Comparison of Laboratory and Field Methods for Biosand Filter Sand Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Sand Samples
2.2. Sand Size Parameter Determination
2.2.1. Sieve Testing
2.2.2. Sand ES and UC Analysis
2.3. Additional Sand Characterization
2.3.1. Density Calculations
2.3.2. Percent Retained
2.3.3. ImageJ
2.4. Statistical Analysis
3. Results
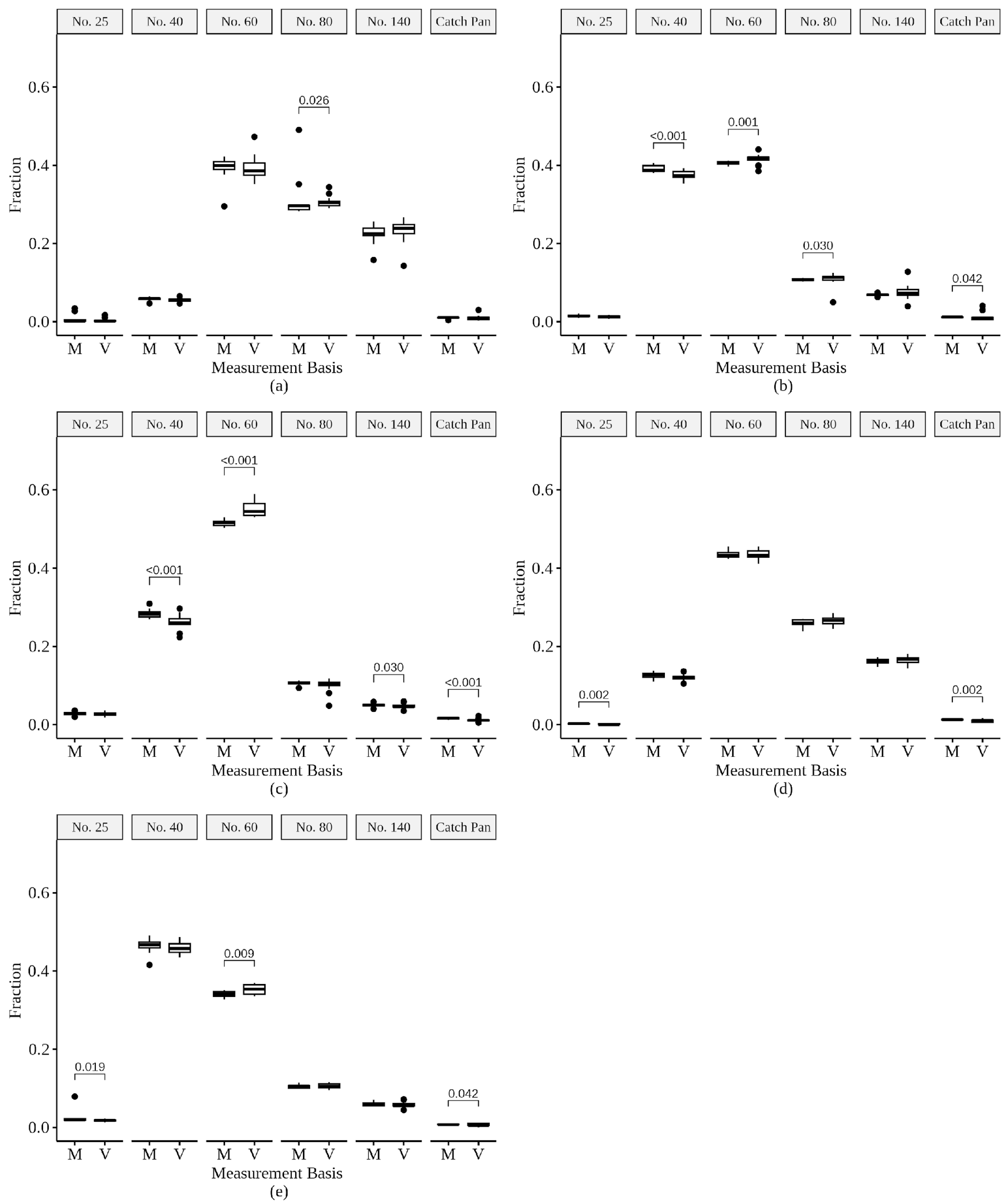
3.1. Mass and Volume Measurement Comparisons
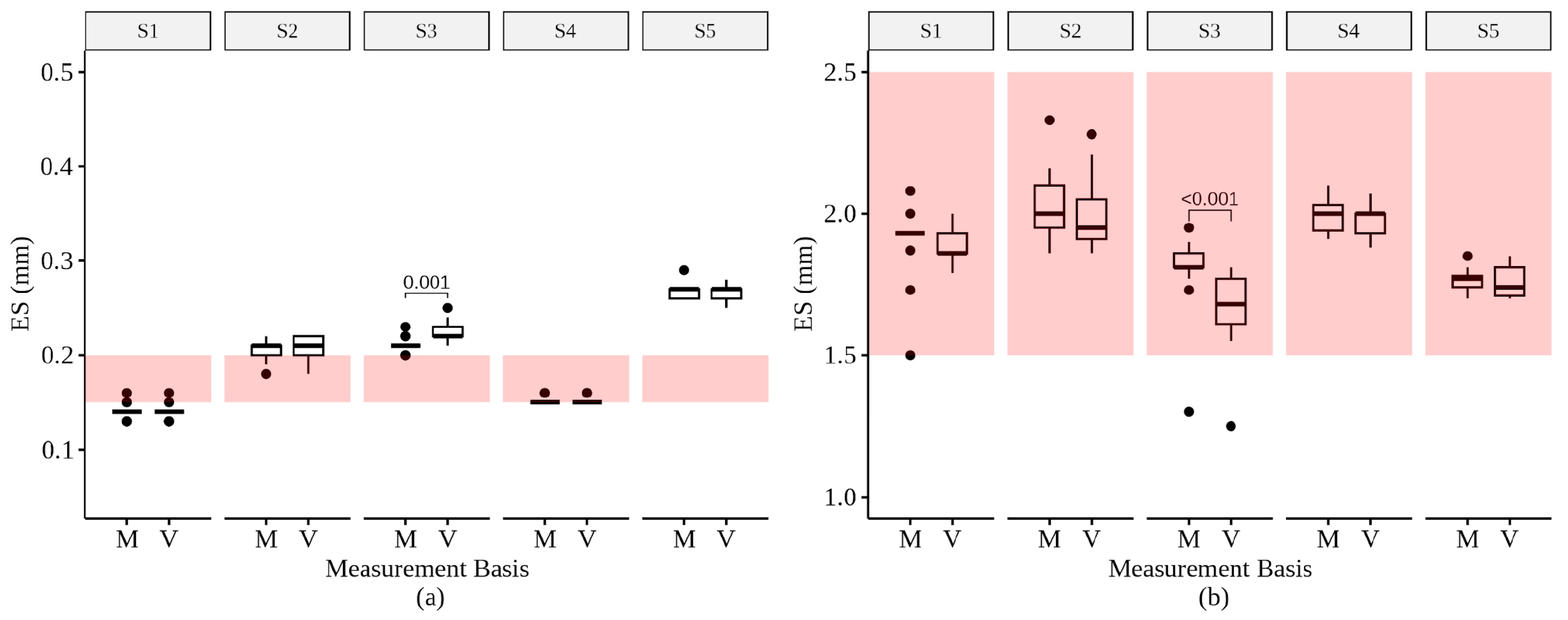
3.2. ES and UC Values
3.3. ES and UC Comparisons
3.4. Sand Characteristic Comparisons
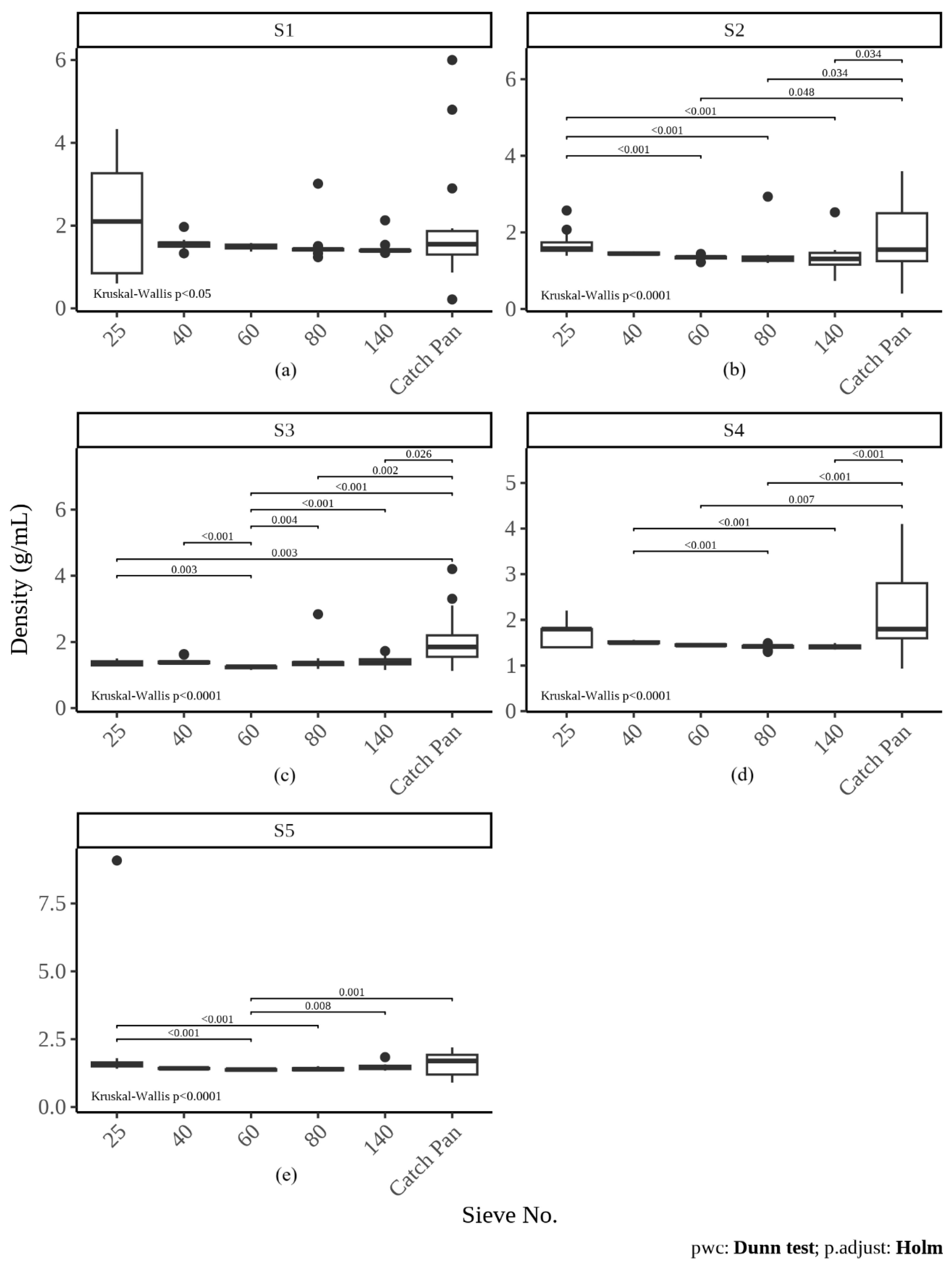
3.4.1. Density
3.4.2. Roundness
3.4.3. Circularity
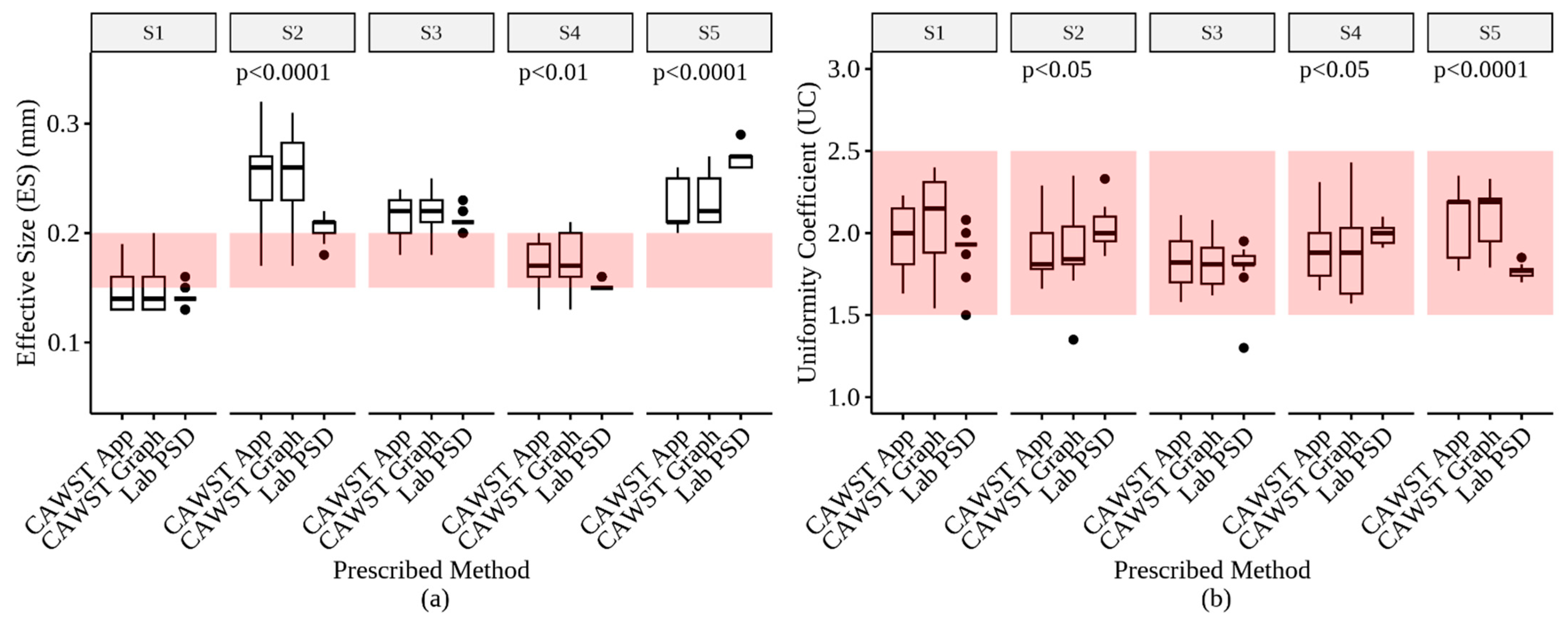
3.5. Prescribed Method Comparisons
