Abstract
Sludge bulking in wastewater treatment is often caused by massive filamentous bacteria. This study aimed to turn such bacteria into a stable system dominated by filamentous biofilms (FBs) by using a continuous flow reactor (CFR) fed with simulated domestic wastewater; to address FBs’ poor solid–liquid separation and uncontrollable sludge retention time (SRT), string carriers were added, SRT was controlled at 30 days, and parameters like mixed liquid suspended solids (MLSS) and sludge volume index (SVI) were monitored. Results showed filamentous Sphaerotilus (68–93% of FBs) self-aggregated as FBs’ reticular skeleton (loose, porous, stable, max 8 cm) with non-filamentous bacteria anchoring; FBs achieved >80% COD/NH4+-N removal despite low MLSS (<1000 mg/L) and SVI > 350 mL/g. The application of carriers increased the proportion of non-filamentous microorganisms to over 80%, reduced SVI to 150–400 mL/g, and increased MLSS to over 2700 mg/L, enabling stable operation. This study challenges the traditional negative perception of filamentous bacteria and opens new prospects for wastewater treatment technology.
1. Introduction
Filamentous bacteria play a complex role in wastewater treatment systems. In the activated sludge process, filamentous bacteria are crucial components of sludge flocs, serving as a skeleton for the attachment of other bacteria via the secreted extracellular polymeric substances (EPS) [1,2,3]. Filamentous bacteria have also been widely mentioned in studies of biofilm and granular sludge formation [4,5,6,7]. Moreover, low levels of filamentous bacteria have been reported to promote the formation [6,7] and stabilization [8,9] of biofilms and aerobic granular sludge systems.
However, negative impacts have been observed with the massive growth of filamentous bacteria in the long-term operation of the above-mentioned processes. In activated sludge, the massive growth of filamentous bacteria can lead to sludge bulking, which is a common threat in wastewater treatment plants around the world [10]. The development of methods to prevent sludge bulking in wastewater treatment has been regarded as an important issue since the invention of the activated sludge process [10,11]. Furthermore, massive growth of filamentous bacteria on the surface of aerobic granular sludge (AGS) causes its gradual filamentation and reduction in the settling performance, and ultimately may lead to system collapse [12].
In fact, the massive growth of filamentous bacteria does not always present a disadvantage to wastewater treatment systems. Conversely, it may have significant potential for wastewater treatment. Slightly bulking could save aeration energy and improve treatment efficiency in the activated sludge system [13]. The structural stability, activity and specific surface area of sludge may be greatly improved after filamentous bacteria grow abundantly in AGS, which in turn may enhance the treatment efficiency [14]. In biofilm research, the phenomenon of filamentous biofilm (FB) formed by the massive growth of filamentous bacteria was also observed, and it was found that the filamentous structure was stable and could exhibit high adsorption efficiency for the pollutants in the wastewater [15].
There are two critical research gaps that remain to be addressed, which severely constrain the practical application of FBs. First, there is a lack of systematic engineering design for FB-dominated systems; existing research treats FBs merely as unintended byproducts of filamentous bacterial overgrowth in existing processes (such as the AGS process or conventional biofilm reactor processes) [14,15], with no studies specifically designing continuous flow reactors (CFRs) dominated by FBs. Second, the core engineering bottlenecks of FBs remain unresolved: their poor solids–liquid separation performance (due to extremely high sludge volume index, SVI) and uncontrollable sludge retention time (SRT) have consistently hindered progress in previous research.
In this study, filamentous biofilms with filamentous bacteria as the skeleton were successfully cultivated in a continuous flow reactor (CFR) and soon became the dominant sludge type in the treatment system. The formation mechanism of FBs was investigated by examining the characteristics, morphological and bacterial community changes during the formation of FBs. And based on the characteristics of FBs, an immobilized culture strategy for FBs was developed by applying string carriers, to achieve effective management and long-term stable operation of FBs in the reactor. This study may promote a breakthrough in the traditional impression of filamentous bacteria in wastewater treatment systems and provide new possibilities for the development and application of novel technologies in wastewater treatment systems.
