Oceanography and Culture Shape Morphometric Divergence in Portunus pelagicus: Defining Actionable Management Units for Climate-Resilient Recreational Fisheries in Asia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Morphometric Variation Analyses
3. Results
3.1. Oceanographic Mirroring of Morphometric Space
3.2. Morphometric Evidence of Population Divergence
| Female | Male | |||
|---|---|---|---|---|
| Variable | First Eigenvector | Second Eigenvector | First Eigenvector | Second Eigenvector |
| CL | 0.75 | −0.08 | 0.35 | 0.75 |
| CP1 | 0.34 | 0.82 | −0.73 | 0.47 |
| CP2 | 0.74 | −0.63 | 0.77 | −0.32 |
| CP3 | 0.83 | −0.32 | 0.51 | −0.75 |
| CP4 | 0.74 | 0.31 | 0.22 | 0.57 |
| CP5 | 0.87 | −0.24 | 0.16 | −0.72 |
| CP6 | 0.73 | −0.65 | 0.78 | −0.43 |
| AB3L | 0.89 | 0.35 | 0.83 | 0.37 |
| AB3RL | 0.87 | 0.34 | 0.78 | 0.34 |
| AB3LL | 0.9 | 0.31 | 0.72 | 0.36 |
| AB3W | 0.79 | 0.09 | 0.37 | 0.53 |
| Eigenvalue | 1.34 | 0.58 | 1.34 | 0.58 |
| Percentage variance | 86% | 11% | 91% | 7% |
3.3. Functional Trait Divergence
| Population Group | Gender | Feature | Growth Rate (b) | Regression Equation Y = MX + C | R2 | Statistical Significance (p-Value) |
|---|---|---|---|---|---|---|
| China Group (CG) | Female | Abdomen (AB3W) | 1.62 | Y = 1.62X + 0.201 | 0.885 | <0.001 |
| Male | 1.58 | Y = 1.58X + 0.187 | 0.872 | <0.001 | ||
| China Subgroup (CSG) | Female | Abdomen (AB3W) | 1.71 | Y = 1.71X + 0.218 | 0.901 | <0.001 |
| Male | 1.65 | Y = 1.65X + 0.195 | 0.892 | <0.001 | ||
| Southeast Asia Group | Female | Abdomen (AB3W) | 1.75 | Y = 1.75X + 0.225 | 0.912 | <0.001 |
| Male | 1.68 | Y = 1.68X + 0.209 | 0.884 | <0.001 |
4. Discussion
4.1. Currents, Provinces, and Constrained Exchange
4.2. Potential Role of Culture as a Selective Driver
4.3. Management Implications: Define and Use Morphological Management Units (MMUs)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Hua, L.; Mei, H.; Wu, X.; Kang, Y.; Zhao, N. Impact of Climate Change on the Dynamic Processes of Marine Environment and Feedback Mechanisms: An Overview. Arch. Computat. Methods Eng. 2024, 31, 3377–3408. [Google Scholar]
- Martínez-Fernández, J.; Banos-González, I.; Esteve-Selma, M. An integral approach to address socio-ecological systems sustainability and their uncertainties. Sci. Total Environ. 2021, 762, 144457. [Google Scholar] [CrossRef] [PubMed]
