Bioremediation of Bacteria in Constructed Wetlands: Role of Endophytic and Rhizosphere Fungi
Abstract
1. Background
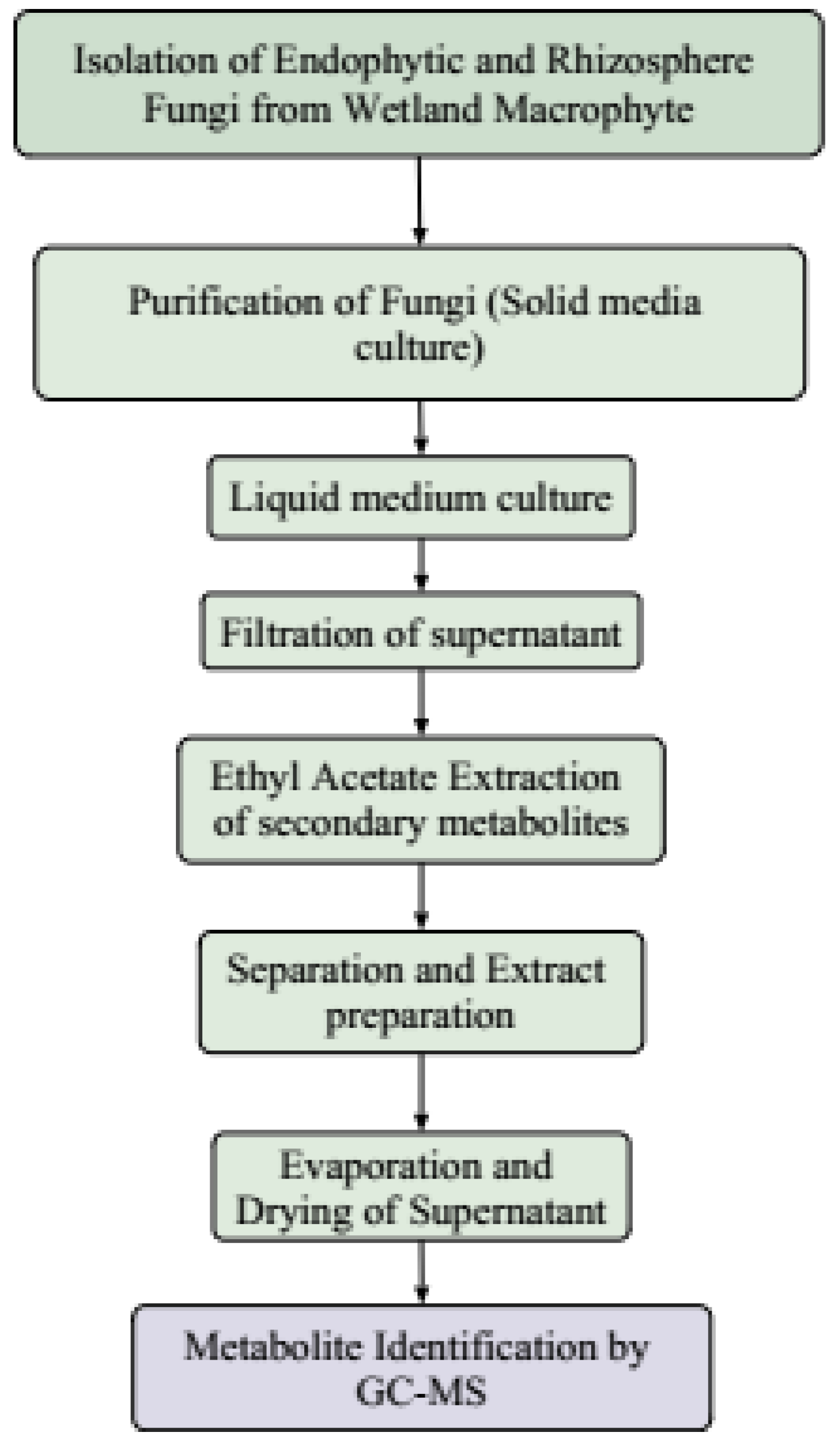
2. Materials and Methods
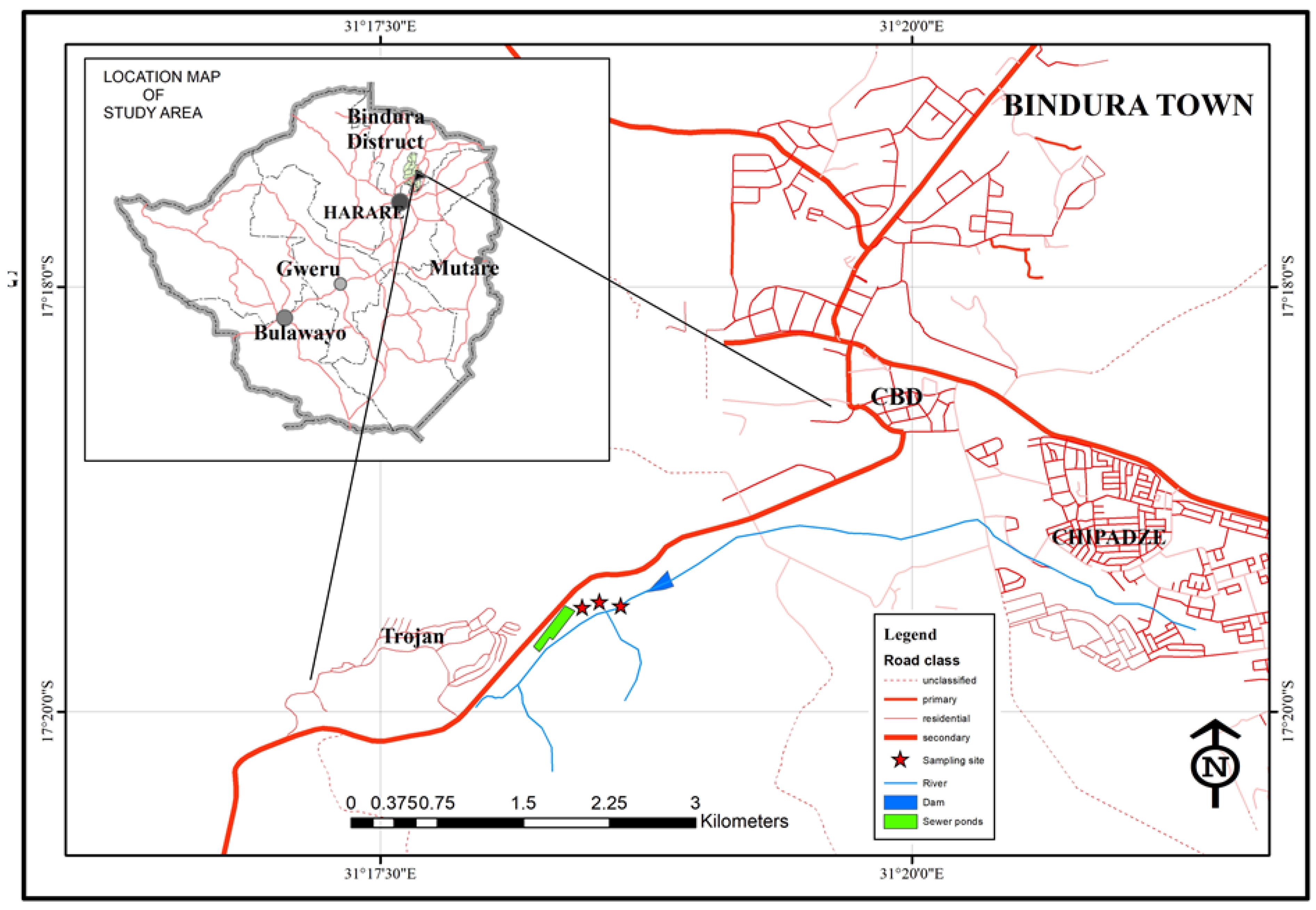
2.1. Study Area
2.2. Endophytic and Rhizosphere Fungi in Macrophytes T. latifolia, C. papyrus and P. mauritianus and Their Antimicrobial Activity