3.6. Filter Performance Pilot Study
4. Discussion
4.1. Impact of Sand Characteristics on Volume Sand Analysis
4.2. Impact of Value Determination Method and Sieve Type on ES and UC
4.3. Implication of Findings for BSF Field Construction
5. Conclusions
- Generally, the use of mass- or volume-based measurements does not affect the ES or UC for a given method. However, non-linear scaling of mass and volume and extreme sand size distributions can affect ES and UC parameters;
- ES and UC values determined with the prescribed CAWST methods (App & Graph with volume-based measurements) generally differ from values determined using the Lab PSD method (using mass-based measurements). For the sands tested in this study, however, there were no cases in which these differences resulted in differential sand selection for a BSF based on the CAWST-recommended ES and UC ranges;
- Differences between CAWST field protocols versus standard lab protocols (e.g., parameter calculation method, sieve type, linear versus smoothed (PSD) line type) may affect ES and UC values and may influence whether a size parameter falls within CAWST’s recommended ES range.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAWST | Centre for Affordable Water and Sanitation Technology |
| ES | Effective Size |
| UC | Uniformity Coefficient |
| BSF | Biosand Filter |
| POU | Point-of-Use |
| mL/min | Milliliters per minute |
| d10 | Diameter 10 |
| d60 | Diameter 60 |
| mm | Millimeter |
| WHO | World Health Organization |
| S1 | Sample 1 |
| S2 | Sample 2 |
| S3 | Sample 3 |
| S4 | Sample 4 |
| S5 | Sample 5 |
| No. | Number |
| PSD | Particle Size Distribution |
| ASTM | American Society for Testing and Materials |
| ISO | International Organization for Standardization |
| g | Gram |
| mL | Milliliter |
| SEM | Scanning Election Microscopy |
| PVC | Polyvinyl Chloride |
| KO | Knock-Out |
| V | Volume |
| P.V. | Pore Volume |
| OD | Outer Diameter |
| ID | Inner Diameter |
| APHA | American Public Health Association |
References
- Centre for Affordable Water and Sanitation Technology Biosand Filter for Technicians 2012. Available online: https://washresources.cawst.org/en/resources/b6be2637/biosand-filter-construction-manual (accessed on 22 December 2024).
