Efficiency and Mechanism of a Hollow Carbon-Based Single-Atom Iron Catalyst in Activating Periodate for Bisphenol a Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
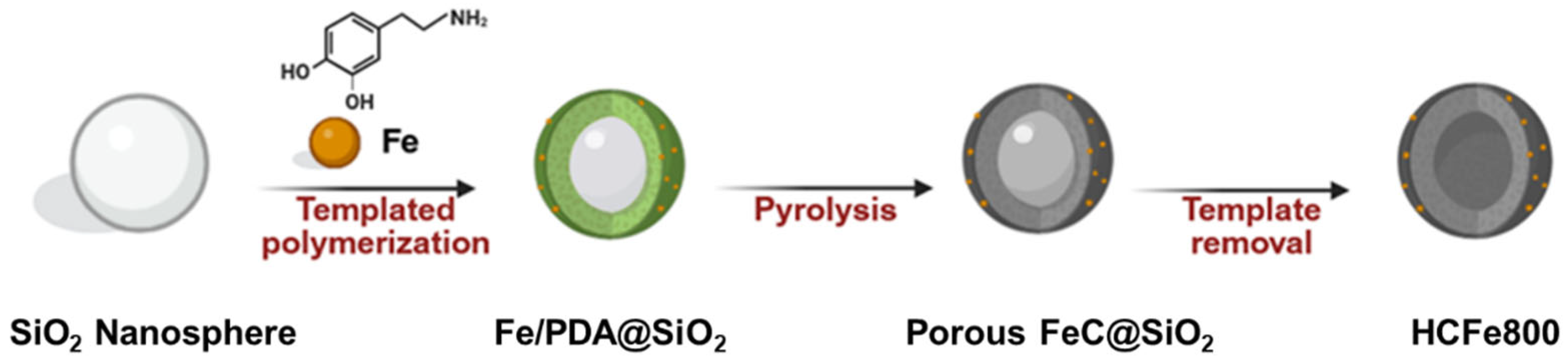
2.2. Synthesis of Catalysts
2.3. Catalytic Degradation Experiments
2.4. Analytical Methods
3. Results and Discussion
3.1. Physicochemical and Structural Properties of the HCFe800 Catalyst
3.2. Excellent BPA Degradation by the Combination of HCFe800 and PI
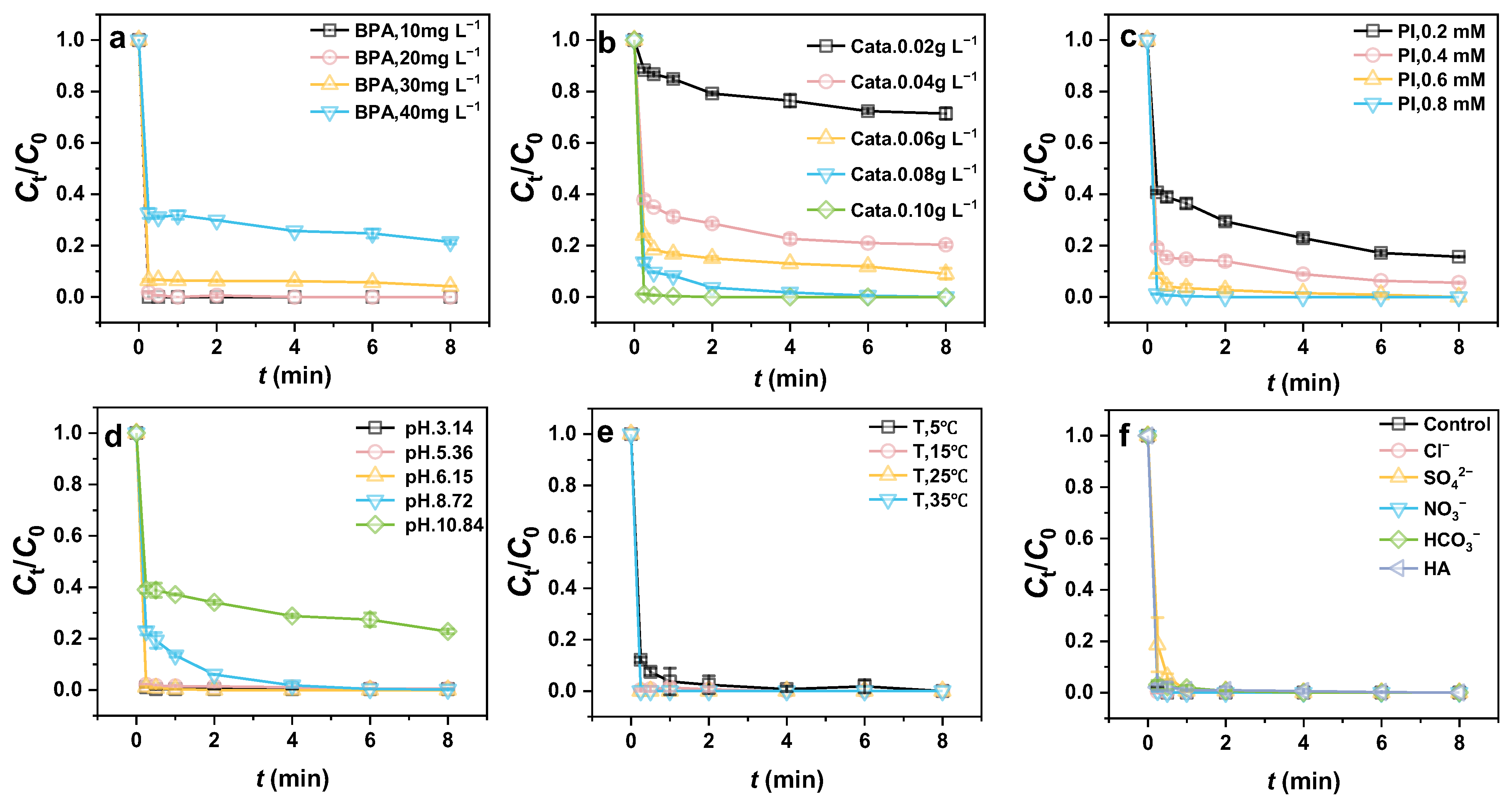
3.3. Study of Factors Influencing BPA Degradation
3.3.1. Effect of the Hollow Structure
3.3.2. Effect of Iron Content in the Catalyst
3.3.3. Effect of Calcination Temperature
3.3.4. Effects of Dosages and Water Quality Conditions
3.4. Catalytic Degradation Mechanism Analysis
3.4.1. Role of ROS and High-Valent Iron
3.4.2. Electrochemical Analysis
3.4.3. Degradation Pathway and Toxicity Evolution of BPA
3.5. Application Feasibility Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, W.X.; Wang, Y.W.; Guo, H. Regulating generation of radical and non-radical in plasma systems for selective degradation of persistent organic pollutants in water with high salinity resistance and slight environmental implication: Regulatory method and selective mechanism. J. Hazard. Mater. 2025, 492, 138138. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Wei, T.; Ding, P.; Liu, L.-M.; Xiong, L.; Tang, J.; Ma, J.; Wang, F.; Liu, H.; Qu, J. Sodium-directed photon-induced assembly strategy for preparing multisite catalysts with high atomic utilization efficiency. J. Am. Chem. Soc. 2023, 145, 1759–1768. [Google Scholar] [CrossRef]
- Tang, Y.G.; Zhang, Z.R.; Wu, F.; Xu, T.; Li, Y.L.; Zhou, X.; Pan, Y.W.; Wu, G.Y. Nanoengineering construction of Fe2O3/g-C3N4 heterojunctions for cooperative enhanced photocatalytic CO2 reduction and pollutant degradation. Environ. Res. 2025, 284, 122279. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.Q.; Huo, Y.D.; Zhang, Y.; Xu, L.J.; Gan, L. Performance and mechanism study of simultaneous removal of carbamazepine and ammonia from water using UV/peroxymonosulfate process. Catalysts 2025, 15, 468. [Google Scholar] [CrossRef]