2. Materials and Methods
2.1. Reactor Setup
As shown in Figure 1, a continuous flow reactor (CFR) was utilized to cultivate FBs. Hydraulic residence time (HRT) for CFR is 3 h. A new CFR was used to cultivate FBs (immobilized) and a small number of FBs were formed after 10 days of operation. A total of 10 days later, string carriers were added to the new CFR. After 50 days of operation, the sludge residence time (SRT) in the reactor was adjusted by removing sludge from the different carriers, and the SRT was maintained at 30 days. Compared with the conventional biofilm carriers, the polyethylene string is characterized by a small diameter (only 0.1 mm), a smooth surface, and a parallel arrangement resulting in a smaller specific surface area and volume. This design not only helps to decrease the overgrowth of attached biofilms, but also reduces the consumption of carrier materials. Furthermore, the flexible structure of FBs allows them to be entangled and stably fixed on the parallel string carriers, and the large gaps between the carriers effectively avoid the clogging problem of traditional carriers. The smaller size of the carriers occupies less space in the reactor, which provides more space for the growth of FBs. This study employed simulated domestic wastewater, with its formulation concentration referenced from actual domestic wastewater collected from sewage pipelines at the Shenzhen campus of Harbin Institute of Technology. The pollutant concentration range of the simulated domestic wastewater is as follows: chemical oxygen demand (COD): 280–350 mg/L; ammonia nitrogen (NH4+-N): 45–55 mg/L; total phosphorus (TP): 4.5–7.2 mg/L.
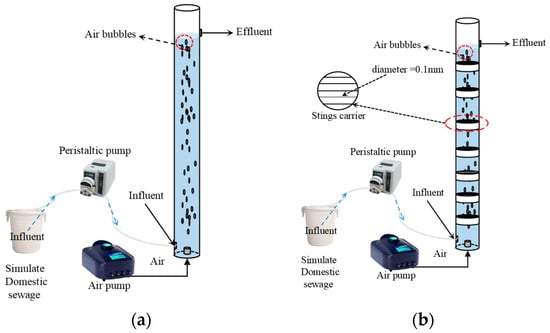
Figure 1.
FBs cultivation process (a) and FBs (immobilized) cultivation process (b).
2.2. Experimental Process
Both the CFR and the filamentous carrier-containing CFR operated for 50 days. Effluent samples were collected from both reactors at 5-day intervals, and sludge samples at 10-day intervals, with subsequent testing of both effluent and sludge.
2.3. Analytical Methods
Water and FBs samples were collected periodically during reactor operation and analyzed for COD, NH4+-N, mixed liquid suspended solids (MLSS), and sludge volume index (SVI) according to standard methods [16]. A thermal extraction procedure was used to isolate the extracellular polymer (EPS) in the FBs [17]. Thirty milliliters of sludge sample was subjected to centrifugation for 10 min at a centrifugal force of 3000× g. After centrifugation, the sludge was resuspended in 30 mL of 0.05% NaCl solution; this resuspended mixture was first sonicated for 2 min at a frequency of 20 kHz, then shaken at 150 rpm for 10 min, and finally subjected to a second sonication treatment for another 2 min. Subsequently, the mixture was centrifuged again for 10 min at 8000× g. The remaining sludge in the centrifuge tube was resuspended once more in 30 mL of 0.05% NaCl solution, followed by sonication for 3 min. After sonication, the mixture was incubated at 80 °C for 30 min, and finally centrifuged for 20 min at 12,000× g; the supernatant from this final centrifugation step was collected as EPS. The EPS composition was assessed by measuring polysaccharide (PS) and protein (PN) contents using the phenol–sulfuric acid method and the Lowry method, respectively [18,19]. The total EPS content was subsequently determined by summing the PS and PN. FBs samples were collected from the CFR on days 10, 30, and 50, and the total DNA from each sample was amplified using the bacterial primers 515 F (5′-GTG CCA GCM GCC GCG G-3′) and 907R (5′-CCG TCA ATT CMT TTR AGT TT-3′) targeting the 16S rRNA gene. All analytical tests (COD, NH4+-N, MLSS, SVI, EPS components) were performed in triplicate (n = 3) for each sample. The data presented in figures are expressed as mean ± standard deviation.
