Abnormal Adsorption Characteristics of Copper, Zinc, and Manganese Ions on Natural Diatomite in a Liquid/Solid Heterogeneous System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Aqueous Solutions of Cu2+, Zn2+, Mn2+
2.3. Adsorption Experiment
2.4. Determination of the Concentration of Heavy Metal Ions
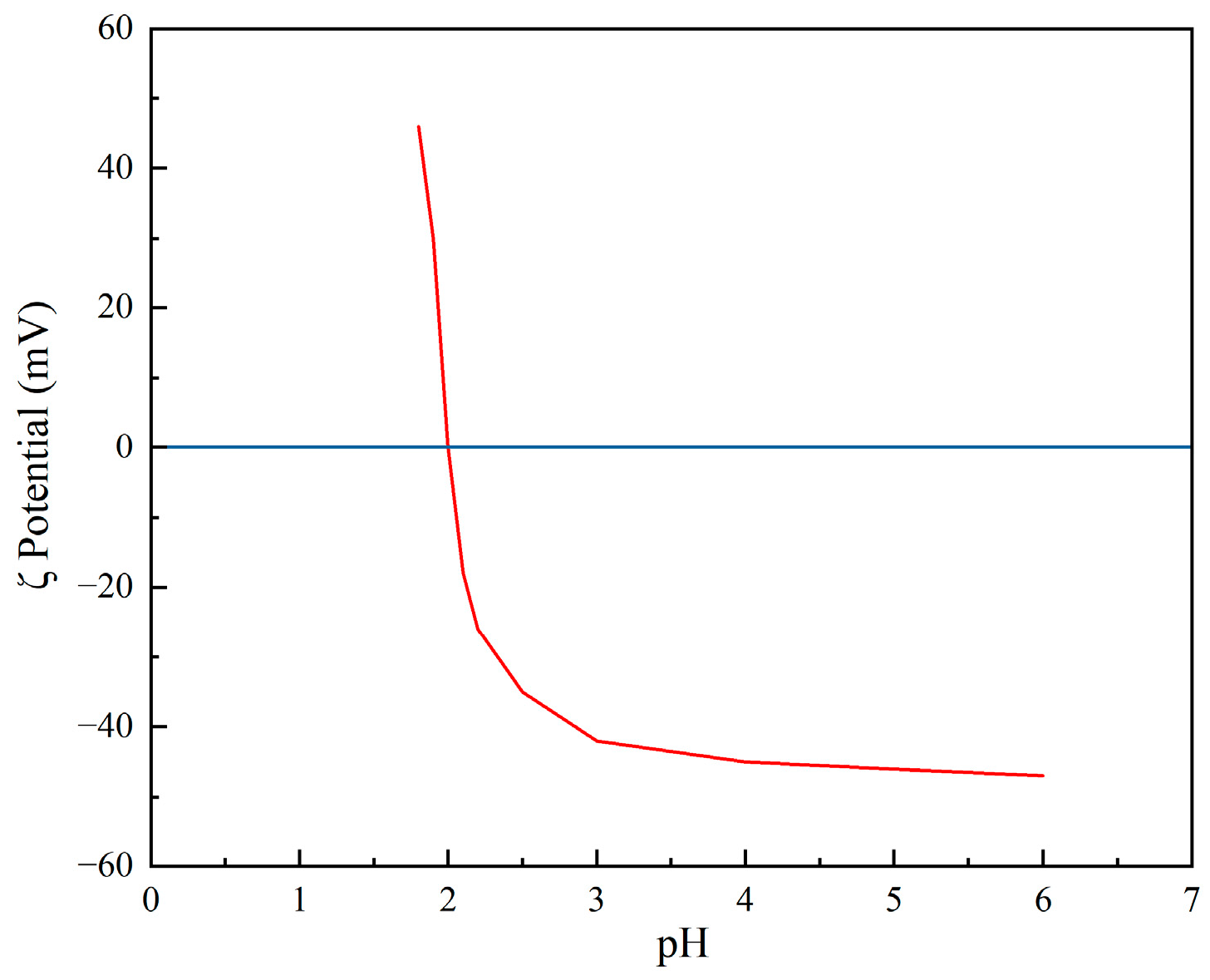
2.5. Determination of Isoelectric Point
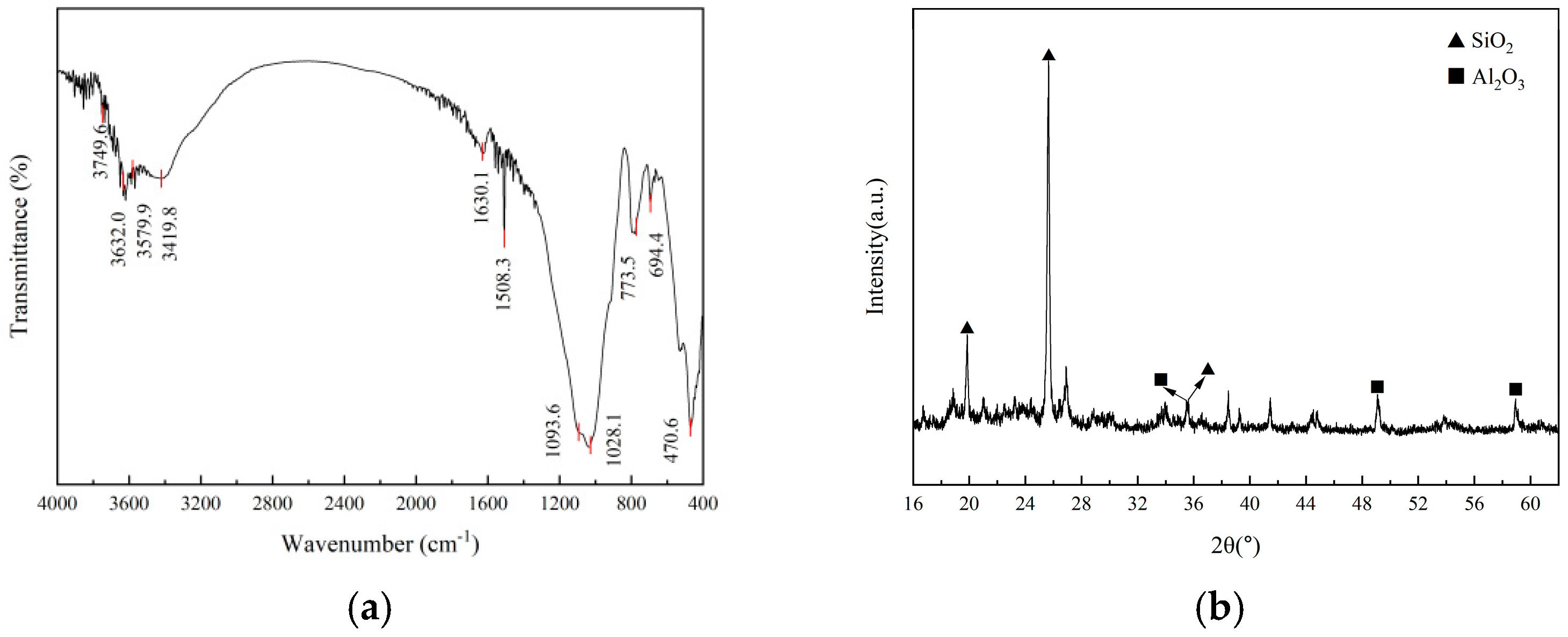
2.6. Characterization of Diatomite
3. Results
3.1. Characterization
3.1.1. Physiochemical Analysis of the Diatomite
3.1.2. Surface Electrochemical Analysis
3.2. Factors Influencing the Cu2+, Zn2+, and Mn2+ Adsorption Process on Diatomite
3.2.1. Effect of Adsorbent Dosage (W0) on the Adsorption Process of Cu2+, Zn2+, and Mn2+ by Diatomite
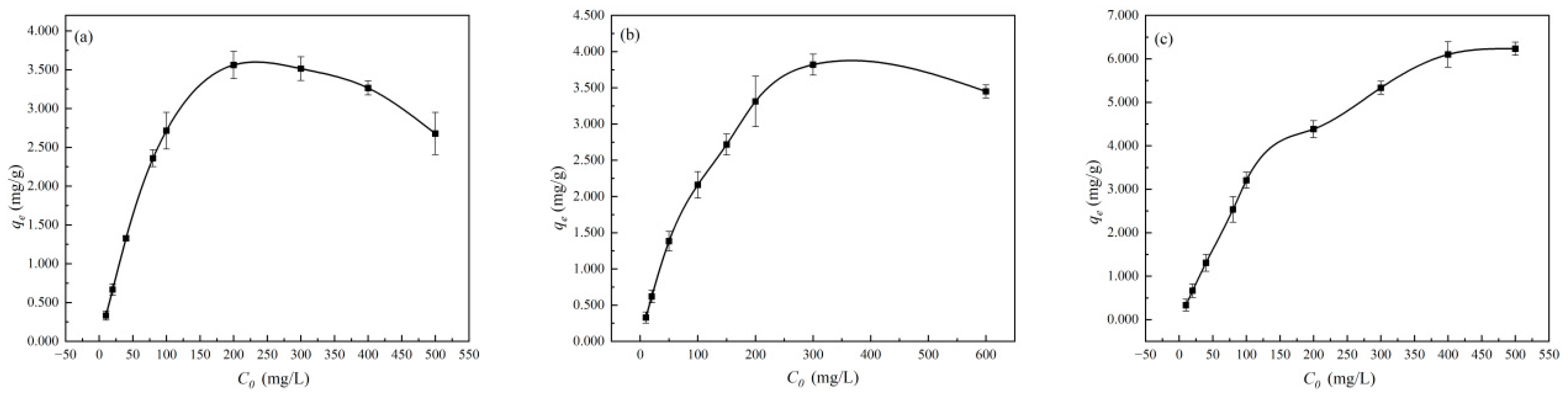
3.2.2. Effect of Initial Ion Concentration (C0) on the Adsorption Process of Cu2+, Zn2+, and Mn2+ by Diatomite
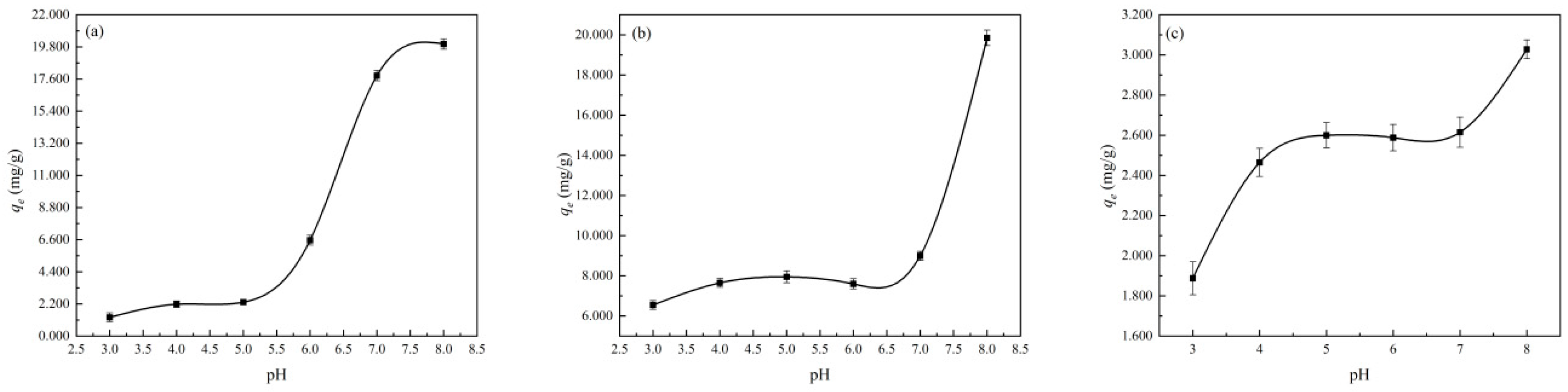
3.2.3. Effect of Initial pH Value of Solution on the Adsorption Process of Cu2+, Zn2+, and Mn2+ by Diatomite
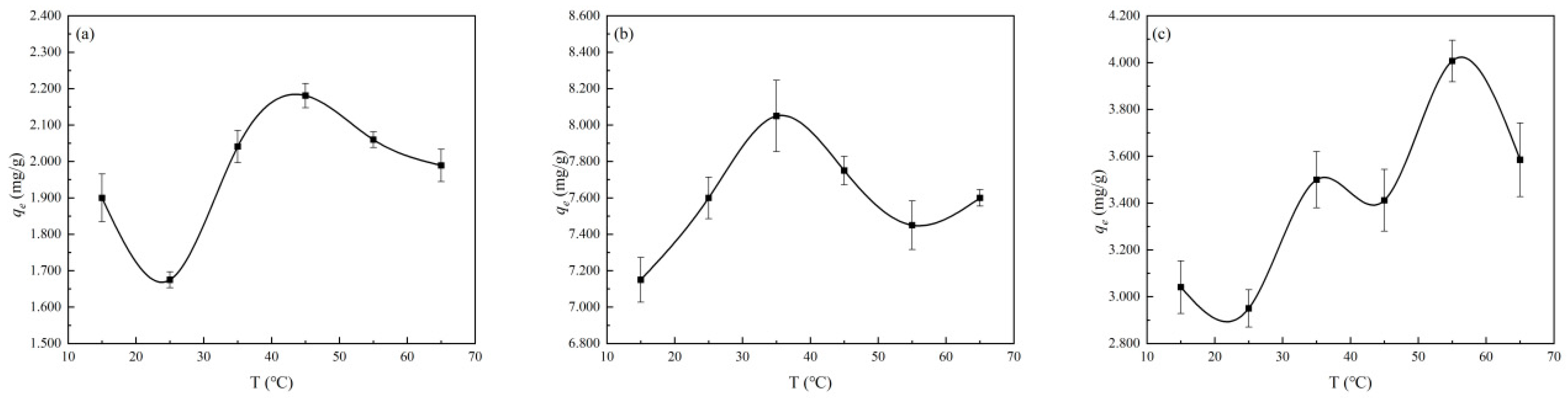
3.2.4. Effect of Solution Temperature (T) on the Adsorption Process of Cu2+, Zn2+, and Mn2+ by Diatomite
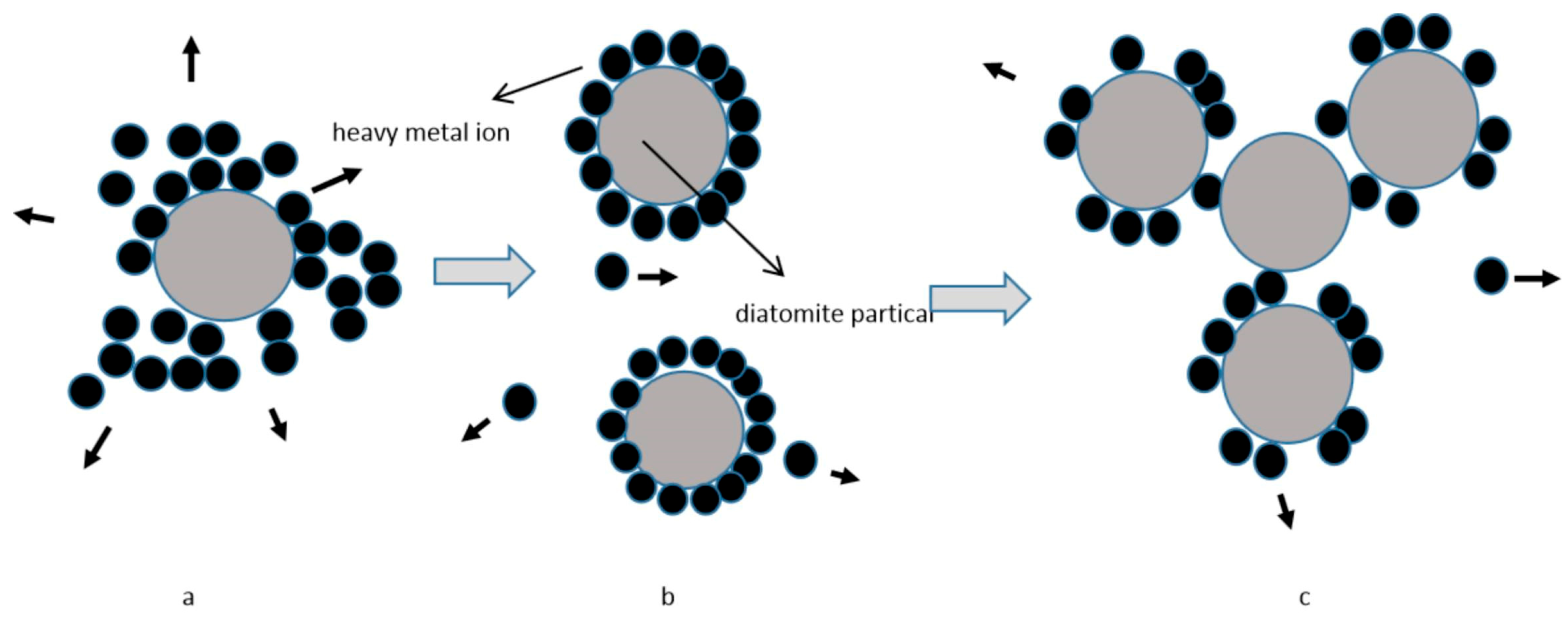
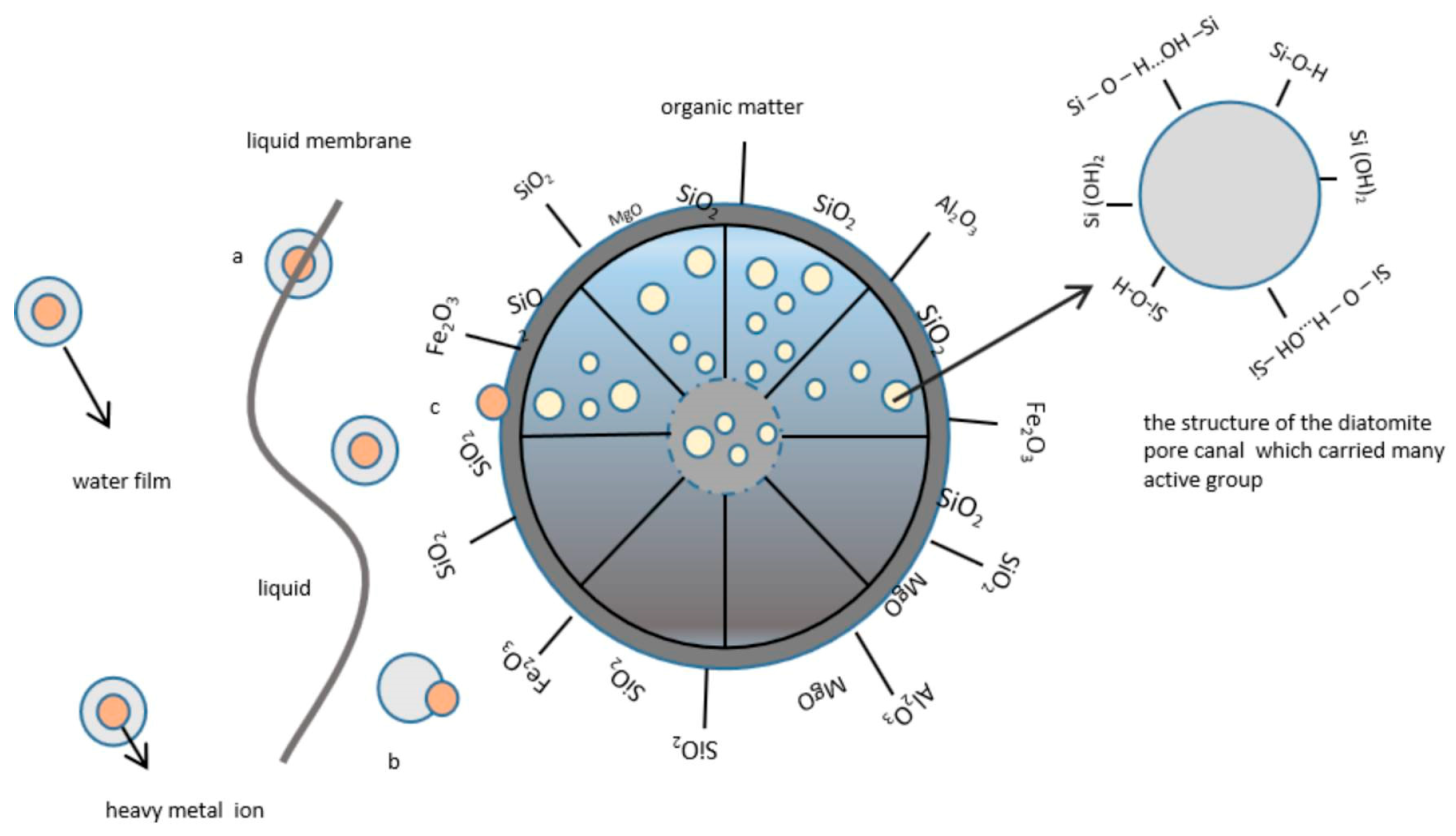
3.3. Adsorption Mechanism of Diatomite
3.3.1. Attributes of the Isotherm Adsorption
3.3.2. Physicochemical Properties of Diatomite Adsorption Processes
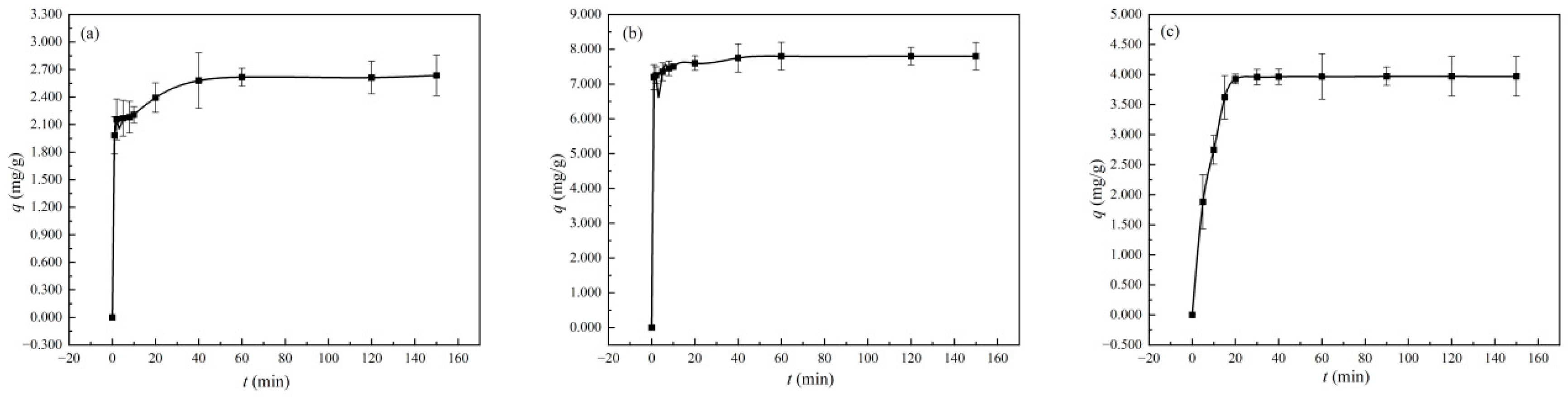
3.3.3. Mass Transfer and Rate-Limiting Step Analysis of Diatomite
3.3.4. Adsorption Kinetics of Diatomite
3.3.5. Adsorption Thermodynamics of Diatomite
4. Discussion
4.1. Adsorbent Dosage and Heavy Metal Adsorption Capacity Exhibit a Non-Linear Relationship
4.2. High Initial Ion Concentration and Low Diatomite Dosage Will Inhibit the Adsorption Capacity of Diatomite
4.3. Multiple Effects of Temperature on the Ability of Diatomite to Adsorb Heavy Metal Ions
4.4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

| Control Procedure | Rate Constant k | ||
|---|---|---|---|
| Cu2+ | Zn2+ | Mn2+ | |
| Liquid Film Diffusion | 0.0601 | 0.0619 | 0.1206 |