- Chen, Z.L.; Liu, C.Y.; Zhang, M.; Li, W.H.; Tian, T.; Qiao, W.C.; Li, J.S. Selective enhancement of degradation capacity of organic pollutants based on low-temperature plasma: The role of specificity of catalysts and reactive oxygen species. Sep. Purif. Technol. 2025, 354, 129140. [Google Scholar] [CrossRef]
- Xu, J.W.; Zhang, Z.Y.; Yang, J.M.; Ma, Y.D.; Han, T.; Quan, G.X.; Zhang, X.D.; Lei, J.Q.; Liu, N. Treatment of pharmaceuticals and personal care products (PPCPs) using periodate-based advanced oxidation technology: A review. Chem. Eng. J. 2025, 512, 162355. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, J.J.; Qiao, J.L.; Chen, J.B.; Batjargal, E.; Tuulaikhuu, B.A.; Qian, Y.J.; Zhou, X.F.; Zhang, Y.L. Metal-based activation of periodate as an advanced oxidation process for water decontamination: A critical review. Chem. Eng. J. 2025, 513, 162949. [Google Scholar] [CrossRef]
- Ling, C.; Wu, S.; Han, J.A.; Dong, T.L.; Zhu, C.Q.; Li, X.W.; Xu, L.J.; Zhang, Y.; Zhou, M.H.; Pan, Y.W. Sulfide-modified zero-valent iron activated periodate for sulfadiazine removal: Performance and dominant routine of reactive species production. Water Res. 2022, 220, 118676. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Li, H.; Wang, J.; Yu, Y. Activation of periodate by C2N3 loaded with metal single-atoms for ultrafast removal of micropollutants: Electron transfer outperforms reactive oxygen species. Chem. Eng. J. 2024, 497, 155052. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, B.; Huang, X.; Ren, W.; Liu, L.; Tian, L.; Chen, Y.; Zhang, L.S.; Sun, Q.; Kang, Z.; et al. Electron transfer mediated activation of periodate by contaminants to generate 1O2 by charge-confined single-atom catalyst. Nat. Commun. 2024, 15, 9549. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wei, W.J.; Zhou, S.Y.; Pan, Y.Z.; Yang, J.; Gan, T.; Zhuang, Z.; Li, W.H.; Zhang, X.; Pan, Y.M.; et al. Phosphorus-Doped Single Atom Copper Catalyst as a Redox Mediator in the Cathodic Reduction of Quinazolinones. Angew. Chem. Int. Ed. Engl. 2025, 64, e202505085. [Google Scholar] [CrossRef]
- Yang, C.J.; Huang, Y.D.; Zhang, Y.Y.; Pan, Y.Z.; Yang, J.; Pan, Y.M.; Gan, T.; Tang, H.T.; Zhang, X.; Li, W.H.; et al. A Mn-Rh dual single-atom catalyst for inducing C-C cleavage: Relay catalysis reversing chemoselectivity in C-H oxidation. Chem. Sci. 2025, 16, 7329–7338. [Google Scholar] [CrossRef]
