A Review of Modification of Carbon-Based Materials Based on Defect Engineering in Capacitive Deionization
Abstract
1. Introduction
2. Intrinsic Defect Engineering
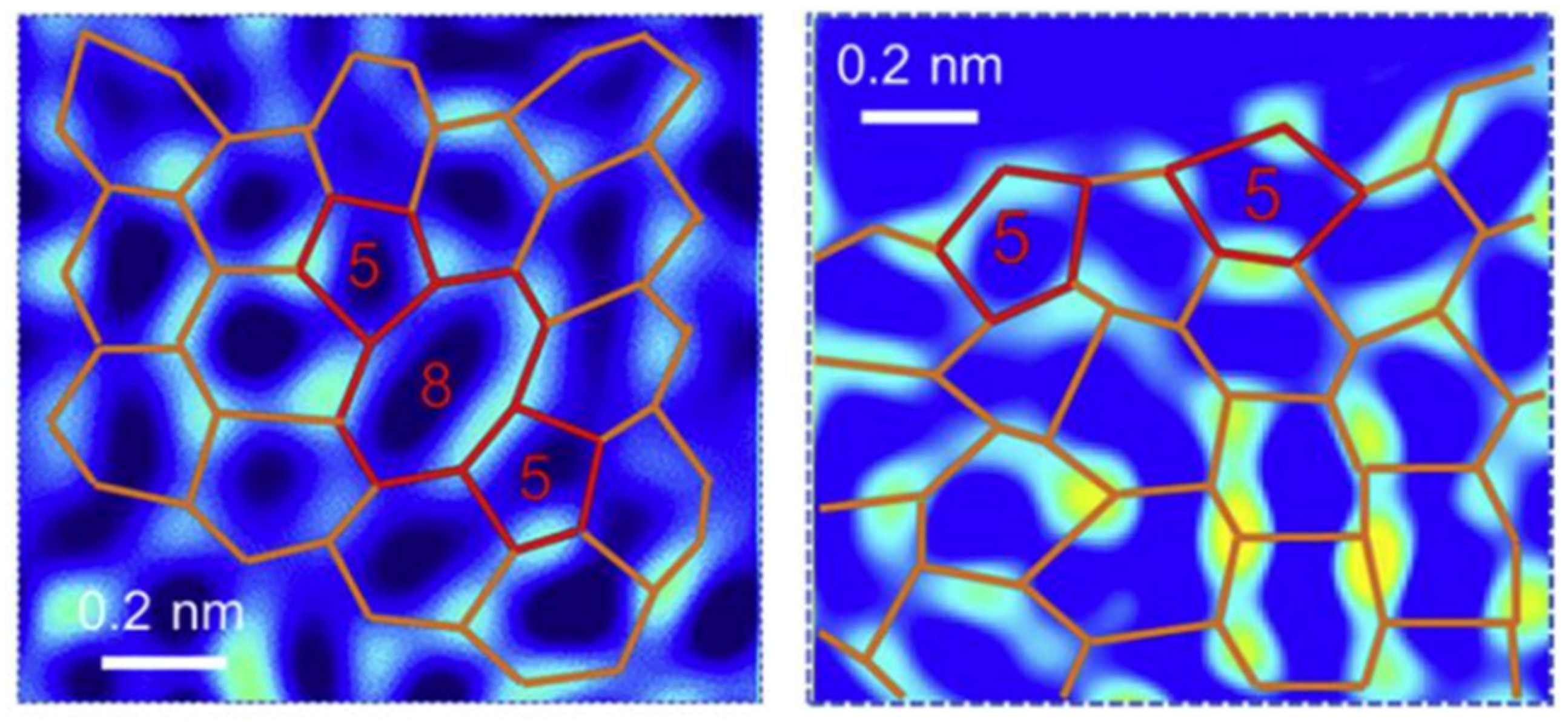
2.1. Topological Defects
2.2. Edge/Vacancy Defects
2.2.1. Microporous Activated Carbon
2.2.2. Mesoporous Carbon
2.2.3. Hierarchical Porous Carbon
2.2.4. Graphene and Fullerenes
3. Extrinsic Defect Engineering
3.1. Doping of Non-Metallic Elements
3.1.1. Nitrogen Doping
3.1.2. Multi-Element Doping
3.2. Doping of Metal Elements
4. Outlook
- (1)
- Limited understanding of the correlation between defect types and adsorption mechanisms
- (2)
- Lack of precise control technology for defect density and distribution
- (3)
- Limited understanding regarding the dynamic evolution and cyclic stability of defects
- (4)
- Lack of research on defect regulation targeting ion selectivity
- (5)
- Engineering challenges in Practical Applications to be addressed
- (1)
- Basic theory of defect engineering: Systematically study the adsorption mechanisms of different defect types and establish a structure–activity relationship model of defect type–density–performance.
- (2)
- Precise control technology: Develop methods, such as ALD and machine learning-assisted design, to achieve precise control of defect density and distribution.
- (3)
- Dynamic stability research: Utilizing in situ characterization techniques to reveal the evolution laws of defects during cycling and optimize the defect–structure co-design.
- (4)
- Selective adsorption optimization: Design defect–functional group collaborative interfaces to enhance the selective adsorption capacity of specific ions.
