Sources and Transport of Dissolved Organic Matter (DOM) in Surface and Groundwater Within a Dominated Greenhouse Agriculture Catchment: Insights from Multi-Tracer
Abstract
1. Introduction
2. Materials and Methods
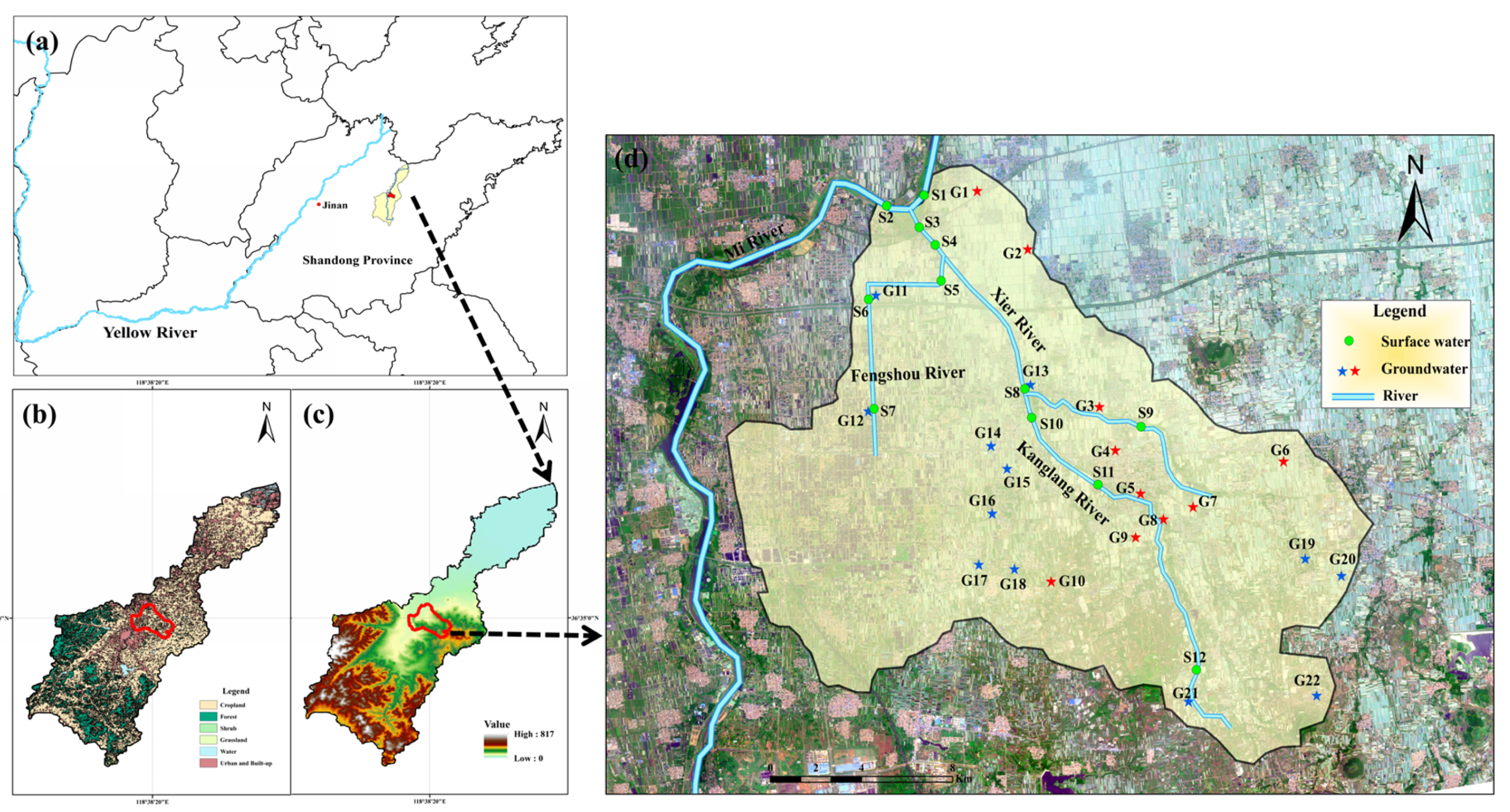
2.1. Study Area
2.2. Field Sampling and Pretreatment
2.3. Analysis of Hydrochemical Indicators
2.4. Analysis of DOM
2.5. Data Analysis Methods of DOM
2.5.1. EEM-PARAFAC and Optical Indices Analysis
2.5.2. 2D-COS Analysis
2.5.3. SOM Analysis
2.6. Statistical Analysis
3. Results and Discussion
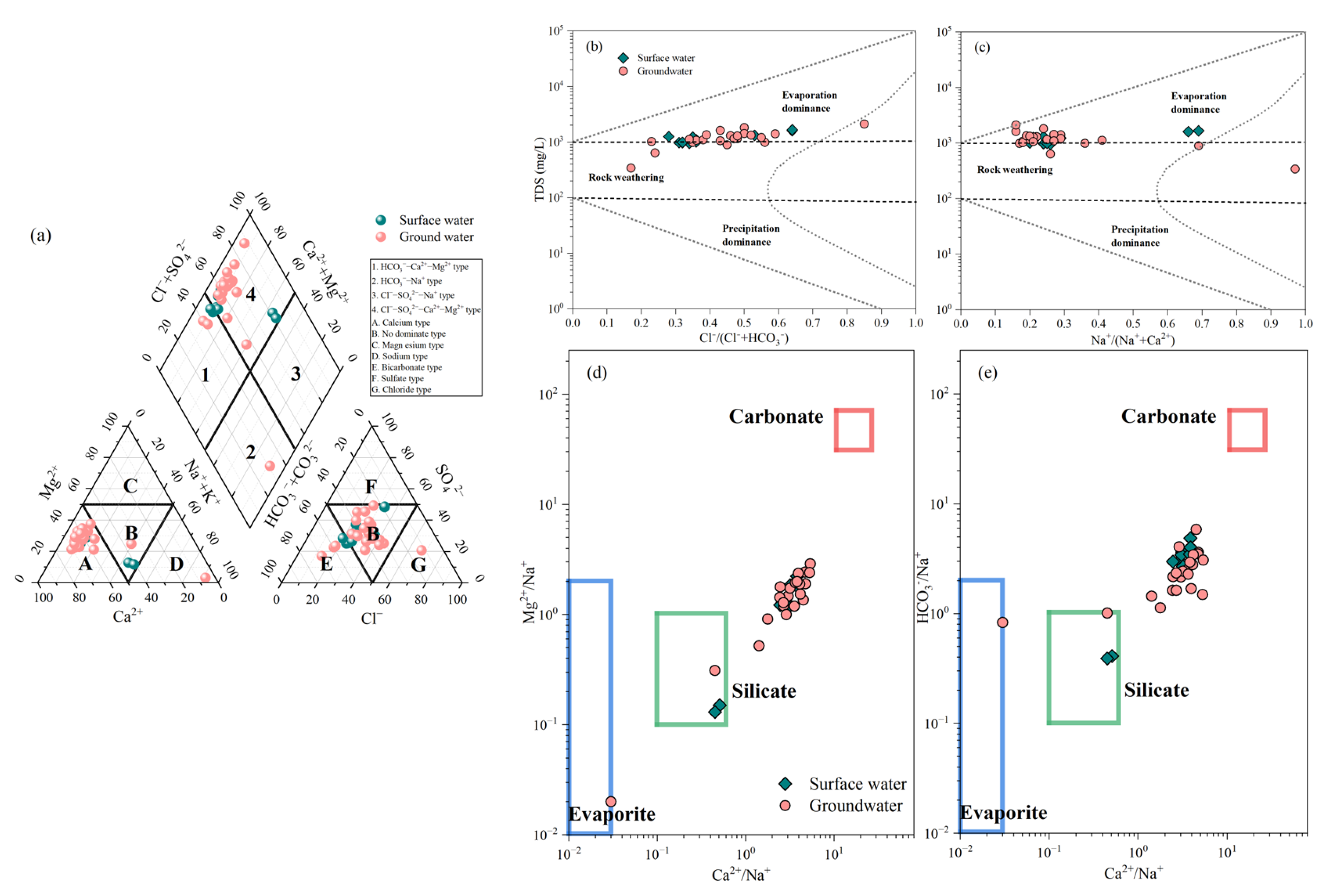
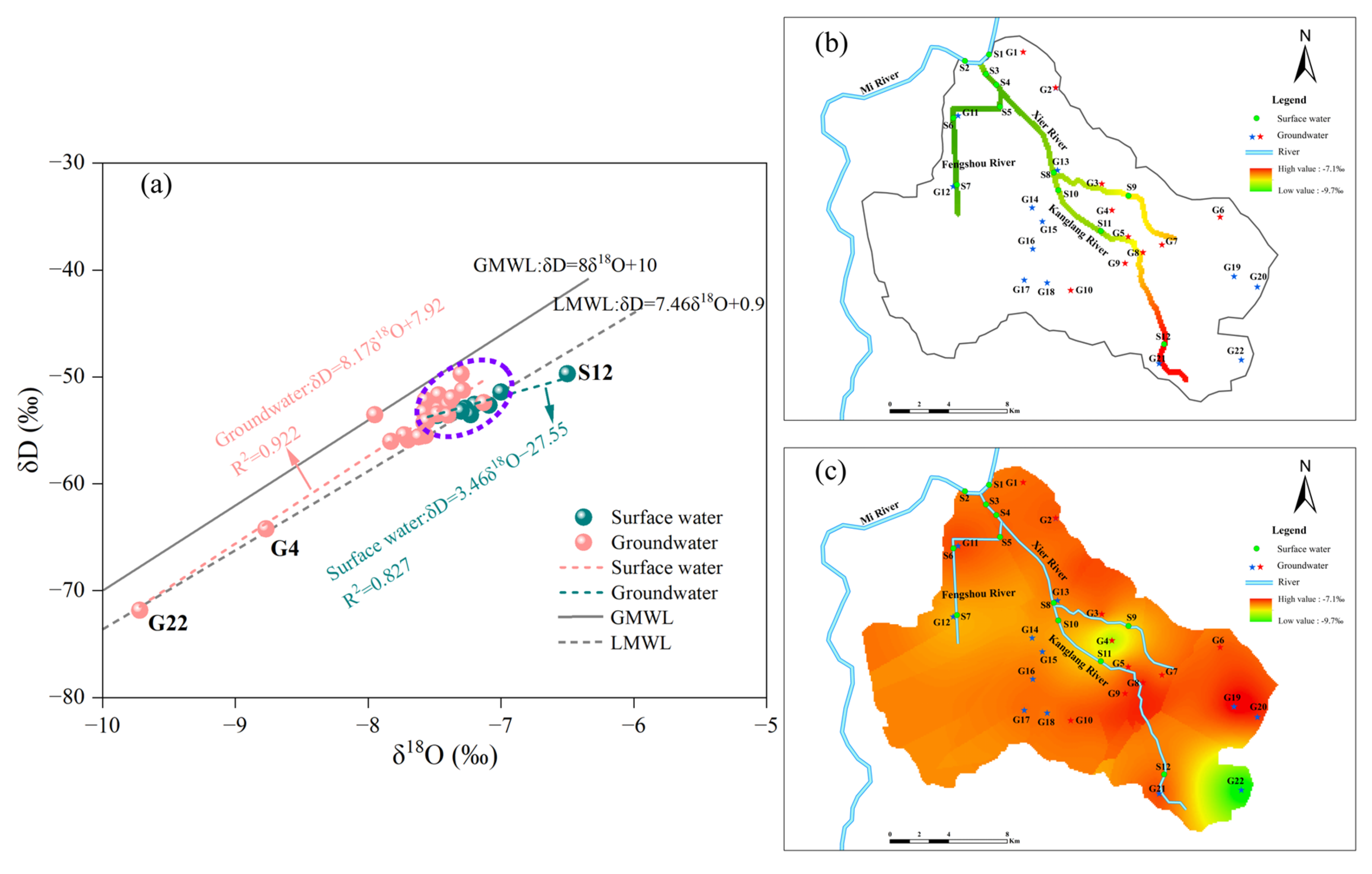
3.1. Surface–Groundwater Interaction Based on Water Chemistry and Isotopes (δD and δ18O)
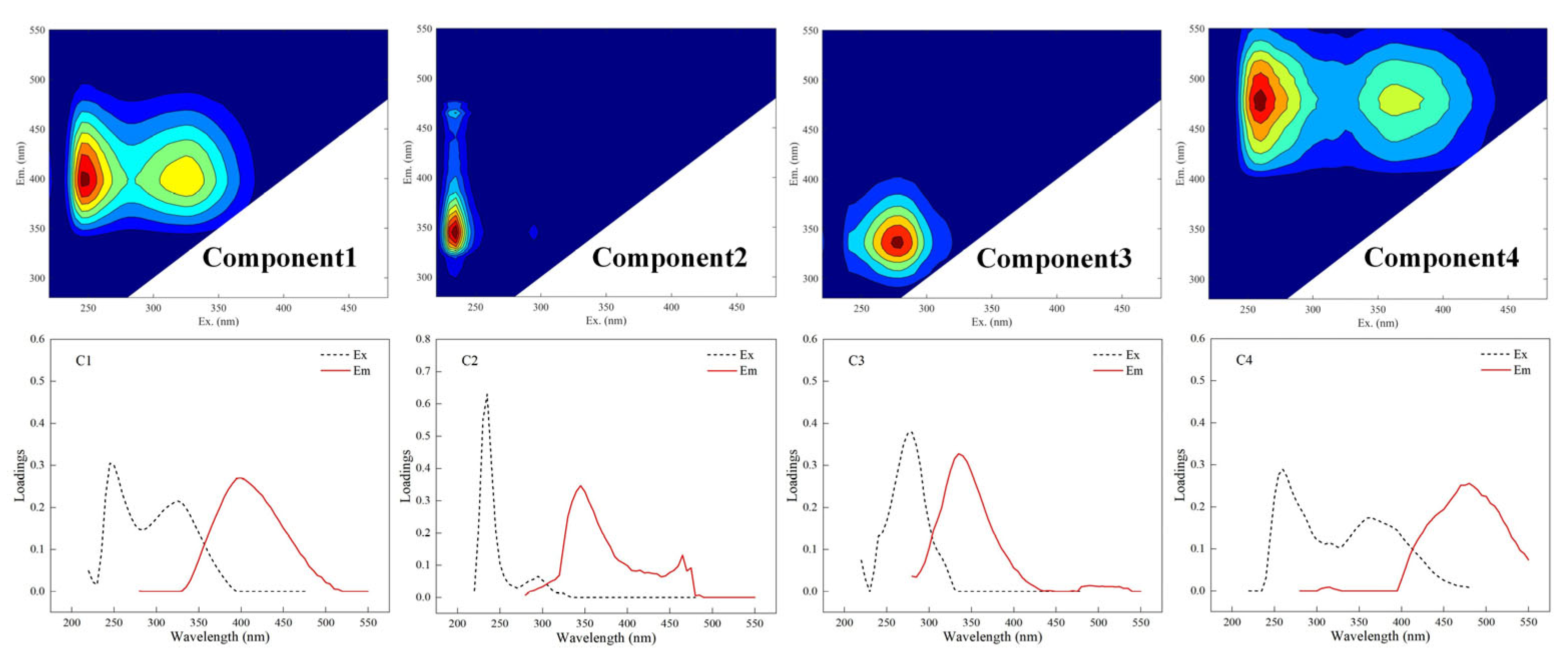
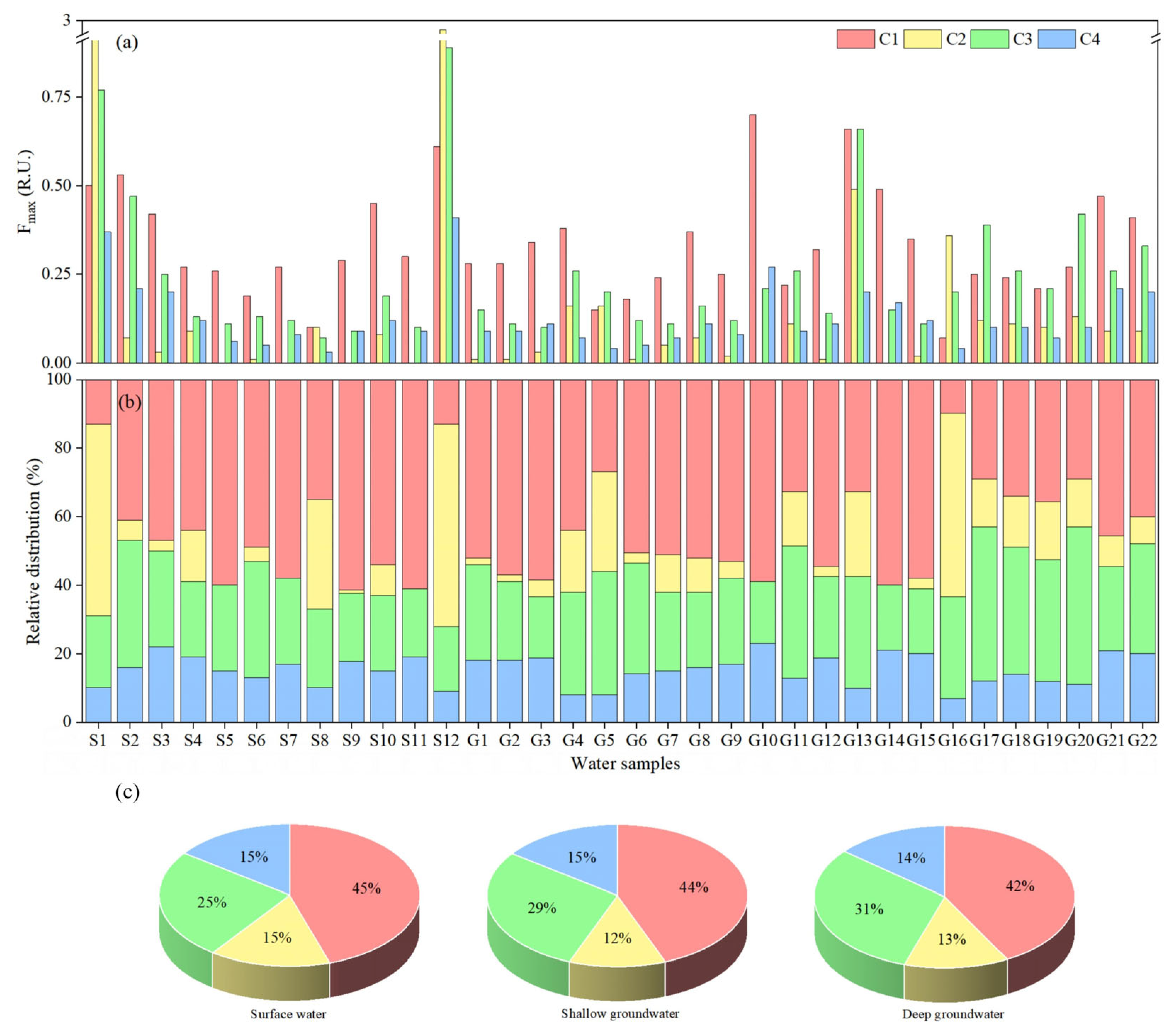
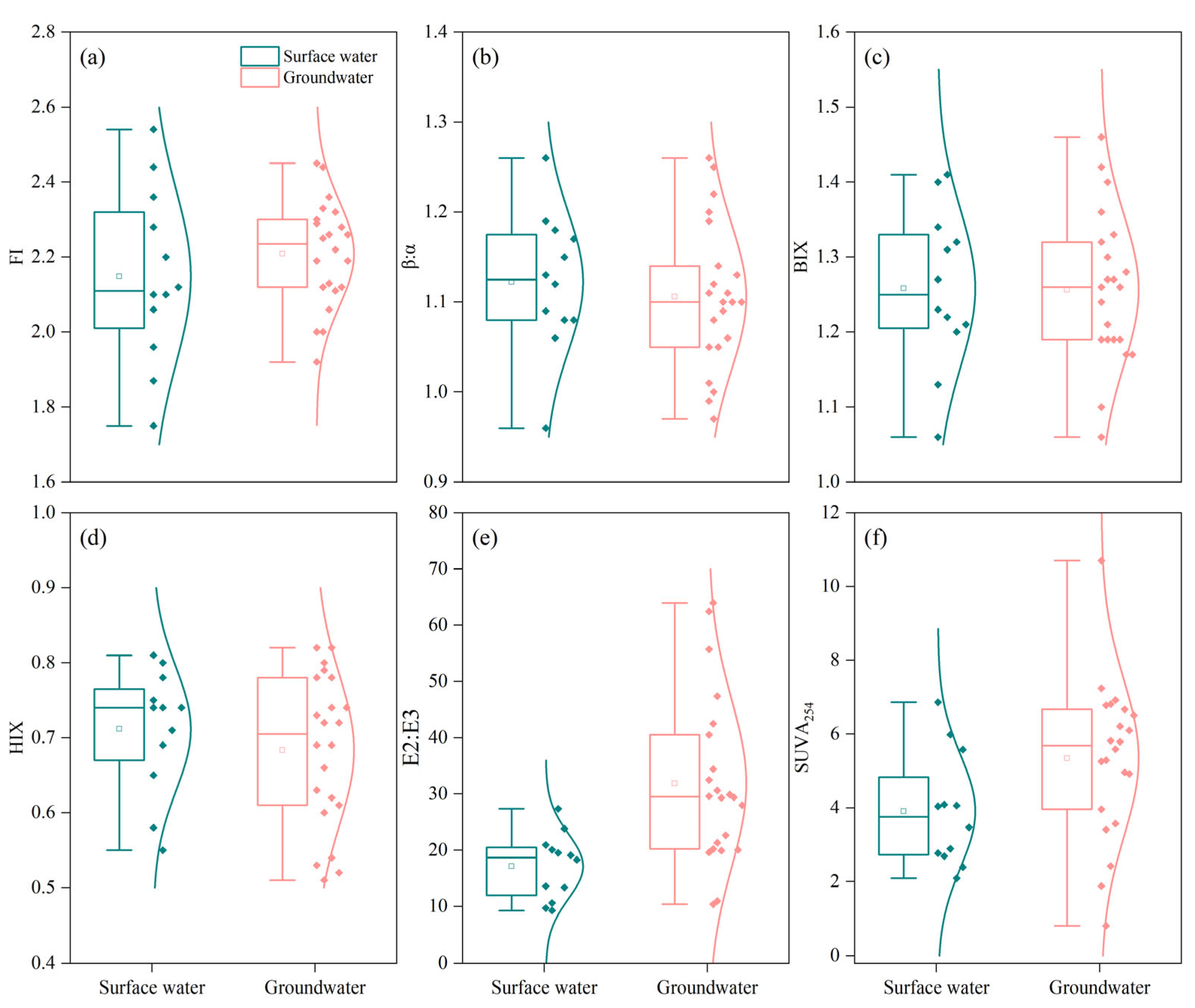
3.2. Variations in DOM Components and Optical Indices Characteristics
| Component | Maximum Ex(nm) | Maximum Em(nm) | Description | Previous Studies | Number of Matches b |
|---|---|---|---|---|---|
| C1 | 245 (325) a | 400 | Microbial humic-like | C2 [68] C2 [9] | 94 |
| C2 | 235 | 345 | Tryptophan-like, produced by human activities | C2 [7] C3 [18] | 14 |
| C3 | 275 | 335 | Tryptophan-like, produced by microbial metabolism | C4 [68] C3 [13] | 96 |
| C4 | 260 (365) | 480 | Terrestrial humic-like | C4 [7] C1 [68] | 80 |
3.3. DOM Components Variation Sequences
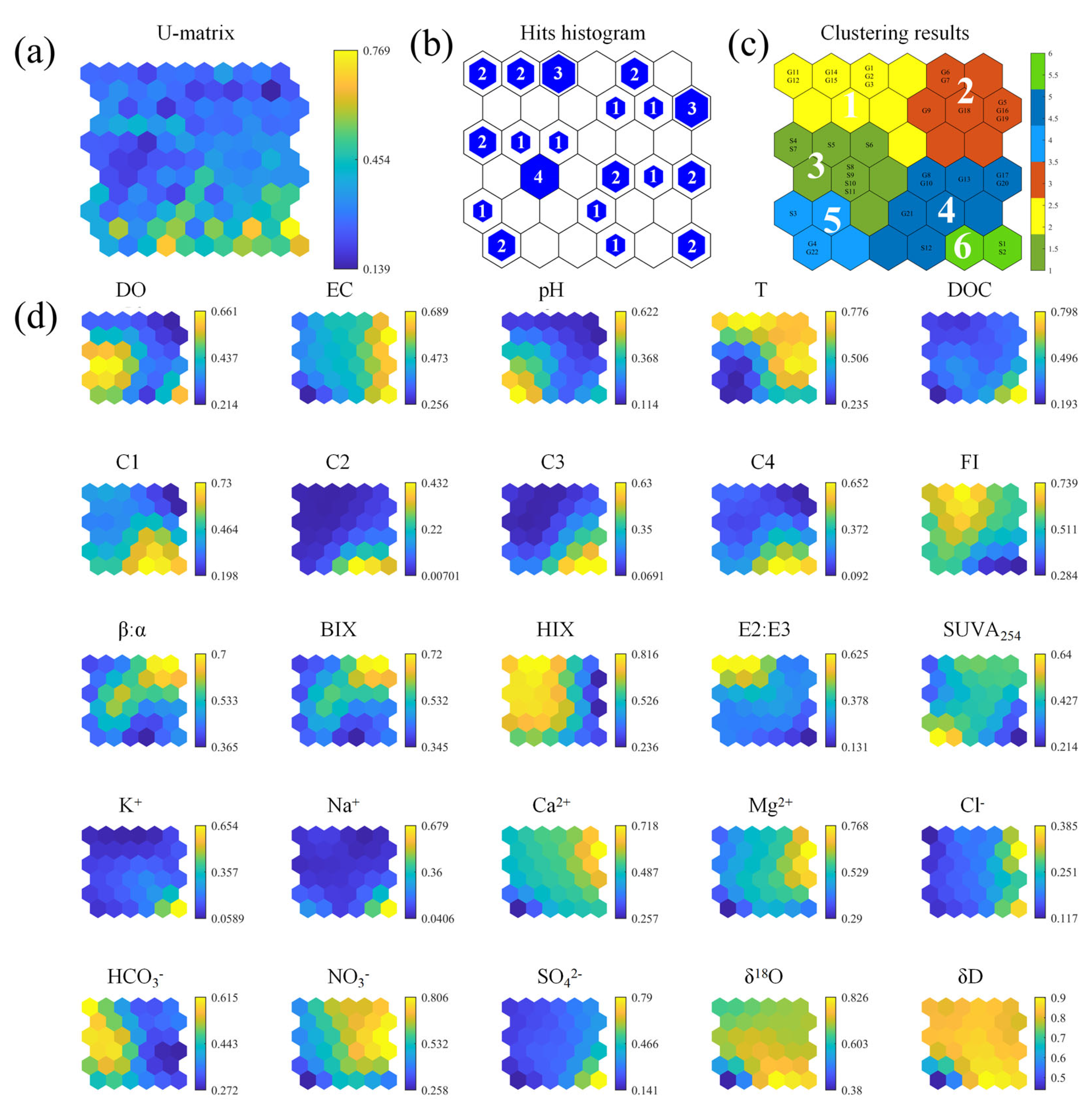
3.4. Classification and Visualization
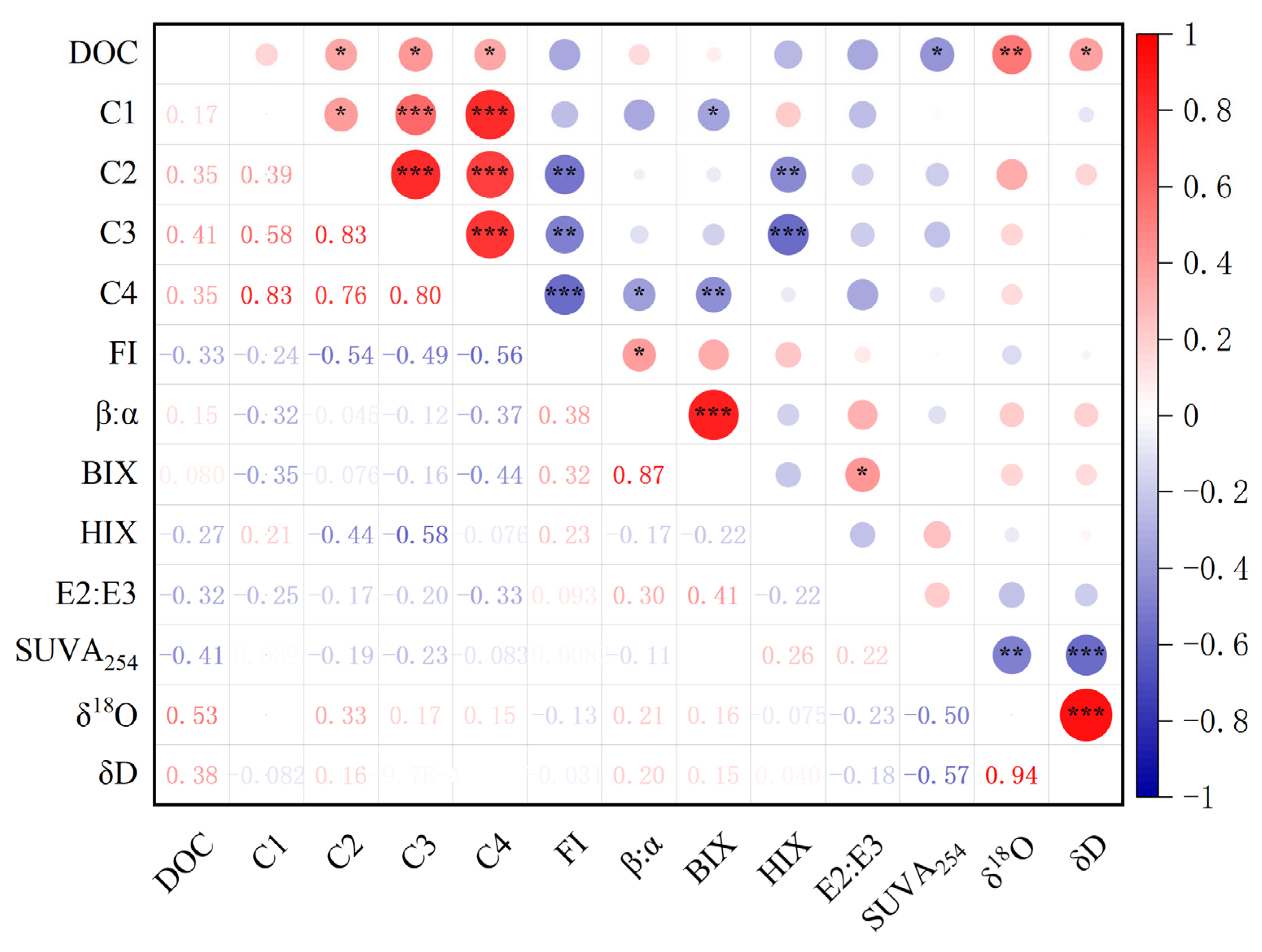
3.5. Results of Pearson’s Correlation Analysis
3.6. Environmental Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.Z.; Dou, Z.X.; Shi, X.J.; Zou, C.Q.; Liu, D.Y.; Wang, Z.Y.; Guan, X.L.; Sun, Y.X.; Wu, G.; Zhang, B.G.; et al. Innovative management programme reduces environmental impacts in Chinese vegetable production. Nat. Food 2020, 2, 47–53. [Google Scholar] [CrossRef]
- Ni, B.; Zhang, W.; Xu, X.C.; Wang, L.G.; Bol, R.; Wang, K.Y.; Hu, Z.J.; Zhang, H.X.; Meng, F.Q. Exponential relationship between N2O emission and fertilizer nitrogen input and mechanisms for improving fertilizer nitrogen efficiency under intensive plastic-shed vegetable production in China: A systematic analysis. Agric. Ecosyst. Environ. 2021, 312, 107353. [Google Scholar] [CrossRef]
- Ti, C.P.; Luo, Y.X.; Yan, X.Y. Characteristics of nitrogen balance in open-air and greenhouse vegetable cropping systems of China. Environ. Sci. Pollut. Res. 2015, 22, 18508–18518. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, Z.-H.; Xi, B.-D.; He, X.-S.; Li, Q.-L.; Qi, Y.-J.; Jin, M.-Y.; Guo, Y. Characteristics of groundwater pollution in a vegetable cultivation area of typical facility agriculture in a developed city. Ecol. Indic. 2019, 105, 709–716. [Google Scholar] [CrossRef]
- Wang, X.Z.; Zou, C.Q.; Gao, X.P.; Guan, X.L.; Zhang, Y.Q.; Shi, X.J.; Chen, X.P. Nitrate leaching from open-field and greenhouse vegetable systems in China: A meta-analysis. Environ. Sci. Pollut. Res. 2018, 25, 31007–31016. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.F.; Li, X.H.; Cao, C.L.; Lin, M.X.; Qiu, Q.L.L.; Xu, Y.Y.; Ren, Y. Compositional variety of dissolved organic matter and its correlation with water quality in peri-urban and urban river watersheds. Ecol. Indic. 2019, 104, 459–469. [Google Scholar] [CrossRef]
