1. Introduction
Evidence of the Quaternary period’s changing climate and environment is preserved in continental deposits and landscape forms. Within the Carpathian Basin, the Danube–Tisza Interfluve in Hungary stands out as a key area for the paleoenvironmental reconstruction. This region, among numerous sand formations and loess sequences, hosts shallow, ephemeral wetlands and saline lakes, which serve as exceptional archives and “natural laboratories” for studying sediment–water interactions under a semi-arid climate [
1,
2,
3,
4,
5]. The region’s inherent sensitivity to climate change is not only a feature of the past; it is a critical contemporary issue, evidenced by increasing desertification in the area [
6,
7,
8,
9].
The substratum of the saline lakes in the Danube–Tisza Interfluve is formed by either Pleistocene loess, aeolian sand of Danubian origin, or a mixture of both [
1,
2,
10,
11]. The lakes are fed mainly by precipitation and, to a lesser extent, by groundwater seeping into the depressions, which process transports significant quantities of salts with diverse chemical compositions into the lakes [
12,
13,
14,
15,
16]. Freshwater carbonate formation (e.g., calcareous mud, freshwater limestone, “honeycomb rock”) occurs in these lakes, which, based on their organic matter content, can be divided into two types: (1) calcite-dominated carbonates, which have a higher organic content, mostly from peat consisting of reed and sedge remains, with their precipitation primarily induced by the CO
2 uptake of vegetation; and (2) dolomite-dominated carbonates, which are poor in organic matter, with their formation resulting mainly from inorganic chemical precipitation driven by evaporation [
1,
2,
11,
12,
17,
18].
This study investigates the mineralogical composition of the lacustrine deposits, with a particular focus on the carbonate-rich layers. The first objective is to provide more extensive and precise data on the major and trace elemental distributions, expanding upon previous knowledge in this area [
19]. A second objective is to establish a geochemical and mineralogical based zonation, which reflects changes in catchment processes, water chemistry, and redox conditions. A subsequent aim is to compare this zonation with existing records [
3,
20,
21,
22,
23] to produce a comprehensive multi-proxy reconstruction of past environmental dynamics.
3. Results
3.1. Mineralogical and Elemental Composition
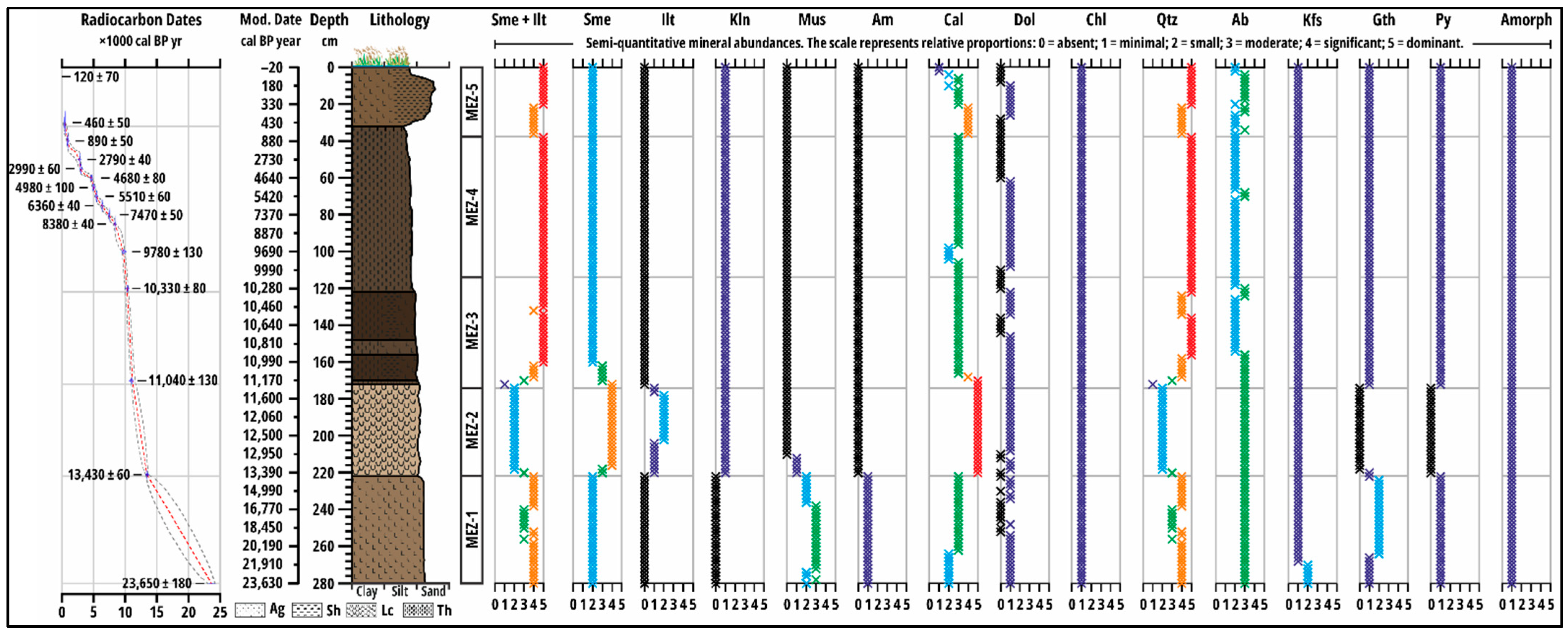
The sedimentary sequence exhibits a complex mineralogical and geochemical architecture, reflecting changes in lake trophic status, detrital input, and post-depositional processes over the last ~23,600 years. Five Mineralogical and Elemental Zones (MEZs) are defined based on the mineralogical and elemental composition of the samples. These broadly correspond to lithostratigraphic zones defined from lake-phase development but with adjusted boundaries to better characterise geochemical variation (
Figure 1 and
Figure 2).
