of Bacterial and Fungal Communities Is Associated with Changes in Abiotic Properties during Pig Manure Composting. Waste Manag. 2021, 131, 350–358. [Google Scholar] [CrossRef]
- Langarica-Fuentes, A.; Zafar, U.; Heyworth, A.; Brown, T.; Fox, G.; Robson, G.D. Fungal Succession in an In-Vessel Composting System Characterized Using 454 Pyrosequencing. FEMS Microbiol. Ecol. 2014, 88, 296–308. [Google Scholar] [CrossRef]
- Mao, H.; Wang, K.; Wang, Z.; Peng, J.; Ren, N. Metabolic Function, Trophic Mode, Organics Degradation Ability and Influence Factor of Bacterial and Fungal Communities in Chicken Manure Composting. Bioresour. Technol. 2020, 302, 122883. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, H.; Wang, B.; Chen, C.; Zou, X.; Cheng, T.; Li, J. Aerobic Co-Composting of Mature Compost with Cattle Manure: Organic Matter Conversion and Microbial Community Characterization. Bioresour. Technol. 2023, 382, 129187. [Google Scholar] [CrossRef]
- Peng, T.; Tang, Y.; Cai, D.; Gu, Y.; Wei, J.; Zhang, J.; Ni, J.; Liu, J.; Ren, X.; Pan, J.; et al. Insights into the Interaction Mechanisms between Microcystin-Degrading Bacteria and Microcystis Aeruginosa. Water Res. 2024, 265, 122241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, X.; Lv, Y.; Zhu, W.; Zhang, H.; Ding, J.; Zhang, X.; Zhu, J.; Ding, Y. Research Progress on Influencing Factors on Compost Maturity and Cyanobacteria Toxin Degradation during Aerobic Cyanobacteria Composting: A Review. Environ. Sci. Pollut. Res. 2022, 29, 70635–70657. [Google Scholar] [CrossRef]
- Hunter, P.D.; Hanley, N.; Czajkowski, M.; Mearns, K.; Tyler, A.N.; Carvalho, L.; Codd, G.A. The Effect of Risk Perception on Public Preferences and Willingness to Pay for Reductions in the Health Risks Posed by Toxic Cyanobacterial Blooms. Sci. Total Environ. 2012, 426, 32–44. [Google Scholar] [CrossRef]
- Li, P.; Cai, Y.; Shi, L.; Geng, L.; Xing, P.; Yu, Y.; Kong, F.; Wang, Y. Microbial Degradation and Preliminary Chemical Characterization of Microcystis Exopolysaccharides from a Cyanobacterial Water Bloom of Lake Taihu. Int. Rev. Hydrobiol. 2009, 94, 645–655. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, M.; Bian, B.; Yang, Z.; Yang, W.; Zhang, L. Full-Scale Thermophilic Aerobic Co-Composting of Blue-Green Algae Sludge with Livestock Faeces and Straw. Sci. Total Environ. 2021, 753, 142079. [Google Scholar] [CrossRef]
- Jiang, J.; Du, J.; Chang, Z.; Jin, H. Changes of Contents of Nutrients and Microcystins during Composting of Blue Algae. Jiangsu J. Agric. Sci. 2012, 28, 314–319. [Google Scholar]
- Si, X.; Tang, S.; Zhao, X.; Wang, S.; Li, Y. Development of Selenium—Enriched Cyanobacteria Organic Fertilizer and Its Application in Bok Choy Planting. Jiangsu J. Agric. Sci. 2021, 37, 340–347. [Google Scholar] [CrossRef]