| Particle Diffusion | 0.0107 | 0.0073 | 0.0153 |
| Adsorption Reaction | 0.0100 | 0.0039 | 0.0124 |
References
- Jiang, Y.; Di, J.; Gao, M.; Dong, Y. Study on the new slow-release carbon source biochemistry and its improvement of SRB on the acid mine drainage treatment. J. Environ. Manag. 2024, 370, 122860. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xu, T.; Hu, S.; Liu, K.; Zeng, Z.; Wu, Q.; Zhou, Y.; He, M.; Cao, X.; Yu, G. Design and implementation of control system for electroplating wastewater treatment by photovoltaic energy sinusoidal alternating current coagulation technology. Process Saf. Environ. Prot. 2024, 182, 405–415. [Google Scholar] [CrossRef]
- Edward, K.; Yuvaraj, K.M.; Kapoor, A. Chitosan-blended membranes for heavy metal removal from aqueous systems: A review of synthesis, separation mechanism, and performance. Int. J. Biol. Macromol. 2024, 279, 134996. [Google Scholar] [CrossRef]
- Biswal, B.K.; Balasubramanian, R. Use of biochar as a low-cost adsorbent for removal of heavy metals from water and wastewater: A review. J. Environ. Chem. Eng. 2023, 11, 110986. [Google Scholar] [CrossRef]
- Luo, K.H.; Tu, H.P.; Chang, H.C.; Yang, C.C.; Weng, W.C.; Chen, T.H.; Yang, C.H.; Chuang, H.Y. Mediation analysis for TNF-α as a mediator between multiple metal exposure and kidney function. Ecotoxicol. Environ. Saf. 2024, 283, 116837. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, X.; Guo, S.; Ma, Y.; Zhang, Y. Associations between patterns of blood heavy metal exposure and health outcomes: Insights from NHANES 2011–2016. BMC Public Health 2024, 24, 558. [Google Scholar] [CrossRef]
- Fontes, A.; Pierson, H.; Bierła, J.B.; Eberhagen, C.; Kinschel, J.; Akdogan, B.; Rieder, T.; Sailer, J.; Reinold, Q.; Cielecka-Kuszyk, J.; et al. Copper impairs the intestinal barrier integrity in Wilson disease. Metabolism 2024, 158, 155973. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Tanaka, K.; Kato-Negishi, M. Zinc, Copper, and Calcium: A Triangle in the Synapse for the Pathogenesis of Vascular-Type Senile Dementia. Biomolecules 2024, 14, 773. [Google Scholar] [CrossRef]