- Ding, H.Y.; Zheng, M.X.; Yan, L.; Zhang, X.Y.; Liu, L.; Sun, Y.Y.; Su, J.; Xi, B.D.; Yu, H.B. Spectral and molecular insights into the variations of dissolved organic matter in shallow groundwater impacted by surface water recharge. Water Res. 2025, 273, 122978. [Google Scholar] [CrossRef]
- Tong, H.; Gao, R.Z.; Yue, C.; Xie, L.M.; Duan, L.M.; Zhu, Y.; Wang, G.Q. Hydrochemical evolution and nitrate sources, migration, and transformation in surface water and groundwater of a typical tributary of the Yellow River. J. Environ. Manag. 2025, 390, 126218. [Google Scholar] [CrossRef]
- Yu, H.; Feng, S.B.; Qiu, H.S.; Liu, J.Y. Interaction between the hydrochemical environment, dissolved organic matter, and microbial communities in groundwater: A case study of a vegetable cultivation area in Huaibei Plain, China. Sci. Total Environ. 2023, 895, 165166. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, J.; Guo, Z.Z.; Li, M.; Wu, H.M. Distributions and interactions of dissolved organic matter and heavy metals in shallow groundwater in Guanzhong basin of China. Environ. Res. 2022, 207, 112099. [Google Scholar] [CrossRef]
- Hu, J.; Kang, L.Y.; Li, Z.L.; Feng, X.H.; Liang, C.F.; Wu, Z.; Zhou, W.; Liu, X.N.; Yang, Y.H.; Chen, L.Y. Photo-produced aromatic compounds stimulate microbial degradation of dissolved organic carbon in thermokarst lakes. Nat. Commun. 2023, 14, 3681. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Cheng, D.D.; Song, J.X.; Zhang, Y.X. Spatiotemporal fate of dissolved organic matter (DOM) in aquatic systems: Drivers, patterns and global implications. J. Hydrol. 2025, 661, 133637. [Google Scholar] [CrossRef]
- Zheng, Y.X.; He, W.; Li, B.H.; Hur, J.; Guo, H.M.; Li, X.M. Refractory Humic-like Substances: Tracking Environmental Impacts of Anthropogenic Groundwater Recharge. Environ. Sci. Technol. 2020, 54, 15778–15788. [Google Scholar] [CrossRef]
- Chen, J.Q.; Zhang, Y.W.; Guo, Y.Q.; Jiang, K.; Li, D.; Zheng, T.H. Differential Molecular Interactions of Imidacloprid with Dissolved Organic Matter in Citrus Soils with Diverse Planting Ages. Agriculture 2025, 15, 997. [Google Scholar] [CrossRef]
- Yao, Y.R.; Zhang, J.Y.; Ma, K.; Li, J.; Hu, X.; Wang, Y.S.; Lin, Y.S.; Fang, F.M.; Li, S.Y. Combination Mechanism of Soil Dissolved Organic Matter and Cu2+ in Vegetable Fields, Forests and Dry Farmland in Lujiang County. Agriculture 2024, 14, 684. [Google Scholar] [CrossRef]
- Ge, J.F.; Qi, Y.L.; Li, C.; Ma, J.F.; Yi, Y.B.; Hu, Q.Z.; Mostofa, K.M.G.; Volmer, D.A.; Li, S.-L. Fluorescence and molecular signatures of dissolved organic matter to monitor and assess its multiple sources from a polluted river in the farming-pastoral ecotone of northern China. Sci. Total Environ. 2022, 837, 154575. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.B.; Zhong, J.; Bao, H.Y.; Mostofa, K.M.G.; Xu, S.; Xiao, H.-Y.; Li, S.-L. The impacts of reservoirs on the sources and transport of riverine organic carbon in the karst area: A multi-tracer study. Water Res. 2021, 194, 116933. [Google Scholar] [CrossRef]