- Josileen, J. Food and feeding of the blue swimmer crab, Portunus pelagicus (Linnaeus, 1758) (Decapoda, Brachyura) from the Gulf of Mannar, Southeast Coast of India. Crustaceana 2011, 84, 1169–1180. [Google Scholar] [CrossRef]
- Chiau, W.Y. The role of religion in coastal resource management: The case of Kupo Island, Penghu (Pescadores), Taiwan. Coast. Manag. 1998, 26, 17–31. [Google Scholar] [CrossRef]
- Gaviglio, A.; Demartini, E.; Mauracher, C.; Pirani, A. Consumer perception of different species and presentation forms of fish: An empirical analysis in Italy. Food Qual. Prefer. 2014, 36, 33–49. [Google Scholar] [CrossRef]
- Lu, Y.M.; Shih, C.H.; Chen, P.C.; Kao, W.C.; Lee, Y.C.; Han, Y.S.; Tzeng, T.D. Genetic variations and expansion of the blue swimmer crab (Portunus pelagicus) in Southeast Asia. J. Mar. Sci. Eng. 2022, 10, 1071. [Google Scholar] [CrossRef]
- Hui, M.; Kraemer, W.E.; Seidel, C.; Nuryanto, A.; Joshi, A.; Kochzius, M. Comparative genetic population structure of three endangered giant clams (Cardiidae: Tridacna species) throughout the Indo-West Pacific: Implications for divergence, connectivity and conservation. J. Mollus. Stud. 2016, 82, 403–414. [Google Scholar] [CrossRef]
- Bernatchez, L.; Wellenreuther, M.; Araneda, C.; Ashton, D.T.; Barth, J.M.; Beacham, T.D.; Maes, G.E.; Martinsohn, J.T.; Miller, K.M.; Naish, K.A.; et al. Harnessing the power of genomics to secure the future of seafood. Trends Ecol. Evol. 2017, 32, 665–680. [Google Scholar] [CrossRef]
- Tzeng, T.D.; Chiu, C.S.; Yeh, S.Y. Morphometric variation in red-spot prawn (Metapenaeopsis barbata) in different geographic waters off Taiwan. Fish. Res. 2001, 53, 211–217. [Google Scholar] [CrossRef]
- Sanford, E.; Kelly, M.W. Local adaptation in marine invertebrates. Annu. Rev. Mar. Sci. 2011, 3, 509–535. [Google Scholar] [CrossRef]
- Tzeng, T.D.; Yeh, S.Y. Permutation tests for difference between two multivariate allometric patterns. Zool. Stud. 1999, 38, 10–18. [Google Scholar]
- Reist, J. An empirical evaluation of several univariate methods that adjust for size variation in morphometric data. Can. J. Zool. 1985, 63, 1429–1439. [Google Scholar] [CrossRef]
- Song, N.; Gao, T.; Ying, Y.; Yanagimoto, T.; Han, Z. Is the Kuroshio Current a strong barrier for the dispersal of the gizzard shad (Konosirus punctatus) in the East China Sea? Mar. Freshw. Res. 2016, 68, 810–820. [Google Scholar] [CrossRef]