- Chang, H.; Zhu, X.; Wu, J.; Guo, D.; Zhang, L.; Feng, Y. Dynamics of Microbial Diversity during the Composting of Agricultural Straw. J. Integr. Agric. 2021, 20, 1121–1136. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Bertilsson, S.; Zhang, W.; Wang, H.; Cong, H.; Cheng, H. Integrating Experiments with Modeling to Understand Key Bacterial Taxa Dynamics after Episodic Mixing of a Stratified Water Column. J. Environ. Manag. 2024, 365, 121651. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, S.; Zhou, J.; Cong, H.; Feng, S.; Sun, F. Relative Contribution of Fungal Communities to Carbon Loss and Humification Process in Algal Sludge Aerobic Composting. Water 2024, 16, 1084. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular Ecological Network Analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Yang, X.; Liu, E.; Zhu, X.; Wang, H.; Liu, H.; Liu, X.; Dong, W. Impact of Composting Methods on Nitrogen Retention and Losses during Dairy Manure Composting. Int. J. Environ. Res. Public Health 2019, 16, 3324. [Google Scholar] [CrossRef]
- Duan, M.; Gu, J.; Wang, X.; Li, Y.; Zhang, S.; Yin, Y.; Zhang, R. Effects of Genetically Modified Cotton Stalks on Antibiotic Resistance Genes, intI1, and intI2 during Pig Manure Composting. Ecotoxicol. Environ. Saf. 2018, 147, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz, N. A Systematic Review of Biochar Use in Animal Waste Composting. Waste Manag. 2019, 88, 291–300. [Google Scholar] [CrossRef]
- Lin, C.; Cheruiyot, N.K.; Bui, X.T.; Ngo, H.H. Composting and Its Application in Bioremediation of Organic Contaminants. Bioengineered 2022, 13, 1073–1089. [Google Scholar] [CrossRef]
- Tran, H.-T.; Lin, C.; Bui, X.-T.; Ngo, H.-H.; Cheruiyot, N.K.; Hoang, H.-G.; Vu, C.-T. Aerobic Composting Remediation of Petroleum Hydrocarbon-Contaminated Soil. Current and Future Perspectives. Sci. Total Environ. 2021, 753, 142250. [Google Scholar] [CrossRef]
- Abdellah, Y.A.Y.; Li, T.; Chen, X.; Cheng, Y.; Sun, S.; Wang, Y.; Jiang, C.; Zang, H.; Li, C. Role of Psychrotrophic Fungal Strains in Accelerating and Enhancing the Maturity of Pig Manure Composting under Low-Temperature Conditions. Bioresour. Technol. 2021, 320, 124402. [Google Scholar] [CrossRef] [PubMed]
- Gou, C.; Wang, Y.; Zhang, X.; Lou, Y.; Gao, Y. Inoculation with a Psychrotrophic-Thermophilic Complex Microbial Agent Accelerates Onset and Promotes Maturity of Dairy Manure-Rice Straw Composting under Cold Climate Conditions. Bioresour. Technol. 2017, 243, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Dias, B.O.; Silva, C.A.; Higashikawa, F.S.; Roig, A.; Sánchez-Monedero, M.A. Use of Biochar as Bulking Agent for the Composting of Poultry Manure: Effect on Organic Matter Degradation and Humification. Bioresour. Technol. 2010, 101, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Evaluation of Humic Substances during Co-Composting of Food Waste, Sawdust and Chinese Medicinal Herbal Residues. Bioresour. Technol. 2014, 168, 229–234. [Google Scholar] [CrossRef]
- Jiménez, E.I.; García, V.P. Determination of Maturity Indices for City Refuse Composts. Agric. Ecosyst. Environ. 1992, 38, 331–343. [Google Scholar] [CrossRef]
- Antunes, L.P.; Martins, L.F.; Pereira, R.V.; Thomas, A.M.; Barbosa, D.; Lemos, L.N.; Silva, G.M.M.; Moura, L.M.S.; Epamino, G.W.C.; Digiampietri, L.A.; et al. Microbial Community Structure and Dynamics in Thermophilic Composting Viewed through Metagenomics and Metatranscriptomics. Sci. Rep. 2016, 6, 38915. [Google Scholar] [CrossRef]
- Dalmasso, G.; Beyrouthy, R.; Brugiroux, S.; Ruppé, E.; Guillouard, L.; Bonnin, V.; Saint-Sardos, P.; Ghozlane, A.; Gaumet, V.; Barnich, N.; et al. Correction: Genes Mcr Improve the Intestinal Fitness of Pathogenic E. coli and Balance Their Lifestyle to Commensalism. Microbiome 2023, 11, 23. [Google Scholar] [CrossRef]