The mineral assemblage of the sequence is dominated by quartz (Qtz) and calcite (Cal), which together account for the majority of the sediment matrix. Quartz is the principal silicate framework mineral. Its relative abundance fluctuates across the sequence, generally decreasing in carbonate-rich zones and increasing where detrital input dominates or carbonate precipitation declines. Calcite, in contrast, shows pronounced maxima in the mesotrophic lake intervals and a gradual decline towards the fen/peatland zones, reflecting the influence of carbonate precipitation and hydrological changes. Minor carbonate, in the form of dolomite (Dol), is detected and exhibits erratic variation, indicating either detrital influx or localized diagenetic formation (
Figure 2).
Feldspar minerals form a secondary component of the framework silicates. Na-feldspar (albite, Ab) is consistently present throughout the sequence, generally increasing in zones where quartz decreases, suggesting compositional complementarity within the detrital load. K-feldspar (Kfs) is present only in minor amounts and demonstrates limited stratigraphic variability. Amphibole (Am) and muscovite (Mus) are restricted to the lower oligotrophic zone, with muscovite reaching a peak in mid-zone sediments before disappearing upward. These minerals are absent in all subsequent zones (
Figure 2).
The phyllosilicate fraction is dominated by smectite (Sme) and smectite–illite mixed-layer phases (Sme+Ilt), particularly where authigenic formation under lacustrine conditions is likely. Chlorite (Chl) exhibits a gradual increase upwards, suggesting progressive detrital contribution or minor diagenetic transformation. Illite (Ilt) and kaolinite (Kln) appear primarily in the mesotrophic and fen/peatland zones, reflecting shifts in detrital supply or weathering intensity. Minor quantities of muscovite (Mus) persist only in the lower oligotrophic zone, while goethite (Gth) and pyrite (Py) occur intermittently, reflecting redox-sensitive conditions, with goethite generally associated with periods of subaerial exposure or oxidation, and pyrite indicative of anoxic conditions. Amorphous material, likely volcanic glass or secondary alteration products, is detected in trace amounts and shows a subtle increase in zones with high detrital influx.
The median abundances of the major rock-forming oxides, expressed in descending order, are SiO
2 > CaO > Al
2O
3 > Fe
2O
3 > MnO
2 > K
2O > MgO > TiO
2 > P
2O
5 > SO
2 (
Figure 1,
Table 1). The geochemical composition mirrors the mineralogical patterns, displaying broad stratigraphic trends and finer-scale variability. SiO
2 dominates throughout, reflecting detrital quartz input, with maxima in oligotrophic and fen intervals and minima in carbonate-rich zones. CaO is highly variable, peaking in mesotrophic, carbonate-rich intervals (MEZ-2) and showing an inverse relationship with SiO
2, highlighting anti-phased detrital versus authigenic carbonate input. Al
2O
3 and K
2O are relatively stable, while Fe
2O
3, MnO
2, TiO
2, and MgO exhibit zone-specific fluctuations linked to detrital load, oxidation state, and carbonate variation. P
2O
5 and SO
2 remain low, with local maxima reflecting organic matter input or sulphide formation (
Figure 1,
Table 1).
