Artificial Water Bodies in Post-Industrial and Urban Landscapes—A Case Study on Assessing Their Potential in Blue–Green Urban Infrastructure
Abstract
1. Introduction
2. Materials and Methods
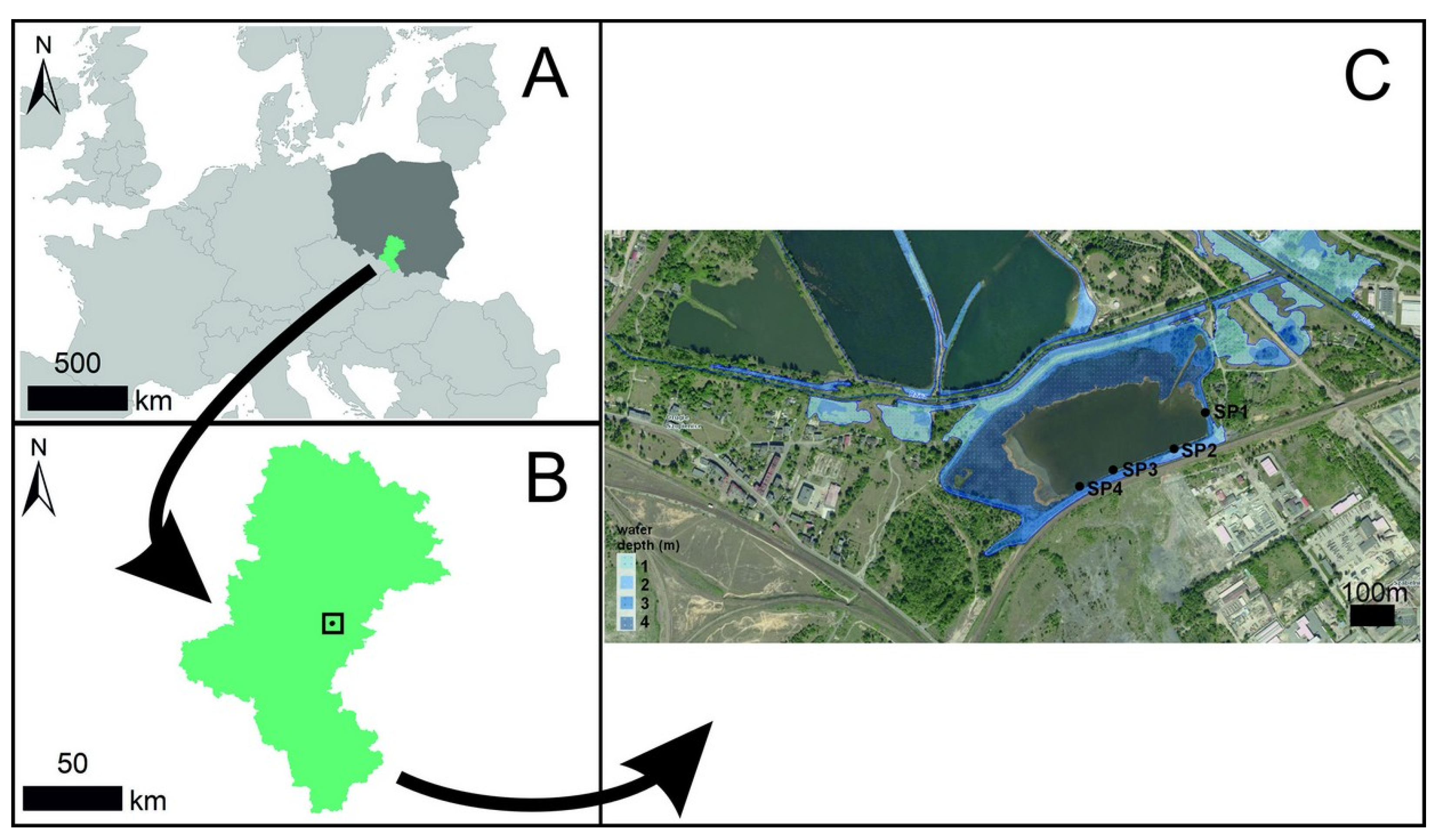
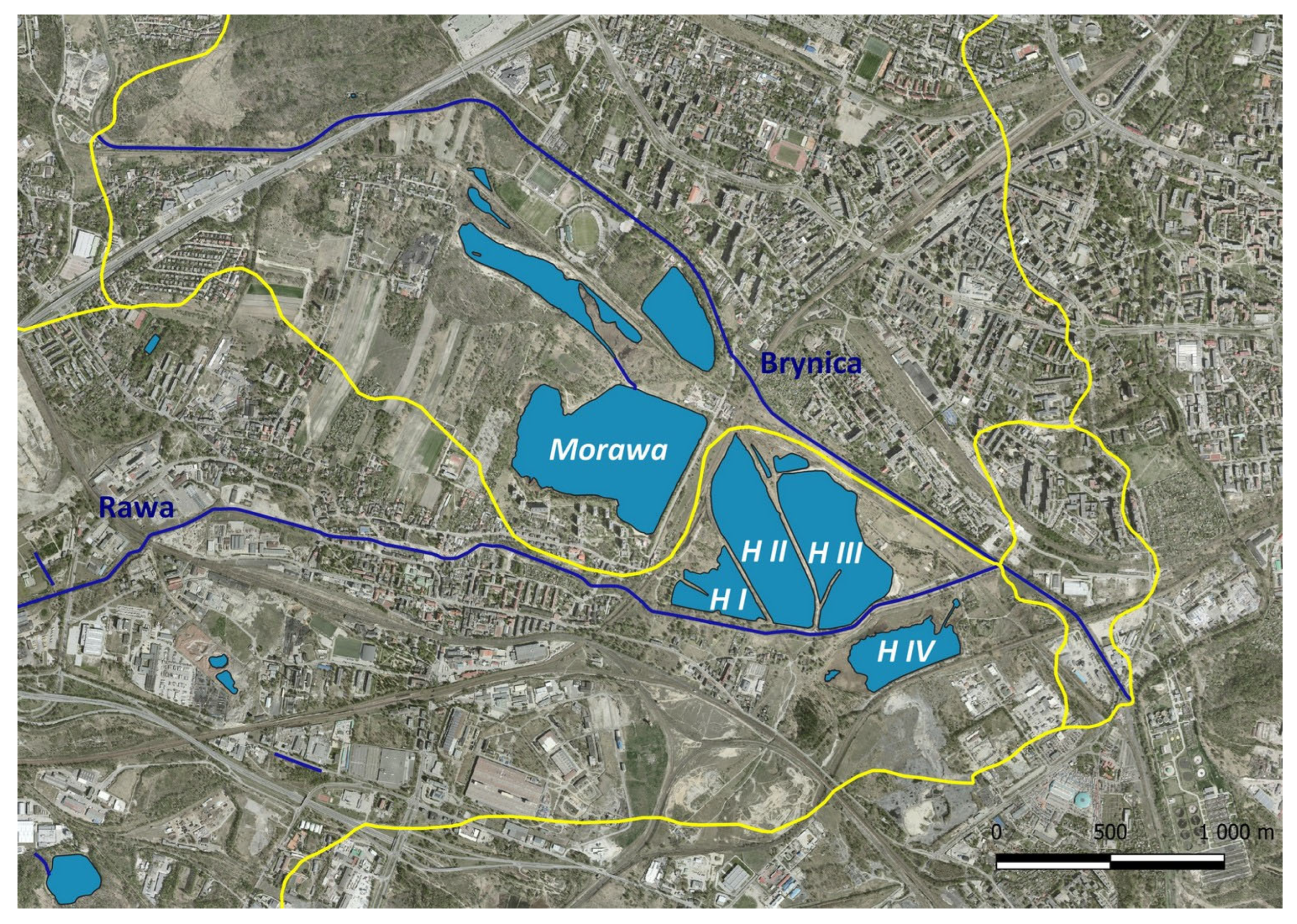
2.1. Study Area
2.2. Reservoir Depth and Topography of the Reservoir Direct Catchment Area
2.3. Physicochemical Parameters of Water Across the Entire Surface of the Reservoir and at Study Plots
2.4. Heavy Metals in Sediment and Fish Samples
2.5. Pollution Indexes Calculation
2.6. Seed Germination and Root Elongation Test
2.7. Phytoplankton
2.8. Macrophytes (Rushes and Submerged Vegetation)
2.9. Macroinvertebrates
2.10. Software Used and Statistical Analyses
3. Results
3.1. Information Obtained from Historical Sources
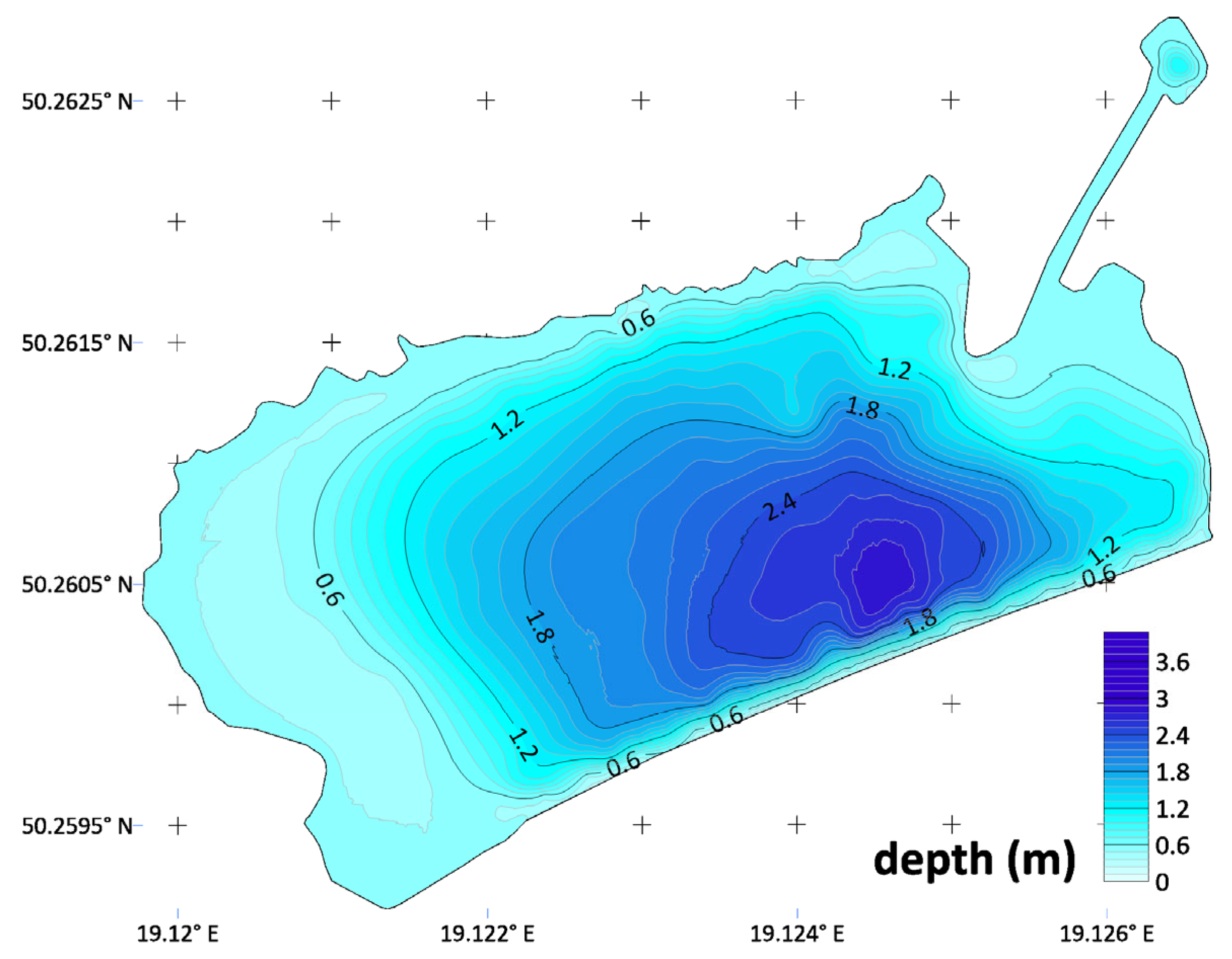
3.2. Bathymetry of the Reservoir and Topography of the Direct Catchment Area
3.3. Physicochemical Parameters
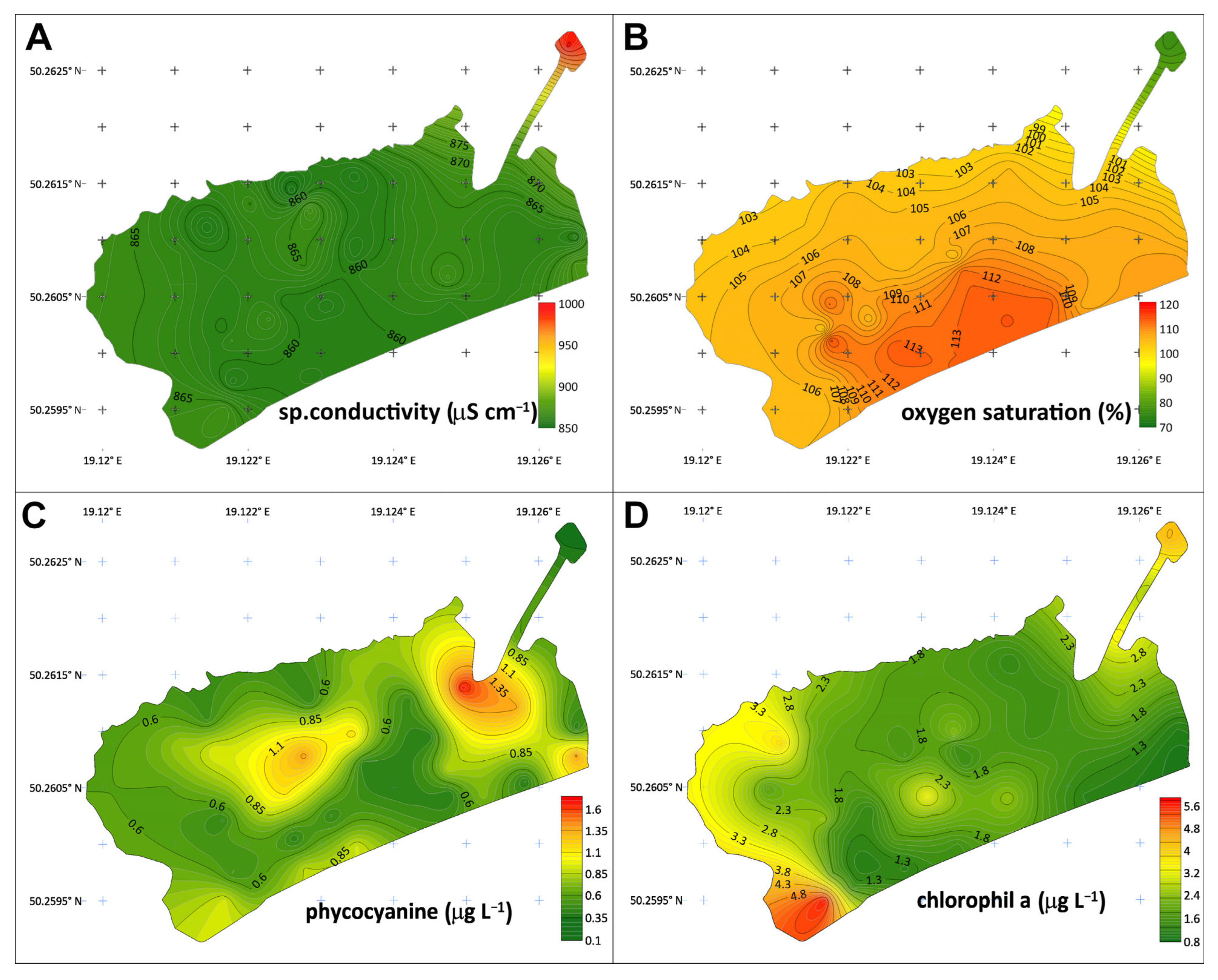
3.3.1. Specific Conductivity
3.3.2. Total Dissolved Solids (TDS)
3.3.3. Oxygen Saturation
3.3.4. Phycocyanin
3.3.5. Chlorophyll-a
3.3.6. pH
3.4. Phytoplankton Analysis
3.5. Macrophyte Structure
3.6. Benthic Macroinvertebrates
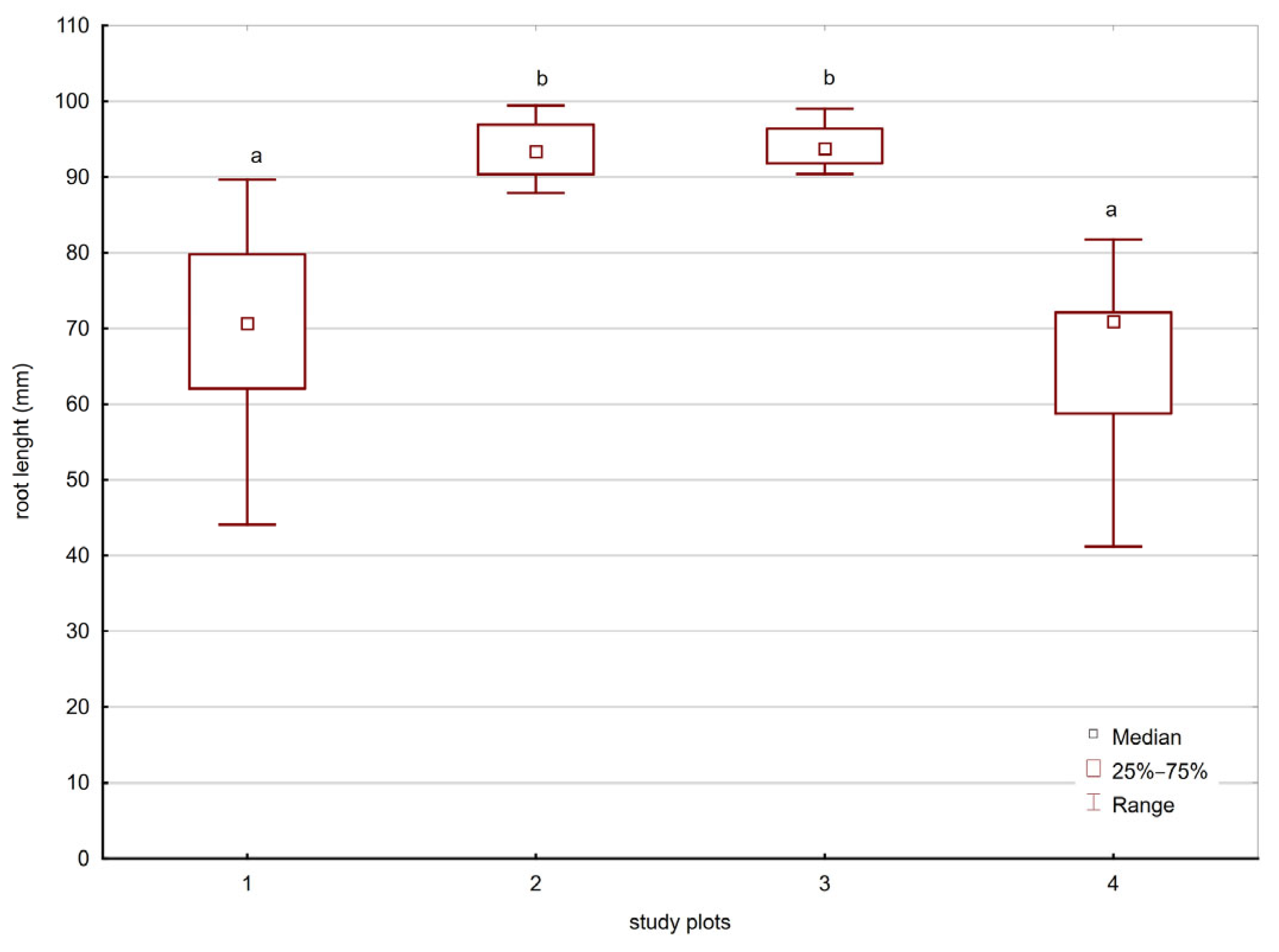
3.7. Germination and Root Elongation Tests
3.8. Metal Concentration and Pollution Indexes
3.9. Metal Concentrations in Fish Tissues
4. Discussion
4.1. Historical Information Data
4.2. Hydrogeomorphological Information Data
4.3. Water Parameters Data
4.4. Biological Parameters Data
4.4.1. Chlorophyll-a
4.4.2. Phycocyanin
4.4.3. Macrophytes
4.4.4. Benthic Macroinvertebrates
4.5. Toxicity Data
4.5.1. Metals in Sediments
4.5.2. Pollution Indexes
4.5.3. Root Elongation
4.6. Decision Support Overview for Post-Industrial Reservoirs Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs, Population Division. World Urbanization Prospects: The 2018 Revision; United Nations: New York, NY, USA, 2018. [Google Scholar]