- Bai, Y.J.; Zhang, S.R.; Mu, E.L.; Zhao, Y.J.; Cheng, L.R.; Zhu, Y.; Yuan, Y.M.; Wang, Y.Y.; Ding, A.Z. Characterizing the spatiotemporal distribution of dissolved organic matter (DOM) in the Yongding River Basin: Insights from flow regulation. J. Environ. Manag. 2023, 325, 116476. [Google Scholar] [CrossRef]
- Jeon, P.; Cho, S.; Hur, J.; Mun, H.; Chae, M.; Cho, Y.; Seok, K.; Hong, S. Close Association between Stream Water Quality and Fluorescence Properties of Dissolved Organic Matter in Agriculture-Dominated Watersheds. Water 2022, 14, 2459. [Google Scholar] [CrossRef]
- Li, S.-L.; Zhang, H.; Yi, Y.B.; Zhang, Y.T.; Qi, Y.L.; Mostofa, K.M.G.; Guo, L.D.; He, D.; Fu, P.Q.; Liu, C.-Q. Potential impacts of climate and anthropogenic-induced changes on DOM dynamics among the major Chinese rivers. Geogr. Sustain. 2023, 4, 329–339. [Google Scholar] [CrossRef]
- Bellmore, R.A.; Harrison, J.A.; Needoba, J.A.; Brooks, E.S.; Kent Keller, C. Hydrologic control of dissolved organic matter concentration and quality in a semiarid artificially drained agricultural catchment. Water Resour. Res. 2015, 51, 8146–8164. [Google Scholar] [CrossRef]
- Wang, Y.H.; Tian, X.G.; Song, T.L.; Jiang, Z.; Zhang, G.L.; He, C.; Li, P. Linking DOM characteristics to microbial community: The potential role of DOM mineralization for arsenic release in shallow groundwater. J. Hazard. Mater. 2023, 454, 131566. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lu, Y.H.; Edmonds, J.W.; Liu, C.K.; Wang, S.; Das, O.; Liu, J.; Zheng, C.M. Hydrological and land use control of watershed exports of dissolved organic matter in a large arid river basin in northwestern China. J. Geophys. Res. Biogeosci. 2016, 121, 466–478. [Google Scholar] [CrossRef]
- Wilson, H.F.; Xenopoulos, M.A. Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat. Geosci. 2008, 2, 37–41. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, Y.H.; Edmonds, J.; Liu, C.K.; Zhang, Q.; Zheng, C.M. Irrigation alters source-composition characteristics of groundwater dissolved organic matter in a large arid river basin, Northwestern China. Sci. Total Environ. 2021, 767, 144372. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Liu, Y.D.; Zhou, A.G.; Zhang, L. Identification of groundwater pollution from livestock farming using fluorescence spectroscopy coupled with multivariate statistical methods. Water Res. 2021, 206, 117754. [Google Scholar] [CrossRef]
- Zafar, R.; Lee, Y.K.; Oh, H.; Hur, J. Tracking microplastic-derived dissolved organic matter in the adsorption of its mixtures with natural organic matter via end-member mixing analysis. Environ. Res. 2025, 283, 122144. [Google Scholar] [CrossRef]
- Cuss, C.W.; Guéguen, C. Relationships between molecular weight and fluorescence properties for size-fractionated dissolved organic matter from fresh and aged sources. Water Res. 2015, 68, 487–497. [Google Scholar] [CrossRef]
