Abstract
This study was conducted to investigate the effect of hydrogen peroxide (H2O2) pretreatment on the anaerobic digestion performance of dairy wastewater. Initial physicochemical characterization revealed that the substrate is highly enriched in volatile solids (approximately 90.67%), indicating its strong potential for anaerobic biodegradation. Given this favorable composition, biochemical methane potential (BMP) assays were performed under mesophilic conditions (37 °C) to quantify biogas and methane generation from the untreated and pretreated dairy effluent. To enhance substrate biodegradability and increase methane yield, an oxidative pretreatment using various doses of H2O2 was applied. This pretreatment aimed to disrupt the complex organic matter and promote the solubilization of chemical oxygen demand (COD), especially in its soluble form (sCOD), which is more readily assimilated by methanogenic microorganisms. The experimental results demonstrated a significant improvement in biogas production efficiency. While the untreated sample yielded approximately 100 mL CH4/g VS, the pretreated substrate achieved a maximum of 168 mL CH4/g VS, marking a substantial enhancement. Gas composition analysis further revealed that methane accounted for nearly 45% of the total biogas produced under optimal conditions. The dosage of 0.2 g H2O2 per g of volatile solids (VS) resulted in the highest improvement in methane production after thermal treatment C1, followed by 1.35 g H2O2/g VS, and then 0.5 g H2O2/g VS. Furthermore, the kinetics of methane production were assessed by fitting the experimental data to the modified Gompertz model. This model enabled the determination of key parameters, such as the maximum specific methane production rate and the duration of the lag phase. The high coefficient of determination (R2) values obtained confirmed the excellent agreement between the experimental data and the model predictions, highlighting the robustness and reliability of the modified Gompertz model in describing the anaerobic digestion process of dairy waste subjected to oxidative pretreatment.
1. Introduction
In recent years, the global urgency to address climate change has prompted numerous countries to commit to reducing greenhouse gas (GHG) emissions. This shift has intensified the search for cleaner and more sustainable energy alternatives capable of mitigating environmental degradation while meeting rising energy demands [1].
Among the various renewable energy sources that have garnered increasing attention are hydrogen, methane, biomass, wind, and solar energy, each offering unique advantages in terms of environmental sustainability and energy yield [2,3]. In this context, biogas production through anaerobic digestion has emerged as an economically viable and energy-efficient solution, capable of simultaneously treating organic waste and generating renewable energy [4,5].
Anaerobic digestion is a multi-step biochemical process facilitated by a complex consortium of microorganisms that operate under oxygen-free conditions [6,7]. This process comprises four successive stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis [8,9,10]. During these stages, complex organic compounds are progressively broken down into simpler molecules, culminating in the generation of energy-rich biogas [11,12]. The biogas produced predominantly contains methane (CH4) and carbon dioxide (CO2), with methane concentrations typically ranging from 50% to 75%, and CO2 comprising 25% to 50%, along with minor quantities of hydrogen sulfide (H2S), ammonia (NH3), hydrogen (H2), and other trace gases [13,14].
Despite its widespread application and benefits, the efficiency of anaerobic digestion is frequently limited by the initial hydrolysis step. During hydrolysis, complex organic matter such as proteins, carbohydrates, and lipids is solubilized into simpler monomers suitable for microbial uptake. However, this step is often slow and inefficient, particularly when the feedstock comprises substrates that are structurally complex or resistant to biological degradation [15,16].
As a result, enhancing the hydrolysis stage is crucial for improving the overall performance of anaerobic digestion systems [11,17]. One promising strategy to overcome the limitations associated with the hydrolysis phase involves the application of pretreatment techniques aimed at increasing substrate solubility and biodegradability.
Thermal, chemical and physical pretreatments are commonly employed to disrupt the physical structure and modify the chemical composition of organic matter, thereby improving its accessibility to microbial enzymes [6,11,12,18].
Among chemical methods, oxidative pretreatments are particularly effective in breaking down recalcitrant compounds, converting them into forms more amenable to microbial degradation [19]. Hydrogen peroxide (H2O2) has been widely studied as an oxidizing agent in pretreatment processes due to its strong oxidative potential and environmentally benign by-products [20,21]. In aqueous environments, H2O2 decomposes into water and oxygen, avoiding the introduction of toxic residues into the treatment system. When applied to organic waste, H2O2 can induce cell lysis, solubilize intracellular contents, and fragment complex molecules, leading to increased levels of soluble chemical oxygen demand (sCOD) and enhanced substrate availability for anaerobic microorganisms [22]. Several studies have demonstrated the effectiveness of H2O2 in improving the biodegradability of various substrates, including sewage sludge and industrial effluents. Its application can significantly enhance the yield of methane by facilitating a higher degree of organic matter conversion. Moreover, H2O2-based pretreatments are relatively simple to implement and operate under mild conditions, making them suitable for integration into existing wastewater treatment infrastructure [23].
Notably, this approach has proven particularly beneficial when dealing with waste streams containing slowly biodegradable or bio-recalcitrant components, such as those found in dairy industry effluents and purification sludge [24]. Dairy wastewater is characterized by high concentrations of organic matter, including proteins, fats, and carbohydrates, which pose challenges for biological treatment processes due to their complex nature. Pretreatment using oxidizing agents like H2O2 can enhance the degradation of these compounds by increasing solubilization and reducing particle size, ultimately improving the efficiency of anaerobic digestion. For example, oxidative disruption of phthalates and similar pollutants in sludge has been shown to increase their bioavailability, facilitating their further degradation by anaerobic consortia [25]. In addition to H2O2, other oxidants such as ozone (O3) have also been utilized in pretreatment processes to achieve substantial rates of organic matter disintegration at ambient temperature and pressure [26].