- Funk, W.C.; McKay, J.K.; Hohenlohe, P.A.; Allendorf, F.W. Harnessing genomics for delineating conservation units. Trends Ecol. Evol. 2012, 27, 489–496. [Google Scholar] [CrossRef]
- Campbell, G.R.; Fielder, D.R. Size at sexual maturity and occurrence of ovigerous females in three species of commercially exploited portunid crabs in S.E. Queensland. Proc. R. Soc. Queensl. 1986, 97, 79–87. [Google Scholar]
- Rasheed, S.; Mustaquim, J. Size at sexual maturity, breeding season and fecundity of three-spot swimming crab Portunus sanguinolentus (Herbst, 1783) (Decapoda, Brachyura, Portunidae) occurring in the coastal waters of Karachi, Pakistan. Fish. Res. 2010, 103, 56–62. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef] [PubMed]
- Sumaila, U.R.; Cheung, W.W.L.; Lam, V.W.Y.; Pauly, D.; Herrick, S. Climate change impacts on the biophysics and economics of world fisheries. Nat. Clim. Chang. 2011, 1, 449–456. [Google Scholar] [CrossRef]
- Ditton, R.B.; Holland, S.M.; Anderson, D.K. Recreational fishing as tourism. Fisheries 2002, 27, 17–24. [Google Scholar] [CrossRef]
- Sayeed, Z.; Sugino, H.; Sakai, Y.; Yagi, N. Consumer preferences and willingness to pay for Mud Crabs in southeast asian countries: A discrete choice experiment. Foods 2021, 10, 2873. [Google Scholar] [CrossRef]
- Klinbunga, S.; Pripue, P.; Khamnamtong, N.; Puanglarp, N.; Tassanakajon, A.; Jarayabhand, P.; Menasveta, P. Genetic diversity and molecular markers of the tropical abalone (Haliotis asinina) in Thailand. Mar. Biotechnol. 2003, 5, 505–517. [Google Scholar] [CrossRef]
- Cadrin, S.X. Advances in morphometric identification of fishery stocks. Rev. Fish. Biol. Fish. 2000, 10, 91–112. [Google Scholar] [CrossRef]
- Begg, G.A.; Friedland, K.D.; Pearce, J.B. Stock identification and its role in stock assessment and fisheries management: An overview. Fish. Res. 1999, 43, 1–8. [Google Scholar] [CrossRef]
- Lai, J.C.Y.; Ng, P.K.L.; Davie, P.J.F. A revision of the Portunus pelagicus (Linnaeus, 1758) species complex (Crustacea: Brachyura: Portunidae), with recognition of four species. Raffles Bull. Zool. 2010, 58, 199–237. [Google Scholar]