Trace elements show distinct stratigraphic trends: Zr peaks indicate detrital zircon input, Rb tracks K-bearing silicates and clays, and Sr reflects carbonate accumulation. Redox-sensitive elements (Cu, Ni, Cr) vary with detrital influx and redox conditions. Pb is confined to the upper fen/peatland intervals, consistent with surface enrichment, while Zn remains low except for sporadic peaks in the uppermost sediments (
Figure 1,
Table 2).
3.2. MEZ-1 (280–224 cm; c. 23,600–13,400 cal BP)
This zone correlates with the oligotrophic lake phase, which is dominated by SiO2 that constitutes over half of the bulk composition (51–59%) and increases stratigraphically upwards. Al2O3 (8.3–9.8%) has a low fluctuation with an overall increasing trend. K2O (1.3–2%) has low fluctuation as well. P2O5 (0.1–0.3%) has an upwards increasing trend. MgO (0.7–1.2%) has a first increasing, then a decreasing trend upwards. Concentrations of CaO (16–23%), MnO2 (1.7–2.8%), SO2 (0.03–0.04%) and TiO2 (0.3–0.9%) display an upward decrease, with minor fluctuations. Fe2O3 (9.3–13%) has relatively low fluctuation before peaking at 246–248 cm, after which its concentration declines. MgO concentrations (0.7–1.2%) first increase and subsequently decrease towards the top of the zone.
Zr is stable at the base (422–442 ppm) before peaking at 272 cm and 248 cm (464 ppm) and then decreasing towards the top of the zone (415 ppm). Rb shows a continuous decline from a maximum of 400 ppm to 90 ppm. Similarly, Cr (138 to 120 ppm), Ni (190 to 144 ppm), and Zn (112 to 12 ppm) all decrease upwards. In contrast, Sr concentrations increase steadily through the zone from 140 to 490 ppm, while Cu also shows a slow increasing trend (115 to 151 ppm).
The mineral assemblage is dominated by primary silicates. Quartz decreases from its maximum at the base before stabilising, while Na-feldspar increases and then stabilises. Muscovite and amphibole are present only in this zone, with muscovite peaking mid-zone before both minerals decrease to zero by the upper boundary. Calcite increases to a stable plateau, while dolomite decreases chaotically. Smectite abundance increases then stabilises, while chlorite increases steadily. Illite and kaolinite are absent. Goethite shows cyclic peaks, while pyrite and amorphous material increase slightly.
3.3. MEZ-2 (222–174 cm; c. 13,400–11,400 cal BP)
This zone corresponds to the mesotrophic, carbonate-rich lake phase, which is characterised by abrupt geochemical shifts that define three distinct sub-units: (I) 222–210 cm, (II) 208–196 cm, and (III) 194–174 cm. This zonation is most pronounced in the anti-phased behaviour of SiO2 and CaO. SiO2 concentrations decrease stepwise from c. 45% to c. 16% through the sub-units, before fluctuating around 18–20%. In contrast, CaO increases steadily from 29% to a maximum of 54% at 178 cm. A sharp, synchronous reversal occurs from 176 cm, where SiO2 peaks starts to increase and CaO starts to decrease. Several other elements, including K2O (peaking at 5.14%), TiO2 (peaking at 1.22%), SO2, and P2O5, attain their maximum concentrations at the base of the zone (222–220 cm) before decreasing upwards. MnO2 peaks later at 208 cm, reaching its highest concentration for the entire sequence (11.36%), then starts to decrease upwards. Fe2O3 (5.02–6.33%), Al2O3 (6.14–16%), and MgO (0.24–0.29%) concentrations also follow the three-stage pattern observed in the major components.