While ozone is highly reactive and effective, it is often associated with higher operational costs and technical complexity. Conversely, H2O2 provides a more practical and cost-effective alternative for small- to medium-scale facilities. Furthermore, in some cases, H2O2 treatment has been reported to result in partial mineralization of the organic content, potentially reducing the residual organic load and improving effluent quality [27]. Numerous studies have shown that thermal pretreatment at high temperatures promotes the biodegradation of effluents with high organic loads. Temperature values around 100 °C are often cited in the literature as being effective in improving the bioavailability of organic matter. The objective is to evaluate the impact of various concentrations of H2O2 on the solubilization of organic matter, methane yield, and the efficiency of volatile solids and COD removal [28]. Previous research has indicated that such pretreatments can boost biogas production by up to 60%, underlining their potential to significantly improve the performance of anaerobic digestion systems when appropriately optimized [29]. This investigation contributes to the growing body of knowledge on the integration of chemical pretreatments in biological waste treatment processes [30]. By systematically evaluating the influence of H2O2 on key parameters such as soluble COD, methane yield, and digestion kinetics, the study aims to identify optimal operating conditions that could be applied in real wastewater treatment plants to improve energy recovery and waste stabilization. This research makes a unique contribution to the energy recovery of agro-food effluents by exploring an innovative thermochemical approach based on a moderate thermal treatment combined with an environmentally friendly oxidizing agent. Numerous studies have shown that thermal pretreatment at high temperatures promotes the biodegradation of effluents with high organic loads.
The combinations of heating at 100 °C and varying doses of hydrogen peroxide (0.2, 0.5, 0.9, and 1.35 g H2O2) have been designed to enhance the bioavailability of organic matter in wastewater from the dairy industry, characterized by its high organic load and complexity. This targeted coupling aims to overcome the limitations often observed during the hydrolysis stage in anaerobic digestion, promoting a more effective disintegration of recalcitrant organic compounds. Unlike many previous studies focused on urban sludge or agricultural waste, the proposed protocol has been applied to a specific effluent derived from milk processing, which has rarely been studied in this form. This sector-specific focus addresses the needs of industries facing complex waste that are difficult to treat using conventional biological methods. With its high reactivity and absence of toxic by-products, hydrogen peroxide allows for increased solubilization of organic matter while maintaining ease of implementation. The value of this strategy also lies in identifying favorable operating conditions for the effective transformation of the material without compromising microbiological activity. By refining the dosage of the oxidant, the work underscores the importance of a trade-off between advanced solubilization and maintaining the biodegradability of the substrate.
This differentiated approach, adapted to the characteristics of the targeted waste, represents a methodological advancement that improves the profitability of anaerobic digestion processes while remaining compatible with existing infrastructure. Finally, the study is distinguished by the integration of an in-depth kinetic analysis to describe the dynamics of methane production.
Modeling according to a sigmoid law (Compertz model) is an approach that gives the work as a whole predictive and technical-scientific value that is likely to promote its transfer to larger-scale applications in the context of sustainable management of agro-industrial effluents.
2. Materials and Methods
2.1. Source of Substrate and Inoculum
The inoculum used in this study was sourced from the Hamma Bouziane Wastewater Treatment Plant (WWTP), located in Constantine, Algeria. The sludge sampling point was situated after secondary treatment at the return line. The substrate was dairy industry wastewater, also obtained from a local dairy plant in Constantine that processes various dairy products including milk, cream, and cheese. Upon collection, the samples were immediately stored at 4 °C to prevent any unwanted biological activity or physicochemical alterations prior to experimentation. The samples will be used within one week.
2.2. Treatment and Preparation of Substrate
To enhance substrate biodegradability and facilitate the hydrolysis phase of anaerobic digestion, a combined thermal-chemical pretreatment was applied. Specifically, the substrate was treated at 100 °C for 1 h [31,32] in the presence of different hydrogen peroxide (H2O2) concentrations: 0.2, 0.5, 0.9, and 1.35 g H2O2 per gram of volatile solids (g−1 VS). A control condition without H2O2 was maintained at ambient temperature (20 °C). Following pretreatment, a comprehensive physicochemical characterization of the liquid phase was performed, including measurements of pH, total solids (TS), volatile solids (VS), alkalinity at pH 6 (TA), total alkalinity at pH 4 (TAC), volatile fatty acids (VFA), total chemical oxygen demand (TCOD), soluble chemical oxygen demand (SCOD), total suspended solids (TSS), and volatile suspended solids (VSS). All the analyses were carried out in accordance with the Standard Methods for the examination of Water and Wastewater Analysis (1998). The initial characteristics of the dairy waste and inoculum are provided in Table 1.

Table 1.
Initial Physicochemical Properties of Dairy Waste and Inoculum.
2.3. Biochemical Methane Potential (BMP) Assay
The anaerobic biodegradability of the treated and untreated samples was evaluated through BMP tests, as outlined in prior studies [33,34]. Each 140 mL batch reactor was filled with an equal ratio of inoculum to substrate (1:1, v/v). To ensure the availability of micronutrients and avoid nutrient deficiencies during the digestion process, 10 mL of a nutrient solution containing essential trace elements was added to each reactor. The nutrient concentrations are listed in Table 2. All experiments were performed in triplicate using serum bottles. The pH in all reactors was adjusted to neutrality (pH ≈ 7) using a sodium hydroxide (NaOH) solution. To establish anaerobic conditions, the headspace of each reactor was purged with pure nitrogen gas (N2) for several minutes. Distilled water was added to standardize the working volume across all bottles. Control reactors (inoculum only) and positive tests (inoculum + substrate) were included. The reactors were then incubated under mesophilic conditions (37 °C) for a period of 34 days. The daily volume of biogas produced was monitored by liquid displacement in a column of acidified water (pH = 2). The experiment was terminated once biogas production became negligible.