- Kao, W.-C.; Shih, C.-H.; Sung, Y.-C.; Chen, P.-C.; Lu, Y.-M.; Han, Y.-S.; Tzeng, T.-D. Morphometric Diversity and Population Structure of the Crucifix Crab (Charybdis feriatus) in East Asian Recreational Fisheries. Water 2025, 17, 688. [Google Scholar] [CrossRef]
- Chen, P.C.; Tzeng, T.D.; Shih, C.H.; Chu, T.J.; Lee, Y.C. Morphometric variation of the oriental river prawn (Macrobrachium nipponense) in Taiwan. Limnologica 2015, 52, 51–58. [Google Scholar] [CrossRef]
- Thorpe, R.S.; Leamy, L. Morphometric studies in inbred and hybrid House Mice (Mus sp.): Multivariate analysis of size and shape. J. Zool. 1983, 199, 421–432. [Google Scholar] [CrossRef]
- Keenan, C.; Davie, P.J.; Mann, D.L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bull. Zool. 1998, 46, 217–245. [Google Scholar]
- Hellberg, M.E. Dependence of gene flow on geographic distance in two solitary corals with different larval dispersal capabilities. Evolution 1996, 50, 1167–1175. [Google Scholar] [CrossRef]
- Kotlik, P.; Berrebi, P. Phylogeography of the barbel (Barbus barbus) assessed by mitochondrial DNA variation. Mol. Ecol. 2001, 10, 2177–2185. [Google Scholar] [CrossRef]
- Yi, S. Willingness-to-pay for sustainable aquaculture products: Evidence from Korean red seabream aquaculture. Sustainability 2019, 11, 1577. [Google Scholar] [CrossRef]
- Soundarapandian, P.; Varadharajan, D.; Boopathi, A. Reproductive biology of the commercially important portunid crab, Portunus pelagicus (Herbst). J. Mar. Sci. Res. Dev. 2013, 3, 2–9. [Google Scholar]
- Bureau of Fisheries. 2020 China Statistical Yearbook; China Statistics Press: Beijing, China, 2020. [Google Scholar]
- Sumpton, W.D. Morphometric growth and fisheries biology of the crab, Charybdis natator (Herbst) in Moreton Bay, Australia (Decapoda, Brachyura). Crustaceana 1990, 59, 113–120. [Google Scholar] [CrossRef]
- Yu, Y.C. Morphometric Studies on Stock Discrimination of Swimming Crab (Charybdis feriatus) in the Offshore Areas of Taiwan. Master’s Thesis, Institute of Oceanography, National Taiwan University, Taipei, Taiwan, 2003; p. 56. (In Chinese). [Google Scholar]
- Adams, D.C.; Rohlf, F.J. Ecological character displacement in Plethodon: Biomechanical differences found from a geometric morphometric study. Proc. Natl. Acad. Sci. USA 2000, 97, 4106–4111. [Google Scholar] [CrossRef]