- Li, M.; Wen, Q.X.; Zhang, Y.M.; Chen, Z.Q. New insights into the transformation of effluent organic matter during Fe(II)-assisted advanced oxidation processes: Parallel factor analysis coupled with self-organizing maps. Water Res. 2022, 221, 118789. [Google Scholar] [CrossRef]
- Zhou, B.; Li, J.N.; Sun, Q.H.; Lei, K. Monsoon-driven DOM dynamics revealed by self-organizing map and end-member mixing analysis: Spatial partitioning of autochthonous and anthropogenic sources in plain river networks. Environ. Res. 2025, 283, 122160. [Google Scholar] [CrossRef]
- Shi, K.; Zhao, Y.T.; Wu, C.B.; Geng, Y.T.; Zhou, S.L.; Chai, B.B. Revealing the distribution characteristics and key driving factors of dissolved organic matter in Baiyangdian Lake inflow rivers from different seasons and sources. Sci. Total Environ. 2024, 951, 175768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Liang, X.Q.; Wang, Z.B.; Xu, L.X. A novel approach combining self-organizing map and parallel factor analysis for monitoring water quality of watersheds under non-point source pollution. Sci. Rep. 2015, 5, 16079. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.M.; Yang, Q.; Cui, H.Y. Insight into the dynamics of dissolved organic matter components under latitude change perturbation. Ecotoxicol. Environ. Saf. 2024, 269, 115734. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Xie, L.N.; Zhang, G.G.; Zhao, Y.; Wei, Z.M. Revealing the Inner Changes of Component Composition Derived from DOM PARAFAC Based on Two-Dimensional Correlation Spectroscopy. Molecules 2022, 27, 7316. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Lee, K.H.; Lim, J.Y.; Kim, J.H.; Eun, B.J.; Lee, S.J.; Park, J.Y.; Oh, H.S.; Oh, J.-M. Revealing spatial-temporal impact of industrial effluent towards DOM in Riverine employing PARAFAC and MW-2D COS. J. Environ. Chem. Eng. 2024, 12, 113412. [Google Scholar] [CrossRef]
- Zhang, X.L.; Nie, L.; Gao, H.J.; Yu, H.B.; Liu, D.P. Applying second derivative synchronous fluorescence spectroscopy combined with Gaussian band fitting to trace variations of DOM fractions along an urban river. Ecol. Indic. 2023, 146, 109872. [Google Scholar] [CrossRef]
- Wang, J.J.; Liang, X.; Ma, B.; Liu, Y.F.; Jin, M.G.; Knappett, P.S.K.; Liu, Y.L. Using isotopes and hydrogeochemistry to characterize groundwater flow systems within intensively pumped aquifers in an arid inland basin, Northwest China. J. Hydrol. 2021, 595, 126048. [Google Scholar] [CrossRef]
- Yi, B.; Liu, J.T.; He, W.; Lü, X.L.; Cao, X.; Chen, X.R.; Zeng, X.J.; Zhang, Y.X. Optical variations of dissolved organic matter due to surface water—Groundwater interaction in alpine and arid Datonghe watershed. Sci. Total Environ. 2023, 864, 161036. [Google Scholar] [CrossRef]
- Martinez, J.L.; Raiber, M.; Cox, M.E. Assessment of groundwater–surface water interaction using long-term hydrochemical data and isotope hydrology: Headwaters of the Condamine River, Southeast Queensland, Australia. Sci. Total Environ. 2015, 536, 499–516. [Google Scholar] [CrossRef]