Zr concentrations increase markedly and become highly variable, peaking at 222 cm (905 ppm), 206–202 cm (1130–1220 ppm), 198-190 cm (1301–1932 ppm), 178 cm (1862 ppm) and reaching a zone maximum of 2163 ppm at 174 cm. Rb drops sharply to c. 50 ppm at 222 cm before beginning a slow, steady increase to 73 ppm. Sr also plummets at the start of the zone (to 21 ppm), showing minor fluctuations and a peak at 212 cm (134 ppm), then a decreasing trend to 27 ppm. Cr concentrations drop at the boundary and continue to decrease (120 to 93 ppm). Conversely, Ni increases abruptly at 222 cm from to a peak of 568 ppm, before beginning a gradual decline. Cu also peaks at the base (235 ppm) and then decreases. Zn remains low throughout the zone (13–23 ppm).
The mineralogy undergoes a fundamental transformation. Calcite abundance increases sharply to a maximum of over 40%, defining the zone. This is accompanied by the complete disappearance of muscovite, amphibole, goethite, and pyrite. Conversely, illite and kaolinite appear for the first time. Smectite abundance increases significantly, while quartz and the combined smectite + illite fraction decrease markedly due to dilution. Chlorite remains stable, while Na-feldspar fluctuates at high levels.
3.4. MEZ-3 (172–116 cm; c. 11,200–10,200 cal BP)
This zone marks the transition from the mesotrophic to eutrophic lake phase and to the onset of fen/peatland conditions, which is generally characterised by a decline in detrital-associated elements. Concentrations of SiO2 (41% to 34%), Al2O3 (13% to 8.45%), K2O (3.42% to 1.68%), MnO2 (7.1% to 2.11%), TiO2 (0.8% to 0.39%), SO2 (0.19% to 0.16%), and P2O5 (declining to 0.09%) all decrease stratigraphically upwards. Conversely, CaO (29% to 42%), MgO (0.26% to 0.62%), and Fe2O3 (6.05% to 7.02%) concentrations increase through the zone.
Zr concentrations remain high and variable, with major peaks at 172–170 cm (1260–1561 ppm) 152–144 cm (860–1114 ppm), 140 cm (2230 ppm), 122 cm (1250 ppm). Sr reverses its previous trend, increasing from a sequence minimum of 11 ppm (164 cm) to 192 ppm. Rb also continues its steady increase (to 110 ppm). Cr continues to decrease to 50 ppm (144 cm) and disappears from the record. Ni continues its slow decline to 124 ppm, while Cu also decreases (217 to 196 ppm). Zn begins to increase from its previously low values, reaching 114 ppm by the top of the zone.
Quartz and the smectite + illite fraction increase substantially as the carbonate influence wanes. Calcite abundance decreases significantly. Smectite content declines from its peak in the previous zone. Goethite and pyrite reappear, and kaolinite stabilises at a low, consistent level.
3.5. MEZ-4 (114–40 cm, c. 10,200–800 cal BP)
This zone represents the lower section of the fen/peatland phase and is characterised by significant, high-frequency geochemical variability, where SiO2 (29–53%) and CaO (21–56%) are displaying a wide and often inverse connection. Fe2O3 is also a notable component (1.18–17%), exhibiting multiple prominent peaks. Al2O3 concentrations (1.25–12%) are generally lower than in the underlying lacustrine zones but display peaks that typically co-vary with those of MnO2, K2O, TiO2, and P2O5. Concentrations of K2O and MnO2 are substantially reduced overall, although MnO2 exhibits a distinct peak at 110 cm (reaching 4.47%) and a secondary peak between 74 and 70 cm (up to 2.32%). P2O5 concentrations remain low throughout this sub-zone (0.01–0.46%). SO2 concentrations exhibit two peaks, one between 96 and 86 cm and a second, higher peak between 76 and 68 cm (reaching a maximum of 0.49%), before declining upwards. MgO concentrations are similarly variable, containing both the sequence minimum (0.01% at 96 cm) and maximum (2.4% at 80 cm).