- Qi, Y.; Si, H.; Jin, X.; Guo, Y.; Xia, J.; He, J.; Deng, X.; Deng, M.; Yao, W.; Hao, C. Changes in serum TIM-3 and complement C3 expression in workers due to Mn exposure. Front. Public Health 2023, 11, 1289838. [Google Scholar] [CrossRef]
- Ko, Y.G. Hybrid method integrating adsorption and chemical precipitation of heavy metal ions on polymeric fiber surfaces for highly efficient water purification. Chemosphere 2024, 363, 142909. [Google Scholar] [CrossRef]
- Jin, H.; Yu, Y.; Chen, X. Electrochemical precipitation for water and wastewater treatment. Process Saf. Environ. Prot. 2024, 184, 1011–1016. [Google Scholar] [CrossRef]
- Kim, J.G.; Ku, J.; Jung, J.; Park, Y.S.; Choi, G.H.; Hwang, S.S.; Lee, J.H.; Lee, A.S. Ion-exchangeable and sorptive reinforced membranes for efficient electrochemical removal of heavy metal ions in wastewater. J. Clean. Prod. 2024, 438, 140779. [Google Scholar] [CrossRef]
- Moore, R.G.; Crawford, J.M. Isolated and Paired Metal Sites in Zeolites Using Solid-State Ion Exchange. Angew. Chem. Int. Ed. 2025, 64, e202505186. [Google Scholar] [CrossRef] [PubMed]
- Wanjiya, M.; Zhang, J.C.; Wu, B.; Yin, M.J.; An, Q.F. Nanofiltration membranes for sustainable removal of heavy metal ions from polluted water: A review and future perspective. Desalination 2024, 578, 117441. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.; Wang, M.; Shen, Q.; Cong, S.; Zhao, Y.; Peng, J.; Pang, H. Metal-organic framework (MOF) membranes for Mg2+/Li+ separation. Sep. Purif. Technol. 2025, 374, 133737. [Google Scholar] [CrossRef]
- Cai, Q.; Shi, C.; Cao, Z.; Li, Z.; Zhao, H.P.; Yuan, S. Electrokinetic bioremediation of trichloroethylene and Cr/As co-contaminated soils with elevated sulfate. J. Hazard. Mater. 2024, 468, 133761. [Google Scholar] [CrossRef]
- Taieb, I.; Ben Younes, S.; Messai, B.; Mnif, S.; Mzoughi, R.; Bakhrouf, A.; Jabeur, C.; Serrano, J.A.A.; Ellafi, A. Isolation, characterization and identification of a new lysinibacillus fusiformis strain zc from metlaoui phosphate laundries wastewater: Bio-treatment assays. Sustainability 2021, 13, 10072. [Google Scholar] [CrossRef]