- Mohajerani, A.; Bakaric, J.; Jeffrey-Bailey, T. The urban heat island effect, its causes, and mitigation, with reference to the thermal properties of asphalt concrete. J. Environ. Manag. 2017, 197, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.; Hess, J.J.; Frumkin, H. Urban form and extreme heat events: Are sprawling cities more vulnerable to climate change than compact cities? Environ. Health Perspect. 2010, 118, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Sierka, E.; Pierzchała, Ł. Role of reservoirs of urban heat island effect mitigation in human settlements: Moderate climate zone. J. Water Land Dev. 2022, 2022, 112–118. [Google Scholar] [CrossRef]
- Sun, Q.H.; Horton, R.M.; Bader, A.D.; Jones, B.; Zhou, L.; Li, T.T. Projections of temperature-related non-accidental mortality in Nanjing, China. Biomed. Environ. Sci. 2019, 32, 134–139. [Google Scholar] [CrossRef]
- Rzętała, M.; Jaguś, A. New lake district in Europe: Origin and hydrochemical characteristics. Water Environ. J. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Kuś, S.; Sierka, E.; Jelonek, I.; Jelonek, Z. Synthetic analysis of thematic studies towards determining the recreational potential of anthropogenic reservoirs. Environ. Ecol. Res. 2022, 10, 355–369. [Google Scholar] [CrossRef]
- Biela, M.; Sierka, E. Using plants of novel ecosystems as resources to create green roofs in cities’ adaptation to the climate change process. Stud. Ecol. Bioethicae 2023, 21, 53–61. [Google Scholar] [CrossRef]
- Costanza, R.; Perrings, C.; Cleveland, C.J. The Development of Ecological Economics; Edward Elgar Publishing Company: Cheltenham, UK, 1997. [Google Scholar]
- Sierka, E.; Stalmachová, B.; Molenda, T.; Chmura, D.; Pierzchała, Ł. Environmental and Socio-Economic Importance of Mining Subsidance Reservoirs; Praha Technical Literature BEN: Prague, Czech Republic, 2012. [Google Scholar]
- Woźniak, G.; Sieka, E.; Wheeler, A. Urban and Industrial Habitats: How Important They Are for Ecosystem Services; Hufnagel, L., Ed.; Ecosystem Services and Global Ecology; IntechOpen: London, UK, 2018; pp. 169–194. [Google Scholar] [CrossRef]