3. Results and Discussion
3.1. Sludge Characteristics
Figure 2 shows the changes in sludge form during reactor operation. By the 10th day of reactor operation, seed sludge was gradually eliminated and FBs began to become dominant as the main sludge form in the system. At the 40th day of operation, the structure of the FBs showed obvious differentiation characteristics.
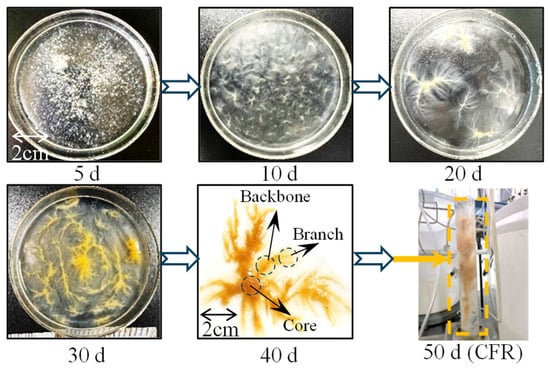
Figure 2.
The formation process of FBs.
The EPS content of the FBs changed significantly during the formation process, as shown in Figure 3a. At the beginning of reactor operation (days 0 to 10), the EPS content rose rapidly to 585 mg/g MLSS, which was significantly higher than that of seed sludge. This may be due to the fact that microorganisms enhance aggregation by secreting large amounts of EPS under unstable operational conditions in the reactor [20]. As the structure of FBs gradually formed, the content of EPS began to gradually decrease to 87 mg/g MLSS at day 55. EPS was more vigorously secreted during the initial growth of sludge, and then tended to stabilize after entering the stabilization period [21]. EPS may play important role in FBs formation. As shown in Figure 3b, the initial mixed liquor suspended solids (MLSS) of the seed sludge was 3450 mg/L. However, on day 10 of reactor operation, the MLSS rapidly decreased to 410 mg/L. This rapid decrease in MLSS was mainly attributed to the fact that the reactor was operated in a continuous flow mode and the sludge–water separation unit was not set. As the size of the FBs increased, the MLSS in the reactor gradually increased, reaching 865 mg/L on day 50. Because of the low MLSS, no sludge discharge operation was performed to maintain stable reactor operation, which also resulted in the inability to calculate the sludge residence time (SRT). SVI is used to measure the settling performance and compressibility of sludge [22]. As shown in Figure 3c, the SVI of the initial seed sludge was about 50 mL/g, indicating great compressibility. At the early stage of reactor operation (day 10), the SVI surged to 2685 mL/g, showing a loose and fluffy sludge structure with poor compressibility. The SVI gradually decreased to 398 mL/g on day 50. It is noteworthy that SVI was always maintained above 350 mL/g, which indicates that FBs have lower compressibility compared to conventional sludge. Adhesion and entanglement of filamentous bacteria with other microorganisms has been reported in other studies [21]. The structure of FBs gradually densified during operation, which in turn led to a gradual decrease in SVI.
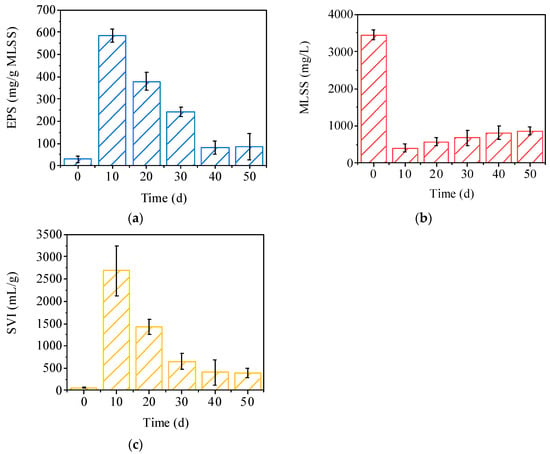
Figure 3.
(a) EPS content during the formation of FBs; (b) MLSS during the formation of FBs; (c) SVI during the formation of FBs.