- Liyanaarachchi, H.; Thambiliyagodage, C.; Lokuge, H.; Vigneswaran, S. Kinetics and thermodynamics study of methylene blue adsorption to sucrose-and urea-derived nitrogen-enriched, hierarchically porous carbon activated by KOH and H3PO4. ACS Omega 2023, 8, 16158–16173. [Google Scholar] [CrossRef]
- Fan, W.; Li, S.; Yuan, Q.; Wu, P.; Zhang, X. Light-driven in-situ synthesis of nano-sulfur and graphene oxide composites for efficient removal of heavy metal ions. J. Hazard. Mater. 2025, 487, 137079. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Abdallah, H.M.; Ali, E.A.; Makhado, E.; Abd El-Ghany, N.A. Recent developments in conductive polysaccharide adsorbent formulations for environmental remediation: A review. Int. J. Biol. Macromol. 2025, 304, 140915. [Google Scholar] [CrossRef]
- Chen, J.; Bao, C.; Chen, M.; Wang, Y.; Xu, X.; Yang, T.; Shang, C.; Zhang, Q. β-cyclodextrin-scaffolded crosslinked poly (ionic liquid) s for ultrafast removal of multiple pollutants: Insight into adsorption performance and mechanism. Chem. Eng. J. 2023, 464, 142526. [Google Scholar] [CrossRef]
- Umejuru, E.C.; Mashifana, T.; Kandjou, V.; Amani-Beni, M.; Sadeghifar, H.; Fayazi, M.; Karimi-Maleh, H.; Sithole, N.T. Application of zeolite based nanocomposites for wastewater remediation: Evaluating newer and environmentally benign approaches. Environ. Res. 2023, 231, 116073. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-based biopolymer hydrogels for heavy metal detection and adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, X.; Ma, K.; Gong, Y.; Peng, H.; Qi, W. PEI-modified chitosan/activated carbon composites for Cu (II) removal from simulated pyrophosphate plating rinsing wastewater. Int. J. Biol. Macromol. 2023, 251, 126429. [Google Scholar] [CrossRef] [PubMed]
- Ravindiran, G.; Rajamanickam, S.; Ramalingam, M.; Hayder, G.; Sathaiah, B.K.; Gaddam, M.K.R.; Muniasamy, S.K.; Arunkumar, P. Conversion of seaweed waste to biochar for the removal of heavy metal ions from aqueous solution: A sustainable method to address eutrophication problem in water bodies. Environ. Res. 2024, 241, 117551. [Google Scholar]