- Pierzchała, Ł. Assessment of the possibility of using remote sensing methods for measuring eutrophication of inland water reservoirs. Inż. Ekol. 2020, 21, 27–32. [Google Scholar] [CrossRef]
- Klusáček, P.; Alexandrescu, F.; Osman, R.; Malý, J.; Kunc, J.; Dvořák, P.; Frantál, B.; Havlíček, M.; Krejčí, T.; Martinát, S.; et al. Good governance as a strategic choice in brownfield regeneration: Regional dynamics from the Czech Republic. Land Use Policy 2018, 73, 29–39. [Google Scholar] [CrossRef]
- Siikamäki, J.; Wernstedt, K. Turning brownfields into greenspaces: Examining incentives and barriers to revitalization. J. Health Politics Policy Law 2008, 33, 559–593. [Google Scholar] [CrossRef]
- Dulias, R. Landscape planning in areas of sand extraction in the Silesian Upland, Poland. Landsc. Urban Plan. 2010, 95, 91–104. [Google Scholar] [CrossRef]
- Stoddard, J.L.; Larsen, D.P.; Hawkins, C.P.; Johnson, R.K.; Norris, R.H. Setting expectations for the ecological condition of streams: The concept of reference condition. Ecol. Appl. 2006, 16, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Pierzchała, Ł.; Sierka, E.; Trząski, L.; Bondaruk, J.; Czuber, B. Evaluation of the suitability of anthropogenic reservoirs in urban space for ecological restoration using submerged plants (Upper Silesia, Poland). Appl. Ecol. Environ. Res. 2016, 14, 277–296. [Google Scholar] [CrossRef]
- Ciecierska, H.; Kolada, A. ESMI: A macrophyte index for assessing the ecological status of lakes. Environ. Monit. Assess. 2014, 186, 5501–5517. [Google Scholar] [CrossRef]
- European Commission. Directive of the European Parliament and of the Council 2000/60/EC. Establishing a Framework for Community Action in the Field of Water Policy. Off. J. Eur. Parliam. 2000, L327. [Google Scholar]
- Barko, J.W.; Adams, M.S.; Clesceri, N.L. Management of submersed aquatic vegetation. J. Aquat. Plant Manag. 1986, 24, 1–10. [Google Scholar]