3.2. Removal Performance
To evaluate the removal capacity of FBs for organic matter and ammonia nitrogen, this study monitored the removal efficiency of ammonia nitrogen (NH4+-N) and chemical oxygen demand (COD) in the reactor. Figure 4a,b shows the variation in removal efficiencies of COD and NH4+-N. The COD removal efficiency fluctuated greatly during the first 20 days of reactor operation, which may be related to the fact that the microbial community was not stabilized at the initial stage of the reactor. As the reactor operation, the COD removal efficiency gradually stabilized and remained above 80% after the 20th day. This indicated that the organic matter removal capacity of the reactor increased significantly with the formation of the FBs. The COD removal may be closely related to the growth of aerobic heterotrophic bacteria in the FBs. Aerobic heterotrophic bacteria effectively reduce COD in water by absorbing organic matter and metabolizing it for decomposition [23]. The removal of NH4+-N by the reactor fluctuated at the beginning of the operation but stabilized above 80% after the 30th day. The significant removal efficiency of FBs on NH4+-N may be related to their increase in biomass and enrichment of ammonia-oxidizing bacteria.
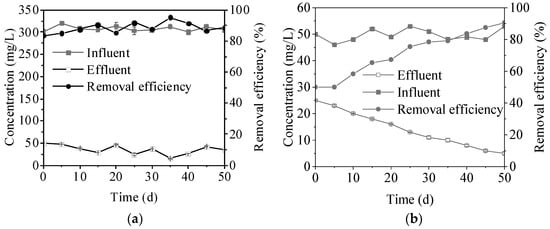
Figure 4.
(a) Variations in COD concentration in influent and effluent samples during the CFR operation; (b) Variations in NH4+-N concentration in influent and effluent samples during the CFR operation.
3.3. Microbial Community
The variation in relative abundance of major genera in the microbial community of FBs is shown in Figure 5, with a decrease in the relative abundance of Sphaerotilus from 92.74% to 68.42%, indicating it may play key role in the formation and stabilization of FBs. Sphaerotilus causes sludge bulking and elevated SVI [24]. In addition, Sphaerotilus can enhance microbial adhesion and floc formation by secreting EPS [25,26]. In conclusion, Sphaerotilus can play a role in the formation and stabilization of FBs by providing an attachment skeleton and secreting EPS, as well as constructing loose structures. With the reactor operation, the microbial community in the FBs underwent significant succession, and the relative abundance of several genera gradually increased. In particular, the abundance of Rhodobacter, Acinetobacter and Gemmobacter gradually increased, indicating that these microorganisms were gradually enriched during the formation and stabilization of FBs. Cloacibacterium and Chryseobacterium have a high abundance in all samples, which may play important roles in the formation and stabilization of FBs. Cloacibacterium is associated with the formation of biofilms and the production of EPS [27,28]. The relative abundance of Cloacibacterium fluctuated between 0.45% and 7.21%. Additionally, the abundance of Chryseobacterium increased significantly from 1.55% to 8.25% from day 10 to day 50. Chryseobacterium is associated with self-aggregation and the production of EPS [29].
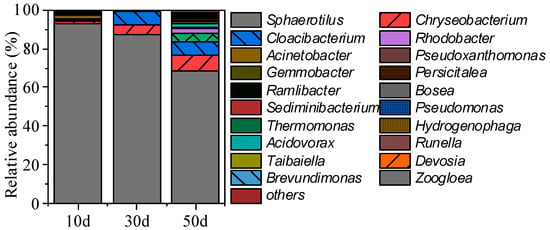
Figure 5.
Changes in microbial community abundance at the genus level in FBs.
3.4. FBs System Construction
In wastewater treatment, rapid sludge–water separation and long-term sludge stabilization are essential to ensure treatment efficiency and system stability. Therefore, it is crucial to design effective cultivation strategies to improve sludge–water separation and achieve rational SRT management. This study proposes an immobilization strategy that is different from the traditional biofilm technology, using polyethylene rope as the immobilization carrier. Compared to MBBR, the immobilized FBs system in this study requires no additional aeration or agitation energy consumption to maintain carrier fluidization. Compared to activated sludge systems, it is easier to cultivate and avoids concerns about sludge bulking caused by filamentous bacteria overgrowth. Compared to traditional biofilm systems, this system requires less immobilized filter media overall, further reducing engineering material costs.