Zr continues to exhibit multiple, sharp peaks, notably at 104 cm (1103 ppm), 100 cm (1690 ppm), 90 cm (1200 ppm), 78 cm (2060 ppm), 58 cm (1560 ppm), 52 cm (1360 ppm), 44 cm (960 ppm). Sr concentrations are highly variable, with a prominent double peak between 56 cm (980 ppm) and 50 cm (1133 ppm), after which it starts to decrease to 619 ppm. Rb fluctuates and shows multiple peaks, greatest between 84 and 76 cm at 370 ppm, before declining. This is the first zone where Pb is detected, appearing between 68 and 60 cm (40–280 ppm) before disappearing again. Cr (21–200 ppm), Ni (20–180 ppm), and Cu (40–330 ppm) are present only intermittently (hiatuses), with fluctuating values. Zn concentrations are also highly varied (6–310 ppm).
Quartz and the smectite + illite fraction remain the dominant components, exhibiting high but relatively stable abundances. Calcite concentrations are low and variable. Smectite content is low and stable. Goethite and pyrite show a slow, steady increase through the zone.
3.6. MEZ-5 (38–0 cm, from c. 800 cal BP to the Present Day)
This zone represents the uppermost section of the fen/peatland phase, where the geochemistry is inferred to reflect recent environmental conditions and ongoing processes at the sediment–water interface. CaO (33–51%) and SiO2 (30–49%) remain the dominant components, with significant contributions from Fe2O3 (2.85–17%) and Al2O3 (0.18–8.44%). Geochemical variability remains high, with most elements exhibiting multiple peaks. MnO2 concentrations are low (0.01–0.86%) but generally co-vary with K2O, Al2O3, Fe2O3, TiO2, and MgO. P2O5 concentrations are generally low (0.03–0.30%) but are punctuated by two peaks at 38–34 cm (3.16%) and 30–26 cm (1.29%). A pronounced geochemical shift occurs at 14 cm. At this horizon, following a period of low concentration from 38 cm downwards, SO2 values drop to near zero. This corresponds to a sharp decrease in Al2O3, K2O, MnO2, P2O5, and MgO, and a concurrent increase in CaO. This trend reverses in the uppermost 4 cm, where concentrations of SO2 (0.41%) increase toward the surface, in concert with MnO2, K2O, Al2O3, Fe2O3, TiO2, and MgO.
Zr values are high but less variable than below, with two smaller peaks at 36 cm (560 ppm) and 26 cm (540 ppm) before stabilising at exactly 860 ppm in the top 14 cm. Sr exhibits peaks (516–660 ppm) before rising to a sequence maximum of 1830 ppm at the top of the sequence. Rb continues its overall decreasing trend, interrupted by a small peak at 14 cm (91 ppm). This zone contains the most significant Pb concentrations, with a median of 320 ppm that perfectly describes the almost stable 38–6 cm interval, and an exceptionally elevated range of 1260–1720 ppm in the uppermost 4 cm. Cr (70–210 ppm) is only present in the lower part of the zone with high peaks, meanwhile Ni (35–70 ppm) is only present at 24–20 cm. Cu (70–210 ppm) and Zn (7–270 ppm) remain highly variable.
Quartz and the combined smectite+illite fraction increase towards the surface. Calcite exhibits a final peak before decreasing sharply in the uppermost sediments. Chlorite and goethite increase towards the surface after a dip. Pyrite steadily increases to the surface.
4. Discussion
4.1. Late Glacial Oligotrophic Lake (c. 23,600–13,400 cal BP Years)
The geochemistry and mineralogy of this zone show a cold, arid, detrital dominated Late Glacial environment. The sediment composition is governed by the influx of physically weathered silicate minerals from the catchment, evidenced by the dominance of silica and the high concentrations of quartz, feldspars, and associated elements like Al2O3, K2O, Rb, Cr, Ni. The mineralogical assemblage provides clear proxies for the prevailing climate. The initial presence of muscovite and amphibole, minerals relatively susceptible to chemical alteration, confirms a landscape where physical weathering predominated. Their gradual disappearance through the zone, however, signals a subtle but significant climatic shift towards conditions that began to enhance chemical weathering, likely a precursor to the major warming that followed this period. The clay mineralogy reinforces this interpretation; the absence of kaolinite is definitive evidence against warm, humid weathering regimes, while the presence of smectite and illite reflects the specific parent rock composition of the catchment.