- Zhu, C.; Cun, F.; Fan, Z.; Nie, Y.; Du, Q.; Liu, F.; Yang, W.; Li, A. Heterogeneous Fe-Co dual-atom catalyst outdistances the homogeneous counterpart for peroxymonosulfate-assisted water decontamination: New surface collision oxidation path and diatomic synergy. Water Res. 2023, 241, 120164. [Google Scholar] [CrossRef]
- Chemakina, I.S.; Ivantsov, M.I.; Kulikova, M.V.; Tretyakov, N.Y.; Elyshev, A.V. Carbon-based catalysts for selective hydrogenation of carbon oxides (methanation). Pet. Chem. 2023, 63, 693–697. [Google Scholar] [CrossRef]
- Ling, C.; Zhang, Z.Y.; Dong, T.L.; Zhu, C.Q.; Xue, Y.Z.; Han, J.A.; Liu, F.Q. Constructing low-temperature-resistant advanced oxidation process by hollow porous carbon-supported single-atom Fe catalyst for efficient cold-water decontamination: Combined kinetic and thermodynamic optimization. Appl. Catal. B-Environ. Energy 2025, 372, 125330. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.Q.; Liu, W.; Chang, C.R.; Tang, H.L.; Li, Z.J.; Chen, W.X.; Jia, C.J.; Yao, T.; Wei, S.Q.; et al. Design of N-coordinated dual-metal sites: A stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 2017, 139, 17281–17284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Mollamahale, Y.B.; Lyu, D.D.; Liang, L.Z.; Yu, F.; Qing, M.; Du, Y.H.; Zhang, X.Y.; Tian, Z.Q.; Shen, P.K. Molecular-level design of Fe-N-C catalysts derived from Fe-dual pyridine coordination complexes for highly efficient oxygen reduction. J. Catal. 2019, 372, 245–257. [Google Scholar] [CrossRef]
- Kang, J.; Duan, X.G.; Wang, C.; Sun, H.Q.; Tan, X.Y.; Tade, M.O.; Wang, S.B. Nitrogen-doped bamboo-like carbon nanotubes with Ni encapsulation for persulfate activation to remove emerging contaminants with excellent catalytic stability. Chem. Eng. J. 2018, 332, 398–408. [Google Scholar] [CrossRef]
- Chen, S.; Li, G.; Zhu, Z.; Zhu, R.; Zhang, J.; Yue, Y.; Li, G.; Zhou, L.; Yan, Z. Single-atom catalysts for lithium-sulfur batteries: Research progress and prospects. J. Energy Chem. 2025, 107, 440–458. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, H.; Liang, Y.; Cao, Y.; Xiao, Y.; Ma, J. Degradation of bisphenol AF in water by periodate activation with FeS (mackinawite) and the role of sulfur species in the generation of sulfate radicals. Chem. Eng. J. 2021, 407, 126738. [Google Scholar] [CrossRef]