- Guo, X.; Feng, S.; Peng, Y.; Li, B.; Zhao, J.; Xu, H.; Meng, X.; Zhai, W.; Pang, H. Emerging insights into the application of metal-organic framework (MOF)-based materials for electrochemical heavy metal ion detection. Food Chem. 2025, 463, 141387. [Google Scholar] [PubMed]
- Zahajská, P.; Opfergelt, S.; Fritz, S.C.; Stadmark, J.; Conley, D.J. What is diatomite? Quat. Res. 2020, 96, 48–52. [Google Scholar] [CrossRef]
- Mazkad, D.; El Idrissi, A.; Marrane, S.E.; Lazar, N.E.; El Ouardi, M.; Dardari, O.; Channab, B.E.; Layachi, O.A.; Farsad, S.; Baqais, A.; et al. An innovative diatomite-polypyrrole composite for highly efficient Cr (VI) removal through optimized adsorption via surface response methodology. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133172. [Google Scholar]
- Tang, X.; Hu, G.; Chen, Z.; Qian, L.; He, C.; Chen, T.; Gao, J.; Zhao, Y.; Han, X. High adsorption of Cu(II) in novel thiacalix[4]arene/acrylic polymer/diatomite fast fabricated by electron beam irradiation: Controllable microstructures, adsorption performance and mechanism. Chem. Eng. J. 2024, 495, 153090. [Google Scholar] [CrossRef]
- Youcef, H.; Al-Senani, G.M.; Al-Qahtani, S.D.; Kiari, M.; Sabantina, L.; Benyoucef, A.D.; Hadjel, M. Highly efficient removal of Cadmium (II) ions from aqueous solutions using an eco-friendly waste-derived carbon-based from date seed and Mn-modified diatomite: Equilibrium, kinetics and characterization studies. Colloids Surf. A Physicochem. Eng. Asp. 2025, 724, 137439. [Google Scholar] [CrossRef]
- Saidi, M.; Reguig, B.A.; El Amine Monir, M.; Althagafi, T.M.; Fatmi, M.; Remil, A.; Zehhaf, A.; Ghebouli, M.A. Kinetics thermodynamics and adsorption study of raw treated diatomite as a sustainable adsorbent for crystal violet dye. Sci. Rep. 2025, 15, 21991. [Google Scholar] [CrossRef]
- Wang, H.; Deng, Q.; Niu, Y.; Wang, X.; Hu, W.; Chen, L. Investigation of mechanical properties and solidification/stabilization mechanisms of Pb2+-contaminated red clay by diatomite-fly-ash-based geopolymer. Constr. Build. Mater. 2025, 492, 142843. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Zhang, P.; Pan, Y.; Huang, Z.; Xiao, H. Remediation of Cd (II) ions in aqueous and soil phases using novel porous cellulose/chitosan composite spheres loaded with zero-valent iron nanoparticles. React. Funct. Polym. 2022, 173, 105210. [Google Scholar] [CrossRef]