- Van Geest, G.J. Macrophyte Succession in Floodplain Lakes: Spatio-Temporal Patterns in Relation to Hydrology, Lake Morphology and Management; Wageningen University and Research: Wageningen, The Netherlands, 2005. [Google Scholar]
- Sumudumali, R.G.I.; Jayawardana, J.M.C.K. A review of biological monitoring of aquatic ecosystems approaches: With special reference to macroinvertebrates and pesticide pollution. Environ. Manag. 2021, 67, 263–276. [Google Scholar] [CrossRef]
- Tachet, H.; Richoux, P.; Bournaud, M.; Usseglio-Polatera, P. Invertébrés D’eau Douce: Systématique, Biologie, Écologie; CNRS Editions: Paris, France, 2010. [Google Scholar]
- Remon, E.; Bouchardon, J.-L.; Le Guédard, M.; Bessoule, J.-J.; Conord, C.; Faure, O. Are plants useful as accumulation indicators of metal bioavailability? Environ. Pollut. 2013, 175, 1–7. [Google Scholar] [CrossRef]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The role of biomarkers in the assessment of aquatic ecosystem health. Integr. Environ. Assess. Manag. 2014, 10, 327–341. [Google Scholar] [CrossRef]
- Palma, P.; Ledo, L.; Alvarenga, P. Ecotoxicological endpoints: Are they useful tools to support ecological status assessment in strongly modified water bodies? Sci. Total Environ. 2016, 541, 119–129. [Google Scholar] [CrossRef]
- Łaszczyca, P.; Nakonieczny, M.; Kostecki, M. Ecotoxicological biotests as tools for continuous monitoring of water quality in dam reservoir. Arch. Environ. Prot. 2023, 49, 25–38. [Google Scholar] [CrossRef]
- Daus, M.; Koberger, K.; Koca, K.; Beckers, F.; Fernández, J.E.; Weisbrod, B.; Dietrich, D.; Gerbersdorf, S.U.; Glaser, R.; Haun, S.; et al. Interdisciplinary Reservoir Management—A Tool for Sustainable Water Resources Management. Sustainability 2021, 13, 4498. [Google Scholar] [CrossRef]
- Absalon, D.; Matysik, M.; Woźnica, A.; Łozowski, B.; Jarosz, W.; Ulańczyk, R.; Babczyńska, A.; Pasierbiński, A. Multi-Faceted Environmental Analysis to Improve the Quality of Anthropogenic Water Reservoirs (Paprocany Reservoir Case Study). Sensors 2020, 20, 2626. [Google Scholar] [CrossRef] [PubMed]