- Kao, W.C.; Chang, P.H.; Shih, C.H.; Chen, P.C.; Tzeng, T.D.; Han, Y.S.; Lu, Y.M. Morphometric differentiation of the swimming crab Portunus sanguinolentus (Herbst, 1783) populations in East Asia: Implications for stock identification and management. Water 2023, 15, 3335. [Google Scholar] [CrossRef]
- Tzeng, C.S. Distribution of the freshwater fishes of Taiwan. J. Taiwan Mus. 1986, 39, 127–146. [Google Scholar]
- Tudela, S. Morphological variability in a Mediterranean, genetically homogeneous population of the European anchovy, Engraulis encrasicolus. Fish. Res. 1999, 42, 229–243. [Google Scholar] [CrossRef]
- Paramo, J.; Saint-Paul, U. Morphological differentiation of southern pink shrimp Farfantepenaeus notialis in Colombian Caribbean Sea. Aquat. Living. Resour. 2010, 23, 95–101. [Google Scholar]
- Rohlf, F.J.; Marcus, L.F. A revolution morphometrics. Trends. Ecol. Evol. 1993, 8, 129–132. [Google Scholar] [CrossRef]
- Bookstein, F.L.; Chernoff, B.; Elder, R.L.; Humphries, J.M.; Smith, G.R.; Strauss, R.E. Morphometrics in Evolutionary Biology: The Geometry of Size and Shape Change with Examples from Fishes, 15th ed.; Academy of National Sciences: Philadelphia, PA, USA, 1985; pp. 20–45. [Google Scholar]
- Reyment, R.A. Morphometrics: An Historical Essay. In Morphometrics for Nonmorphometricians; Elewa, A.M.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 9–24. [Google Scholar]
- Lachenbruch, P.A.; Goldstein, M. Discriminant analysis. Biometrics 1979, 35, 69–85. [Google Scholar]
- Lu, Y.M.; Shih, C.H.; Chen, P.C.; Kao, W.C.; Lee, Y.C.; Han, Y.S.; Tzeng, T.D. Phylogeography and genetic structure of the swimming crabs Portunus sanguinolentus (Herbst, 1783) in East Asia. J. Mar. Sci. Eng. 2022, 10, 281. [Google Scholar] [CrossRef]
- Dineshbabu, A.P.; Sreedhara, B.; Muniyappa, Y. Fishery and stock assessment of Portunus pelagicus (Herbst) from south Karnataka coast, India. J. Mar. Biol. Assoc. India 2007, 49, 134–140. [Google Scholar]
- Sugama, K.; Benzie, J.A.H.; Ballment, E. Genetic variation and population groups of the giant tiger prawn, Penaeus monodon, in Indonesia. Aquaculture 2002, 205, 37–48. [Google Scholar] [CrossRef]
- Yosho, I.; Hirose, T.; Shirai, S. Bathymetric distribution of beni-zuwai crab Chionoecetes japonicus in the northern part of the Sea of Japan. Fish. Sci. 2009, 75, 1417–1429. [Google Scholar] [CrossRef]