- Baumgartner, J.; Murcott, S.; Ezzati, M. Reconsidering ‘Appropriate Technology’: The Effects of Operating Conditions on the Bacterial Removal Performance of Two Household Drinking-Water Filter Systems. Environ. Res. Lett. 2007, 2, 024003. [Google Scholar] [CrossRef]
- Tsafack, H.N.; Djoko, G.R.P.; Akwa, T.E.; Kwekap, J.W.; Wamba, F.R.; Temgoua, E. Assessment of Filtration Capacity of Different Filters Used in the West Region of Cameroon for Quality Improvement of Drinking Water. Sustain. Water Resour. Manag. 2024, 10, 159. [Google Scholar] [CrossRef]
- Eniola, J.O.; Sizirici, B. Assessing the Sustainability of Modified Biosand Filters Using Life Cycle Assessment, Cost and Performance Factors. Environ. Dev. Sustain. 2024. [Google Scholar] [CrossRef]
- Ojomo, E.; Elliott, M.; Goodyear, L.; Forson, M.; Bartram, J. Sustainability and Scale-up of Household Water Treatment and Safe Storage Practices: Enablers and Barriers to Effective Implementation. Int. J. Hyg. Environ. Health 2015, 218, 704–713. [Google Scholar] [CrossRef]
- Benson, M.; Noor, A.; Moreau, J.-L. Clean Water for All Building Filtration Systems in Rural Uganda. Glob. Res. Acad. Synerg. 2025, 3, 11–22. [Google Scholar]
- Brown, J.; Clasen, T. High Adherence Is Necessary to Realize Health Gains from Water Quality Interventions. PLoS ONE 2012, 7, e36735. [Google Scholar] [CrossRef]
- Chan, C.C.V.; Neufeld, K.; Cusworth, D.; Gavrilovic, S.; Ngai, T. Investigation of the Effect of Grain Size, Flow Rate and Diffuser Design on the CAWST Biosand Filter Performance. Int. J. Serv. Learn. Eng. 2015, 10, 1–23. [Google Scholar] [CrossRef]
- Duran Romero, D.A.; De Almeida Silva, M.C.; Chaúque, B.J.M.; D. Benetti, A. Biosand Filter as a Point-of-Use Water Treatment Technology: Influence of Turbidity on Microorganism Removal Efficiency. Water 2020, 12, 2302. [Google Scholar] [CrossRef]
- Freitas, B.L.S.; Terin, U.C.; Fava, N.M.N.; Maciel, P.M.F.; Garcia, L.A.T.; Medeiros, R.C.; Oliveira, M.; Fernandez-Ibañez, P.; Byrne, J.A.; Sabogal-Paz, L.P. A Critical Overview of Household Slow Sand Filters for Water Treatment. Water Res. 2022, 208, 117870. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Baker, D.L. Recent Advances in Household Biosand Filter Design. In Progress in Slow Sand and Alternative Biofiltration Processes; IWA Publishing: London, UK, 2014; pp. 319–330. ISBN 978-1-78040-638-1. [Google Scholar]
- Dwivedi, A.H.; Nidheesh, P.V. Comparison of Various Low-Cost Household Drinking Water Treatment Filters, Mechanisms and Applications: A Review. Water Air Soil Pollut. 2025, 236, 331. [Google Scholar] [CrossRef]
- Jenkins, M.W.; Tiwari, S.K.; Darby, J. Bacterial, Viral and Turbidity Removal by Intermittent Slow Sand Filtration for Household Use in Developing Countries: Experimental Investigation and Modeling. Water Res. 2011, 45, 6227–6239. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.-L.; Fane, A.G.; Krantz, W.B.; Lim, T.-T. Emergency Water Supply: A Review of Potential Technologies and Selection Criteria. Water Res. 2012, 46, 3125–3151. [Google Scholar] [CrossRef] [PubMed]
- Centre for Affordable Water and Sanitation Technology Sand Grain Size Analysis (Sieve Analysis) 2012. Available online: https://washresources.cawst.org/en/resources/ea0b7613/biosand-filter-sand-grain-size-analysis-instructions?queryId=75d9b0305d9f57540c6cc781b5b1cb32 (accessed on 22 December 2024).