- Zhao, J.N.; Chen, J.W.; Wang, Q.; Xiong, R.X.; Ma, J. Activation of periodate by biocarbon-supported multiple modified nanoscale iron for the degradation of bisphenol A in high-temperature aqueous solution. Environ. Sci. Pollut. Res. 2024, 31, 24263–24281. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Zhang, W.; Qin, C.; Yan, Y.; Gao, Z.Q.; Zhu, L.; Tang, C.M.; Jin, C.; Yang, S.G. Fe confined in Prophyrin-based porous organic polymer as an efficient periodate activator for nonradical pathway removal of contaminants. Sep. Purif. Technol. 2023, 317, 123868. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, W.; Lin, X.; Wang, Y.; Lu, X.; Yu, Y.; Gu, H.; Heng, S.; Zhang, H.; Ma, J. Periodate activation with stable MgMn2O4 spinel for bisphenol A removal: Radical and non-radical pathways. Chem. Eng. J. 2023, 459, 141574. [Google Scholar] [CrossRef]
- Dai, J.; Wang, Z.; Chen, K.; Ding, D.; Yang, S.; Cai, T. Applying a novel advanced oxidation process of biochar activated periodate for the efficient degradation of bisphenol A: Two nonradical pathways. Chem. Eng. J. 2023, 453, 139889. [Google Scholar] [CrossRef]
- Qian, K.; Chen, H.; Li, W.L.; Ao, Z.M.; Wu, Y.N.; Guan, X.H. Single-atom Fe catalyst outperforms its homogeneous counterpart for activating peroxymonosulfate to achieve effective degradation of organic contaminants. Environ. Sci. Technol. 2021, 55, 7034–7043. [Google Scholar] [CrossRef]
- Gao, Y.W.; Zhu, Y.; Li, T.; Chen, Z.H.; Jiang, Q.K.; Zhao, Z.Y.; Liang, X.Y.; Hu, C. Unraveling the high-activity origin of single-atom iron catalysts for organic pollutant oxidation via peroxymonosulfate activation. Environ. Sci. Technol. 2021, 55, 8318–8328. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Wang, W.Y.; Nie, M.H.; Yan, C.X.; Wang, P.; Ding, M.J. Visible light-mediated activation of periodate for bisphenol A degradation in the presence of Fe3+ and gallic acid at neutral pH. Chem. Eng. J. 2024, 479, 147541. [Google Scholar] [CrossRef]
- Li, R.X.; Wang, J.Q.; Wu, H.; Zhu, Z.Y.; Guo, H.G. Periodate activation for degradation of organic contaminants: Processes, performance and mechanism. Sep. Purif. Technol. 2022, 292, 120928. [Google Scholar] [CrossRef]
- Zhang, M.; Ruan, J.; Wang, X.; Shao, W.; Chen, Z.; Chen, Z.; Gu, C.; Qiao, W.; Li, J. Selective oxidation of organic pollutants based on reactive oxygen species and the molecular structure: Degradation behavior and mechanism analysis. Water Res. 2023, 246, 120697. [Google Scholar] [CrossRef]