Table 2.
Nutrient and Trace Element Composition Added to BMP Reactors [35].
2.4. Biogas Measurement
Biogas production was measured daily using the water displacement technique in acidified water (pH = 2) [15]. During the incubation, all reactors were manually agitated once per day to ensure homogeneity and reduce the risk of stratification. Methane yield was calculated by subtracting the gas production in control reactors (inoculum only) from that of test reactors (inoculum + substrate). To determine the methane and carbon dioxide content in the biogas, a potassium hydroxide (KOH) absorption method was used [6,29]. A known volume of the headspace gas (V1) was extracted with a syringe and injected into a secondary serum bottle containing 20 g/L of KOH solution. The bottle was shaken vigorously for 3–4 min to allow complete absorption of CO2 and H2S. The residual gas volume (V2), which primarily consisted of CH4 (>99.9%), was then quantified. This method enabled the estimation of methane concentration in the produced biogas.
3. Results and Discussion
3.1. Physicochemical Characterization of the Substrate Following H2O2 Pretreatment
The effect of hydrogen peroxide (H2O2) pretreatment on the physicochemical properties of the substrate was assessed and summarized in Table 3. The pretreatment was carried out using different H2O2 doses (0.2, 0.5, 0.9, and 1.35 g H2O2/g VS) combined with a thermal treatment at 100 °C for 1 h. The untreated sludge (C0) was maintained at room temperature (20 °C) and served as the control. The pH values showed a slight acidification with increasing H2O2 concentration, indicating the possible generation of acidic oxidation by-products during pretreatment. The total solids (TS) values fluctuated among the treated samples, with the highest TS content (12.00 g/L) observed at 1.35 g H2O2/g VS (1.35), suggesting a concentration effect or incomplete hydrolysis at higher doses. Conversely, volatile solids (VS) did not follow a clear trend, although a slight reduction was observed at intermediate concentrations. The VS/TS ratio, indicative of the organic fraction in the substrate, dropped from 78% (C1, thermal treatment alone) to 57.5% (1.35), reflecting organic matter mineralization due to oxidative cleavage. Suspended solids parameters (TSS and VSS) exhibited a significant decline in the early treatments, particularly at 0.5 g H2O2/g VS (0.5), where TSS dropped to 5.3 g/L. This indicates an efficient disruption of particulate matter and solubilization into the liquid phase. However, VSS levels remained relatively high, especially at higher doses, suggesting partial degradation of organic particles. The alkalinity at pH 6 and 4 decreased with increasing H2O2 concentration, potentially due to consumption of buffer compounds or release of acidic intermediates. COD measurements revealed a clear trend: total COD (CODt) decreased by approximately 12–18% following pretreatment. This decline is attributed either to the reaction of organic matter with the oxidant or to losses during handling, as some material may adhere to the reactor walls [36]. More importantly, the soluble COD (CODs) increased with H2O2 addition, rising from 5.11 g/L in the untreated sample to 6.42 g/L at 0.5. This enhancement in CODs reflects the successful breakdown of complex molecules into smaller, soluble fractions, improving substrate bioavailability [33]. These findings are in agreement with previous studies reporting the release of intracellular contents (e.g., N, P, carbohydrates) following cell wall disruption under oxidative stress [34,37].

Table 3.
Effect of the oxidizing agent (H2O2) on the solubilization of the substrate.
These adjustments allow for a better understanding of the complex dynamics between H2O2 concentrations, variations in TSS and VSS, as well as the mechanisms of volatile solids degradation. Our results indicate that managing H2O2 concentrations is crucial for optimizing the degradation of organic matter while maintaining a balance between the oxidative disintegration of solids and the preservation of microbial activity essential for effective biodegradation.
For the COD, we can say that the decrease in total COD can be attributed to handling-related factors, while the increase in soluble COD indicates a successful degradation of complex compounds, thereby facilitating their bioavailability. These results, supported by previous references, enhance our understanding of the processes involved and highlight the importance of rigorous sample management to obtain reliable data.
3.2. Effect of H2O2 Concentration on Solubilization Indices
To quantify solubilization efficiency, the ratios TSS/TS (equivalent to the proportion of particulate matter), CODs/CODt, and VSS/TSS (an indicator of the quality and biodegradability of organic matter) were calculated (Table 4). The use of hydrogen peroxide (H2O2) in wastewater treatment has significant effects on the degradation of organic matter. The suspended solids (SS) ratio, which estimates the particulate fraction of total solids, decreased with the increase in H2O2 concentration, dropping from 82% in untreated sludge to 63% and 55% for the respective dosages of 0.2 and 0.5. This indicates a pronounced release of soluble organic matter [37]. Similarly, for the pretreated samples, this ratio significantly decreased, particularly at the lower doses (0.2 and 0.5), falling from 97% in the control to approximately 69.81%. This trend suggests partial oxidation or conversion of organic matter into non-volatile compounds, likely due to increased mineralization [27,37,38]. At an intermediate concentration of 0.5 g H2O2/g VS, effective solubilization of volatile solids (VSS) can lead to a decrease in the VSS/TSS ratio, thereby improving the bioavailability of soluble organic compounds [38]. However, at higher concentrations (0.9 and 1.35), it has been observed that incomplete oxidation and reaggregation of organic fragments can occur, which may explain the increase in the VSS/TSS ratio at these levels [38] These results underscore the importance of managing H2O2 concentrations to optimize the degradation of organic matter in wastewater treatment processes.