- Ji, X.; Zhao, C.; Lv, Y.; Yang, J.; Li, B. Influence of Particle Size of River Sand on the Decontamination Process in the Slow Sand Filter Treatment of Micro-Polluted Water. Water 2022, 14, 100. [Google Scholar] [CrossRef]
- Chaúque, B.J.M.; Issufo, M.; Benitez, G.B.; Cossa, V.C.; Chaúque, L.G.H.; Stauber, C.E.; Benetti, A.D.; Rott, M.B. Why Do Low-Cost Point-of-Use Water Treatment Technologies Succeed or Fail in Combating Waterborne Diseases in the Field? A Systematic Review. J. Environ. Chem. Eng. 2023, 11, 110575. [Google Scholar] [CrossRef]
- Brown, J.; Proum, S.; Sobsey, M.D. Sustained Use of a Household-Scale Water Filtration Device in Rural Cambodia. J. Water Health 2009, 7, 404–412. [Google Scholar] [CrossRef]
- Cho, G.-C.; Dodds, J.; Santamarina, J.C. Particle Shape Effects on Packing Density, Stiffness, and Strength: Natural and Crushed Sands. J. Geotech. Geoenviron. Eng. 2006, 132, 591–602. [Google Scholar] [CrossRef]
- Cubrinovski, M.; Ishihara, K. Maximum and Minimum Void Ratio Characteristics of Sands. Soils Found. 2002, 42, 65–78. [Google Scholar] [CrossRef]
- Guan, W.; Qi, Q.; Zhang, Z.; Nan, S. Effect of Sand Particle Size on Microstructure and Mechanical Properties of Gypsum-Cemented Similar Materials. Materials 2020, 13, 765. [Google Scholar] [CrossRef]
- Maeda, K.; Miura, K. Relative Density Dependency of Mechanical Properties of Sands. Soils Found. 1999, 39, 69–79. [Google Scholar] [CrossRef]
- Polakowski, C.; Sochan, A.; Bieganowski, A.; Ryzak, M.; Földényi, R.; Tóth, J. Influence of the Sand Particle Shape on Particle Size Distribution Measured by Laser Diffraction Method. Int. Agrophysics 2014, 28, 195–200. [Google Scholar] [CrossRef]
- ASTM C136/C136M-19; ASTM International Standard Test Method for Sieve Analysis of Fine and Coarse Aggregates. ASTM: West Conshohocken, PA, USA, 2019. [CrossRef]
- ASTM D6913-04; ASTM International Standard Test Methods for Particle-Size Distribution (Gradation) of Soils Using Sieve Analysis. ASTM: West Conshohocken, PA, USA, 2004. [CrossRef]
- ISO 11277:2020; ISO Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation. ISO: Geneva, Switzerland, 2020.
- Ferreira, T.; Rasband, W. ImageJ User Guide. Available online: https://imagej.net/ij/docs/guide/ (accessed on 22 December 2024).