- Zhong, X.-Z.; Ma, S.-C.; Wang, S.-P.; Wang, T.-T.; Sun, Z.-Y.; Tang, Y.-Q.; Deng, Y.; Kida, K. A Comparative Study of Composting the Solid Fraction of Dairy Manure with or without Bulking Material: Performance and Microbial Community Dynamics. Bioresour. Technol. 2018, 247, 443–452. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, T.; Liu, X.; Zhang, W. Simultaneous Oxidation and Adsorption of As(III) from Water by Cerium Modified Chitosan Ultrafine Nanobiosorbent. J. Hazard. Mater. 2016, 308, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gharechahi, J.; Salekdeh, G.H. A Metagenomic Analysis of the Camel Rumen’s Microbiome Identifies the Major Microbes Responsible for Lignocellulose Degradation and Fermentation. Biotechnol. Biofuels 2018, 11, 216. [Google Scholar] [CrossRef]
- Steger, K.; Jarvis, Å.; Smårs, S.; Sundh, I. Comparison of Signature Lipid Methods to Determine Microbial Community Structure in Compost. J. Microbiol. Methods 2003, 55, 371–382. [Google Scholar] [CrossRef]
- Wei, H.; Wang, L.; Hassan, M.; Xie, B. Succession of the Functional Microbial Communities and the Metabolic Functions in Maize Straw Composting Process. Bioresour. Technol. 2018, 256, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Sun, Q.; Xu, D.; Zhang, Z.; Chen, D.; Li, C.; Shen, Q.; Shen, B. Succession of Bacterial Communities during Composting Process as Detected by 16S rRNA Clone Libraries Analysis. Int. Biodeterior. Biodegrad. 2013, 78, 58–66. [Google Scholar] [CrossRef]
- Finore, I.; Feola, A.; Russo, L.; Cattaneo, A.; Di Donato, P.; Nicolaus, B.; Poli, A.; Romano, I. Thermophilic Bacteria and Their Thermozymes in Composting Processes: A Review. Chem. Biol. Technol. Agric. 2023, 10, 7. [Google Scholar] [CrossRef]
- Rathinam, N.K.; Bibra, M.; Salem, D.R.; Sani, R.K. Bioelectrochemical Approach for Enhancing Lignocellulose Degradation and Biofilm Formation in Geobacillus Strain WSUCF1. Bioresour. Technol. 2020, 295, 122271. [Google Scholar] [CrossRef]
- Qin, X.; Zou, J.; Yang, K.; Li, J.; Wang, X.; Tu, T.; Wang, Y.; Yao, B.; Huang, H.; Luo, H. Deciphering the Efficient Cellulose Degradation by the Thermophilic Fungus Myceliophthora thermophila Focused on the Synergistic Action of Glycoside Hydrolases and Lytic Polysaccharide Monooxygenases. Bioresour. Technol. 2022, 364, 128027. [Google Scholar] [CrossRef]
- Muhammad, N.; Avila, F.; Kim, S.-G. Comparative Genome Analysis of the Genus Marivirga and Proposal of Two Novel Marine Species: Marivirga arenosa sp. nov., and Marivirga salinae sp. nov. BMC Microbiol. 2024, 24, 245. [Google Scholar] [CrossRef]
- Azim, K.; Elbakhouch, Y.; Tabrika, I.; Elame, F.; Bouizgarne, B.; Aboutayeb, R.; Dihazi, A. Suppressive Effect of Different Compost Extracts Against Fusarium oxysporum f. sp. ciceri: Causal Agent of Fusarium Wilt of Chickpea (Cicer arietinum). In Microbial Biotechnology for Sustainable Agriculture; Springer: Singapore, 2024; Volume 2, pp. 309–330. ISBN 978-981-9723-55-3. [Google Scholar]