3.4.1. Sludge Properties
Figure 6 demonstrates the change in SVI of immobilized FBs in the reactor, which gradually decreased with the operation and stably maintained below 400 mL/g. Carrier application greatly decreased the SVI, suggesting that carriers promote the compressibility of the FBs structure. It may be attributed to the fact that the carrier enhances filamentous bacterial intertwining and facilitates other microbial aggregation. The SVI of immobilized FBs was still higher than 150 mL/g, indicating that they maintained a relatively loose sludge structure. The loose structure facilitates the transfer of nutrients, oxygen, and cellular secretions in the sludge, thus contributing to the maintenance of FBs stability. As the operation progressed, MLSS gradually increased and reached to 2700 mg/L. Compared to the results of Figure 3b, the MLSS of immobilized FBs was markedly higher than that of FBs, suggesting that carrier application promoted sludge retention through immobilization.
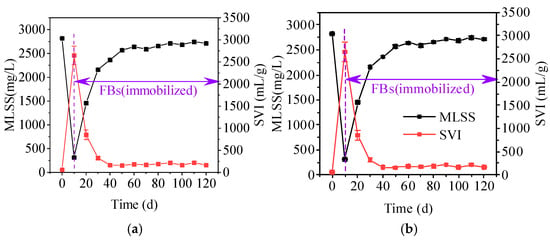
Figure 6.
The changes in sludge properties changes during the treatment of actual domestic wastewater. (a) MLSS; (b) SVI.
3.4.2. Removal Performance of Immobilized FBs
Figure 7a shows the removal efficiency of immobilized FBs for COD. The COD removal efficiency remained stable above 80%, indicating that the immobilized FBs were able to maintain a long-term organic matter treatment effect. Figure 7b demonstrates the NH4+-N removal efficiency of immobilized FBs, which also stabilized above 90% with reactor operation. It was shown that immobilized FBs were able to remove NH4+-N consistently and efficiently. Therefore, the immobilization strategy not only achieves the successful cultivation of FBs in CFRs, but also exhibits stability efficiency in long-term operation. In the future, through parameter optimization, FBs may achieve higher treatment efficiency. Due to variations in hydraulic retention time (HRT) and influent concentration across different studies, it is difficult to directly compare treatment performance with AS and AGS. However, in studies involving AS and AGS, the removal rates for ammonia nitrogen and COD in simulated domestic wastewater typically remain stable between 80% and 100% [30,31].
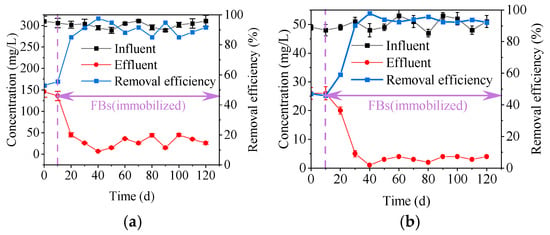
Figure 7.
(a) COD treatment effectiveness; (b) NH4+-N treatment effectiveness.
3.4.3. Microbial Community of Immobilized FBs
The Alpha diversity of immobilized FBs on days 10, 30 and 50 is shown in Figure 8a,b. From day 10 to day 50, the Shannon value gradually increased, indicating that the diversity of microorganisms in immobilized FBs was increasing. As CFR operation, Chao values increased, indicating the gradual increase in community richness in immobilized FBs. In addition, the increase in Chao values of immobilized FBs was higher than that of FBs, indicating that string carriers have a positive role in promoting microbial immobilization and growth.
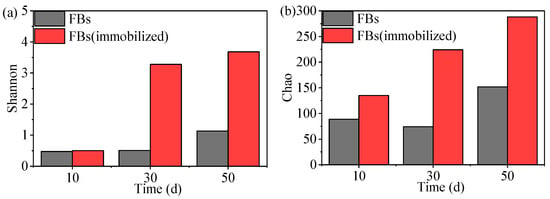
Figure 8.
Dynamics of alpha diversity indices (a) Shannon; (b) Chao in FBs.