- Machowski, R.; Rzetala, M.A.; Rzetala, M.; Solarski, M. Anthropogenic enrichment of the chemical composition of bottom sediments of water bodies in the neighborhood of a non-ferrous metal smelter (Silesian Upland, Southern Poland). Sci. Rep. 2019, 9, 51027. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Jeliazkova, E.A.; Kovacheva, N.; Dzhurmanski, A. Metal uptake by medicinal plant species grown in soils contaminated by a smelter. Environ. Exp. Bot. 2008, 64, 207–216. [Google Scholar] [CrossRef]
- Wójcik, M.; Sugier, P.; Siebielec, G. Metal accumulation strategies in plants spontaneously inhabiting Zn-Pb waste deposits. Sci. Total Environ. 2014, 487, 313–322. [Google Scholar] [CrossRef]
- Prychepa, M.; Hrynevych, N.; Martseniuk, V.; Potrokhov, O.; Vodianitskyi, O.; Khomiak, O.; Rud, O.; Kytsokon, L.; Sliusarenko, A.; Dunaievska, O.; et al. Rudd (Scardinius erythrophthalmus L., 1758) as a bioindicator of anthropogenic pollution in freshwater bodies. Ukr. J. Ecol. 2021, 11, 253–260. [Google Scholar]
- Sula, E.; Aliko, V.; Barceló, D.; Faggio, C. Combined effects of moderate hypoxia, pesticides and PCBs upon crucian carp fish (Carassius carassius) from a freshwater lake: An in situ ecophysiological approach. Aquat. Toxicol. 2020, 228, 105644. [Google Scholar] [CrossRef]
- Ballabio, C.; Jones, A.; Panagos, P. Cadmium in topsoils of the European Union—An analysis based on LUCAS topsoil database. Sci. Total Environ. 2023, 912, 168710. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water, and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef]
- Oorts, K.; Smolders, E.; Lanno, R.; Chowdhury, M.J. Bioavailability and ecotoxicity of lead in soil: Implications for setting ecological soil quality standards. Environ. Toxicol. Chem. 2021, 40, 1950–1963. [Google Scholar] [CrossRef]
- Journal of Laws of the Republic of Poland. Dz.U. 2016 poz. 1395. 2016. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20160001395 (accessed on 2 April 2025).