- (5)
- Engineering application: Explore low-cost and large-scale defect introduction processes and conduct long-term operational stability tests.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H.; Xu, X.; Gao, X.; Li, L.; Pan, L. Design of three-dimensional faradic electrode materials for high-performance capacitive deionization. Coord. Chem. Rev. 2024, 510, 215835. [Google Scholar] [CrossRef]
- Nordstrand, J.; Dutta, J. Theory of bipolar connections in capacitive deionization and principles of structural design. Electrochim. Acta 2022, 430, 141066. [Google Scholar] [CrossRef]
- Gao, M.; Chen, W. Engineering strategies toward electrodes stabilization in capacitive deionization. Coord. Chem. Rev. 2024, 505, 215695. [Google Scholar] [CrossRef]
- Gong, S.; Liu, H.; Zhao, F.; Zhang, Y.; Xu, H.; Li, M.; Qi, J.; Wang, H.; Li, C.; Peng, W.; et al. Vertically Aligned Bismuthene Nanosheets on MXene for High-Performance Capacitive Deionization. ACS Nano 2023, 17, 4843–4853. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.; Wang, Y.; Liang, W.; Yang, Z.; Chen, Y.; Deng, W.; Wang, Z.; Ao, T.; Chen, W. Flexible nitrogen-doped carbon nanofiber-reinforced hierarchical hollow iron oxide nanorods as a binder-free electrode for efficient capacitive deionization. Desalination 2023, 549, 116360. [Google Scholar] [CrossRef]
- Guo, Z.; Shen, G.; Wang, Z.; Ma, Q.; Zhang, L.; Xiao, B.; Yan, Y.; Zheng, Y.; Liu, Y.; Yuan, X. Integrating FeOOH with bacterial cellulose-derived 3D carbon nanofiber aerogels for fast and stable capacitive deionization based on accelerating chloride insertion. Desalination 2024, 576, 117329. [Google Scholar] [CrossRef]
- Wang, L.; Lin, S.H. Mechanism of Selective Ion Removal in Membrane Capacitive Deionization for Water Softening. Environ. Sci. Technol. 2019, 53, 5797–5804. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, Y.; Fang, R.; Wang, J. Flexible structural engineering of PPy-NiCo-LDH@Mxene for improved capacitive deionization and efficient hard water softening process. Sep. Purif. Technol. 2022, 280, 119828. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, H.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Selective Pseudocapacitive Deionization of Calcium Ions in Copper Hexacyanoferrate. ACS Appl. Mater. Interfaces 2020, 12, 41437–41445. [Google Scholar] [CrossRef]
- Tang, W.; Wang, X.; Zeng, G.; Liang, J.; Li, X.; Xing, W.; He, D.; Tang, L.; Liu, Z. Electroassisted adsorption of Zn(II) on activated carbon cloth in batch-flow mode: Experimental and theoretical investigations. Environ. Sci. Technol. 2019, 53, 2670–2678. [Google Scholar] [CrossRef]
- Zhang, B.; Yi, Q.; Qu, W.; Zhang, K.; Lu, Q.; Yan, T.; Zhang, D. Titanium Carbon Oxide Flakes with Tunable Interlayer Spacing for Efficient Capacitive Deionization. Adv. Funct. Mater. 2024, 34, 2401332. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhao, H.; Quan, X.; Chen, S.; Yu, H. Enhanced adsorption of ionizable antibiotics on activated carbon fiber under electrochemical assistance in continuous-flow modes. Water Res. 2018, 134, 162–169. [Google Scholar] [CrossRef]
- Lim, J.; Lee, H.; Lee, S.; Hong, S. Capacitive deionization incorporating a fluidic MOF-CNT electrode for the high selective extraction of lithium. Desalination 2024, 578, 117403. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Q.; Zhao, M.; Lu, S.; Liang, D. Prussian blue analogue based integrated membrane electrodes for desalination and selective removal of ammonium ions in a rocking-chair capacitive deionization. Chem. Eng. J. 2024, 482, 148923. [Google Scholar] [CrossRef]
- Chen, D.; Yang, L.; Zhang, Z.; Wang, Y.; Ouyang, D.; Zhu, H.; Yin, J. Iron nanoparticle embedded carbon nanofibers as flexible electrodes for selective chloride ions capture in capacitive deionization. Desalination 2024, 573, 117175. [Google Scholar] [CrossRef]
- Tauk, M.; Folaranmi, G.; Cretin, M.; Bechelany, M.; Sistat, P.; Zhang, C.; Zaviska, F. Recent advances in capacitive deionization: A comprehensive review on electrode materials. J. Environ. Chem. Eng. 2023, 11, 111368. [Google Scholar] [CrossRef]
- Xu, X.; Eguchi, M.; Asakura, Y.; Pan, L.; Yamauchi, Y. Metal-organic framework derivatives for promoted capacitive deionization of oxygenated saline water. Energy Environ. Sci. 2023, 16, 1815–1820. [Google Scholar] [CrossRef]
- Miao, L.; Wang, Z.; Gao, M.; Peng, J.; Chen, Y.; Chen, F.; Chen, W.; Ao, T. A green potassium citrate activation strategy via one-step synthesis of 3D porous carbon for capacitive deionization. Sep. Purif. Technol. 2024, 346, 127510. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Z.; Ma, Q.; Shen, G.; Xiao, B.; Zhang, L.; Li, Q.; Liu, Y.; Yuan, X. Combining Bismuth nanoclusters embedded 3D carbon nanofiber Aerogels: Towards fast and ultra-durable faradic capacitive deionization. Chem. Eng. J. 2024, 482, 149028. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, K.X.; Gan, Y.H.; Zhang, K.G.; Zhang, J.; Luo, J.; Xu, H.; Chen, Z.H.; Yang, W.Z.; Li, H.L.; et al. An integrated capacitive deionization and photocatalysis system for efficient ammonium removal from low-concentration wastewater. J. Water Process Eng. 2024, 58, 104912. [Google Scholar] [CrossRef]
- Pastushok, O.; Zhao, F.; Ramasamy, D.L.; Sillanpää, M. Nitrate removal and recovery by capacitive deionization (CDI). Chem. Eng. J. 2019, 375, 121943. [Google Scholar] [CrossRef]
- Xing, Z.; Xuan, X.; Hu, H.; Li, M.; Gao, H.; Alowasheeir, A.; Jiang, D.; Zhu, L.; Li, Z.; Kang, Y.; et al. Particle size optimization of metal–organic frameworks for superior capacitive deionization in oxygenated saline water. Chem. Commun. 2023, 59, 4515–4518. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Mo, X.; Li, K. The design of nitrogen-doped core-shell-structured mesopore-dominant hierarchical porous carbon nanospheres for high-performance capacitive deionization. Environ. Sci. Nano 2020, 7, 3575–3586. [Google Scholar] [CrossRef]