- Reddy, M.M.; Macdonald, A.H.; Groeneveld, J.C.; Schleyer, M.H. Phylogeography of the scalloped spiny-lobster Panulirus Homarus rubellus in the southwest Indian Ocean. J. Crust. Biol. 2014, 34, 773–781. [Google Scholar] [CrossRef]
- Asche, F.; Guillen, J. The importance of fishing method, gear and origin: The Spanish hake market. Mar. Policy 2012, 36, 365–369. [Google Scholar] [CrossRef]
- Chien, C.J. From Sea Crabs to Wanli Crab: The Reterritorialization of Quality and Resource Governance. Master’s Thesis, Department of Geography, National Taiwan Normal University, Taipei, China, 2024; p. 132. (In Chinese). [Google Scholar]
- Pacana, N.R. The Moro in the Moro Moro: Hegemonic Representation in the Linambay Plays in Cebu. PQCS 2007, 35, 87–99. [Google Scholar]
- Yan, X.; Zhang, C. The connotation of Chinese crab culture: A comprehensive review from the perspectives of literature, art, and diet. Front. Mar. Sci. 2025, 12, 1556758. [Google Scholar] [CrossRef]
- Mirera, O.D. Trends in exploitation, development and management of artisanal mud crab (Scylla serrata-Forsskal-1775) fishery and small-scale culture in Kenya: An overview. Ocean. Coast. Manag. 2011, 54, 844–855. [Google Scholar] [CrossRef]
- Supungul, P.; Sootanan, P.; Klinbunga, S.; Kamonrat, W.; Jarayabhand, P.; Tassanakajon, A. Microsatellite polymorphism and the population groups of the black tiger shrimp (Penaeus monodon) in Thailand. Mar. Biotechnol. 2000, 2, 339–347. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Grimes, C.B.; Johnson, A.G.; Fable, W.A., Jr. Delineation of king mackerel (Scomberomorus cavalla) stocks along the US east coast and in the Gulf of Mexico. In Proceedings of the Stock Identification Workshop; Kumpf, H.E., Vaught, R.N., Grimes, C.B., Johnson, A.G., Nakamura, E.L., Eds.; NOAA Technical Memorandum NMFS-SEFC: Panama City Beach, FL, USA, 1987; Volume 199, pp. 186–187. [Google Scholar]
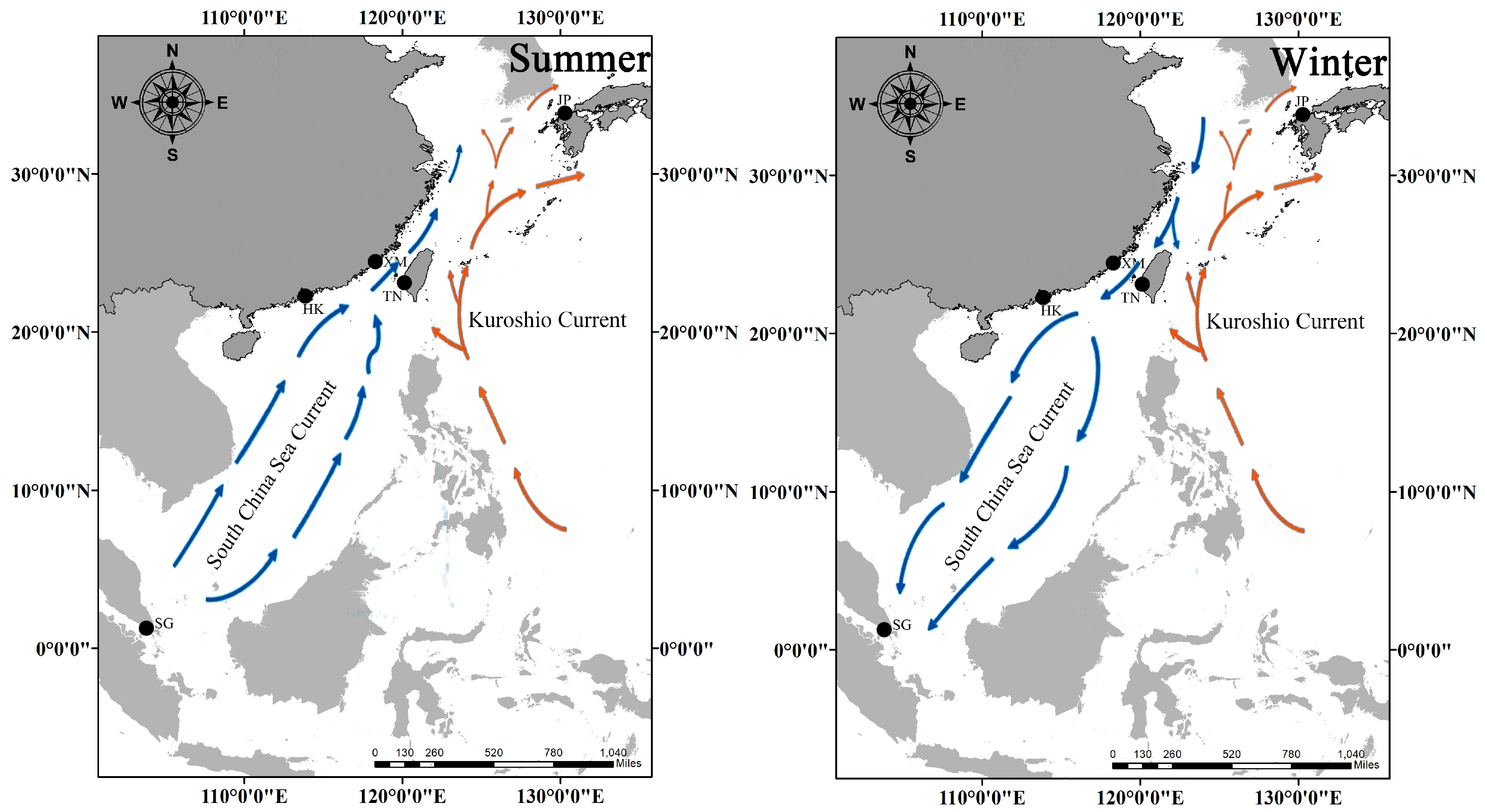
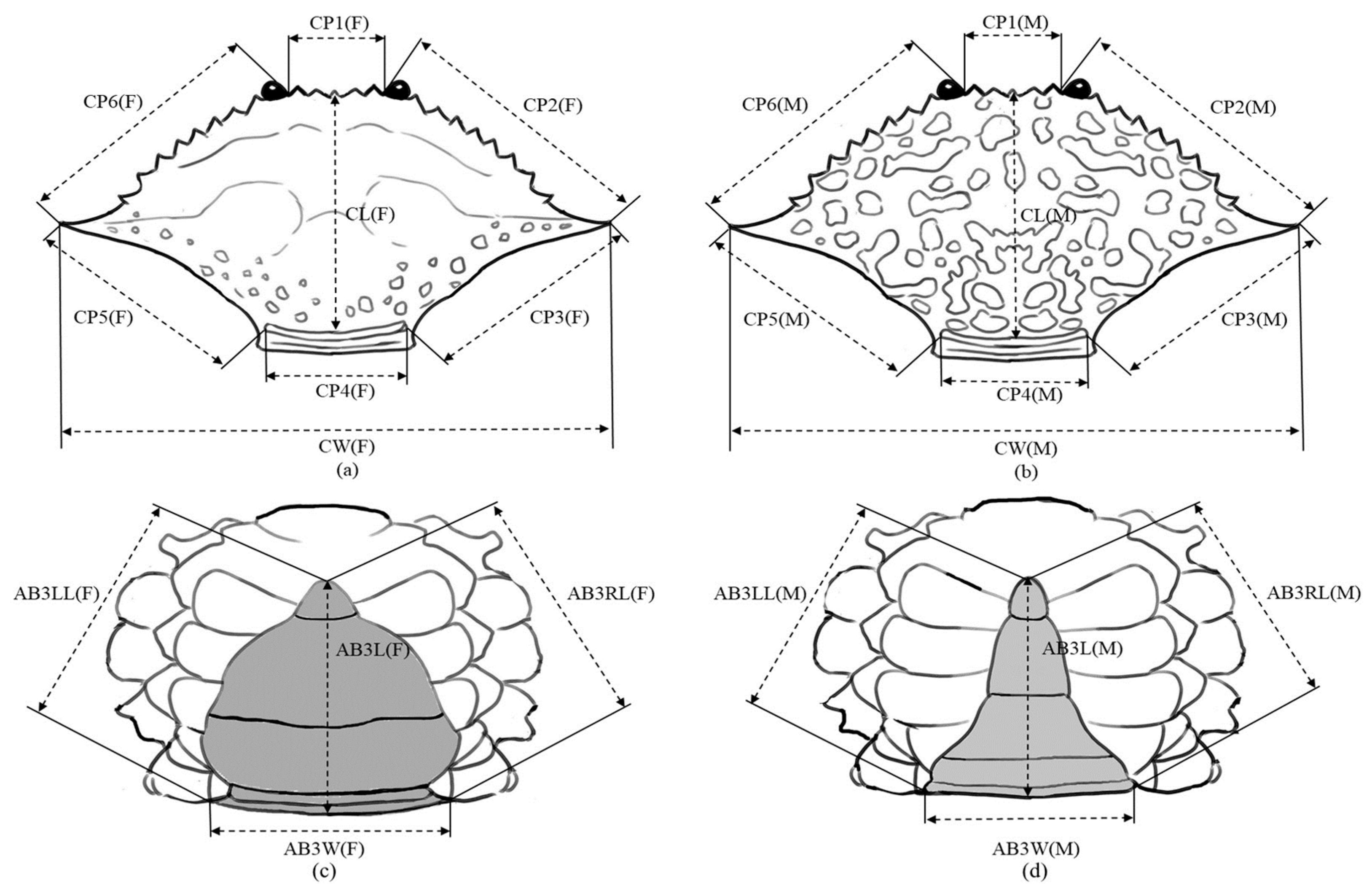



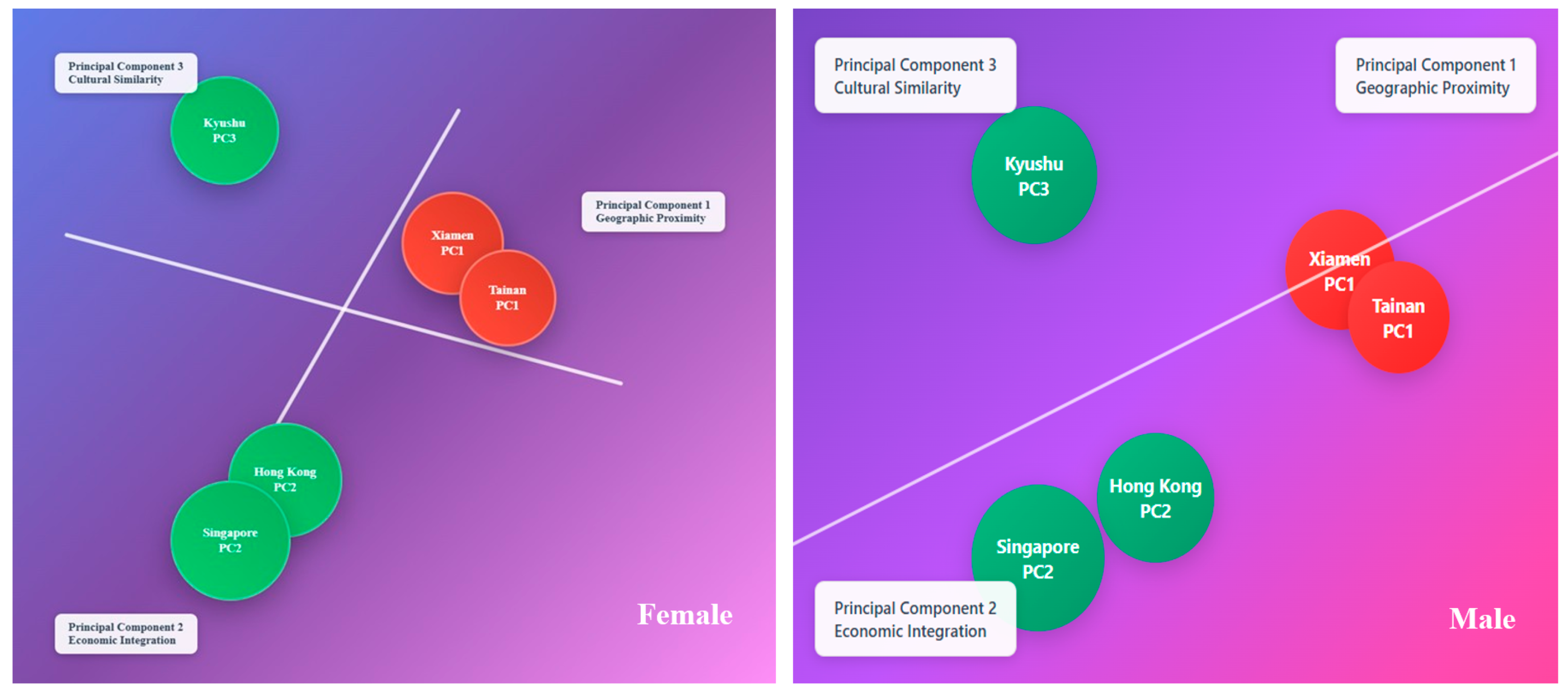
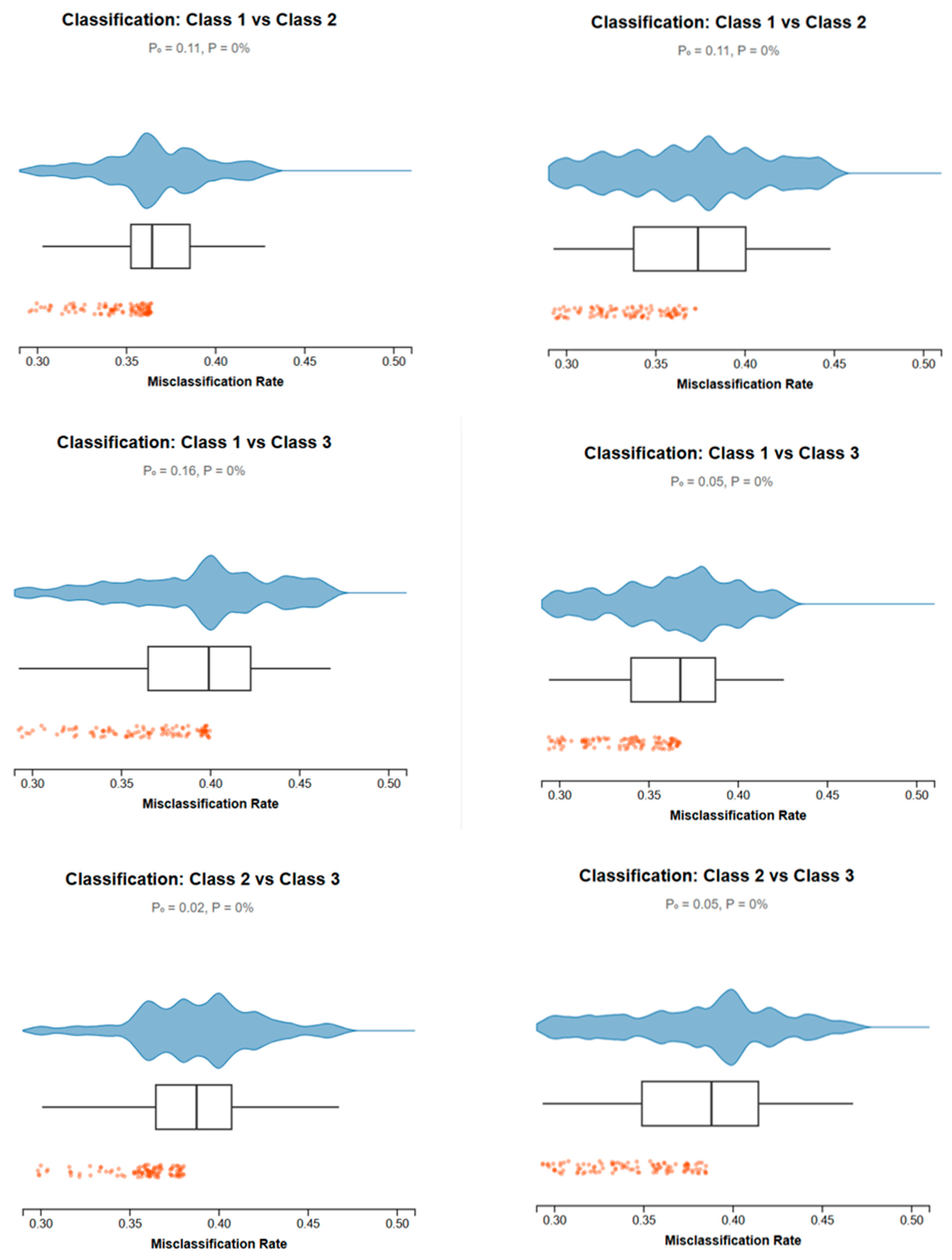
| Area Code | Sampling Site | n | Sex | Sampling Date | CW (mm) | Depth Range (m) | SST Range (°C) | Salinity (psu) | Substrate Type | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | |||||||||
| KS | Kyushu | 54 | F | January 2024 | 113.13 (18.76) | 66.42–156.43 | 15–35 | 16.2–18.5 | 34.1–34.3 | Sandy-muddy with shell fragments |