- PN EN 15204: 2006; Water Quality-Guidance Standard on the Enumeration of Phytoplankton Using Inverted Microscopy (UTERMHL TECHNIQUE). Polish Committee for Standardization: Warsaw, Poland, 2013.
- Grulich, V.; Vydrová, A. Metodika Odběru a Zpracování Vzorku Makrofyt Stojatých Vod; Masaryk Water Research Institute: Prague, Czech Republic, 2006. [Google Scholar]
- Schaumburg, J.; Schranz, C.; Hofmann, G.; Stelzer, D.; Schneider, S.; Schmedtje, U. Macrophytes and phytobenthos as indicators of ecological status in German lakes—A contribution to the implementation of the Water Framework Directive. Limnologica 2004, 34, 302–314. [Google Scholar] [CrossRef]
- Godzik, S.; Kubiesa, P.; Staszewski, T.; Szdzuj, J. Ecological problems of the Katowice administrative district. In Biomarkers: A Pragmatic Basis for Remediation of Severe Pollution in Eastern Europe; Springer: Dordrecht, The Netherlands, 1999; pp. 49–73. [Google Scholar] [CrossRef]
- Rzętała, M. Anthropogenic Water Reservoirs in Poland. In Springer Water; Springer: Cham, Switzerland, 2021; pp. 59–89. [Google Scholar] [CrossRef]
- Ulańczyk, R.; Łozowski, B.; Woźnica, A.; Absalon, D.; Kolada, A. Water Quality and Ecosystem Modelling: Practical Application on Lakes and Reservoirs. In Springer Water; Springer: Cham, Switzerland, 2021; pp. 173–189. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Su, B.; Xiao, D.-D.; Ye, L.-Y. Study on pH value and its variation characteristics of the main rivers into Dianchi lake under the anthropogenic and natural processes, Yunnan, China. J. Inf. Optim. Sci. 2017, 38, 1197–1210. [Google Scholar] [CrossRef]
- Dewangan, S.K.; Toppo, D.N.; Kujur, A. Investigating the impact of pH levels on water quality: An experimental approach. Int. J. Res. Appl. Sci. Eng. Technol. 2023, 11, 756–759. [Google Scholar] [CrossRef]
- Pinheiro, J.P.S.; Windsor, F.M.; Wilson, R.W.; Tyler, C.R. Global variation in freshwater physico-chemistry and its influence on chemical toxicity in aquatic wildlife. Biol. Rev. 2021, 96, 1528–1546. [Google Scholar] [CrossRef]
- Kannel, P.R.; Lee, S.; Lee, Y.S.; Kanel, S.R.; Khan, S.P. Application of water quality indices and dissolved oxygen as indicators for river water classification and urban impact assessment. Environ. Monit. Assess. 2007, 132, 93–110. [Google Scholar] [CrossRef]
- Geist, D.R.; Linley, T.J.; Cullinan, V.; Deng, Z. The effects of total dissolved gas on chum salmon fry survival, growth, gas bubble disease, and seawater tolerance. N. Am. J. Fish. Manag. 2013, 33, 200–215. [Google Scholar] [CrossRef]
- Schiedek, D.; Sundelin, B.; Readman, J.W.; Macdonald, R.W. Interactions between climate change and contaminants. Mar. Pollut. Bull. 2007, 54, 1845–1856. [Google Scholar] [CrossRef]
- Iglesias, M.C.A. A review of recent advances and future challenges in freshwater salinization. Limnetica 2020, 39, 185–211. [Google Scholar] [CrossRef]
- Cunillera-Montcusí, D.; Beklioğlu, M.; Cañedo-Argüelles, M.; Jeppesen, E.; Ptacnik, R.; Amorim, C.A.; Arnott, S.E.; Berger, S.A.; Brucet, S.; Dugan, H.A.; et al. Freshwater salinisation: A research agenda for a saltier world. Trends Ecol. Evol. 2022, 37, 440–453. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines on Recreational Water Quality: Coastal and Fresh Waters; World Health Organization: Geneva, Switzerland, 2021; Volume 1.
- Ahn, C.Y.; Joung, S.H.; Yoon, S.K.; Oh, H.M. Alternative alert system for cyanobacterial bloom, using phycocyanin as a level determinant. J. Microbiol. 2007, 45, 98–104. [Google Scholar] [PubMed]
- Sierka, E.; Bujok, M.; Stalmachova, B.; Horaczek, T. Fluorescence parameters of chlorophyll a halophytes as a response to salinity of post-mining subsidence reservoirs. J. Water Land Dev. 2022, 2022, 164–170. [Google Scholar] [CrossRef]
- Cieplok, A.; Krodkiewska, M.; Franiel, I.; Starzak, R.; Sowa, M.; Spyra, A. The role of habitat protection in maintaining the diversity of aquatic fauna in rural and industrial areas. Water 2022, 14, 3983. [Google Scholar] [CrossRef]
- Krodkiewska, M.; Strzelec, M.; Spyra, A.; Lewin, I. The impact of environmental factors on benthos communities and freshwater gastropod diversity in urban sinkhole ponds in roadside and forest contexts. Landsc. Res. 2019, 44, 477–492. [Google Scholar] [CrossRef]
- Mackintosh, T.J.; Davis, J.A.; Thompson, R.M. The influence of urbanisation on macroinvertebrate biodiversity in constructed stormwater wetlands. Sci. Total Environ. 2015, 536, 527–537. [Google Scholar] [CrossRef]
- Hill, M.J.; Biggs, J.; Thornhill, I.; Briers, R.A.; Gledhill, D.G.; White, J.C.; Wood, P.J.; Hassall, C. Urban ponds as an aquatic biodiversity resource in modified landscapes. Glob. Change Biol. 2017, 23, 986–999. [Google Scholar] [CrossRef]
- Cieplok, A.; Spyra, A.; Czerniawski, R. Globally invasive Potamopyrgus antipodarum (Gray, 1843)—An indicator of the degraded water systems in relation to native aquatic invertebrates. Ecol. Indic. 2023, 156, 111194. [Google Scholar] [CrossRef]
- Hill, M.J.; Ryves, D.B.; White, J.C.; Wood, P.J. Macroinvertebrate diversity in urban and rural ponds: Implications for freshwater biodiversity conservation. Biol. Conserv. 2016, 201, 50–59. [Google Scholar] [CrossRef]
- European Council. Consolidated Text: Commission Decision of 3 May 2000 Replacing Decision 94/3/EC Establishing a List of Wastes Pursuant to Article 1(a) of Council Directive 75/442/EEC on Waste and Council Decision 94/904/EC Establishing a List of Hazardous Waste. 2003. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02000D0532-20231206 (accessed on 2 April 2025).