- Zhang, L.S.; Jiang, X.H.; Zhong, Z.A.; Tian, L.; Sun, Q.; Cui, Y.T.; Lu, X.; Zou, J.P.; Luo, S.L. Carbon Nitride Supported High-Loading Fe Single-Atom Catalyst for Activating of Peroxymonosulfate to Generate 1O2 with 100% Selectivity. Angew. Chem. Int. Ed. 2021, 60, 21751–21755. [Google Scholar] [CrossRef]
- Chen, M.; Chen, J.H.; Chen, C.C.; Zhang, C.B.; He, H. Distinct photocatalytic charges separation pathway on CuOx modified rutile and anatase TiO2 under visible light. Appl. Catal. B-Environ. Energy 2022, 300, 120735. [Google Scholar] [CrossRef]
- Javed, H.; Metz, J.; Eraslan, T.C.; Mathieu, J.; Wang, B.; Wu, G.; Tsai, A.L.; Wong, M.S.; Alvarez, P.J.J. Discerning the Relevance of Superoxide in PFOA Degradation. Environ. Sci. Technol. Lett. 2020, 7, 653–658. [Google Scholar] [CrossRef]
- Yu, M.; Yang, J.; Chen, J.; Luo, H.; Ma, H.; Pu, S. Enhancement of 2,4,6-trichlorophenol degradation in groundwater via natural pyrite-activated percarbonate: Mechanistic insights and efficiency evaluation. J. Environ. Chem. Eng. 2025, 13, 116048. [Google Scholar] [CrossRef]
- Hu, J.; Zou, Y.; Li, Y.; Yu, Z.; Bao, Y.; Lin, L.; Li, B.; Li, X.-Y. Periodate activation by atomically dispersed Mn on carbon nanotubes for the production of iodate radicals and rapid degradation of sulfadiazine. Chem. Eng. J. 2023, 472, 144862. [Google Scholar] [CrossRef]
- Li, Y.; Yang, T.; Qiu, S.H.; Lin, W.Q.; Yan, J.T.; Fan, S.S.; Zhou, Q. Uniform N-coordinated single-atomic iron sites dispersed in porous carbon framework to activate PMS for efficient BPA degradation via high-valent iron-oxo species. Chem. Eng. J. 2020, 389, 124382. [Google Scholar] [CrossRef]
- Di, L.; Wang, T.; Lu, Q.W.; Lu, J.J.; Zhang, Y.T.; Zhou, Y.; Zhou, Y.B. Efficient PMS activation toward degradation of bisphenol A by metal-free nitrogen-doped hollow carbon spheres. Sep. Purif. Technol. 2024, 339, 126740. [Google Scholar] [CrossRef]
- Zheng, J.; Lin, Q.; Liu, Y.; Fan, X.; Xu, K.; Ma, Y.; He, J.; Fu, H. Peroxymonosulfate activation by Mg-introduced Fe-N carbon nanotubes to accelerate sulfamethoxazole degradation: Singlet oxygen-dominated nonradical pathway. Chem. Eng. J. 2023, 452, 139233. [Google Scholar] [CrossRef]