- Akhter, A.; Hage-Ahmed, K.; Soja, G.; Steinkellner, S. Potential of Fusarium Wilt-Inducing Chlamydospores, in Vitro Behaviour in Root Exudates and Physiology of Tomato in Biochar and Compost Amended Soil. Plant Soil 2016, 406, 425–440. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, Y.; Wang, Z.; Diao, C.; Liu, Z. The Fungi–Bacteria Interaction Mechanism of Microbial Consortium During Efficient Lignin Degradation Based on Metabolomics Analysis. Molecules 2025, 30, 508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q. Competition and Interaction between DNRA and Denitrification in Composting Ecosystems: Insights from Metagenomic Analysis. Bioresour. Technol. 2023, 381, 129140. [Google Scholar] [CrossRef]
- Carlson, C.; Singh, N.K.; Bibra, M.; Sani, R.K.; Venkateswaran, K. Pervasiveness of UVC254-Resistant Geobacillus Strains in Extreme Environments. Appl. Microbiol. Biotechnol. 2018, 102, 1869–1887. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lin, Y.; Liu, C.; Zhang, H.; Lin, C. Improving the Utilization of Flammulina velutipes Waste during Biochar-Amended Composting: Emphasis on Bacterial Communities. Agronomy 2024, 14, 1046. [Google Scholar] [CrossRef]
- Yang, S.-H.; Seo, H.-S.; Oh, H.-M.; Kim, S.-J.; Lee, J.-H.; Kwon, K.K. Brumimicrobium mesophilum sp. nov., Isolated from a Tidal Flat Sediment, and Emended Descriptions of the Genus Brumimicrobium and Brumimicrobium glaciale. Int. J. Syst. Evol. Microbiol. 2013, 63, 1105–1110. [Google Scholar] [CrossRef]
- Jurado, M.M.; Suárez-Estrella, F.; Vargas-García, M.C.; López, M.J.; López-González, J.A.; Moreno, J. Evolution of Enzymatic Activities and Carbon Fractions throughout Composting of Plant Waste. J. Environ. Manag. 2014, 133, 355–364. [Google Scholar] [CrossRef]
- Motyl, M.R.; McKinley, G.; Janda, J.M. In Vitro Susceptibilities of Aeromonas Hydrophila, Aeromonas Sobria, and Aeromonas Caviae to 22 Antimicrobial Agents. Antimicrob. Agents Chemother. 1985, 28, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Davó, A.; Dias, T.; Gomes, S.E.; Rodrigues, S.; Parera-Valadez, Y.; Borralho, P.M.; Pereira, F.; Rodrigues, C.M.P.; Santos-Sanches, I.; Gaudêncio, S.P. The Madeira Archipelago As a Significant Source of Marine-Derived Actinomycete Diversity with Anticancer and Antimicrobial Potential. Front. Microbiol. 2016, 7, 1594. [Google Scholar] [CrossRef]
- Leadbeater, D.R.; Oates, N.C.; Bennett, J.P.; Li, Y.; Dowle, A.A.; Taylor, J.D.; Alponti, J.S.; Setchfield, A.T.; Alessi, A.M.; Helgason, T.; et al. Mechanistic Strategies of Microbial Communities Regulating Lignocellulose Deconstruction in a UK Salt Marsh. Microbiome 2021, 9, 48. [Google Scholar] [CrossRef]
- Ruben, M.; Marchant, H.; Wietz, M.; Gentz, T.; Strauss, J.; Koch, B.P.; Mollenhauer, G. Microbial Communities Degrade Ancient Permafrost-Derived Organic Matter in Arctic Seawater. J. Geophys. Res. Biogeosci. 2024, 129, e2023JG007936. [Google Scholar] [CrossRef]
- Friedrich, J.; Gradišar, H.; Mandin, D.; Chaumont, J.P. Screening Fungi for Synthesis of Keratinolytic Enzymes. Lett. Appl. Microbiol. 1999, 28, 127–130. [Google Scholar] [CrossRef]
- Aoki, M.; Andoh, T.; Ueki, T.; Masuyoshi, S.; Sugawara, K.; Oki, T. BU-4794F, A New β-1, 3-Glucan Synthase Inhibitor. J. Antibiot. 1993, 46, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Cha, I.-T.; Song, E.-J.; Seok, Y.J.; Lee, H.; Park, I.; Lee, Y.K.; Roh, S.W.; Choi, H.-J.; Nam, Y.-D.; Seo, M.-J. Draft Genome Sequence of a Denitrifying Bacterium Paracoccus Marcusii PAMC 22219 Isolated from Arctic Marine Sediment. Mar. Genom. 2015, 21, 27–29. [Google Scholar] [CrossRef]