Figure 9 shows changes in the relative abundance of the bacterial genera. The relative abundance of microorganisms changed significantly as the reactor operation was carried out. This suggests that the community underwent significant succession during the operation. After immobilization, the relative abundance of Sphaerotilus decreased significantly and remained below 20% after day 30. This suggests that the application of the carrier greatly facilitated the growth and aggregation of non-filamentous microorganisms. The regulatory effect of polyethylene rope carriers on FBs systems and microorganisms manifests across two interconnected dimensions: On the one hand, it provides abundant attachment sites for non-filamentous microorganisms, significantly enhancing their fixation and growth during the early cultivation phase through these sites; on the other hand, the carrier promotes the formation of denser FBs structures. These structures create localized anoxic microenvironments, establishing suitable conditions for the growth and metabolism of denitrifying bacteria, thereby driving their rapid proliferation. In addition, this carrier-induced enrichment of non-filamentous microorganisms may enable FBs systems to overcome the risks of ecological instability or vulnerability to disturbances that are associated with long-term dependence on a single dominant species (e.g., Sphaerotilus in non-immobilized FBs).
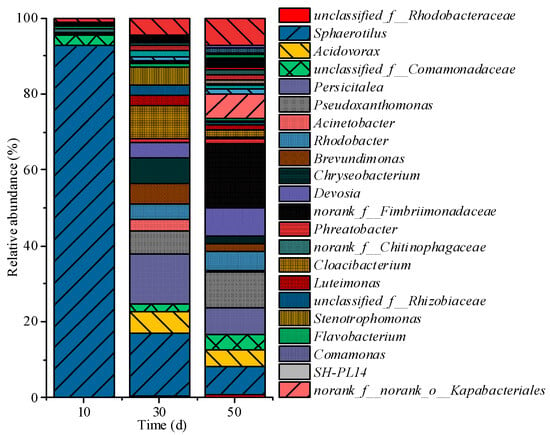
Figure 9.
Dynamics of genus-level microbial community abundance.
4. Scalability Potential, Limitations, and Linkages to the Biocircular Economy
The polyethylene rope carriers used in this study is low-cost and readily available, significantly reducing engineering investment costs for large-scale system deployment. Combined with a simple continuous-flow reactor design, this system can be flexibly adapted to municipal and industrial wastewater treatment scenarios. However, current research has only preliminarily identified the potential application advantages of FBs carriers. Numerous issues require further investigation, such as verifying the system’s long-term operational stability (>1 year) and optimizing reactor operating modes and structural designs. Additionally, FBs carriers inherently possess high EPS content, a characteristic that demonstrates significant potential for subsequent sludge resource utilization, including carbon source recovery and bioflocculant preparation.
5. Conclusions
The massive growth of filamentous bacteria can form a wastewater treatment system dominated by FBs in the CFR. FBs have an SVI value of more than 350 mL/g and a flexible, loose, and porous morphology, and they have good removal performance, removing more than 80% of the COD and NH4+-N. The filamentous bacteria consist of the skeleton of the FBs, and other bacteria adhere to the skeleton by secreting EPS. According to the sludge characteristics of FBs, the immobilization culture strategy of FBs was established, and the immobilized FBs could be effectively managed and showed stable treatment effects. In conclusion, this study can help to break through the traditional conception of the phenomenon of filamentous bacteria mass growth in wastewater treatment systems. It provides a possibility for the development and application of novel technologies in wastewater treatment.
Author Contributions
Writing—original draft preparation, T.S.; writing—review and editing, J.L. and X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Inner Mongolia Autonomous Region Natural Science Foundation Project, grant number 2025QN05057; the Shenzhen Science and Technology Innovation Commission, grant number GJHZ20220913143007014; and the Postdoctoral Fellowship Program of CPSF, grant number GZC20240763.