- Xing, W.; Luo, K.; Liang, J.; Su, C.; Tang, W. Urchin-like core-shell tungsten oxide@carbon composite electrode for highly efficient and stable water desalination via hybrid capacitive deionization (HCDI). Chem. Eng. J. 2023, 477, 147268. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, Z.; Hao, C.; Deng, Y.; Li, X.; Li, K.; Zhao, Y. A review of modification of carbon electrode material in capacitive deionization. RSC Adv. 2019, 9, 24401. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Zhang, W.; Yi, S.; Chen, H.; Su, Z.; Niu, B.; Zhang, Y.; Long, D. Defect engineering in carbon materials for electrochemical energy storage and catalytic conversion. Mater. Adv. 2023, 4, 835–867. [Google Scholar] [CrossRef]
- Han, Y.; Yan, X.; Wu, Q.; Xu, H.; Li, Q.; Du, A.; Yao, X. Defect-Derived Catalysis Mechanism of Electrochemical Reactions in Two-Dimensional Carbon Materials. Small Struct. 2023, 4, 2300036. [Google Scholar] [CrossRef]
- Gao, H.; Liu, J.; Zhang, Z.; Lu, Y.; Chen, R.; Huang, Y.-C.; Xie, C.; Qiu, M.; Wu, T.; Wang, J.; et al. Electrochemical etching induced high-valence cobalt with defects site for boosting electrochemical water splitting. Chem. Eng. J. 2023, 463, 142224. [Google Scholar] [CrossRef]
- Zhu, J.; Mu, S. Defect Engineering in Carbon-Based Electrocatalysts: Insight into Intrinsic Carbon Defects. Adv. Funct. Mater. 2020, 30, 2001097. [Google Scholar] [CrossRef]
- Huo, S.; Zhang, X.; Liang, B.; Zhao, Y.; Li, K. Synthesis of interconnected hierarchically porous carbon networks with excellent diffusion ability based on NaNO3 crystal-assisted strategy for high performance supercapacitors. J. Power Sources 2020, 450, 227612. [Google Scholar] [CrossRef]
- Xu, H.; Li, M.; Li, C.; Wang, H.; Zhao, F.; Qi, J.; Peng, W.; Liu, J. Modulating the surfaces functional groups and defects in carbon via salt template method for high-performance capacitor deionization. Sep. Purif. Technol. 2024, 330, 125433. [Google Scholar] [CrossRef]
- Xu, H.; Li, M.; Gong, S.; Zhao, F.; Yan, Y.; Li, C.; Qi, J.; Wang, Z.; Hu, Y.; Wang, H.; et al. Fluorine-induced porous carbon nanosheets with abundant edge-defects for high-performance capacitive deionization. Desalination 2022, 538, 115919. [Google Scholar] [CrossRef]
- Liang, M.; Rem, Y.; Cui, J.; Zhang, X.; Xing, S.; Lei, J.; He, M.; Xie, H.; Deng, L.; Yu, F.; et al. Order-in-disordered ultrathin carbon nanostructure with nitrogen-rich defects bridged by pseudographitic domains for high-performance ion capture. Nat. Commun. 2024, 15, 6437. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Luo, W.; Yan, L.; Guo, C.; Ding, X.; Gong, X.; Jia, D.; Xu, M.; Ai, L.; Guo, N.; et al. Constructing the quinonyl groups and structural defects in carbon for supercapacitor and capacitive deionization applications. J. Colloid Interface Sci. 2023, 645, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Feng, S.; Wang, L.; Jia, D.; Guo, N.; Xu, M.; Ai, L.; Ma, Q.; Zhang, Q.; Wang, Z. Defect-rich hierarchical porous carbon prepared by homogeneous activation for high performance capacitive deionization. Desalination 2023, 564, 116766. [Google Scholar] [CrossRef]
- Peng, W.; Wang, W.; Qi, M.; Miao, Y.; Huang, Y.; Yu, F. Enhanced capacitive deionization of defect-containing MoS2/graphene composites through introducing appropriate MoS2 defect. Electrochim. Acta 2021, 383, 138363. [Google Scholar] [CrossRef]
- Zhang, D.T.; Tian, W.J.; Chu, M.L.; Zhao, J.; Zou, M.Y.; Jiang, J.F. B-doped graphitic carbon nitride as a capacitive deionization electrode material for the removal of sulfate from mine wastewater. J. Taiwan Inst. Chem. Eng. 2023, 145, 104829. [Google Scholar] [CrossRef]
- Niu, J.; Shao, R.; Liang, J.; Dou, M.; Li, Z.; Huang, Y.; Wang, F. Biomass-derived mesopore-dominant porous carbons with large specific surface area and high defect density as high performance electrode materials for Li-ion batteries and supercapacitors. Nano Energy 2017, 36, 322–330. [Google Scholar] [CrossRef]
- Guo, R.; Lv, C.; Xu, W.; Sun, J.; Zhu, Y.; Yang, X.; Yang, D. Effect of intrinsic defects of carbon materials on the sodium storage performance. Adv. Energy Mater. 2020, 10, 1903652. [Google Scholar] [CrossRef]
- Huo, S.; Song, X.; Zhao, Y.; Ni, W.; Wang, H.; Li, K. Insight into the significant contribution of intrinsic carbon defects for the high-performance capacitive desalination of brackish water. J. Mater. Chem. A 2020, 8, 19927–19937. [Google Scholar] [CrossRef]
- Huo, S.; Zhang, P.; He, M.; Zhang, W.; Liang, B.; Zhang, M.; Wang, H.; Li, K. Sustainable development of ultrathin porous carbon nanosheets with highly accessible defects from biomass waste for high-performance capacitive desalination. Green Chem. 2021, 23, 8554–8565. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Ye, M.; Zhang, X.; Zhang, H.; Wang, G.; Zhang, Y. Hierarchically porous poly (amidoxime)/bacterial cellulose composite aerogel for highly efficient scavenging of heavy metals. J. Colloid Interface Sci. 2021, 600, 752–763. [Google Scholar] [CrossRef]
- Xiao, L.; Lu, H.; Fang, Y.; Sushko, M.L.; Cao, Y.; Ai, X.; Yang, H.; Liu, J. Low-defect and low-porosity hard carbon with high coulombic efficiency and high capacity for practical sodium ion battery anode. Adv. Energy Mater. 2018, 8, 1703238. [Google Scholar] [CrossRef]
- Lombardi, F.; Lodi, A.; Ma, J.; Liu, J.; Slota, M.; Narita, A.; Myers, W.; Müllen, K.; Feng, X.; Bogani, L. Quantum units from the topological engineering of molecular graphenoids. Science 2019, 366, 1107–1110. [Google Scholar] [CrossRef]
- Toh, C.-T.; Zhang, H.; Lin, J.; Mayorov, A.S.; Wang, Y.-P.; Orofeo, C.M.; Ferry, D.B.; Andersen, H.; Kakenov, N.; Guo, Z.; et al. Synthesis and properties of free-standing monolayer amorphous carbon. Nature 2020, 577, 199–203. [Google Scholar] [CrossRef]