In-lake conditions were predominantly oligotrophic and well-oxygenated. However, the iron mineralogy reveals a more complex redox story than simple, persistent oxygenation. The co-existence of both goethite (an iron oxyhydroxide indicating oxic conditions) and pyrite (an iron sulphide indicating anoxic conditions) points to a system with distinct redox micro-environments. The water column itself was likely oxic, allowing for the formation of goethite. The pronounced, cyclic peaks in goethite abundance suggest dynamic, pulsed inputs of dissolved iron from the catchment or periodic enhancements of bottom-water oxygenation. Simultaneously, the minor but persistent presence of pyrite indicates that sulphate reduction was occurring within anoxic niches, likely just below the sediment–water interface where microbial activity, fuelled by a limited supply of organic matter, could deplete oxygen locally.
The carbonate mineralogy also tracks environmental change. Initially low but stable calcite levels are consistent with an oligotrophic lake, with most carbonate being detrital (like the co-occurring dolomite). The subsequent increase in calcite, coupled with the disappearance of dolomite, suggests a shift in the catchment’s erosional sources and possibly the first hints of increasing in-lake productivity, a trend that would accelerate dramatically in the next zone.
4.2. Late Glacial Interstadial Productivity Maximum (c. 13,400–11,200 cal BP Years)
This period marks the warming of the Bølling-Allerød interstadial. The geochemical and mineralogical signatures shift fundamentally, driven by rapid climatic amelioration. The primary response was a massive increase in lake productivity. This ‘algal bloom’ consumed vast amounts of dissolved CO
2, drastically increasing water pH and triggering the precipitation of enormous quantities of fine-grained calcite [
11,
12,
34,
35]. This is recorded by the sharp increase in CaO to a sequence maximum of 54% and calcite becoming the single most dominant mineral.
This immense autogenic carbonate production had a diluting effect on all other sediment components. The decreases in SiO2, Al2O3, Rb, and minerals like quartz and clays are not indicative of reduced input, but rather of being overwhelmed by the sheer volume of precipitated calcite. The geochemical changes at the base of this zone—with sharp peaks in K2O, MnO2, TiO2, SO2, Ni, and Cu— reflects metal scavenging and precipitation at a migrating chemocline in a newly stratified water column. The Cu, however, may also be post-depositional, having been leached from the overlying strata. The complete disappearance of both goethite and pyrite suggests the sedimentary system was entirely reset, with carbonate chemistry now dictating geochemical processes. The complete disappearance of muscovite and amphibole, coupled with the first appearance of kaolinite and illite, signals a fundamental switch in the catchment’s weathering regime from physical to intense chemical weathering under warmer and more humid conditions. This period of general warming and humidity appears to have been punctuated by intense aridity. The dramatic increase and high variability of Zr, which is a proxy for the dense mineral zircon that requires high energy for transport, is strong evidence for enhanced aeolian activity. During dry seasons or multi-annual droughts, reduced vegetation cover on the exposed Danube River alluvial plains would have allowed strong winds to mobilize and transport sand-sized sediment, including zircon, into the basin. This points to a climate of extremes, not simple uniform warmth.
4.3. Paleoenvironmental Transition and Eutrophication (c. 11,200–10,200 cal BP Years)
This transitional zone captures the critical environmental shift at the dawn of the Holocene, chronicling the final stage of the open-water lake and its rapid terrestrialisation into a fen. The primary driver of this transformation was the establishment of a warmer, more stable climate, which initiated a cascade of interconnected responses in both the surrounding catchment and the water body itself.
The geochemical signature reflects a landscape undergoing rapid stabilisation. The marked decline in detrital-associated elements (SiO2, Al2O3, K2O, TiO2) and their corresponding trace elements (Ni, Cu) does not signify a reduction in weathering, but rather a fundamental change in erosional processes. The expansion of forests and dense ground cover across the catchment, fostered by the warmer climate, effectively anchored soils and reduced the physical transport of mineral matter into the basin. The disappearance of Cr from the record further underscores this shift in sediment sourcing.
Simultaneously, the lake itself was becoming shallower and significantly more productive—a process of natural eutrophication. The constant presence of and increase in CaO, MgO, and Sr points towards a carbonate-rich mud typical of shallow, productive, hardwater wetlands. This process is often biologically mediated by macrophytes such as
Chara, which encrust themselves with calcite in sunlit shallow waters [
22,
23].