- e Silva, D.C.T.; da Silva, M.L.M.; de Farias, P.H.M.; Galvao, C.C.; dos Santos Costa, E.M.; Melo, R.A.; Medeiros, E.B.M.; de Lima Filho, N.M. Synthesis and characterization of polyaniline, sucrose octaacetate and chitosan blend for removal of remazol black by adsorption: Equilibrium, kinetics, and regeneration. Int. J. Biol. Macromol. 2025, 289, 138863. [Google Scholar]
- Pedroza, R.H.; David, C.; Lodeiro, P.; Rey-Castro, C. Interactions of humic acid with pristine poly (lactic acid) microplastics in aqueous solution. Sci. Total Environ. 2024, 908, 168366. [Google Scholar]
- Jing, Q.; Wang, Y.; Chai, L.; Tang, C.; Huang, X.; Guo, H.; Wang, W.; You, W. Adsorption of copper ions on porous ceramsite prepared by diatomite and tungsten residue. Trans. Nonferrous Met. Soc. China 2018, 28, 1053–1060. [Google Scholar] [CrossRef]
- Zhu, J.; Lei, M.J.; Wang, P.; Zhang, W.L.; Chen, Y. Preparation of poly-hydroxy-aluminum pillared diatomite and characteristics of Cu2+, Zn2+ adsorption on the pillar in aqueous solutions. Huan Jing Ke Xue Huanjing Kexue 2016, 37, 3177–3185. [Google Scholar]
- Yang, X.; Zhou, Y.; Hu, J.; Zheng, Q.; Zhao, Y.; Lv, G.; Liao, L. Clay minerals and clay-based materials for heavy metals pollution control. Sci. Total Environ. 2024, 954, 176193. [Google Scholar] [CrossRef]
- Alshameri, A.; He, H.; Zhu, J.; Xi, Y.; Zhu, R.; Ma, L.; Tao, Q. Adsorption of ammonium by different natural clay minerals: Characterization, kinetics and adsorption isotherms. Appl. Clay Sci. 2018, 159, 83–93. [Google Scholar] [CrossRef]


| Physical Property | Chemical Composition | ||||||
|---|---|---|---|---|---|---|---|
| Bulk Density (g/cm3) | Specific Surface Area (m2/g) | Particle Size (M) | Pore Size (nm) | Content of SiO2 (%) | Content of Al2O3 (%) | Content of Fe2O3 (%) | LOI (%) |
| 0.57 | 58.00 | 7.5 | 30–400 | 64.80 | 16.40 | 2.91 | 3.10 |
| Ions | Temperature | Freundlich Model | Langmuir Model | Temkin Model | D-R Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | kF | n | R2 | kL | qm | R2 | KTe | B | R2 | Β | qm | ||