- Li, H.; Zou, L.; Pan, L.; Sun, Z. Novel Graphene-Like Electrodes for Capacitive Deionization. Environ. Sci. Technol. 2010, 44, 8692–8697. [Google Scholar] [CrossRef]
- Nie, C.; Pan, L.; Li, H.; Chen, T.; Lu, T.; Sun, Z. Electrophoretic deposition of carbon nanotubes film electrodes for capacitive deionization. J. Electroanal. Chem. 2012, 666, 85–88. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Zheng, X.; Lv, W.; Wang, M.; Yang, Q.-H.; Kang, F. Adsorption of Lead(II) Ions from Aqueous Solution on Low-Temperature Exfoliated Graphene Nanosheets. Langmuir 2011, 27, 7558–7562. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Mao, X.; Zhang, L.; Liu, D.; Song, L.; Yan, X.; Chen, J.; Yang, D.; Zhou, J.; et al. A Directional Synthesis for Topological Defect in Carbon. Chem 2020, 6, 2009–2023. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Q. Nanocarbon for Oxygen Reduction Electrocatalysis: Dopants, Edges, and Defects. Adv. Mater. 2017, 29, 1604103. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Wang, Z.X.; Yu, Y.S.; Hu, Y. Localized Electron Density Regulation Effect for Promoting Solid-Liquid Ion Adsorption to Enhance Areal Capacitance of Micro-Supercapacitors. Small 2023, 19, 2302489. [Google Scholar] [CrossRef]
- Sharma, S.; Joshi, A.; Tomar, A.K.; Sahu, V.; Singh, G.; Sharma, R.K. Vacancies and Edges: Enhancing Supercapacitive Performance Metrics of Electrode Materials. J. Energy Storage 2020, 31, 101614. [Google Scholar] [CrossRef]
- Huo, S.; Nie, W.; Song, X.; Zhang, M.; Wang, H.; Li, K. Synergistic Effect of Doping and Defect Interactions in Carbon for High-performance Capacitive Deionization. Sep. Purif. Technol. 2022, 281, 119807. [Google Scholar] [CrossRef]
- Mitui, N.; Tomita, T.; Oda, H. Removal of Electrolytes from Dilute Aqueous Solutions Using Activated Carbon Electrodes. TANSO 2000, 194, 243–247. [Google Scholar] [CrossRef]
- Hu, C.-C.; Wang, C.-C.; Wu, F.-C.; Tseng, R.-L. Characterization of pistachio shell-derived carbons activated by a combination of KOH and CO2 for electric double-layer capacitors. Electrochim. Acta 2007, 52, 2498–2505. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. A short review of activated carbon assisted electrosorption process: An overview, current stage and future prospects. J. Hazard. Mater. 2009, 170, 552–559. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Jung, D.H. Characteristics of mesoporous activated carbon prepared from oxygen annealed pitch using dry air and its application to capacitive deionization electrode. J. Ind. Eng. Chem. 2024, 137, 195–206. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, J.; Cui, X.; Li, J.; Zhu, Z. Highly mesoporous carbon nanofiber electrodes with ultrahigh specific surface area for efficient capacitive deionization. Carbon 2023, 201, 920–929. [Google Scholar] [CrossRef]
- Wang, G.; Qian, B.; Dong, Q.; Yang, J.; Zhao, Z.; Qiu, J. Highly mesoporous activated carbon electrode for capacitive deionization. Sep. Purif. Technol. 2013, 103, 216–221. [Google Scholar] [CrossRef]
- Li, L.; Zou, L.; Song, H.; Morris, G. Ordered mesoporous carbons synthesized by a modified sol-gel process for electrosorptive removal of sodium chloride. Carbon 2009, 47, 775–781. [Google Scholar] [CrossRef]
- Yang, J.; Zou, L. Recycle of calcium waste into mesoporous carbons as sustainable electrode materials for capacitive deionization. Microporous Mesoporous Mater. 2014, 183, 91–98. [Google Scholar] [CrossRef]
- Liu, T.; Serrano, J.; Elliott, J.; Yang, X.; Cathcart, W.; Wang, Z.; He, Z.; Liu, G. Exceptional capacitive deionization rate and capacity by block copolymer-based porous carbon fibers. Sci. Adv. 2020, 6, eaaz0906. [Google Scholar] [CrossRef]
- Noonan, O.; Liu, Y.; Huang, X.; Yu, C. Layered graphene/mesoporous carbon heterostructures with improved mesopore accessibility for high performance capacitive deionization. J. Mater. Chem. A 2018, 6, 14272–14280. [Google Scholar] [CrossRef]
- Xu, X.; Tan, H.; Wang, Z.; Wang, C.; Pan, L.; Kaneti, Y.V.; Yang, T.; Yamauchi, Y. Extraordinary capacitive deionization performance of highly-ordered mesoporous carbon nano-polyhedra for brackish water desalination. Environ. Sci.-Nano 2019, 6, 981–989. [Google Scholar] [CrossRef]
- Han, L.; Karthikeyan, K.G.; Anderson, M.A.; Gregory, K.B. Exploring the impact of pore size distribution on the performance of carbon electrodes for capacitive deionization. J. Colloid Interface Sci. 2014, 430, 93–99. [Google Scholar] [CrossRef]
- Tsouris, C.; Mayes, R.; Kiggans, J.; Sharma, K.; Yiacoumi, S.; DePaoli, D.; Dai, S. Mesoporous Carbon for Capacitive Deionization of Saline Water. Environ. Sci. Technol. 2011, 45, 10243–10249. [Google Scholar] [CrossRef]
- Porada, S.; Weinstein, L.; Dash, R.; van der Wal, A.; Bryjak, M.; Gogotsi, Y.; Biesheuvel, P.M. Water Desalination Using Capacitive Deionization with Microporous Carbon Electrodes. ACS Appl. Mater. Interfaces 2012, 4, 1194–1199. [Google Scholar] [CrossRef]
- Baroud, T.N.; Giannelis, E.P. Role of Mesopore Structure of Hierarchical Porous Carbons on the Electrosorption Performance of Capacitive Deionization Electrodes. ACS Sustain. Chem. Eng. 2019, 7, 7580–7596. [Google Scholar] [CrossRef]
- Yeh, C.-L.; Hsi, H.-C.; Li, K.-C.; Hou, C.-H. Improved performance in capacitive deionization of activated carbon electrodes with a tunable mesopore and micropore ratio. Desalination 2015, 367, 60–68. [Google Scholar] [CrossRef]
- Wang, S.; Chen, D.; Zhang, Z.-X.; Hu, Y.; Quan, H. Mesopore dominated capacitive deionization of N-doped hierarchically porous carbon for water purification. Sep. Purif. Technol. 2022, 290, 120912. [Google Scholar] [CrossRef]
- Zang, X.; Xue, Y.; Ni, W.; Li, C.; Hu, L.; Zhang, A.; Yang, Z.; Yan, Y.-M. Enhanced Electrosorption Ability of Carbon Nanocages as an Advanced Electrode Material for Capacitive Deionization. ACS Appl. Mater. Interfaces 2020, 12, 2180–2190. [Google Scholar] [CrossRef]