- Li, L.; Iskander, M. Evaluation of Roundness Parameters in Use for Sand. J. Geotech. Geoenviron. Eng. 2021, 147, 04021081. [Google Scholar] [CrossRef]
- Napotnik, J.A.; Baker, D.; Jellison, K.L. Effect of Sand Bed Depth and Medium Age on Escherichia Coli and Turbidity Removal in Biosand Filters. Environ. Sci. Technol. 2017, 51, 3402–3409. [Google Scholar] [CrossRef]
- World Health Organization (Ed.) Guidelines for Drinking-Water Quality; Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-004506-4. [Google Scholar]
- Blott, S.J.; Pye, K. Particle Shape: A Review and New Methods of Characterization and Classification. Sedimentology 2008, 55, 31–63. [Google Scholar] [CrossRef]
- Rahmadyanti, E.; Palupi, A.E. Rainwater Treatment with Bio-Slow Sand Filtration for Sustainable Water Supply. J. Ecol. Eng. 2025, 26, 190–200. [Google Scholar] [CrossRef]
- Rao, D.R.J. Evolving High Rate Filter and Use of Crushed Stone as Filter Media. J. Inst. Eng. 1981, 61, 92–96. [Google Scholar]
- Tellen, V.; Nkeng, G.; Dentel, S. Improved Filtration Technology for Pathogen Reduction in Rural Water Supplies. Water 2010, 2, 285–306. [Google Scholar] [CrossRef]
- Centre for Affordable Water and Sanitation Technology Wash That Sand. Available online: https://washresources.cawst.org/en/resources/06a575a3/wash-that-sand (accessed on 22 December 2024).
- APHA 2130 TURBIDITY; Standard Methods for the Examination of Water and Waste Water. American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012.
- Fogel, D.; Isaac-Renton, J.; Guasparini, R.; Moorehead, W.; Ongerth, J. Removing Giardia and Cryptosporidium by Slow Sand Filtration. J.-Am. Water Work. Assoc. 1993, 85, 77–84. [Google Scholar] [CrossRef]

| Sand | Sand Characterization Parameter 1 | Notes | ||||
|---|---|---|---|---|---|---|
| Effective Size (ES) | Uniformity Coefficient (UC) | Overall Density 2 (mg/L) | Roundness (Unitless) | Circularity (Unitless) | ||
| S1 | Med. = 0.14 s.d. = 0.01 n = 17 | Med. = 1.93 s.d. = 0.14 n = 17 | Med. = 1.47 s.d. = 0.12 n = 17 | Med. = 0.70 s.d. = 0.14 n = 159 | Med. = 0.68 s.d. = 0.08 n = 159 | |
| S2 | Med. = 0.21 s.d. = 0.01 n = 17 | Med. = 2.00 s.d. = 0.11 n = 17 | Med. = 1.42 s.d. = 0.04 n = 17 | Med. = 0.65 s.d. = 0.17 n = 225 | Med. = 0.57 s.d. = 0.11 n = 225 | Small, dead insects and shell fragments observed in sample |
| S3 | Med. = 0.21 s.d. = 0.01 n = 17 | Med. = 1.81 s.d. = 0.14 n = 17 | Med. = 1.34 s.d. = 0.04 n = 17 | Med. = 0.70 s.d. = 0.15 n = 720 | Med. = 0.65 s.d. = 0.14 n = 720 | |
| S4 | Med. = 0.15 s.d. = 0.00 n = 17 | Med. = 2.00 s.d. = 0.06 n = 17 | Med. = 1.51 s.d. = 0.29 n = 17 | Med. = 0.68 s.d. = 0.15 n = 150 | Med. = 0.69 s.d. = 0.08 n = 150 | Recovered from deconstructed BSFs |
| S5 | Med. = 0.27 s.d. = 0.01 n = 17 | Med. = 1.77 s.d. = 0.04 n = 17 | Med. = 1.50 s.d. = 0.04 n = 17 | Med. = 0.67 s.d. = 0.14 n = 187 | Med. = 0.55 s.d. = 0.09 n = 187 | |
| Characteristic | Method | |||
|---|---|---|---|---|
| CAWST PSD | Prescribed Methods | |||
| CAWST Graph | CAWST App | Lab PSD (Control Method) | ||
| Recommender (Prescriber) | N/A | CAWST | CAWST | Generally accepted laboratory method |
| Recommended Measurement Basis | N/A | Volume | Volume | Mass |