- Zhang, S.R.; Bai, Y.J.; Wen, X.; Ding, A.Z.; Zhi, J.H. Seasonal and downstream alterations of dissolved organic matter and dissolved inorganic ions in a human-impacted mountainous tributary of the Yellow River, China. Environ. Sci. Pollut. Res. 2018, 25, 17967–17979. [Google Scholar] [CrossRef]
- Hudson, N.; Baker, A.; Reynolds, D. Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—A review. River Res. Appl. 2007, 23, 631–649. [Google Scholar] [CrossRef]
- Zepp, R.G.; Sheldon, W.M.; Moran, M.A. Dissolved organic fluorophores in southeastern US coastal waters: Correction method for eliminating Rayleigh and Raman scattering peaks in excitation–emission matrices. Mar. Chem. 2004, 89, 15–36. [Google Scholar] [CrossRef]
- Huo, P.; Gao, P.C. Seasonal changes of dissolved CO2 and its linkage with optical characteristics of DOM in groundwater in agricultural areas. J. Hydrol. 2024, 643, 131927. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhang, H.Z.; Song, J.X.; Cheng, D.D. More sensitive signatures of DOM are stimulated by socioeconomic-related anthropogenic influences in a typical loess watershed. J. Hydrol. 2025, 657, 133106. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P.; Bro, R. OpenFluor-an online spectral library of auto-fluorescence by organic compounds in the environment. Anal. Methods 2014, 6, 658–661. [Google Scholar] [CrossRef]
- Ni, M.F.; Jiang, S.H.; Li, S.Y. Spectroscopic indices trace spatiotemporal variability of dissolved organic matter in a river system with Karst characteristic. J. Hydrol. 2020, 590, 125570. [Google Scholar] [CrossRef]
- Li, P.H.; Hur, J. Utilization of UV-Vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 131–154. [Google Scholar] [CrossRef]
- Chen, W.; Teng, C.-Y.; Qian, C.; Yu, H.-Q. Characterizing Properties and Environmental Behaviors of Dissolved Organic Matter Using Two-Dimensional Correlation Spectroscopic Analysis. Environ. Sci. Technol. 2019, 53, 4683–4694. [Google Scholar] [CrossRef]
- Liu, M.X.; Han, X.K.; Liu, C.-Q.; Guo, L.D.; Ding, H.; Lang, Y.C. Differences in the spectroscopic characteristics of wetland dissolved organic matter binding with Fe3+, Cu2+, Cd2+, Cr3+ and Zn2+. Sci. Total Environ. 2021, 800, 149476. [Google Scholar] [CrossRef]
- Li, K.; Yang, S.P.; Wang, H.Y.; Wu, Z.B.; Liang, Y.S.; Gong, X.M.; Peng, X.; Qin, P.F. Molecular spectra and docking simulations investigated the binding mechanisms of tetracycline onto E. coli extracellular polymeric substances. Talanta 2024, 276, 126231. [Google Scholar] [CrossRef]
- Li, W.J.; Liu, G.B.; Lei, M.; Zhou, Y.Y.; Cui, H.J.; Du, H.H. Spectral fingerprints of DOM-tungsten interactions: Linking molecular binding to conformational changes. J. Hazard. Mater. 2025, 483, 136649. [Google Scholar] [CrossRef]
- Noda, I. Two-dimensional correlation spectroscopy (2DCOS) analysis of polynomials. J. Mol. Struct. 2016, 1124, 53–60. [Google Scholar] [CrossRef]
- Noda, I. Frontiers of Two-Dimensional Correlation Spectroscopy. Part 1. New concepts and noteworthy developments. J. Mol. Struct. 2014, 1069, 3–22. [Google Scholar] [CrossRef]