Table 4.
Substrate characterization after H2O2 pretreatment.
Regarding CODs/CODt, the highest ratio (96.96%) was observed for the C3 treatment (0.5 g H2O2/g VS), indicating maximal solubilization of total organic matter into the liquid phase. This confirms the effectiveness of intermediate H2O2 dosing for maximizing bioavailable carbon. At lower (0.2) or higher concentrations (0.9 and 1.35), this ratio declined, suggesting either insufficient solubilization or secondary reactions leading to recalcitrant by-products. These results align with observations by other researchers who noted the optimal solubilization window depends on the balance between oxidation intensity and substrate concentration.
In conclusion, intermediate doses of H2O2, particularly at 0.5 g H2O2/g VS, maximize the solubilization of total solids and the bioavailability of carbon, as evidenced by the increase in CODs/CODt ratios and the decrease in TSS/TS and VSS/TSS ratios.
3.3. Biogas and Methane Production Performance
Figure 1 illustrates the total biogas production during the 34-day anaerobic digestion period for all pretreatment conditions. The thermal treatment alone (C1) achieved the highest biogas yield (154.1 mL/g VS), surpassing both untreated and pretreated samples with H2O2. The thermal treatment alone (C1) yielded a higher biogas production than some high-dose oxidative treatments. This is due to the fact that high oxidant doses can generate inhibitory by-products (phenolic compounds, furfurals, and excess organic acids) and increase the redox potential, which are unfavorable conditions for methanogenic microorganisms. In contrast, heating alone releases biodegradable substrates without producing such inhibitors. Among the H2O2-treated groups, the test at 0.9 g H2O2/g VS (C4) produced 152.6 mL/g VS, followed closely by C2 (0.2), C3 (0.5), and C5 (1.35) thus produced (142, 137 and 127.2 mL/g VS successively. The lowest biogas production (120 mL/g VS) was recorded for the untreated control (C0), highlighting the benefit of pretreatment. The trends in methane production, illustrated in Figure 2, reveal a similar pattern. The maximum methane yield of 90 mL/g VS was observed for both C1 and C4, corresponding to a methane yield of 11.30 m3/g COD. This yield is closely followed by C2 (0.2), C3 (0.5), and C5 (1.35), which produced 142, 137, and 127.2 mL/g VS, translating to 15.94 m3/g COD, 12.80 m3/g COD, and 12.76 m3/g COD, respectively. The lowest methane production of 120 mL/g VS (9.20 m3/g DCO) was recorded for the untreated control (C0), highlighting the clear advantages of pretreatment.
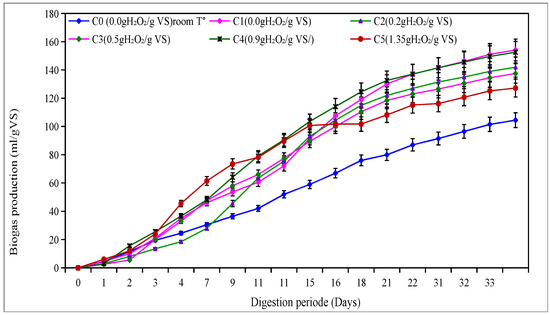
Figure 1.
Accumulative biogas produced in mesophilic phase.
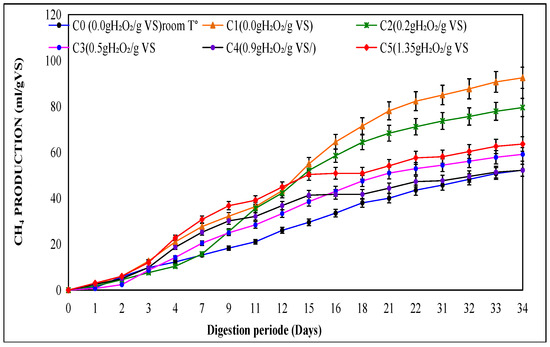
Figure 2.
Accumulative methane produced in a mesophilic phase.
While C2 and C3 also showed favorable results, it is particularly noteworthy that C5 (1.35 g H2O2/g VS), despite demonstrating enhanced solubilization, experienced a plateau in methane production. This phenomenon is likely due to the formation of inhibitory by-products at elevated oxidant dosages, such as organic acids and other toxic compounds resulting from excessive oxidation [11] All treatments exhibited a comparable lag phase, indicating no significant impact of pretreatment on microbial acclimatization. However, the rate of methane generation post-lag phase increased with H2O2 dose up to 0.5 g/g VS, confirming the role of H2O2 in enhancing hydrolysis and subsequent methanogenesis.
3.4. Enhancement of Methane Production Through H2O2 Pretreatment
During the incubation period, the average percentage of methane in the produced biogas from different tests was 50%, 56%, 41%, 60%, 43%, and 50%, corresponding to C0 (0.0 g H2O2/g VS) room T°, C1 (0.0 g H2O2/g VS), C2 (0.2 g H2O2/g VS), C3 (0.5 g H2O2/g VS), C4 (0.9 g H2O2/g VS) and C5 (1.35 g H2O2/g VS), respectively.