- Wang, S.; Wang, G.; Wu, T.; Zhang, Y.; Zhan, F.; Wang, Y.; Wang, J.; Fu, Y.; Qiu, J. BCN nanosheets templated by g-3N4 for high performance capacitive deionization. J. Mater. Chem. A 2018, 6, 14644–14650. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Xia, D.; Hu, L.; Li, P.; Tan, L.; Wang, Y.; He, C.; Shu, D.; Xie, X. High-performance water desalination of heteroatom nitrogen- and sulfur-codoped open hollow tubular porous carbon electrodes via capacitivedeionization. Environ. Sci.-Nano 2019, 6, 3359–3373. [Google Scholar] [CrossRef]
- Dianbudiyanto, W.; Liu, S. Outstanding performance of capacitive deionization by a hierarchically porous 3D architectural graphene. Desalination 2019, 468, 114069. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Kim, J.H.; Park, H.S. Hierarchically open-porous nitrogen-incorporated carbon polyhedrons derived from metal-organic frameworks for improved CDI performance. Chem. Eng. J. 2020, 382, 122996. [Google Scholar] [CrossRef]
- Zong, M.; Zhang, Y.; Li, K.; Lv, C.; Tian, P.; Zhao, Y.; Liang, B. Zeolitic imidazolate framework-8 derived two-dimensional N-doped amorphous mesoporous carbon nanosheets for efficient capacitive deionization. Electrochim. Acta 2020, 329, 135089. [Google Scholar] [CrossRef]
- Tang, K.; Chang, J.; Cao, H.; Su, C.; Li, Y.; Zhang, Z.; Zhang, Y. Macropore- and Micropore-Dominated Carbon Derived from Poly(vinyl alcohol) and Polyvinylpyrrolidone for Supercapacitor and Capacitive Deionization. ACS Sustain. Chem. Eng. 2017, 5, 11324–11333. [Google Scholar] [CrossRef]
- Cuong, D.V.; Wu, P.-C.; Liu, N.-L.; Hou, C.-H. Hierarchical porous carbon derived from activated biochar as an eco-friendly electrode for the electrosorption of inorganic ions. Sep. Purif. Technol. 2020, 242, 116813. [Google Scholar] [CrossRef]
- Liu, M.; Xu, M.; Xue, Y.; Ni, W.; Huo, S.; Wu, L.; Yang, Z.; Yan, Y.-M. Efficient Capacitive Deionization Using Natural Basswood-Derived, Freestanding, Hierarchically Porous Carbon Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 31260–31270. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, T.; Fang, J.; Shen, J.; Shi, L.; Zhang, D. Enhanced capacitive deionization of saline water using N-doped rod-like porous carbon derived from dual-ligand metal-organic frameworks. Environ. Sci.-Nano 2020, 7, 926–937. [Google Scholar] [CrossRef]
- Xu, X.; Yang, T.; Zhang, Q.; Xia, W.; Ding, Z.; Eid, K.; Abdullah, A.M.; Hossain, S.A.; Zhang, S.; Tang, J.; et al. Ultrahigh capacitive deionization performance by 3D interconnected MOF-derived nitrogen-doped carbon tubes. Chem. Eng. J. 2020, 390, 124493. [Google Scholar] [CrossRef]
- Chao, L.; Liu, Z.; Zhang, G.; Song, X.; Lei, X.; Noyong, M.; Simon, U.; Chang, Z.; Sun, X. Enhancement of capacitive deionization capacity of hierarchical porous carbon. J. Mater. Chem. A 2015, 3, 12730–12737. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, T.; Chen, G.; Shi, L.; Zhang, D. High Salt Removal Capacity of Metal-Organic Gel Derived Porous Carbon for Capacitive Deionization. ACS Sustain. Chem. Eng. 2017, 5, 11637–11644. [Google Scholar] [CrossRef]
- Wang, T.; Chen, C.; Guan, Z.; Tao, T.; Xiao, Q.; Zhu, J. Chemical reduction-induced defect-rich bismuth oxide-reduced graphene oxide anode for high-performance supercapacitors. J. Colloid Interface Sci. 2025, 677, 45–54. [Google Scholar] [CrossRef]
- Dolbin, A.V.; Khlistyuck, M.V.; Esel’son, V.B.; Gavrilko, V.G.; Vinnikov, N.A.; Basnukaeva, R.M.; Maluenda, I.; Maser, W.K.; Benito, A.M. The Effect of the Thermal Reduction Temperature on the Structure and Sorption Capacity of Reduced Graphene Oxide Materials. Appl. Surf. Sci. 2016, 361, 213–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.; Yang, X. Edge-charged functionalized graphene oxide nanoslit for water desalination and ion-sieving. Desalination 2025, 615, 119248. [Google Scholar] [CrossRef]
- Wang, F.; Mao, J. Role of oxygen vacancy inducer for graphene in graphene-containing anodes. Front. Chem. Sci. Eng. 2023, 17, 326–333. [Google Scholar] [CrossRef]
- Zhang, W.; van Dijk, B.; Wu, L.; Maheu, C.; Tudor, V.; Hofmann, J.P.; Jiang, L.; Hetterscheid, D.; Schneider, G. Role of Vacancy Defects and Nitrogen Dopants for the Reduction of Oxygen on Graphene. ACS Catal. 2024, 14, 11065–11075. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, S.; Du, X.; Hong, S.; Zhao, S.; Chen, Y.; Chen, X.; Song, H. Boosting the Electrical Double-Layer Capacitance of Graphene by Self-Doped Defects through Ball-Milling. Adv. Funct. Mater. 2019, 29, 1901127. [Google Scholar] [CrossRef]
- Podlivaev, A.I.; Openov, L.A. Specific features of the formation of defects in fullerene C46. Phys. Solid State 2012, 54, 1507–1513. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Sivanesan, I. A Comprehensive Compilation of Graphene/Fullerene Polymer Nanocomposites for Electrochemical Energy Storage. Polymers 2023, 15, 701. [Google Scholar] [CrossRef]
- Yu, A.; Peng, Z.; Li, Y.; Zhu, L.; Peng, P.; Li, F. Fullerene-Derived Carbon Nanotubes and Their Electrocatalytic Properties in Oxygen Reduction and Zn–Air Batteries. Appl. Mater. Interfaces 2022, 14, 42337–42346. [Google Scholar] [CrossRef]
- Li, N.; Guo, K.; Li, M.; Shao, X.; Du, Z.; Bao, L.; Yu, Z.; Li, X. Fullerene Fragment Restructuring: How Spatial Proximity Shapes Defect-Rich Carbon Electrocatalysts. Am. Chem. Soc. 2023, 145, 24580–24589. [Google Scholar] [CrossRef]
- Oreshkin, A.I.; Bakhtizin, R.Z.; Muzychenko, D.A.; Oreshkin, S.; Petukhov, M.N.; Panov, V. Adsorption of Fluorinated Fullerene Molecules on Metallic and Semiconducting Surfaces. Mosc. Univ. Phys. Bull. 2021, 76, 117–125. [Google Scholar] [CrossRef]
- Uwayid, R.; Diesendruck, C.E.; Suss, M.E. Comparison of Electrosorption Capacity of various carbon electrodes. Environ. Sci.-Wat. Res. 2022, 8, 949–956. [Google Scholar]