- Hong, P.; Wu, Z.; Yang, D.; Zhang, K.; He, J.; Li, Y.; Xie, C.; Yang, W.; Yang, Y.; Kong, L.; et al. Efficient generation of singlet oxygen (1O2) by hollow amorphous Co/C composites for selective degradation of oxytetracycline via Fenton-like process. Chem. Eng. J. 2021, 421, 129594. [Google Scholar] [CrossRef]
- Shang, Y.; Kan, Y.; Xu, X. Stability and regeneration of metal catalytic sites with different sizes in Fenton-like system. Chin. Chem. Lett. 2023, 34, 108278. [Google Scholar] [CrossRef]






| Catalysts | Mass (g L−1) | pH0 | T (°C) | C0 BPA (mmol·L−1) | PI Dosage (mmol·L−1) | k (min−1) | knorm-1 (L·g−1·min−1) | knorm-2 (L·g−1·min−1) | Refs |
|---|---|---|---|---|---|---|---|---|---|
| FeS | 1.0 | 5.5 | / | 0.02 | 1.0 | 0.0356 | 0.0356 | 0.001 | [20] |
| S-(nFe0-Ni)/BC | 0.2 | 4.0 | 65 | 0.02 | 1.0 | 0.0167 | 0.0835 | 0.002 | [21] |
| Fe@PrPOP | 0.1 | 4.0 | 20 | 0.005 | 0.5 | 0.3838 | 3.838 | 0.038 | [22] |
| MgMn2O4 | 0.1 | 7 | 20 | 0.01 | 1.0 | 0.05461 | 0.5461 | 0.005 | [23] |
| BC800 | 0.3 | 7 | / | 0.022 | 0.5 | 0.0419 | 0.1397 | 0.006 | [24] |
| HC800 | 0.1 | 6.25 | 25 | 0.088 | 0.8 | 0.558 | 5.58 | 0.614 | This work |
| HCFe800 | 0.1 | 6.25 | 25 | 0.088 | 0.8 | 5.094 | 50.94 | 5.603 | This work |
| knorm-1; knorm-2 = | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, C.; Yuan, M.; Gao, S.; Xue, Y.; Pan, Y. Efficiency and Mechanism of a Hollow Carbon-Based Single-Atom Iron Catalyst in Activating Periodate for Bisphenol a Degradation. Water 2025, 17, 2705. https://doi.org/10.3390/w17182705
Ling C, Yuan M, Gao S, Xue Y, Pan Y. Efficiency and Mechanism of a Hollow Carbon-Based Single-Atom Iron Catalyst in Activating Periodate for Bisphenol a Degradation. Water. 2025; 17(18):2705. https://doi.org/10.3390/w17182705
Chicago/Turabian StyleLing, Chen, Mengyue Yuan, Shang Gao, Yuzhu Xue, and Yuwei Pan. 2025. "Efficiency and Mechanism of a Hollow Carbon-Based Single-Atom Iron Catalyst in Activating Periodate for Bisphenol a Degradation" Water 17, no. 18: 2705. https://doi.org/10.3390/w17182705
APA StyleLing, C., Yuan, M., Gao, S., Xue, Y., & Pan, Y. (2025). Efficiency and Mechanism of a Hollow Carbon-Based Single-Atom Iron Catalyst in Activating Periodate for Bisphenol a Degradation. Water, 17(18), 2705. https://doi.org/10.3390/w17182705