| °C | mg∙g−1 | L∙mg−1 | mg∙g−1 | L∙mg−1 | J∙mol−1 | mol2∙J−2 | mg∙g−1 | ||||||
| Cu2+ | 25 | 0.928 | 0.781 | 2.506 | 0.993 | 0.254 | 4.335 | 0.983 | 3.892 | 3514.92 | 0.857 | 2.0 × 10−7 | 2.949 |
| 40 | 0.914 | 0.775 | 2.672 | 0.993 | 0.277 | 4.010 | 0.995 | 4.729 | 4022.90 | 0.874 | 1.8 × 10−7 | 2.799 | |
| 55 | 0.891 | 0.775 | 2.848 | 0.994 | 0.302 | 3.724 | 0.995 | 6.039 | 4607.06 | 0.890 | 1.6 × 10−7 | 2.654 | |
| Zn2+ | 25 | 0.921 | 1.399 | 3.248 | 0.849 | 3.946 | 3.031 | 0.953 | 37.892 | 4371.23 | 0.817 | 3.0 × 10−8 | 3.139 |
| 40 | 0.911 | 1.467 | 3.343 | 0.809 | 4.126 | 3.111 | 0.968 | 45.853 | 4424.204 | 0.845 | 2.7 × 10−8 | 3.290 | |
| 55 | 0.933 | 1.539 | 3.532 | 0.796 | 9.424 | 2.793 | 0.937 | 75.272 | 4763.88 | 0.799 | 1.6 × 10−8 | 3.130 | |
| Mn2+ | 25 | 0.997 | 0.599 | 2.769 | 0.876 | 1.226 | 1.844 | 0.912 | 4.379 | 5039.29 | 0.614 | 8.2 × 10−8 | 2.078 |
| 40 | 0.990 | 0.650 | 3.213 | 0.838 | 2.809 | 1.642 | 0.918 | 9.195 | 6495.98 | 0.607 | 4.0 × 10−8 | 1.899 | |
| 55 | 0.982 | 0.542 | 3.113 | 0.637 | 1.360 | 1.571 | 0.937 | 5.915 | 6937.87 | 0.621 | 6.5 × 10−8 | 1.724 | |
| Ions | Temperature | n | KL | RL max | RL min | Β | E |
|---|---|---|---|---|---|---|---|
| °C | mol2∙J−2 | kJ∙mol−1 | |||||
| Cu2+ | 25 | 2.506 | 0.254 | 0.282 | 0.013 | 2.0 × 10−7 | 2.236 |
| 40 | 2.672 | 0.277 | 0.265 | 0.012 | 1.8 × 10−7 | 2.357 | |
| 55 | 2.848 | 0.302 | 0.249 | 0.011 | 1.6 × 10−7 | 2.500 | |
| Zn2+ | 25 | 3.248 | 3.946 | 0.025 | 0.001 | 3.0 × 10−8 | 5.774 |
| 40 | 3.343 | 4.126 | 0.024 | 0.001 | 2.7 × 10−8 | 6.086 | |
| 55 | 3.532 | 9.424 | 0.010 | 0.000 | 1.6 × 10−8 | 7.906 | |
| Mn2+ | 25 | 2.769 | 1.226 | 0.075 | 0.003 | 8.2 × 10−8 | 3.492 |
| 40 | 3.213 | 2.809 | 0.034 | 0.001 | 4.0 × 10−8 | 5.000 | |
| 55 | 3.113 | 1.360 | 0.068 | 0.002 | 6.5 × 10−8 | 3.922 |
| Models and Linear Expressions | Parameters | Cu2+ | Zn2+ | Mn2+ |
|---|---|---|---|---|
| First-order kinetics equation ln(qe − qt) = lnqe − k1t | R2 | 0.815 | 0.809 | 0.652 |
| k1 | 0.039 | 0.030 | 0.049 | |
| qe | 0.510 (2.635) 1 | 0.362 (7.810) 1 | 0.283 (3.971) 1 | |