The effectiveness of hydrogen peroxide (H2O2) as a pretreatment agent was further evaluated by comparing the methane production of pretreated dairy waste samples to that of untreated ones. The enhancement in methane yield was quantified using the following expression [34]:
Among the different H2O2 dosages investigated—namely 0.2, 0.5, 0.9, and 1.35 g H2O2/g VS—the latency phase revealed that the most significant increases in methane production were observed at doses of 0.9 and 1.35 g H2O2/g VS. These experimental conditions resulted in methane yields approximately 1.7 to 2 times higher than those of the untreated control (see Figure 3). The observed improvements are attributed to an increased solubilization of organic matter, as well as an enhanced bioavailability of biodegradable components, a phenomenon resulting from the oxidative breakdown of complex molecular structures. Regarding the exponential phase, it was found that the thermal pretreatment alone, as well as the pretreatment corresponding to the dose of 0.9 g H2O2/g VS, produced the most favorable results. These observations align with previous research that has highlighted the positive impact of oxidative pretreatment on enhancing anaerobic biodegradability by increasing the accessibility of organic substrates to microbial attacks [33,34].
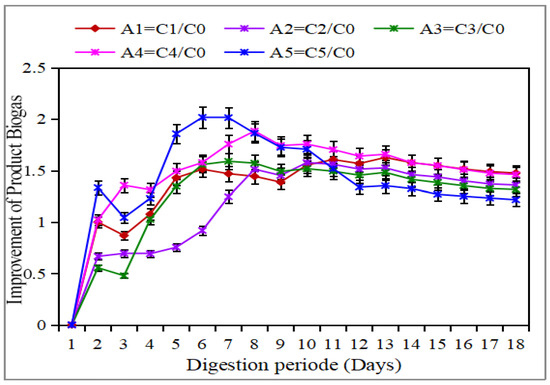
Figure 3.
Improvement in biogas with different conditions.
3.5. Post-Digestion Characterization of Digestate
To assess the effectiveness of anaerobic digestion after pretreatment, the physicochemical characteristics of the digestate were evaluated in terms of solids removal and chemical oxygen demand (COD) reduction.
3.5.1. Removal Efficiency of Total and Volatile Solids
The digestion performance was first assessed through the removal of total solids (TS) and volatile solids (VS). Across the different pretreatment conditions, TS removal efficiency ranged from 9% to 11%, indicating the degradation of suspended solids during anaerobic digestion. In parallel, the VS removal efficiency varied significantly from 34.61% to 62.18%, with the highest degradation observed for the sample pretreated at 0.9 g H2O2/g VS. This outcome supports the hypothesis that H2O2 enhances hydrolysis and subsequent microbial conversion of organic fractions to biogas.
3.5.2. COD Removal Performance
Substantial reductions in both total COD (CODt) and soluble COD (CODs) were recorded post-digestion. As shown in Table 5, the CODt removal reached up to 80.87%, whereas CODs removal peaked at 80.46%. These results demonstrate the effectiveness of oxidative pretreatment in improving substrate biodegradability. In comparison, the untreated control showed CODt and CODs removals of 77.16% and 68.88%, respectively, underscoring the enhancement introduced by pretreatment. These improvements are attributed to the oxidative breakdown of complex macromolecules into simpler, more accessible intermediates, which facilitate subsequent microbial degradation during anaerobic digestion.

Table 5.
Characterization of the liquid phase (substrate + Inoculum) of reactors after incubation.
3.6. Kinetic Modelling
To better understand the methane production dynamics during anaerobic digestion of pretreated dairy waste, a kinetic study was performed by fitting experimental data from biochemical methanogenic potential (BMP) tests using the modified Gompertz model. This empirical model, widely used to model microbial processes, allows predicting cumulative methane production as a function of time and extracting key kinetic parameters. Three key parameters were determined by this model: (i) the maximum methanogenic potential (Vmax), expressed in mL of CH4 per gram of volatile matter (g VS); (ii) the maximum methane production rate (Rmax), in mL CH4·g−1 VS·day−1; and (iii) the lag phase duration (λ), which represents the time required for microorganisms to adapt before significantly producing methane. These parameters were calculated using OriginPro 2021 software, according to the modified Gompertz model equation:
where M(t) is the cumulative methane production at time t, e being the base of the natural logarithm (≈2.718), Vmax is the methane production potential, Rmax is the maximum daily methane production rate, and λ is the lag phase duration (t).
The model follows a typical sigmoidal shape, characteristic of growth-limited biological processes. The cumulative methane production data were fitted to the above equation for each pretreatment condition applied. The parameters estimated from these fits are presented in Table 6, while the fitting curves are illustrated in Figure 4. The excellent correlation between experimental data and calculated values (R2 > 0.98) confirms the relevance of the Gompertz model to describe biogas production kinetics.

Table 6.
Comparison of experimental data and Gompertz estimate of the kinetic parameters.
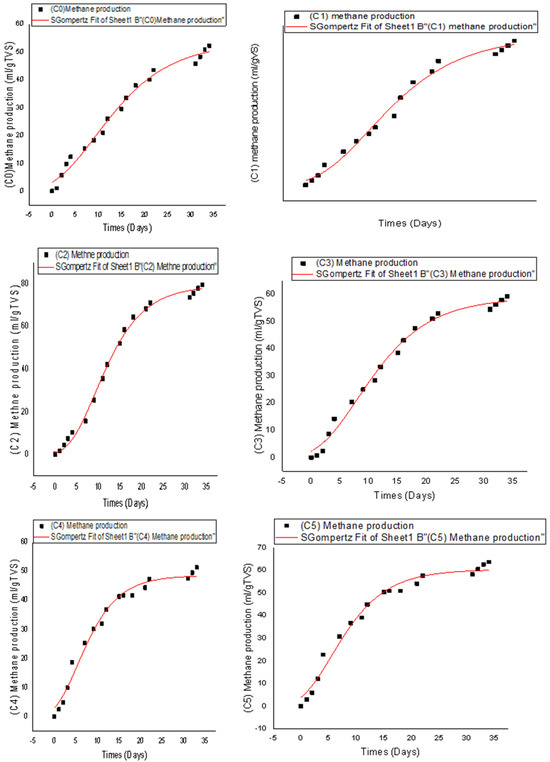
Figure 4.