- Journal of Laws of the Republic of Poland. Dz.U. 2015 poz. 1277. 2015. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150001277 (accessed on 2 April 2025).
- Baud-Grasset, F.; Baud-Grasset, S.; Safferman, S.I. Evaluation of the bioremediation of a contaminated soil with phytotoxicity tests. Chemosphere 1993, 26, 1365–1374. [Google Scholar] [CrossRef]


| Study Plots | Oxygen Concentration (mg L−1) | Oxygen Saturation (%) | Specific Conductivity (uS cm−1) | TDS (mg L−1) | pH |
|---|---|---|---|---|---|
| 1 | 8.33 | 97 | 685 | 377 | 8.1 |
| 2 | 7.74 | 91 | 685 | 377 | 8.0 |
| 3 | 8.12 | 98 | 687 | 377 | 7.9 |
| 4 | 6.63 | 72 | 690 | 380 | 7.3 |
| Study Plot | Phytoplankton | Macrophytes | Benthic Macroinvertebrates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Taxa | Shannon–Wiener Index | Biomass (mg L−1) | Number of Taxa | Plant Cover (%) | Shannon–Wiener Index | Total Density (Individuals m−2) | Biomass (g m−2) | Shannon–Wiener Index | Number of Taxa | Dominant Taxa | |
| 1 | 51 | 1.497 | 1211.7 | 3 | 30 | 1.169 | 1287 | 3.031 | 1.709 | 10 | Chironomidae, Caenidae, Potamopyrgus antipodarum (invasive species) |
| 2 | 28 | 0.979 | 895.7 | 4 | 40 | 1.094 | 1473 | 9.414 | 1.295 | 12 | Chironomidae |
| 3 | 46 | 1.325 | 2578.3 | 4 | 30 | 1.613 | 3962 | 3.640 | 0.676 | 7 | Chironomidae |
| 4 | 32 | 1.027 | 2277.6 | 6 | 30 | 1.271 | 525 | 2.895 | 2.070 | 13 | Chironomidae |
| Study Plots | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| metal concentration (mg kg dry mass−1) | Cd | 2.65 | 0.55 | 0.40 | 3.85 |
| Zn | 573.20 | 103.35 | 81.30 | 607.85 | |
| Pb | 70.45 | 29.40 | 20.20 | 91.75 | |
| CFp | Cd | 0.23 | 0.06 | 0.04 | 0.42 |
| Zn | 0.48 | 0.10 | 0.20 | 0.65 | |
| Pb | 0.12 | 0.06 | 0.04 | 0.18 | |
| CF | Cd | 7.19 | 1.72 | 1.25 | 12.97 |
| Zn | 8.74 | 1.88 | 3.57 | 11.74 | |
| Pb | 3.05 | 1.47 | 0.89 | 4.59 | |
| PLIp | 0.24 | 0.07 | 0.07 | 0.37 | |
| PLI | 1.97 | 0.57 | 0.54 | 3.03 | |
| Method | Advantages | Disadvantages | Required Resources |
|---|---|---|---|
| Historical informative data groups (irreplaceable, it is a starting point for rational decisions regarding further research of the reservoir) | |||
| Analysis of available documents regarding the reservoir (including maps, official studies, reports, literature) | possibility to determine historical pressures and adapt the scope of further research accordingly to the situation of the reservoir, the ability to eliminate unnecessary tests and focus resources on essential analyses | potential lack of documentation or difficulty in obtaining it, problems with data being up-to-date | Digitized analog data, including historical data, requiring a time-consuming process of selecting the information contained within them. |
| Hydro- and geomorphological informative data groups | |||
| Document and map analysis | ease to obtain information | potential lack of current data, digitalization of analog maps may be necessary | Digitized data based on collected documentation materials, which do not require financial investment but may be time-consuming to access. |
| Bathymetric measurements, measurements of water inflows and outflows | high accuracy of research, up-to-date data | research requiring floating equipment (may be remote in some applications) and access to specialized software, experience in field required | Results obtained based on data analysis with expected accuracy, dependent on the available floating research equipment, reservoir size, and the nature of the reservoir. |
| Mathematical water flow modeling | enable modeling of scenarios related to various water supply to the reservoir, combined with chemical and biological models, they enable the representation of the entire ecosystem | model calibration and testing required | Results of time-consuming modeling analyses using licensed/non-licensed (free) software, along with their verification and validation. Specialized knowledge is required. |
| Physicochemical water parameters informative data groups | |||
| Physicochemical probe measurements, chlorophyll-a and phycocyanin measurements using multiparameter probes | the possibility of conducting ad hoc—patrol monitoring, depending on the need, measurement can be performed from: a shore, a pier or a floating object | unaccredited measurements, probe calibration required | Results of water property analysis obtained in real-time, in situ (in the field) using specialized probes by a technician, some probes associated with high costs of purchasing probes, which can be reduced through rental. |
| Remote sensing (chlorophyll-a) measurement | enables obtaining quantitative data on a local, regional, and global scale, characterized by the repeatability of observations | the need to calibrate and adapt the model to local conditions, limited application possibilities in reservoirs with aquatic plants, chlorophyll contained in vascular plants significantly interferes or prevents the measurement of chlorophyll in phytoplankton biomass, dependent on weather conditions (cloud cover over the reservoir prevents measurement), in the case of inland waters, high biological and optical complexity makes its application difficult | Results of a multi-step, time-consuming research procedure as well as standardization, which are associated with high costs. |
| Laboratory measurements | possibility of accreditation of tests, high accuracy (however, not necessary in preliminary and screening tests), possibility of testing unusual parameters | the need to secure, store and transport of samples, the duration of the test may be long | Results of parameters of water and other elements of the reservoir, such as sediment, obtained through laboratory analyses. |
| Biological informative data groups | |||
| Phytoplankton laboratory measurement | it is possible to obtain precise information about trophy status | requires direct field sampling | Results of studies conducted both in the field and in the laboratory throughout the entire growing season, with the involvement of a specialist and laboratory infrastructure. |
| Macrophytes analysis | allows obtaining information on the trophy of the reservoir water and coastal zone, the potential to block surface runoff, and the possibility of creating habitats for birds and fish | requires expertise and direct field sampling from the reservoir area and the coastal zone | Results of time-consuming studies conducted throughout the entire growing season with the involvement of a specialist, utilizing citizen science data. |