| Second-order kinetics equation t/qt = 1/k2·1/qe2 + t/qe | R2 | 0.998 | 0.997 | 0.999 |
| qe | 2.650 (2.635) 1 | 7.819 (7.810) 1 | 4.065 (3.971) 1 | |
| k2 | 0.309 | 0.418 | 0.098 | |
| Elovich equation qt = a + blnt | R2 | 0.799 | 0.754 | 0.641 |
| a | 1.973 | 7.165 | 1.800 | |
| b | 0.139 | 0.140 | 0.512 | |
| Double constant equation logqt = logk3 + mlogt | R2 | 0.899 | 0.953 | 0.909 |
| m | 0.058 | 0.019 | 0.173 | |
| k3 | 1.995 | 7.173 | 1.913 |
| Ions | ΔG° | ΔH° (kJ∙mol−1) | ΔS° | ||||||
|---|---|---|---|---|---|---|---|---|---|
| kJ∙mol−1 | J∙mol−1∙K−1 | ||||||||
| 298 K | 313 K | 328 K | 298 K | 313 K | 328 K | 298 K | 313 K | 328 K | |
| Cu2+ * | −1.783 | −1.989 | −2.255 | 2.896 | 2.896 | 2.896 | 15.672 | 15.672 | 15.672 |
| Zn2+ * | −5.890 | −6.480 | −7.096 | 6.091 | 6.091 | 6.091 | 40.190 | 40.190 | 40.190 |
| Mn2+ * | −0.846 | −0.905 | −1.635 | 0.008 | 0.008 | 0.008 | 3.583 | 3.583 | 3.583 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; He, Q.; Lei, M.; Han, J.; Wang, J.; Li, W.; Xiao, Y.; Huang, H.; Huang, X.; Zhu, J. Abnormal Adsorption Characteristics of Copper, Zinc, and Manganese Ions on Natural Diatomite in a Liquid/Solid Heterogeneous System. Water 2025, 17, 2782. https://doi.org/10.3390/w17182782
Wang J, He Q, Lei M, Han J, Wang J, Li W, Xiao Y, Huang H, Huang X, Zhu J. Abnormal Adsorption Characteristics of Copper, Zinc, and Manganese Ions on Natural Diatomite in a Liquid/Solid Heterogeneous System. Water. 2025; 17(18):2782. https://doi.org/10.3390/w17182782
Chicago/Turabian StyleWang, Jieying, Qihao He, Mingjing Lei, Jing Han, Jiacheng Wang, Wenmin Li, Ying Xiao, Hongchun Huang, Xindeng Huang, and Jian Zhu. 2025. "Abnormal Adsorption Characteristics of Copper, Zinc, and Manganese Ions on Natural Diatomite in a Liquid/Solid Heterogeneous System" Water 17, no. 18: 2782. https://doi.org/10.3390/w17182782
APA StyleWang, J., He, Q., Lei, M., Han, J., Wang, J., Li, W., Xiao, Y., Huang, H., Huang, X., & Zhu, J. (2025). Abnormal Adsorption Characteristics of Copper, Zinc, and Manganese Ions on Natural Diatomite in a Liquid/Solid Heterogeneous System. Water, 17(18), 2782. https://doi.org/10.3390/w17182782