Fitting of the Gompertz model for cumulative methane production for all pretreatment tests.
Analysis of the results reveals that the sample subjected only to heat treatment at 100 °C without the addition of hydrogen peroxide (C1) has the highest methanogenic potential (Vmax = 94.3 mL/g VS). This indicates that the effect of temperature alone is sufficient to improve the bioavailability of organic matter by promoting hydrolysis and cell disintegration, often limiting steps in anaerobic digestion. The highest methane production rate (Rmax = 15.36 mL/g VS·d) is observed for condition C2 (0.17 g H2O2/g VS), suggesting that moderate oxidative pretreatment combined with heat optimizes the accessibility of solubilized substrates by methanogenic microorganisms. In contrast, when the H2O2 concentration is increased (C4 and C5), a slight decrease in Vmax and Rmax is observed, probably due to excessive mineralization or the formation of toxic by-products partially inhibiting the active biomass [37,38]. Regarding the lag phase (λ), it remains overall short under all conditions, ranging from 0.03 to 0.93 days. This rapid biological activation demonstrates the good adaptation of microbial consortia, particularly when the substrates are adequately pretreated. The sample that received the highest dose of H2O2 (C5) shows minimal latency (λ = 0.03 days), which could be explained by an early release of readily biodegradable compounds following oxidation. Overall, these results highlight the importance of an optimal balance in pretreatment intensity. A moderate dosage of H2O2 effectively improves the solubility and digestibility of the substrate without degrading the usable organic fraction. Low-concentration thermal–oxidative coupling therefore proves promising for maximizing both the yield and the kinetics of methane production from organic waste from the dairy industry.
4. Conclusions
The experimental investigation aimed to evaluate the anaerobic biodegradability of sludge sourced from the Ibn Ziad wastewater treatment plant through mesophilic anaerobic digestion, with a particular focus on the effect of oxidative pretreatment using hydrogen peroxide (H2O2) combined with thermal treatment at 100 °C. The primary objective was to enhance biogas production and improve the solubilization of organic matter. The results clearly demonstrate that the integration of H2O2 as an oxidizing agent with thermal activation significantly enhances the anaerobic digestibility of the sludge. Indeed, biogas yields increased by a factor of 1.7 to 2 compared to untreated sludge during the first 9 days of digestion. Among the different concentrations tested, the dosage of 0.2 g H2O2 per g of volatile solids (VS) resulted in the highest improvement in methane production after thermal treatment C1, followed by 1.35 g H2O2/g VS, and then 0.5 g H2O2/g VS. Regarding substrate solubilization, the treatment with 0.5 g H2O2/g VS achieved the best balance between organic matter disintegration and microbial compatibility, reaching a solubilization efficiency of approximately 45%, without excessive mineralization or potential inhibitory effects. Furthermore, kinetic modeling using the modified Gompertz equation provided an excellent fit to the experimental data, with coefficients of determination (R2) consistently exceeding 0.98 across all experimental conditions. This strong correlation confirms the predictive reliability of the model in describing methane production dynamics and highlights the effectiveness of the applied pretreatment strategies.
In conclusion, it was observed that thermal treatment alone is the most effective method for valorizing sewage sludge, surpassing the use of hydrogen peroxide (H2O2) in terms of solubilization and methane recovery under mesophilic conditions. Although hydrogen peroxide may have positive effects, the doses studied in this manuscript did not show the same advantages as thermal treatment. It is possible that its effectiveness could be improved at lower doses. However, it is important to recognize certain limitations of this study. The results are based on laboratory tests, and their applicability to large-scale systems needs to be confirmed. Additionally, the long-term effects of using H2O2 on the anaerobic microflora and the environment should be examined in more detail. Therefore, further research aimed at optimizing this approach, including studies on energy balance and economic feasibility, would be beneficial for large-scale applications.
Author Contributions
Conceptualization, B.K., K.D., M.T., R.T., M.Z., S.L., M.S.O., J.Z., A.A. and H.T.; Data curation, M.T., R.T. and M.Z.; Formal analysis, B.K., M.T., R.T., M.Z., S.L., M.S.O., J.Z., A.A. and H.T.; Funding acquisition, S.L. and M.S.O.; Investigation, B.K., K.D., M.T., R.T., M.Z., S.L., M.S.O., J.Z., A.A. and H.T.; Methodology, B.K., K.D., M.T., R.T., M.Z., M.S.O., J.Z., A.A. and H.T.; Resources, B.K., K.D., M.T., M.Z., S.L., M.S.O., J.Z., A.A. and H.T.; Software, B.K., K.D., R.T., M.Z., S.L. and J.Z.; Supervision, M.Z., J.Z., A.A. and H.T.; Validation, B.K., K.D., M.T., R.T., M.Z., M.S.O., J.Z., A.A. and H.T.; Visualization, B.K., K.D., S.L., M.S.O., J.Z., A.A. and H.T.; Writing—original draft, B.K., M.T. and R.T.; Writing—review & editing, K.D., M.Z., S.L., M.S.O., J.Z., A.A. and H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ongoing Research Funding Program (ORF-2025-710), King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
The original contributions of this study are available in the article. For additional information, please contact the corresponding authors.