Data Availability Statement
The data in this study have been explained in the article. For detailed data, please contact the first author or corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilén, B.M.; Jin, B.; Lant, P. The influence of key chemical constituents in activated sludge on surface and flocculating properties. Water Res. 2003, 37, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, L.; Li, H.; Fang, F.; Yan, P.; Chen, Y.; Guo, J.; Ma, T.; Shen, Y. The branched chains and branching degree of exopolysaccharides affecting the stability of anammox granular sludge. Water Res. 2020, 178, 115818. [Google Scholar] [CrossRef]
- Sezgin, M.; Jenkins, D.; Parker, D.S. A unified theory of filamentous activated sludge bulking. J. Water Pollut. Control. Fed. 1978, 50, 362–381. [Google Scholar]
- Liang, Y.; Pan, Z.; Guo, T.; Feng, H.; Yan, A.; Ni, Y.; Li, J. Filamentous bacteria and stalked ciliates for the stable structure of aerobic granular sludge treating wastewater. Int. J. Environ. Res. Public Health 2022, 19, 15747. [Google Scholar] [CrossRef]
- Zinatizadeh, A.A.L.; Ghaytooli, E. Simultaneous nitrogen and carbon removal from wastewater at different operating conditions in a moving bed biofilm reactor (mbbr): Process modeling and optimization. J. Taiwan Inst. Chem. Eng. 2015, 53, 98–111. [Google Scholar] [CrossRef]
- Geng, M.; You, S.; Guo, H.; Ma, F.; Xiao, X.; Zhang, J. Impact of fungal pellets dosage on long-term stability of aerobic granular sludge. Bioresour. Technol. 2021, 332, 125106. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Ren, H.Q.; Geng, J.J.; Xu, K.; Huang, H.; Ding, L.L. Physicochemical characteristics and microbial community evolution of biofilms during the start-up period in a moving bed biofilm reactor. Bioresour. Technol. 2015, 180, 345–351. [Google Scholar] [CrossRef]
- Wang, H.; Song, Q.; Wang, J.; Zhang, H.; He, Q.; Zhang, W.; Song, J.; Zhou, J.; Li, H. Simultaneous nitrification, denitrification and phosphorus removal in an aerobic granular sludge sequencing batch reactor with high dissolved oxygen: Effects of carbon to nitrogen ratios. Sci. Total Environ. 2018, 642, 1145–1152. [Google Scholar] [CrossRef]
- Xiao, X.; Ma, F.; You, S.; Guo, H.; Zhang, J.; Bao, X.; Ma, X. Direct sludge granulation by applying mycelial pellets in continuous-flow aerobic membrane bioreactor: Performance, granulation process and mechanism. Bioresour. Technol. 2022, 344, 126233. [Google Scholar] [CrossRef]
- Ren, X.; Guo, L.; Chen, Y.; She, Z.; Gao, M.; Zhao, Y.; Shao, M. Effect of magnet powder (Fe3O4) on aerobic granular sludge (ags) formation and microbial community structure characteristics. ACS Sustain. Chem. Eng. 2018, 6, 9707–9715. [Google Scholar] [CrossRef]
- Zhang, D.; Li, W.; Hou, C.; Shen, J.; Jiang, X.; Sun, X.; Li, J.; Han, W.; Wang, L.; Liu, X. Aerobic granulation accelerated by biochar for the treatment of refractory wastewater. Chem. Eng. J. 2017, 314, 88–97. [Google Scholar] [CrossRef]
- Martins Antonio, M.P.; Pagilla, K.; Heijnen, J.J.; van Loosdrecht, M.C. Filamentous bulking sludge—A critical review. Water Res. 2004, 38, 793–817. [Google Scholar] [CrossRef]
- Wágner, D.S.; Ramin, E.; Szabo, P.; Dechesne, A.; Plósz, B.G. Microthrix parvicella abundance associates with activated sludge settling velocity and rheology–quantifying and modelling filamentous bulking. Water Res. 2015, 78, 121–132. [Google Scholar] [CrossRef]