| Benthic macroinvertebrates analysis | provides information on the condition of the tank and the food base, including for fish | requires expertise and long-term study | Results of time-consuming, full-season studies conducted using field and laboratory equipment, with the involvement of a specialist. |
| eDNA analysis | relatively simple and quick method of species identification, enables obtaining information about biodiversity, does not cause any disruption to the ecosystem | risk of sample contamination, does not provide information about population structure, size, sex ratio, age, individuals condition, potential for false positives and negatives, leading to potential misinterpretations of data, the degradation of eDNA in water, especially in warm and turbid waters, lack of standardized protocols and methodologies can result in variations in results, lack of standardized protocols, large datasets demand advanced bioinformatics skills and resources for proper handling and interpretation | Results of multi-stage studies conducted using specialized equipment in a molecular laboratory, involving a specialist and the use of costly reagents. |
| Mathematical ecosystem modeling based on biological parameters | enable the prediction of phenomena in the reservoir | comprehensive reservoir testing required to calibrate and test the model | Verified and validated results of time-consuming mathematical modeling analyses based on previously collected biological data. The analysis must be conducted using licensed software by a specialist. An alternative is free software. |
| Toxicological informative data groups | |||
| Metals in sediments (or in other environmental samples like soil or water) measurement | relatively easily available material, relatively accessible laboratories and methods | no significant disadvantages of the method | Results of standard analyses of environmental samples conducted by environmental laboratories. |
| Metals in tissues measurement | relatively easily available material, relatively accessible laboratories and methods | requires a precise selection of objects (plant or animal species and their tissues or fragments) so that the results obtained are representative of the analyzed environment, this may require the involvement of specialists in ecotoxicology or environmental toxicology, potential need for bioethical approvals | Results of standard analyses of environmental samples, such as animal organs, which require additional manual procedures for sample preparation. |
| Pollution indexes | if based on data collected using time- and money-effective methods (as metal concentrations or other effective data) and referred to the standard or in any other normalized reference data, they provide very valuable information for the decision-making process | no significant disadvantages of the method | Results of time-consuming analyses based on historical data and reference studies, along with their rapid interpretation using basic computational software. |
| Certified biotests for environmental toxicology | tests based on toxicity parameters specific for standardized model species/forms of plants and animals within relatively short time provide information on acute toxicity of environmental samples, if exact contents of potentially toxic substances or chemicals are not necessary | usually require well-qualified personnel, working exclusively on this task, capable of competent interpretation of markers of changes in model individuals, occurring under the influence of potentially polluted/harmful environmental samples | Results of costly certified biotests conducted according to the manufacturer’s instructions or alternative non-certified tests; time-consuming single measurements, with a methodology requiring repetitions. |
| Non-certified toxicity tests (e.g., Phytotoxkit-like tests) | germination and root elongation tests do not need certified seeds, if include unpolluted reference samples tests are easy for interpretation and do not require much individual working time, substrate toxicity demonstrated by a simple and rapid reaction of plant bioindicators | the plant may not be sensitive to a given type of toxic substance or the concentrations may be too low for it to react noticeably, potential possibility of hormesis | Results of rapid toxicity test analyses that do not require advanced equipment but adhere to testing procedures developed using free software. |
| Other specific informative data groups Other specific measurements due to the land use history or neighboring objects (e.g., old waste dumps, arable fields). This situation requires starting the process of decision making from the first step recommended, i.e., historical analysis of the area which is irreplaceable in this proposed analysis. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krodkiewska, M.; Łozowski, B.; Sierka, E.; Nadgórska-Socha, A.; Woźnica, A.; Feist, B.; Babczyńska, A. Artificial Water Bodies in Post-Industrial and Urban Landscapes—A Case Study on Assessing Their Potential in Blue–Green Urban Infrastructure. Water 2025, 17, 2862. https://doi.org/10.3390/w17192862
Krodkiewska M, Łozowski B, Sierka E, Nadgórska-Socha A, Woźnica A, Feist B, Babczyńska A. Artificial Water Bodies in Post-Industrial and Urban Landscapes—A Case Study on Assessing Their Potential in Blue–Green Urban Infrastructure. Water. 2025; 17(19):2862. https://doi.org/10.3390/w17192862
Chicago/Turabian StyleKrodkiewska, Mariola, Bartosz Łozowski, Edyta Sierka, Aleksandra Nadgórska-Socha, Andrzej Woźnica, Barbara Feist, and Agnieszka Babczyńska. 2025. "Artificial Water Bodies in Post-Industrial and Urban Landscapes—A Case Study on Assessing Their Potential in Blue–Green Urban Infrastructure" Water 17, no. 19: 2862. https://doi.org/10.3390/w17192862
APA StyleKrodkiewska, M., Łozowski, B., Sierka, E., Nadgórska-Socha, A., Woźnica, A., Feist, B., & Babczyńska, A. (2025). Artificial Water Bodies in Post-Industrial and Urban Landscapes—A Case Study on Assessing Their Potential in Blue–Green Urban Infrastructure. Water, 17(19), 2862. https://doi.org/10.3390/w17192862