- Wang, K.; Zheng, B.; Lee, J.; Schuelke, T.; Jin, H.; Fan, Q.H. Enhanced Capacitance and Desalination Performance with Plasma-Activated Biochar Electrodes. Energy Technol. 2023, 11, 2300043. [Google Scholar] [CrossRef]
- Gao, X.; Omosebi, A.; Landon, J.; Liu, K. Voltage-Based Stabilization of Microporous Carbon Electrodes for Inverted Capacitive Deionization. J. Phys. Chem. C 2018, 122, 1158–1168. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Tewari, S. Capacitive deionization: Processes, materials and state of the technology. J. Electroanal. Chem. 2018, 813, 178–192. [Google Scholar] [CrossRef]
- Gao, K.; Wang, B.; Tao, L.; Cunning, B.V.; Zhang, Z.; Wang, S.; Ruoff, R.S.; Qu, L. Efficient Metal-Free Electrocatalysts from N-Doped Carbon Nanomaterials: Mono-Doping and Co-Doping. Adv. Mater. 2019, 31, 1805121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xi, B.; Huang, M.; Shi, L.; Huang, S.; Guo, N.; Li, D.; Ju, Z.; Xiong, S. Defect-selectivity and “Order-in-Disorder” engineering in carbon for durable and fast potassium storage. Adv. Mater. 2022, 34, 2018621. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, W.; Chen, X.; Peng, Y.; Song, Y.; Lv, C.; Liu, H.; Sun, J.; Yuan, D.; Li, X.; et al. Defect-rich nitrogen doped Co3O4/C porous nanocubes enable high-efficiency bifunctional oxygen electrocatalysis. Adv. Funct. Mater. 2019, 29, 1902875. [Google Scholar] [CrossRef]
- Kim, J.; Choi, M.S.; Shin, K.H.; Kota, M.; Kang, Y.; Lee, S.; Lee, J.Y.; Park, H.S. Rational design of carbon nanomaterials for electrochemical sodium storage and capture. Adv. Mater. 2019, 31, 803444. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Doping of Carbon Materials for Metal-Free Electrocatalysis. Adv. Mater. 2019, 31, 1804672. [Google Scholar] [CrossRef]
- Huo, S.; Ni, W.; Zhao, Y.; Song, X.; Zhao, Y.; Li, K.; Wang, H.; Zhang, M. Highly efficient atomically dispersed Co-N active sites in porous carbon for high-performance capacitive desalination of brackish water. J. Mater. Chem. A 2021, 9, 3066–3076. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, W.; Gu, Y.; Liao, T.; Sun, Z. Metal-Nitrogen-Doped Carbon Materials as Highly Efficient Catalysts: Progress and Rational Design. Adv. Sci. 2020, 7, 2001069. [Google Scholar] [CrossRef]
- Huo, S.; Zhao, Y.; Zong, M.; Liang, B.; Zhang, X.; Khan, I.U.; Li, K. Enhanced supercapacitor and capacitive deionization boosted by constructing inherent N and P external defects in porous carbon framework with a hierarchical porosity. Electrochim. Acta 2020, 353, 136523. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, J.; Han, J.; Yan, T.; Shi, L.; Zhang, D. N, P, S co-doped hollow carbon polyhedra derived from MOF-based core-shell nanocomposites for capacitive deionization. J. Mater. Chem. A 2018, 6, 15245–15252. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Tian, P.; Wang, L.; Li, K.; Lv, C.; Liang, B. Nitrogen-rich mesoporous carbons derived from zeolitic imidazolate framework-8 for efficient capacitive deionizatio. Electrochim. Acta 2019, 321, 134665. [Google Scholar] [CrossRef]
- Feng, B.; Khan, Z.U.; Khan, W.U. 3D graphene-supported N-doped hierarchically porous carbon for capacitive deionization of saline water. Environ. Sci.-Nano 2023, 10, 1163–1176. [Google Scholar] [CrossRef]
- Meng, F.; Ding, Z.; Xu, X.; Liu, Y.; Lu, T.; Pan, L. Metal organic framework-derived nitrogen-doped porous carbon sustained Prussian blue analogues for efficient and fast hybrid capacitive deionization. Sep. Purif. Technol. 2023, 317, 123899. [Google Scholar] [CrossRef]
- Shi, M.; Hong, X.; Liu, C.; Qiang, H.; Wang, F.; Xia, M. Green double organic salt activation strategy for one-step synthesis of N-doped 3D hierarchical porous carbon for capacitive deionization. Chem. Eng. J. 2023, 453, 139764. [Google Scholar] [CrossRef]
- Chen, C.; Liu, A.; Fei, C.; Hui, B.; Li, Y.; Guan, D.; Ju, D. High-performance nitrogen-doped porous carbon electrode materials for capacitive deionization of Industrial salt-contaminated wastewater. Desalination 2023, 565, 116863. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Tu, Y.-H.; Yang, Y.-H.; Wang, J.-A.; Hu, C.-C. Improved performance and long-term stability of activated carbon doped with nitrogen for capacitive deionization. Desalination 2020, 481, 114362. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Tang, J.; Hou, S.; Hossain, S.A.; Pan, L.; Yamauchi, Y. High performance capacitive deionization electrodes based on ultrathin nitrogen-doped carbon/graphene nano-sandwiches. Chem. Commun. 2017, 53, 10784–10787. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Sun, N.; Zhang, X.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Biomass-derived N-doped porous carbon as electrode materials for Zn-air battery powered capacitive deionization. Chem. Eng. J. 2018, 334, 1270–1280. [Google Scholar] [CrossRef]
- Xu, X.T.; Pan, L.K.; Liu, Y.; Lu, T.; Sun, Z. Enhanced capacitive deionization performance of graphene by nitrogen doping. J. Colloid Interface Sci. 2015, 45, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Lu, Y.; Jiang, J.; Chen, X.; Wu, Y.; Zhu, C.; Li, Y. Constructing a high-performance nitrogen-doped three-dimensional framework graphene material for efficient capacitive deionization. Desalination 2024, 576, 117382. [Google Scholar] [CrossRef]
- Qian, M.; Duan, M.; Gong, Z. Nitrogen-doped self-shrinking porous 3D graphene capacitor deionization electrode. Int. J. Energy Res. 2019, 43, 7583–7593. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Liu, T.; Sun, Z.; Chua, D.H.C.; Pan, L. Nitrogen-doped electrospun reduced graphene oxide-carbon nanofiber composite for capacitive deionization. RSC Adv. 2015, 5, 34117–34124. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, H.; Zhu, H.; Ma, Y.; Song, X. Structural and spectral characterizations of mono-nitrogen doped C70 fullerene by soft X-ray spectroscopy. Chem. Phys. 2024, 590, 112523. [Google Scholar] [CrossRef]