- Zhu, G.; Wu, X.; Ge, J.P.; Liu, F.; Zhao, W.G.; Wu, C. Influence of mining activities on groundwater hydrochemistry and heavy metal migration using a self-organizing map (SOM). J. Clean. Prod. 2020, 257, 120664. [Google Scholar] [CrossRef]
- Yan, C.X.; Liu, H.H.; Sheng, Y.R.; Huang, X.; Nie, M.H.; Huang, Q.; Baalousha, M. Fluorescence characterization of fractionated dissolved organic matter in the five tributaries of Poyang Lake, China. Sci. Total Environ. 2018, 637–638, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Cuss, C.W.; Guéguen, C. Analysis of dissolved organic matter fluorescence using self-organizing maps: Mini-review and tutorial. Anal. Methods 2016, 8, 716–725. [Google Scholar] [CrossRef]
- Zhou, P.; Huang, J.L.; Pontius, R.G.; Hong, H.S. New insight into the correlations between land use and water quality in a coastal watershed of China: Does point source pollution weaken it? Sci. Total Environ. 2016, 543, 591–600. [Google Scholar] [CrossRef]
- Deng, Y.D.; Lu, Y.; Du, X.Q.; Ye, X.Y.; Feng, J. Identifying spatial patterns and driving factors of anthropogenic impacts on the groundwater environment based on groundwater chemical kinetics. J. Clean. Prod. 2025, 486, 144436. [Google Scholar] [CrossRef]
- Li, P.Y.; Wu, J.H.; Qian, H. Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ. Earth Sci. 2012, 69, 2211–2225. [Google Scholar] [CrossRef]
- Xing, S.P.; Zhang, C.R.; Guo, H.M.; Sheng, Y.Z.; Liu, X.Y. Hydrologic changes induced by groundwater abstraction lead to arsenic mobilization in shallow aquifers. J. Hazard. Mater. 2024, 480, 136133. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, Q.M.; Peng, W.H.; Liu, X.H. Source apportionment and natural background levels of major ions in shallow groundwater using multivariate statistical method: A case study in Huaibei Plain, China. J. Environ. Manag. 2022, 301, 113806. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, B.; Whittier, R.B. Solute sources and water mixing in a flashy mountainous stream (Pahsimeroi River, U.S. Rocky Mountains): Implications on chemical weathering rate and groundwater–surface water interaction. Chem. Geol. 2015, 391, 123–137. [Google Scholar] [CrossRef]
- Yang, Y.J.; Yuan, X.F.; Deng, Y.M.; Xie, X.J.; Gan, Y.Q.; Wang, Y.X. Seasonal dynamics of dissolved organic matter in high arsenic shallow groundwater systems. J. Hydrol. 2020, 589, 125120. [Google Scholar] [CrossRef]
- Tian, Y.; Zheng, Y.; Wu, B.; Wu, X.; Liu, J.; Zheng, C.M. Modeling surface water-groundwater interaction in arid and semi-arid regions with intensive agriculture. Environ. Model. Softw. 2015, 63, 170–184. [Google Scholar] [CrossRef]
- Liu, J.R.; Song, X.F.; Yuan, G.F.; Sun, X.M.; Liu, X.; Wang, S.Q. Characteristics of δ18O in precipitation over Eastern Monsoon China and the water vapor sources. Chin. Sci. Bull. 2009, 55, 200–211. [Google Scholar] [CrossRef]