| 52 | M | 115.06 (12.46) | 78.24–134.12 | Sandy-muddy with shell fragments | ||||||
| XM | Xiamen | 56 | F | October 2023 | 116.15 (15.25) | 88.38–152.00 | 8–25 | 22.8–24.1 | 33.8–34.2 | Muddy-sandy, high organic content |
| 48 | M | 112.02 (14.06) | 78.24–134.12 | |||||||
| TN | Tainan | 53 | F | November 2023 | 105.80 (6.53) | 91.59–122.09 | 12–28 | 24.5–26.2 | 34.0–34.4 | Rocky-sandy, coarse sediment |
| 52 | M | 101.35 (7.04) | 91.24–117.97 | |||||||
| HK | Hongkong | 55 | F | October 2023 | 114.64 (8.12) | 98.69–152.06 | 10–30 | 25.1–26.8 | 33.5–34.1 | Muddy with gravel patches |
| 50 | M | 118.72 (7.76) | 103.76–132.84 | |||||||
| SG | Singapore | 56 | F | January 2024 | 139.68 (5.82) | 119.40–153.25 | 5–20 | 28.3–29.7 | 33.2–33.8 | Fine mud, high silt content |
| 49 | M | 121.48 (9.41) | 91.64–137.03 | |||||||
| (A) Female | |||||||||||
| Variable | CL | CP1 | CP2 | CP3 | CP4 | CP5 | CP6 | AB3L | AB3RL | AB3LL | AB3W |
| CL | 0.81 ** | 0.83 ** | 0.85 ** | 0.86 ** | 0.83 ** | 0.82 ** | 0.92 ** | 0.92 ** | 0.92 ** | 0.93 ** | |
| CP1 | 0.19 ** | 0.49 ** | 0.17 | 0.79 ** | 0.67 ** | 0.47 ** | 0.80 ** | 0.80 ** | 0.80 ** | 0.77 ** | |
| CP2 | 0.59 ** | −0.24 ** | 0.92 ** | 0.72 ** | 0.92 ** | 0.98 ** | 0.82 ** | 0.83 ** | 0.83 ** | 0.84 ** | |
| CP3 | 0.56 ** | 0.13 | 0.79 ** | 0.81 ** | 0.97 ** | 0.90 ** | 0.88 ** | 0.88 ** | 0.89 ** | 0.86 ** | |
| CP4 | 0.71 ** | 0.55 ** | 0.33 ** | 0.54 ** | 0.81 ** | 0.72 ** | 0.89 ** | 0.86 ** | 0.86 ** | 0.81 ** | |
| CP5 | 0.57 ** | 0.24 ** | 0.78 ** | 0.92 ** | 0.58 ** | 0.91 ** | 0.88 ** | 0.87 ** | 0.88 ** | 0.85 ** | |
| CP6 | 0.59 ** | −0.27 ** | 0.94 ** | 0.78 ** | 0.35 ** | 0.77 ** | 0.81 ** | 0.81 ** | 0.82 ** | 0.84 ** | |
| AB3L | 0.59 ** | 0.51 ** | 0.46 ** | 0.59 ** | 0.71 ** | 0.65 ** | 0.43 ** | 0.99 ** | 0.99 ** | 0.94 ** | |
| AB3RL | 0.55 ** | 0.48 ** | 0.45 ** | 0.56 ** | 0.61 ** | 0.63 ** | 0.41 ** | 0.95 ** | 0.99 ** | 0.95 ** | |
| AB3LL | 0.58 ** | 0.47 ** | 0.47 ** | 0.60 ** | 0.65 ** | 0.65 ** | 0.44 ** | 0.96 ** | 0.97 ** | 0.95 ** | |
| AB3W | 0.46 ** | 0.26 ** | 0.55 ** | 0.54 ** | 0.46 ** | 0.56 ** | 0.53 ** | 0.75 ** | 0.78 ** | 0.79 ** | |
| (B) Male | |||||||||||
| Variable | CL | CP1 | CP2 | CP3 | CP4 | CP5 | CP6 | AB3L | AB3RL | AB3LL | AB3W |
| CL | 0.38 | 0.94 ** | 0.96 ** | 0.94 ** | 0.97 ** | 0.93 ** | 0.99 ** | 0.98 ** | 0.98 ** | 0.98 ** | |