- He, Q.; Zhang, J.; Gao, S.; Chen, L.; Lyu, W.; Zhang, W.; Song, J.; Hu, X.; Chen, R.; Wang, H.; et al. A comprehensive comparison between non-bulking and bulking aerobic granular sludge in microbial communities. Bioresour. Technol. 2019, 294, 122151. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Peng, Y.; Wang, S.; Yang, X.; Wang, Z.; Zhu, A. Stable limited filamentous bulking through keeping the competition between floc-formers and filaments in balance. Bioresour. Technol. 2012, 103, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhang, X.; Li, J. The formation and distinct characteristics of aerobic granular sludge with filamentous bacteria in low strength wastewater. Bioresour. Technol. 2022, 360, 127409. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, A.; Zhao, B.; Xue, S.; Xu, A. Single-stage mbbr using novel carriers to remove nitrogen in rural domestic sewage: The effect of carrier structure on biofilm morphology and snd. J. Environ. Chem. Eng. 2022, 10, 108267. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Foundation: Alexandria, VA, USA, 2005. [Google Scholar]
- Zou, J.; Li, Y.; Zhang, L.; Wang, R.; Sun, J. Understanding the impact of influent nitrogen concentration on granule size and microbial community in a granule-based enhanced biological phosphorus removal system. Bioresour. Technol. 2015, 177, 209–216. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Hamza, R.A.; Zaghloul, M.S.; Tay, J.H. Aerobic granular sludge membrane bioreactor (agmbr): Extracellular polymeric substances (eps) analysis. Water Res. 2019, 156, 305–314. [Google Scholar] [CrossRef]
- Taherzadeh, D.; Picioreanu, C.; Horn, H. Mass transfer enhancement in moving biofilm structures. Biophys. J. 2012, 102, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Sagastume, F.; Allen, D.G. Effects of temperature transient conditions on aerobic biological treatment of wastewater. Water Res. 2003, 37, 3590–3601. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Pan, J.; Wu, S.; Qian, M.; He, Z.; Wang, B.; Li, J. Rapid control of activated sludge bulking and simultaneous acceleration of aerobic granulation by adding intact aerobic granular sludge. Sci. Total Environ. 2019, 674, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, C.; Fu, L.; Cui, B.; Zhou, D. Social network of filamentous sphaerotilus during activated sludge bulking: Identifying the roles of signaling molecules and verifying a novel control strategy. Chem. Eng. J. 2023, 454, 140109. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Zheng, X.; Liu, Q.; Xiang, W.; Qu, J.; Yang, C. Microbial community structure analysis in a hybrid membrane bioreactor via high-throughput sequencing. Chemosphere 2021, 282, 130989. [Google Scholar] [CrossRef]
- Gu, Y.-Q.; Li, T.-T.; Li, H.-Q. Biofilm formation monitored by confocal laser scanning microscopy during startup of mbbr operated under different intermittent aeration modes. Process Biochem. 2018, 74, 132–140. [Google Scholar] [CrossRef]
- He, Q.; Chen, L.; Zhang, S.; Chen, R.; Wang, H.; Zhang, W.; Song, J. Natural sunlight induced rapid formation of water-born algal-bacterial granules in an aerobic bacterial granular photo-sequencing batch reactor. J. Hazard. Mater. 2018, 359, 222–230. [Google Scholar] [CrossRef]
- Quartaroli, L.; Silva, C.M.; Silva, L.C.F.; Lima, H.S.; de Paula, S.O.; Dias, R.S.; Carvalho, K.B.; Souza, R.S.; Bassin, J.P.; da Silva, C.C. Effect of the gradual increase of salt on stability and microbial diversity of granular sludge and ammonia removal. J. Environ. Manag. 2019, 248, 109273. [Google Scholar] [CrossRef]
- Zhang, B.; Lens, P.N.; Shi, W.; Zhang, R.; Zhang, Z.; Guo, Y.; Bao, X.; Cui, F. Enhancement of aerobic granulation and nutrient removal by an algal–bacterial consortium in a lab-scale photobioreactor. Chem. Eng. J. 2018, 334, 2373–2382. [Google Scholar] [CrossRef]
- Brudey, T.; Largitte, L.; Jean-Marius, C.; Tant, T.; Dumesnil, P.C.; Lodewyckx, P. Adsorption of lead by chemically activated carbons from three lignocellulosic precursors. J. Anal. Appl. Pyrolysis 2016, 120, 450–463. [Google Scholar] [CrossRef]
- Castellanos, R.M.; Dias, J.M.R.; Bassin, I.D.; Dezotti, M.; Bassin, J.P. Effect of sludge age on aerobic granular sludge: Addressing nutrient removal performance and biomass stability. Process Saf. Environ. Prot. 2021, 149, 212–222. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).