Acknowledgments
The authors are grateful to the Ongoing Research Funding Program (ORF-2025-710), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
All authors declare that they do not have a conflict of interest.
Abbreviations
| H2O2 | hydrogen peroxide |
| MSS | mineral suspended solids (g/L) |
| SCOD | soluble chemical oxygen demand (gO2/L) |
| TA | alkalinity |
| TAC | total alkalinity |
| TS | total solid (g/L) |
| TSS | total suspended solids (g/L) |
| VS | total volatile solids (g/L) |
| VSS | volatile suspended solids (g/L) |
| TCOD | total chemical oxygen demand (gO2/L) |
References
- Li, S.; Xie, Q.; Yang, M.; Wu, N.; Lian, Y.; Fang, C. Degradation of leachate and high concentration emerging pollutant tetracycline through electro oxidation. J. Environ. Sci. 2026, 159, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zhang, X.; Tang, X.; Jiang, L.; He, R. Uncovering anaerobic oxidation of methane and active microorganisms in landfills by using stable isotope probing. Environ. Res. 2025, 271, 121139. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Song, Y.; Yan, Y.; Chen, W.; Ren, T.; Ma, A.; Li, S.; Jia, Y. Characterization of an epilactose-producing cellobiose 2-epimerase from Clostridium sp. TW13 and reutilization of waste milk. Food Chem. 2025, 480, 143948. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Effect of substrate pretreatment on biogas production through anaerobic digestion of food waste. Int. J. Hydrogen Energy 2017, 42, 26522–26528. [Google Scholar] [CrossRef]
- Bensegueni, C.; Kheireddine, B.; Khalfaoui, A.; Amrouci, Z.; Bouznada, M.O.; Derbal, K. Optimization of Biogas and Biomethane Yield from Anaerobic Conversion of Pepper Waste Using Response Surface Methodology. Sustainability 2025, 17, 2688. [Google Scholar] [CrossRef]
- Rokaya, B.; Kerroum, D.; Hayat, Z.; Panico, A.; Ouafa, A.; Pirozzi, F. Biogas production by an anaerobic digestion process from orange peel waste and its improvement by limonene leaching: Investigation of H2O2 pre-treatment effect. Energy Sources A Recovery Util. Environ. Eff. 2024, 46, 2704–2712. [Google Scholar] [CrossRef]
- Pradeshwaran, V.; Sundaramoorthy, V.; Saravanakumar, A. A comprehensive review on biogas production from food waste: Exploring cutting-edge technologies and innovations. Biomass Bioenergy 2024, 188, 107336. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from microalgae: Technologies, challenges and opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Kanchanasuta, S.; Pisutpaisal, N. Waste utilization of palm oil decanter cake on biogas fermentation. Int. J. Hydrogen Energy 2016, 41, 15661–15666. [Google Scholar] [CrossRef]
- Naik, G.P.; Poonia, A.K.; Chaudhari, P.K. Pretreatment of lignocellulosic agricultural waste for delignification, rapid hydrolysis, and enhanced biogas production: A review. J. Indian Chem. Soc. 2021, 98, 100147. [Google Scholar] [CrossRef]
- Derbal, K. Evaluation of ultrasonic pretreatment on anaerobic digestion of citrus orange peel waste for methane production. J. New Technol. Mater. 2019, 8, 76–81. [Google Scholar]
- Lorenzo Acosta, Y.; Obaya Abreu, M.C. La digestión anaerobia. In Aspectos teóricos, Parte I; ICIDCA Sobre Los Deriv. Caña Azúcar: Ciudad de La Habana, Cuba, 2005; Volume XXXIX, pp. 35–48. [Google Scholar]
- Kheireddine, B.; Derbal, K.; Bencheikh-Lehocine, M. Effect of starting ph on the produced methane from dairy wastewater in thermophilic phase. Chem. Eng. Trans. 2014, 38, 511–516. [Google Scholar] [CrossRef]
- Gujer, W.; Zehnder, A.J.B. Conversion Processes in Anaerobic Digestion. Water Sci. Technol. 1983, 15, 127–167. [Google Scholar] [CrossRef]
- Takashima, M. Examination on Process Configurations Incorporating Thermal Treatment for Anaerobic Digestion of Sewage Sludge. J. Environ. Eng. 2008, 134, 543–549. [Google Scholar] [CrossRef]
- Kumar, G.; Sivagurunathan, P.; Thi, N.B.D.; Zhen, G.; Kobayashi, T.; Kim, S.-H.; Xu, K. Evaluation of different pretreatments on organic matter solubilization and hydrogen fermentation of mixed microalgae consortia. Int. J. Hydrogen Energy 2016, 41, 21628–21640. [Google Scholar] [CrossRef]
- Chikani-Cabrera, K.D.; Fernandes, P.M.B.; Tapia-Tussell, R.; Parra-Ortiz, D.L.; Hernández-Zárate, G.; Valdez-Ojeda, R.; Alzate-Gaviria, L. Improvement in Methane Production from Pelagic Sargassum Using Combined Pretreatments. Life 2022, 12, 1214. [Google Scholar] [CrossRef]
- Kheiredine, B.; Derbal, K.; Charbit, K.; Maryem, A. Biogas production by an anaerobic co-digestion process from olive mill waste: Effect of ultrasonic pre-treatment. Desalination Water Treat. 2022, 246, 139–145. [Google Scholar] [CrossRef]