| Effective Size (ES) (and d60) value determination method | A PSD graph where sieve mesh size versus percent sand finer was fit with a smooth curve using a computer program (e.g., Microsoft Excel). | A reusable, hand-drawn graph (provided by CAWST and included with the hand-held sieve package) where sieve mesh size versus percent sand finer were connected linearly. In this study, each ES and d60 were calculated as the average of four users’ hand-drawn results. | Automatically calculated with a mobile app created by CAWST in which one input is entered for each sieve in CAWST’s sieve column. Each input is the cumulative volume (mL) of sand retained on that sieve (i.e., the sum of the volume retained on that sieve and all sieves with a larger mesh). | A PSD graph where sieve mesh size versus percent sand finer was fit with a smooth curve using a computer program (e.g., Microsoft Excel). |
| Uniformity coefficient (UC) value determination method | UC = d60/ES | UC = d60/ES | Automatically calculated with a mobile app created by CAWST. | UC = d60/ES |
| Sieve type | CAWST hand-held | CAWST hand-held | CAWST hand-held | Laboratory-grade |
| Sand | Effective Size (ES) (mm) | Uniformity Coefficient (UC) | ||||
|---|---|---|---|---|---|---|
| CAWST App | CAWST Graph | Lab PSD | CAWST App | CAWST Graph | Lab PSD | |
| S1 | Med. = 0.14 s.d. = 0.02 n = 17 | Med. = 0.14 s.d. = 0.03 n = 17 | Med. = 0.14 s.d. = 0.01 n = 17 | Med. = 2.00 s.d. = 0.22 n = 17 | Med. = 2.15 s.d. = 0.30 n = 17 | Med. = 1.93 s.d. = 0.14 n = 17 |
| S2 | Med. = 0.26 s.d. = 0.04 n = 17 | Med. = 0.26 s.d. = 0.05 n = 17 | Med. = 0.21 s.d. = 0.01 n = 17 | Med. = 1.81 s.d. = 0.18 n = 17 | Med. = 1.84 s.d. = 0.23 n = 17 | Med. = 2.00 s.d. = 0.11 n = 17 |
| S3 | Med. = 0.22 s.d. = 0.02 n = 17 | Med. = 0.22 s.d. = 0.02 n = 17 | Med. = 0.21 s.d. = 0.01 n = 17 | Med. = 1.82 s.d. = 0.16 n = 17 | Med. = 1.81 s.d. = 0.13 n = 17 | Med. = 1.81 s.d. = 0.14 n = 17 |
| S4 | Med. = 0.17 s.d. = 0.02 n = 17 | Med. = 0.17 s.d. = 0.02 n = 17 | Med. = 0.15 s.d. = 0.00 n = 17 | Med. = 1.88 s.d. = 0.17 n = 17 | Med. = 1.88 s.d. = 0.27 n = 17 | Med. = 2.00 s.d. = 0.06 n = 17 |
| S5 | Med. = 0.21 s.d. = 0.02 n = 17 | Med. = 0.22 s.d. = 0.02 n = 17 | Med. = 0.27 s.d. = 0.01 n = 17 | Med. = 2.19 s.d. = 0.18 n = 17 | Med. = 2.19 s.d. = 0.18 n = 17 | Med. = 1.77 s.d. = 0.04 n = 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbott, N.; Hudson, A.; Brown, S.; Foley, A.; Jellison, K. Comparison of Laboratory and Field Methods for Biosand Filter Sand Characterization. Water 2025, 17, 2706. https://doi.org/10.3390/w17182706
Abbott N, Hudson A, Brown S, Foley A, Jellison K. Comparison of Laboratory and Field Methods for Biosand Filter Sand Characterization. Water. 2025; 17(18):2706. https://doi.org/10.3390/w17182706
Chicago/Turabian StyleAbbott, Nora, Ava Hudson, Sean Brown, Ann Foley, and Kristen Jellison. 2025. "Comparison of Laboratory and Field Methods for Biosand Filter Sand Characterization" Water 17, no. 18: 2706. https://doi.org/10.3390/w17182706
APA StyleAbbott, N., Hudson, A., Brown, S., Foley, A., & Jellison, K. (2025). Comparison of Laboratory and Field Methods for Biosand Filter Sand Characterization. Water, 17(18), 2706. https://doi.org/10.3390/w17182706