- Li, W.; Kuang, X.; Tang, J.; Liu, X.; Lei, H.; Guo, Q.; Yan, X.; Liu, X.; Deng, B.; Chen, H. Self-Assembled Fullerene-Based Materials for Energy-Related Applications. ACS Appl. Energy Mater. 2025, 8, 8756–8780. [Google Scholar] [CrossRef]
- Tian, Z.; Ni, K.; Chen, G.; Zeng, W.; Tao, Z.; Ikram, M.; Zhang, Q.; Wang, H.; Sun, L. Incorporating Pyrrolic and Pyridinic Nitrogen into a Porous Carbon made from C60 Molecules to Obtain Superior Energy Storage. Adv. Mater. 2017, 29, 1603414. [Google Scholar]
- Tian, S.; Wu, J.; Zhang, X.; Ostrikov, K.K.; Zhang, Z. Capacitive deionization with nitrogen-doped highly ordered mesoporous carbon electrodes. Chem. Eng. J. 2020, 380, 122514. [Google Scholar] [CrossRef]
- Gu, X.; Yang, Y.; Hu, Y.; Hu, M.; Huang, J.; Wang, C. Nitrogen-doped graphene composites as efficient electrodes with enhanced capacitive deionization performance. RSC Adv. 2014, 4, 63189–63199. [Google Scholar] [CrossRef]
- Xu, X.; Pan, L.; Liu, Y.; Lu, T.; Sun, Z.; Chua, D.H.C. Facile synthesis of novel graphene sponge for high performance capacitive deionization. Sci. Rep. 2015, 5, 8458. [Google Scholar] [CrossRef]
- Fu, F.; Yang, D.; Zhao, B.; Fan, Y.; Liu, W.; Lou, H.; Qiu, X. Boosting capacitive performance of N, S co-doped hierarchical porous lignin-derived carbon via self-assembly assisted template-coupled activation. J. Colloid Interface Sci. 2023, 640, 698–709. [Google Scholar] [CrossRef]
- Wu, G.; Wang, H.; Huang, L.; Huang, L.; Yan, J.; Chen, X.; Xiao, Y.; Liu, X.; Zhang, H. Gas exfoliation induced N, S-doped porous 2D carbon nanosheets for effective removal of copper ions by capacitive deionization. Desalination 2023, 565, 116881. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Liu, S.; Li, J. Laser-induced nitrogen and phosphorus-doped spongy carbon with graphene wings derived from lignin for significantly enhanced capacitance deionization performance. Sep. Purif. Technol. 2024, 351, 128072. [Google Scholar] [CrossRef]
- Wu, J.; Xuan, X.; Zhang, S.; Li, Z.; Li, H.; Zhao, B.; Ye, H.; Xiao, Z.; Zhao, X.; Xu, X.; et al. N, P-doped carbon nanorings for high-performance capacitive deionization. Chem. Eng. J. 2023, 473, 145421. [Google Scholar] [CrossRef]
- Guo, J.; Xu, X.; Hill, J.P.; Wang, L.; Dang, J.; Kang, Y.; Li, Y.; Guan, W.; Yamauchi, Y. Graphene-carbon 2D heterostructures with hierarchically-porous P, N-doped layered architecture for capacitive deionization. Chem. Sci. 2021, 12, 10334–10340. [Google Scholar] [CrossRef]
- Qiang, H.; Shi, M.; Wang, F.; Xia, M. Biomass-based N/P co-doped hierarchical porous carbon fabricated by a facile dual physico-chemical activation strategy for efficient capacitive deionization. Sep. Purif. Technol. 2024, 333, 125915. [Google Scholar] [CrossRef]
- Ma, X.; Song, X.; Ning, G.; Hou, L.; Kan, Y.; Xiao, Z.; Li, W.; Ma, G.; Gao, J.; Li, Y. S-Doped Porous Graphene Microspheres with Individual Robust Red-Blood-Cell-Like Microarchitecture for Capacitive Energy Storage. Ind. Eng. Chem. Res. 2017, 56, 9524–9532. [Google Scholar] [CrossRef]
- Fu, Q.; Sun, S.; Lu, K.; Li, N.; Dong, Z. Boron-doped carbon dots: Doping strategies, performance effects, and applications. Chin. Chem. Lett. 2024, 35, 109136. [Google Scholar] [CrossRef]
- Yang, L.; Cao, Z.; Chen, D.; Zhang, Z.; Aihemaiti, A.; Gao, X.; Zhu, H.; Yin, J. Boron enriched edge-nitrogen defective carbon network toward high-capacity capacitive deionization. Chem. Eng. J. 2024, 489, 151214. [Google Scholar] [CrossRef]
- Xie, Z.; Shang, X.; Yang, J.; Hu, B.; Nie, P.; Jiang, W.; Liu, J. 3D interconnected boron- and nitrogen-codoped carbon nanosheets decorated with manganese oxides for high-performance capacitive deionization. Carbon 2020, 158, 184–192. [Google Scholar] [CrossRef]
- Cuong, D.V.; Hiep, N.; Hoa, T.T.H.; Nguyen, V.A.; Hou, C.H.; Fan, C.S.; Van Truc, N. Development of a capacitive deionization stack with highly porous oxygen-doped carbon electrodes for brackish water desalination in remote coastal areas. Mater. Chem. Phys. 2023, 307, 128165. [Google Scholar] [CrossRef]
- Fang, Z.; Peng, Y.; Zhou, X.; Zhu, L.; Wang, Y.; Dong, X.; Xia, Y. Fluorinated Carbon Materials and the Applications in Energy Storage Systems. ACS Appl. Energy Mater. 2022, 5, 3966–3978. [Google Scholar] [CrossRef]
- Huo, S.; Zhao, Y.; Zong, M.; Liang, B.; Zhang, X.; Khan, I.; Song, X.; Li, K. Boosting supercapacitor and capacitive deionization performance of hierarchically porous carbon by polar surface and structural engineering. J. Mater. Chem. A 2020, 8, 2505. [Google Scholar] [CrossRef]
- Hu, X.; Min, X.; Wang, H.; Li, X.; He, Y.; Yang, W. Enhanced capacitive deionization boosted by Co and N co-doping in carbon materials. Sep. Purif. Technol. 2021, 266, 118590. [Google Scholar] [CrossRef]
- Gao, T.; Liu, Z.; Li, H. Heteroatom doping modified hierarchical mesoporous carbon derived from ZIF-8 for capacitive deionization with enhanced salt removal rate. Sep. Purif. Technol. 2020, 231, 115918. [Google Scholar] [CrossRef]
- Wang, H.; You, H.; Wu, G.; Huang, L.; Yan, J.; Liu, X.; Ma, Y.; Wu, M.; Zeng, Y.; Yu, J.; et al. Co/Fe co-doped ZIF-8 derived hierarchically porous composites as high-performance electrode materials for Cu2+ ions capacitive deionization. Chem. Eng. J. 2023, 460, 141621. [Google Scholar] [CrossRef]
- Xu, X.; Tang, J.; Kaneti, Y.V.; Tan, H.; Chen, T.; Pan, L.; Yang, T.; Bando, Y.; Yamauchi, Y. Unprecedented capacitive deionization performance of interconnected iron–nitrogen-doped carbon tubes in oxygenated saline water. Mater. Horiz. 2020, 7, 1404–1412. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.H.; Zhang, W.X.; Zhang, M.; Qi, J.W.; Qian, J.S.; Sun, X.Y.; Yuliarto, B.; Na, J.; Park, T.; et al. Nitrogen, phosphorus co-doped eave-like hierarchical porous carbon for efficient capacitive deionization. J. Mater. Chem. A 2021, 9, 12807–12817. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, P.; Wang, Y.; He, M.M.; Zhang, W.Z.; Xu, Z.K.; Li, K.X. Uniformly dispersed Fe-N active centers on hierarchical carbon electrode for high-performance capacitive deionization. ACS Sustain. Chem. Eng. 2023, 11, 8847–8857. [Google Scholar] [CrossRef]