- Rivero, J.C.; Suidan, M. Effect of H2O2 dose on the thermo-oxidative co-treatment with anaerobic digestion of excess municipal sludge. Water Sci. Technol. 2006, 54, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Hangri, S.; Derbal, K.; Benalia, A.; Policastro, G.; Panico, A.; Pizzi, A. Enhancing Biomethane Yield from Microalgal Biomass via Enzymatic Hydrolysis: Optimization and Predictive Modeling Using RSM Approach. Processes 2025, 13, 2086. [Google Scholar] [CrossRef]
- Singh, P.K.; Mohanty, P.; Mishra, S.; Adhya, T.K. Food Waste Valorisation for Biogas-Based Bioenergy Production in Circular Bioeconomy: Opportunities, Challenges, and Future Developments. Front. Energy Res. 2022, 10, 903775. [Google Scholar] [CrossRef]
- Rivero, J.A.C.; Madhavan, N.; Suidan, M.T.; Ginestet, P.; Audic, J. Oxidative Co-Treatment Using Hydrogen Peroxide with Anaerobic Digestion of Excess Municipal Sludge. Water Environ. Res. 2006, 78, 691–700. [Google Scholar] [CrossRef]
- Valo, A.; Carrère, H.; Delgenès, J.P. Thermal, chemical and thermo-chemical pre-treatment of waste activated sludge for anaerobic digestion. J. Chem. Technol. Biotechnol. 2004, 79, 1197–1203. [Google Scholar] [CrossRef]
- Gonzalez, A.; Hendriks, A.; van Lier, J.; de Kreuk, M. Pre-treatments to enhance the biodegradability of waste activated sludge: Elucidating the rate limiting step. Biotechnol. Adv. 2018, 36, 1434–1469. [Google Scholar] [CrossRef]
- Barnabé, S.; Brar, S.; Tyagi, R.; Beauchesne, I.; Surampalli, R. Pre-treatment and bioconversion of wastewater sludge to value-added products—Fate of endocrine disrupting compounds. Sci. Total. Environ. 2009, 407, 1471–1488. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Wu, P.-S.; Chung, Y.-C. Coupled biological and photo-Fenton pretreatment system for the removal of di-(2-ethylhexyl) phthalate (DEHP) from water. Bioresour. Technol. 2009, 100, 4531–4534. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.; Deleris, S.; Geaugey, V.; Ginestet, P.; Paul, E. A comparative study between mechanical, thermal and oxidative disintegration techniques of waste activated sludge. Water Sci. Technol. 2002, 46, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Sayoud, S.; Derbal, K.; Panico, A.; Pontoni, L.; Fabbricino, M.; Pirozzi, F.; Benalia, A. The Effect of Hydrogen Peroxide on Biogas and Methane Produced from Batch Mesophilic Anaerobic Digestion of Spent Coffee Grounds. Fermentation 2025, 11, 60. [Google Scholar] [CrossRef]
- Edeline, F. L’Épuration Biologique des Eaux: Théorie & Technologie des Réacteurs; Cebedoc: Villeurbanne, France, 1993; ISBN 2870800304. [Google Scholar]
- Dhavaleswarapu, R.K.; Hoysall, C.N.; Srinivasaiah, D. Thermal-alkaline pretreatment of different biomass to understand the interplay of composition of biomass, biogas yields, rates and mass balance. Bioresour. Technol. Rep. 2025, 30, 102162. [Google Scholar] [CrossRef]
- Bernet, N.; Béline, F. Challenges and innovations on biological treatment of livestock effluents. Bioresour. Technol. 2009, 100, 5431–5436. [Google Scholar] [CrossRef]
- Walter, W.G. Standard methods for the examination of water and wastewater (11th ed.). Am. J. Public Health Nations Health 1961, 51, 940. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jeong, E.; Oh, S.-E.; Shin, H.-S. Combined (alkaline+ultrasonic) pretreatment effect on sewage sludge disintegration. Water Res. 2010, 44, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Bougrier, C.; Albasi, C.; Delgenès, J.P.; Carrere, H. Effect of ultrasonic, thermal and ozone pre-treatments on waste activated sludge solubilisation and anaerobic biodegradability. Chem. Eng. Process. Process. Intensif. 2006, 45, 711–718. [Google Scholar] [CrossRef]
- Siraj, S.; Islam, M.M.; Das, P.C.; Masum, S.M.; Jahan, I.A.; Shajahan, M.D. Removal of chromium from Tannery effluent using chitosan-charcoal composite. J. Bangladesh Chem. Soc. 2012, 25, 53–61. [Google Scholar] [CrossRef]
- Bougrier, C. Optimisation du Procédé de Méthanisation par Mise en Place d’un Co-Traitement Physico-Chimique: Application au Gisement de Biogaz Représenté par les Boues D’épuration des Eaux Usées. 2005. Available online: https://agris.fao.org/search/en/providers/122439/records/6474721b2d3f560f80aab12f (accessed on 10 July 2025).
- Zhang, R.; Lu, Q.; Zhang, C.; Chen, Y.; Li, D.; Qi, W.; Ping, Q.; Li, Y. Fungal pretreatment as a promising approach for simultaneous recovery of phosphorus and carbon resource from garden waste: Performance and mechanism. Water Res. X 2025, 29, 100330. [Google Scholar] [CrossRef]
- Sohn, S.; Kim, M.K.; Lee, Y.M.; Sohn, E.J.; Choi, G.Y.; Chae, S.H.; Zoh, K.D. Removal characteristics of 53 micropollutants during ozonation, chlorination, and UV/H2O2 processes used in drinking water treatment plant. Chemosphere 2024, 352, 141360. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).