| CP1 | 0.12 | 0.71 ** | 0.76 ** | 0.36 | 0.78 ** | 0.68 ** | 0.83 ** | 0.82 ** | 0.81 ** | 0.45 | |
| CP2 | 0.15 * | −0.73 ** | 0.97 ** | 0.85 ** | 0.97 ** | 0.99 ** | 0.96 ** | 0.96 ** | 0.96 ** | 0.43 | |
| CP3 | −0.32 ** | −0.65 ** | 0.62 ** | 0.85 ** | 0.99 ** | 0.97 ** | 0.97 ** | 0.47 | 0.47 | 0.45 | |
| CP4 | 0.56 ** | −0.12 | 0.15 * | −0.34 ** | 0.86 ** | 0.85 ** | 0.91 ** | 0.40 | 0.41 | 0.49 | |
| CP5 | −0.39 ** | −0.27 ** | 0.19 ** | 0.64 ** | −0.59 ** | 0.97 ** | 0.48 | 0.48 | 0.48 | 0.95 ** | |
| CP6 | −0.05 | −0.86 ** | 0.79 ** | 0.64 ** | 0.10 | 0.27 ** | 0.96 ** | 0.96 ** | 0.96 ** | 0.42 | |
| AB3L | 0.55 ** | −0.38 ** | 0.47 ** | 0.18 ** | 0.36 ** | −0.09 | 0.42 ** | 0.99 ** | 0.99 ** | 0.97 ** | |
| AB3RL | 0.43 ** | −0.31 ** | 0.32 ** | 0.12 | 0.09 | 0.057 | 0.34 ** | 0.80 ** | 1.00 ** | 0.97 ** | |
| AB3LL | 0.37 ** | −0.25 ** | 0.26 ** | 0.05 | 0.13 | 0.02 | 0.32 ** | 0.72 ** | 0.88 ** | 0.97 ** | |
| AB3W | 0.44 ** | 0.04 | 0.06 | −0.12 | 0.09 | −0.22 ** | −0.01 | 0.42 ** | 0.51 ** | 0.47 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-C.; Shih, C.-H.; Tzeng, T.-D.; Huang, C.-H.; Zhang, G.-M. Oceanography and Culture Shape Morphometric Divergence in Portunus pelagicus: Defining Actionable Management Units for Climate-Resilient Recreational Fisheries in Asia. Water 2025, 17, 2783. https://doi.org/10.3390/w17182783
Chen P-C, Shih C-H, Tzeng T-D, Huang C-H, Zhang G-M. Oceanography and Culture Shape Morphometric Divergence in Portunus pelagicus: Defining Actionable Management Units for Climate-Resilient Recreational Fisheries in Asia. Water. 2025; 17(18):2783. https://doi.org/10.3390/w17182783
Chicago/Turabian StyleChen, Po-Cheng, Chun-Han Shih, Tzong-Der Tzeng, Chi-Hui Huang, and Gui-Mei Zhang. 2025. "Oceanography and Culture Shape Morphometric Divergence in Portunus pelagicus: Defining Actionable Management Units for Climate-Resilient Recreational Fisheries in Asia" Water 17, no. 18: 2783. https://doi.org/10.3390/w17182783
APA StyleChen, P.-C., Shih, C.-H., Tzeng, T.-D., Huang, C.-H., & Zhang, G.-M. (2025). Oceanography and Culture Shape Morphometric Divergence in Portunus pelagicus: Defining Actionable Management Units for Climate-Resilient Recreational Fisheries in Asia. Water, 17(18), 2783. https://doi.org/10.3390/w17182783