- Martinez, J.; Colán, M.; Castillón, R.; Ramos, P.G.; Paria, R.; Sánchez, L.; Rodríguez, J.M. Fabrication of Activated Carbon Decorated with ZnO Nanorod-Based Electrodes for Desalination of Brackish Water Using Capacitive Deionization Technology. Int. J. Mol. Sci. 2023, 24, 1409. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, Z.R.; Li, X.A.; Qin, D.Y.; Jin, M.Y.; Tong, Y.Z.D.; Li, R. Enhancement of Zn-N-C charge-directed rearrangement for high-performance selectivity of heavy metal ions in capacitive deionization. Desalination 2023, 557, 116597. [Google Scholar] [CrossRef]
- Xu, Y.; Zhong, Z.; Zeng, X.; Zhao, Y.; Deng, Y.; Chen, Y. Novel Materials for Heavy Metal Removal in Capacitive Deionization. Appl. Sci. 2023, 13, 5635. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Yan, L.; Wu, B.C.; Wei, D.; Ouyang, B.X.; Chen, P.; Zhang, T.Z.; Wang, H.Y.; Huang, L. Metal cobalt dot-doped carbon structures with specific exposure surfaces for high-performance capacitive deionization. J. Environ. Chem. Eng. 2024, 12, 112189. [Google Scholar] [CrossRef]










| Defect Type | Electrode Material | Experimental Conditions | Electroadsorption Capacity (mg·g−1) | Ref. | ||
|---|---|---|---|---|---|---|
| Voltage | Concentration (mg·L−1) | Ion Type | ||||
| Topological Defects | Graphene nanoribbons | 1.2 | 500 | NaCl | 10.84 | [95] |
| Edge defects | Plasma-activated biochar | 1.2 | 68 | NaCl | 6.5 | [96] |
| Vacancy defects | Activated carbon | 0.4 | 580 | NaCl | 7.2 | [97] |
| Vacancy defects | N-doped carbon nanosheets | 1.2 | 100 | NaCl | 32 | [32] |
| Vacancy defects | Carbon nanotubes | 1.2 | 500 | NH4Cl | 460 | [98] |
| Doping Element | Electrode Material | Experimental Conditions | Electroadsorption Capacity (mg·g−1) | Ref. | ||
|---|---|---|---|---|---|---|
| Voltage | Concentration (mg·L−1) | Ion Type | ||||
| N | Nitrogen-doped hierarchical porous carbon | 1.2 | 500 | NaCl | 24.17 | [80] |
| N, P | N/P co-doped eave-like hierarchical porous carbon | 1.2 | 500 | NaCl | 24.14 | [143] |
| N, Fe | Fe-N-doped hierarchical carbon | 1.2 | 500 | NaCl | 28.88 | [144] |
| N, Fe | 3D interconnected Fe-N-doped carbon tubes | 1.2 | 500 | NaCl | 40.70 | [142] |
| Zn | ZnO-decorated activated carbon | 1.2 | 23,400 | NaCl | 123.66 | [145] |
| Zn | Single-atom Zn-doped N-doped carbon | 1.2 | 500 | Mn2+ | 280 mg/g | [146] |
| S | S-doped activated carbon | 1.2 | 1000 | Cr(VI) | 90.2 | [147] |
| Co, N | Co-N-doped carbon | 1.2 | 20 | Cr3+ | 15.20 | [148] |
| 1.2 | 40 | Pb2+ | 20.91 | |||
| Aspect | Intrinsic Defects | Extrinsic Defects |
|---|---|---|
| Defect type | topological defects, vacancy/edge defects | Substitutional or interstitial doping with heteroatoms |
| Formation method | High-temperature treatment, chemical activation, plasma etching | Pyrolysis doping, hydrothermal synthesis, electrochemical deposition |
| Effect on structure | Enhances specific surface area and pore volume; introduces lattice irregularities | Alters electronic structure via heteroatom incorporation; may form hybridized networks |
| Surface chemistry | Increases oxygen-containing functional groups (e.g., -COOH, -OH), conducive to ion diffusion | Introduces polar heteroatom groups, conducive to surface wettability and chemical activity |
| Conductivity | May reduce conductivity due to disrupted conjugation | Improves conductivity via charge carrier modulation (e.g., N-doping) |
| Electrosorption performance | High ion adsorption capacity via enhanced double-layer capacitance | Improved adsorption capacity and selectivity through Faradaic reactions |
| Cycle stability | Prone to structural degradation during cycling due to structural collapse | Exhibits better stability due to robust heteroatom-carbon bonds |
| Typical application scenarios | Desalination of high-concentration water (such as seawater desalination) Selective ion separation based on pore size sieving (such as Cs+/K+ separation) | Removal of low-concentration heavy metal ions (such As Cu2+, As3+); Selective adsorption of target ions in complex water quality (such as phosphate recovery) |
| Challenges and limitations | The defect density is difficult to control precisely, which may lead to structural instability; Selectivity depends on physical sieving and has limited ability to distinguish ions of similar sizes | The doping uniformity and stability need to be optimized; excessive doping may reduce the conductivity; Some heteroatom precursors (such as precious metals) are relatively expensive, which limits their large-scale application |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Liu, R.; Fang, J.; Chen, F.; Huo, S. A Review of Modification of Carbon-Based Materials Based on Defect Engineering in Capacitive Deionization. Water 2025, 17, 2478. https://doi.org/10.3390/w17162478
Zhao Y, Liu R, Fang J, Chen F, Huo S. A Review of Modification of Carbon-Based Materials Based on Defect Engineering in Capacitive Deionization. Water. 2025; 17(16):2478. https://doi.org/10.3390/w17162478
Chicago/Turabian StyleZhao, Yubo, Rupeng Liu, Jinfeng Fang, Feiyong Chen, and Silu Huo. 2025. "A Review of Modification of Carbon-Based Materials Based on Defect Engineering in Capacitive Deionization" Water 17, no. 16: 2478. https://doi.org/10.3390/w17162478
APA StyleZhao, Y., Liu, R., Fang, J., Chen, F., & Huo, S. (2025). A Review of Modification of Carbon-Based Materials Based on Defect Engineering in Capacitive Deionization. Water, 17(16), 2478. https://doi.org/10.3390/w17162478