- Du, Y.; Deng, Y.M.; Ma, T.; Xu, Y.; Tao, Y.Q.; Huang, Y.W.; Liu, R.; Wang, Y.X. Enrichment of Geogenic Ammonium in Quaternary Alluvial–Lacustrine Aquifer Systems: Evidence from Carbon Isotopes and DOM Characteristics. Environ. Sci. Technol. 2020, 54, 6104–6114. [Google Scholar] [CrossRef]
- Zeng, X.J.; Zheng, Y.X.; Chen, X.R.; Cao, X.; He, W.; Jiang, B.; Li, B.H.; Guo, H.M. Molecular Responses of Dissolved Organic Matter to Anthropogenic Groundwater Recharge: Characteristics, Transformations, and Sensitive Molecules. Environ. Sci. Technol. 2023, 57, 7789–7799. [Google Scholar] [CrossRef]
- Singh, S.; Dutta, S.; Inamdar, S. Land application of poultry manure and its influence on spectrofluorometric characteristics of dissolved organic matter. Agric. Ecosyst. Environ. 2014, 193, 25–36. [Google Scholar] [CrossRef]
- Harjung, A.; Schweichhart, J.; Rasch, G.; Griebler, C. Large-scale study on groundwater dissolved organic matter reveals a strong heterogeneity and a complex microbial footprint. Sci. Total Environ. 2023, 854, 158542. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Liu, L.L.; Wang, Z.P.; Lin, K.F. Using EEM-PARAFAC to identify and trace the pollution sources of surface water with receptor models in Taihu Lake Basin, China. J. Environ. Manag. 2022, 321, 115925. [Google Scholar] [CrossRef]
- Du, C.; Chang, Z.B.; Tang, W.Z.; Hao, Z.N.; Zhang, M.Y.; Zhang, X.Y.; Liu, M.X.; Zhang, H. Molecular insights into the seasonal variation of fluorescent dissolved organic matter in a hydrologically isolated aquatic continuum. J. Hydrol. 2025, 661, 133620. [Google Scholar] [CrossRef]
- Graham, P.W.; Baker, A.; Andersen, M.S. Dissolved Organic Carbon Mobilisation in a Groundwater System Stressed by Pumping. Sci. Rep. 2015, 5, 18487. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.H.; Zhao, Y.; Wei, D.; Zhang, D.Y.; Wei, Z.M.; Wu, J.Q. Insight into transformation of dissolved organic matter in the Heilongjiang River. Environ. Sci. Pollut. Res. 2018, 26, 3340–3349. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.H.; Yu, Y.; Huang, X. Comparison of Organic Matter Properties and Disinfection By-Product Formation between the Typical Groundwater and Surface Water. Water 2022, 14, 1418. [Google Scholar] [CrossRef]
- Ding, H.Y.; Su, J.; Sun, Y.Y.; Yu, H.B.; Zheng, M.X.; Xi, B.D. Insight into spatial variations of DOM fractions and its interactions with microbial communities of shallow groundwater in a mesoscale lowland river watershed. Water Res. 2024, 258, 121797. [Google Scholar] [CrossRef]
- Wilson, G.J.L.; Lu, C.; Lapworth, D.J.; Kumar, A.; Ghosh, A.; Niasar, V.J.; Krause, S.; Polya, D.A.; Gooddy, D.C.; Richards, L.A. Spatial and seasonal controls on dissolved organic matter composition in shallow aquifers under the rapidly developing city of Patna, India. Sci. Total Environ. 2023, 903, 166208. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Z.-H.; Xi, B.-D.; Li, W.-T.; Xu, Y.-Q.; Zhao, H.-Z.; Chen, Z.-Q.; He, X.-S.; Xing, B. Molecular structure and evolution characteristics of dissolved organic matter in groundwater near landfill: Implications of the identification of leachate leakage. Sci. Total Environ. 2021, 787, 147649. [Google Scholar] [CrossRef]