The system was no longer overwhelmed by calcite precipitation, allowing other sedimentary processes to re-assert themselves. The rebound in the abundance of quartz and the combined smectite+illite fraction reflects the relative decrease in carbonate dilution. Crucially, the reappearance of goethite and pyrite signifies the re-establishment of complex redox gradients. As the fen developed and organic matter accumulated, distinct oxic zones (favouring goethite) and anoxic zones (favouring pyrite) formed within the sediment profile, a hallmark of productive wetland ecosystems. The stabilisation of kaolinite at a low but consistent level confirms that the catchment was now under a long-term, warm, and humid Holocene weathering regime. However, the persistent high concentrations and sharp peaks of Zr introduce a vital nuance. These Zr peaks, representing aeolian transport of the heavy mineral zircon, indicate that significant, periodic droughts still affected the wider region.
4.4. Established Holocene Fen Environment (c. 10,200–800 cal BP Years)
This long period represents the mature, established fen ecosystem, and its geochemistry is primarily a record of local hydrological variability rather than major regional climate shifts. The defining characteristic of this zone is the high-frequency, inverse cycling of CaO/calcite and SiO
2/quartz. This pattern is a classic signature of a fluctuating water table in a wetland environment [
36,
37]. During wetter climatic phases or periods of higher groundwater levels, shallow, open-water pools would have expanded across the fen. These pools, rich in dissolved calcium and bicarbonate, would have supported blooms of calcareous mud-producing algae and charophytes, leading to the deposition of sediments rich in CaO and Sr. Conversely, during drier phases, the water table would have lowered, exposing the peat and calcareous mud surfaces at the margins of the pools to desiccation, oxidation, and erosion. Runoff from these erosional events would have delivered siliciclastic material (quartz, clays) and soil-derived elements into the remaining water bodies, resulting in layers enriched in SiO
2, Al
2O
3, and Fe
2O
3.
The intermittent presence of trace metals like Cr, Ni, and Cu likely reflects these fluctuating redox conditions, where metals could be mobilized during oxidative periods and re-precipitated under anoxic conditions. The sharp peaks in Fe
2O
3 may represent the formation of “bog iron” [
38] during particularly intense or prolonged dry spells.
Despite the dominance of local processes, the fen remained connected to the wider regional climate system. The continued occurrence of multiple, sharp Zr peaks throughout this ~9000-year period demonstrates that episodic aridity and strong winds were a persistent feature of the Holocene climate in this region. These events were significant enough to transport dense mineral dust from the Danube floodplains, layering a regional aeolian signal on top of the local hydrological record. The first, transient appearance of Pb in this zone is intriguing; while it could represent a natural enrichment event associated with a specific soil-forming process, its timing makes it a candidate for a very early, low-level anthropogenic signal, possibly from distant, pre-industrial smelting activities whose atmospheric signature was captured by the fen. The slow, steady increase in pyrite throughout this zone also reflects the long-term process of peat accumulation and the establishment of permanent anoxia deep within the sediment profile.
4.5. Recent Fen Dynamics and Anthropogenic Influence (c. 800 cal BP Years to Present Days)
The geochemistry of the uppermost zone is unequivocally dominated by the signature of human activity, marking the local onset of the Anthropocene. The system’s natural cycles are sharply disrupted by external inputs. The anomalous P
2O
5 peaks, are far beyond natural background levels [
39] and are clear markers of nutrient loading from modern agricultural fertilisers. The Pb profile provides the most striking evidence of industrial impact. Its near-absence in deeper sediments followed by a sharp increase from the mid-20th century, culminating in extreme concentrations of over 1700 ppm in the top few centimetres, is a classic, unambiguous signature of atmospheric pollution from leaded petrol combustion and industrial emissions.
The coherent enrichment of Sr in the same surficial layer further supports an anthropogenic source, as both elements are common tracers of industrial and combustion-derived particulates. These impacts are superimposed on local land-use changes. The pronounced geochemical discontinuity at 14 cm, followed by the surficial increase in SO2 (from acid rain), redox-sensitive metals (Fe, Mn), and detrital proxies (Al, K, Ti), likely reflects the combined effects of historical peat mining and modern drainage schemes. These activities would have drastically altered the local hydrology, lowered the water table, and exposed previously anoxic sediments to oxidation, thereby mobilising metals and altering the sediment chemistry, a process clearly captured in the final few centimetres of the core.