- Su, J.F.; Yang, S.; Huang, T.L.; Li, M.; Liu, J.R.; Yao, Y.X. Enhancement of the Denitrification in Low C/N Condition and Its Mechanism by a Novel Isolated Comamonas sp. YSF15. Environ. Pollut. 2020, 256, 113294. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Paszti, S.; Takahashi, K.; Toyofuku, M.; Pessi, G.; Eberl, L. Mapping of the Denitrification Pathway in Burkholderia Thailandensis by Genome-Wide Mutant Profiling. J. Bacteriol. 2020, 202, e00304-20. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Shi, G.; Fan, G.; Tong, F.; Liu, L.; Li, J.; Gao, Y. The Role of Symbiotic Nitrogen-Fixing Bacteria, Rhizobium and Sinorhizobium, as “Bridges” in the Rhizosphere of Legumes after Fomesafen Application. Appl. Soil Ecol. 2025, 209, 106013. [Google Scholar] [CrossRef]
- Qin, X.; Bao, R.; Huang, W.; Li, Q. Facilitating the Enzymatic Hydrolysis of Polysaccharides by Carbohydrate Active Enzymes and Enhanced Humification Process with Microbial Consortium Revealed by Metagenomics Analysis during Cow Manure-Straw Composting. J. Environ. Chem. Eng. 2025, 13, 115428. [Google Scholar] [CrossRef]
- Wu, M.; Tao, Y.; Zeng, Q.; Pan, Z.; Zhang, H.; Yin, Z.; Li, W.; Liu, Y.; Li, X.; Qiu, Z. Deciphering the Driving Mechanism of Microbial Community for Rapid Stabilization and Lignocellulose Degradation during Waste Semi-Aerobic Bioreactor Landfilling with Multifunctional Microbial Inoculum. Waste Manag. 2025, 194, 88–103. [Google Scholar] [CrossRef]
- Kulyashov, M.; Peltek, S.E.; Akberdin, I.R. A Genome-Scale Metabolic Model of 2,3-Butanediol Production by Thermophilic Bacteria Geobacillus icigianus. Microorganisms 2020, 8, 1002. [Google Scholar] [CrossRef]
- Shamshitov, A.; Satkevičiūtė, E.; Decorosi, F.; Viti, C.; Supronienė, S. Phenotypic Profiling of Selected Cellulolytic Strains to Develop a Crop Residue-Decomposing Bacterial Consortium. Microorganisms 2025, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Dal Bello, M.; Abreu, C.I. Temperature Structuring of Microbial Communities on a Global Scale. Curr. Opin. Microbiol. 2024, 82, 102558. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Zhu, W.; Zheng, Z.; Wu, H.; Cong, H.; Feng, S. Material Conversion, Microbial Community Composition, and Metabolic Functional Succession During Algal Sludge Composting. Water 2025, 17, 2904. https://doi.org/10.3390/w17192904
Zhou M, Zhu W, Zheng Z, Wu H, Cong H, Feng S. Material Conversion, Microbial Community Composition, and Metabolic Functional Succession During Algal Sludge Composting. Water. 2025; 17(19):2904. https://doi.org/10.3390/w17192904
Chicago/Turabian StyleZhou, Manting, Wenjing Zhu, Zhenrong Zheng, Hainan Wu, Haibing Cong, and Shaoyuan Feng. 2025. "Material Conversion, Microbial Community Composition, and Metabolic Functional Succession During Algal Sludge Composting" Water 17, no. 19: 2904. https://doi.org/10.3390/w17192904
APA StyleZhou, M., Zhu, W., Zheng, Z., Wu, H., Cong, H., & Feng, S. (2025). Material Conversion, Microbial Community Composition, and Metabolic Functional Succession During Algal Sludge Composting. Water, 17(19), 2904. https://doi.org/10.3390/w17192904