- Cuss, C.W.; Shi, Y.X.; McConnell, S.M.; Guéguen, C. Changes in the fluorescence composition of multiple DOM sources over pH gradients assessed by combining parallel factor analysis and self-organizing maps. J. Geophys. Res. Biogeosci. 2014, 119, 1850–1860. [Google Scholar] [CrossRef]
- Jin, X.C.; Chen, X.Q.; Gao, L.M.; Wu, Y.F.; Lu, H.S.; Yuan, M.H.; Cui, J.H.; Wei, F.Y. Fluorescence Analysis of River DOM Spectra Using PARAFAC in Combination with a Self-Organizing Map to Distinguish Organic Matter Sources. Int. J. Environ. Res. 2024, 18, 20. [Google Scholar] [CrossRef]
- Yang, X.L.; Yu, X.B.; Cheng, J.R.; Zheng, R.Y.; Wang, K.; Dai, Y.X.; Tong, N.J.; Chow, A.T. Impacts of land-use on surface waters at the watershed scale in southeastern China: Insight from fluorescence excitation-emission matrix and PARAFAC. Sci. Total Environ. 2018, 627, 647–657. [Google Scholar] [CrossRef]
- Qin, X.; Wang, H.S.; Gong, J.S.; Ye, Y.H.; Zhou, K.; Xu, N.Z.; Li, L.; Li, J. Multivariate Statistics and Hydrochemistry Combined to Reveal the Factors Affecting Shallow Groundwater Evolution in a Typical Area of the Huaibei Plain, China. Water 2025, 17, 962. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, S.; Taxak, A.K.; Pandey, R.; Yu, Z.-G. Nature rejuvenation: Long-term (1989–2016) vs short-term memory approach based appraisal of water quality of the upper part of Ganga River, India. Environ. Technol. Innov. 2020, 20, 101164. [Google Scholar] [CrossRef]
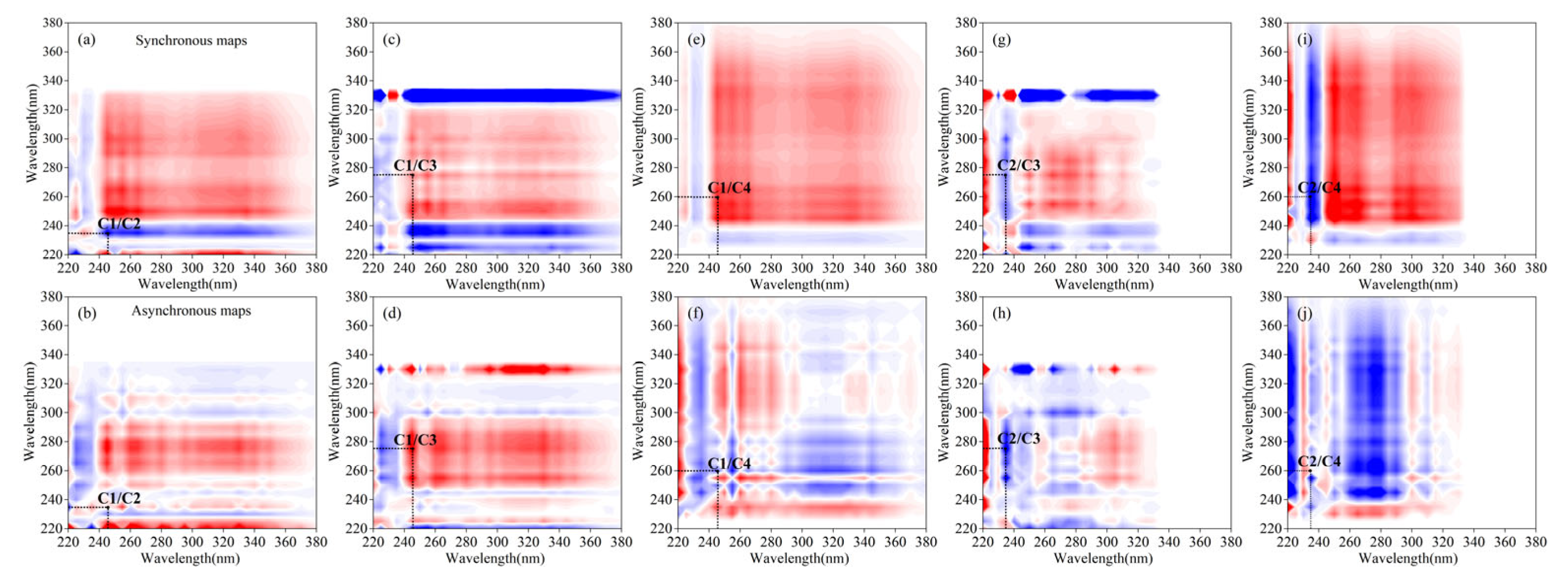
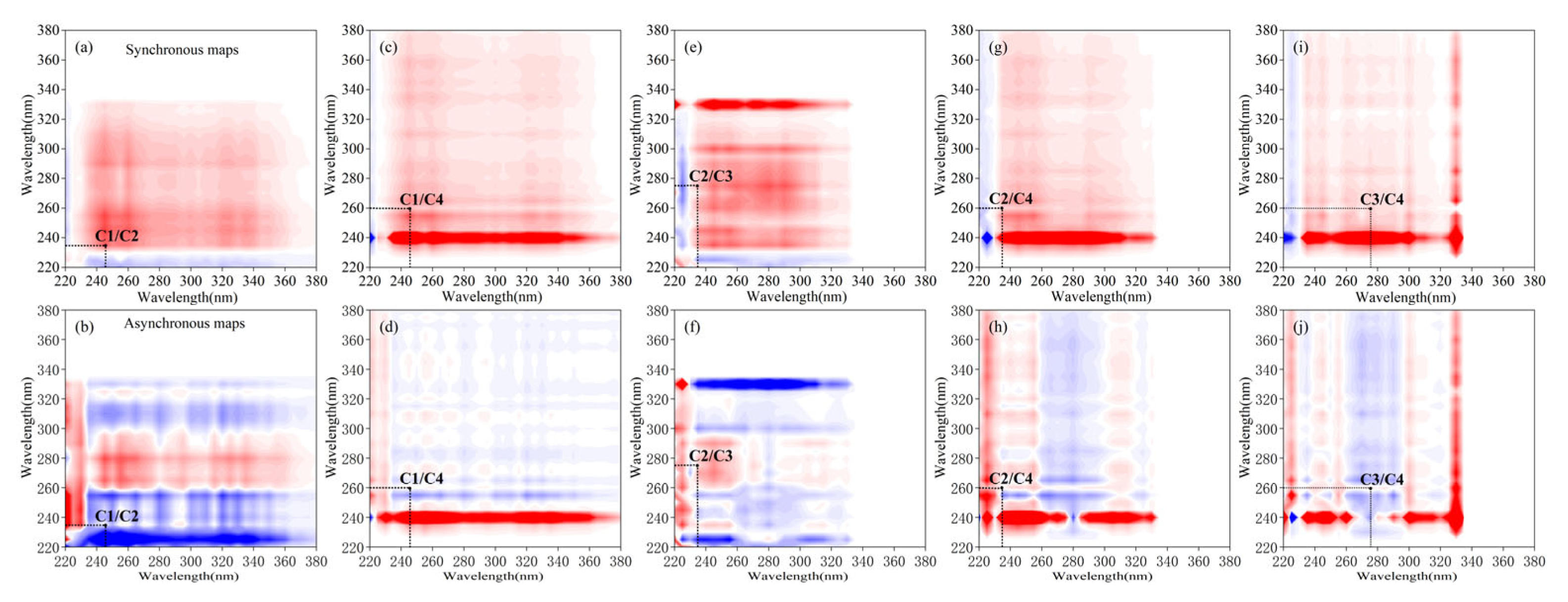
| Disturbance | Components | 275 (C3) | 260 (C4) | 245 (C1) | 235 (C2) |
|---|---|---|---|---|---|
| PR | 275 (C3) | + | +(−) | +(+) | −(−) |
| 260 (C4) | + | +(−) | −(+) | ||
| 245 (C1) | + | −(+) | |||
| 235 (C2) | + | ||||
| VR | 275 (C3) | + | +(+) | +(+) | +(+) |
| 260 (C4) | + | +(+) | +(+) | ||
| 245 (C1) | + | +(+) | |||
| 235 (C2) | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Song, S.; Xu, W.; Yue, F.-J. Sources and Transport of Dissolved Organic Matter (DOM) in Surface and Groundwater Within a Dominated Greenhouse Agriculture Catchment: Insights from Multi-Tracer. Water 2025, 17, 2681. https://doi.org/10.3390/w17182681
Wang H, Song S, Xu W, Yue F-J. Sources and Transport of Dissolved Organic Matter (DOM) in Surface and Groundwater Within a Dominated Greenhouse Agriculture Catchment: Insights from Multi-Tracer. Water. 2025; 17(18):2681. https://doi.org/10.3390/w17182681
Chicago/Turabian StyleWang, Haoyang, Shuang Song, Wei Xu, and Fu-Jun Yue. 2025. "Sources and Transport of Dissolved Organic Matter (DOM) in Surface and Groundwater Within a Dominated Greenhouse Agriculture Catchment: Insights from Multi-Tracer" Water 17, no. 18: 2681. https://doi.org/10.3390/w17182681
APA StyleWang, H., Song, S., Xu, W., & Yue, F.-J. (2025). Sources and Transport of Dissolved Organic Matter (DOM) in Surface and Groundwater Within a Dominated Greenhouse Agriculture Catchment: Insights from Multi-Tracer. Water, 17(18), 2681. https://doi.org/10.3390/w17182681