2.2.1. Collection of Wetland Macrophytes
2.2.2. Isolation of Endophytic and Rhizosphere Fungi
2.2.3. Isolation of Fungi from Macrophyte Rhizosphere
2.2.4. Preliminary Screening of Endophytic and Rhizosphere Fungi for Antimicrobial Activity
2.3. Antimicrobial Activity of Endophytic and Rhizosphere Fungi Extracts
2.3.1. Preparation of Ethyl Acetate Extracts by Fermentation of Endophytic and Rhizosphere Fungi
2.3.2. Antimicrobial Activity of Fungal Crude Extracts
2.3.3. Identification of Secondary Metabolites Produced by Endophytic Fungi
2.3.4. Data Analysis
3. Results
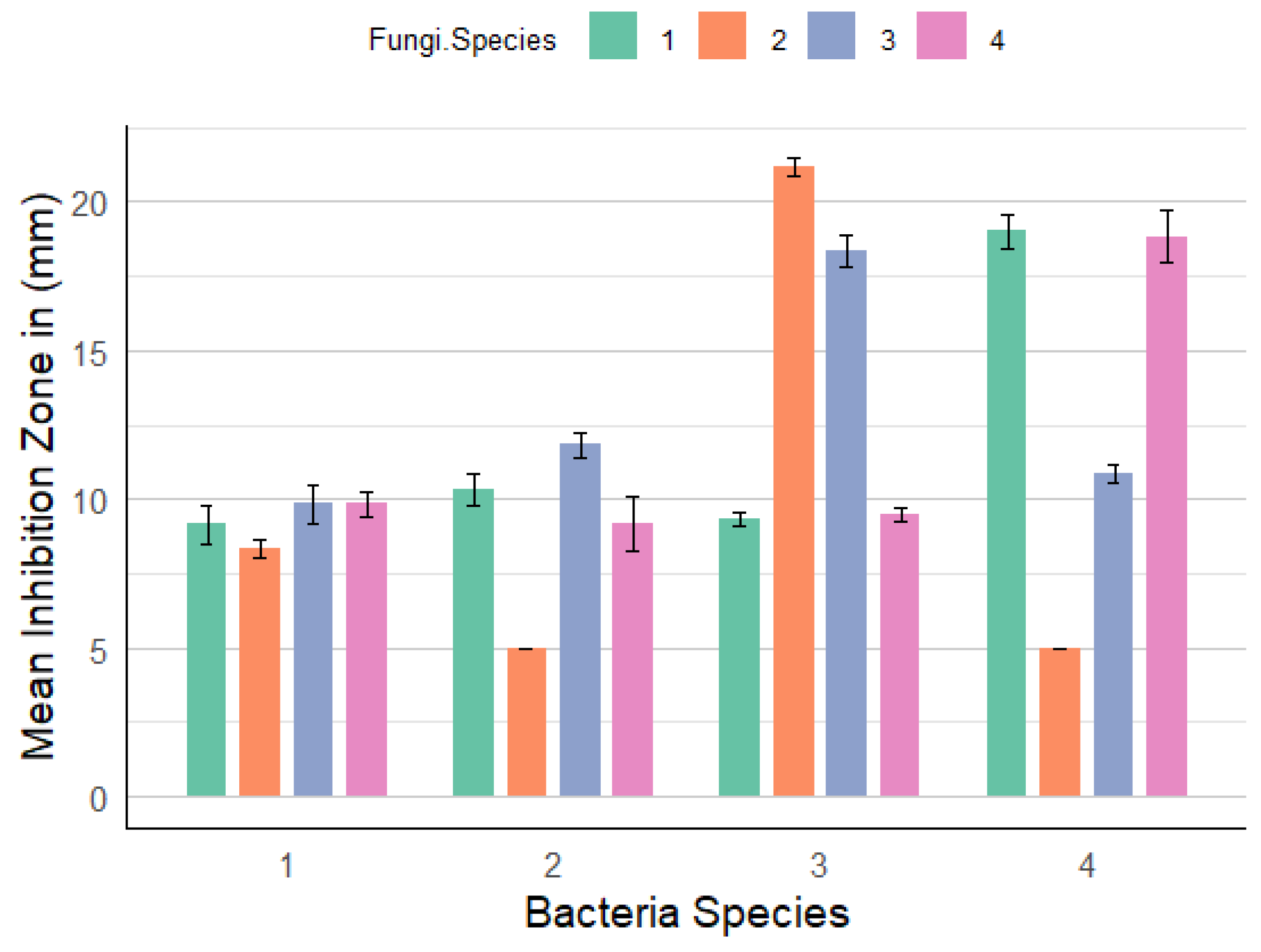
3.1. Antimicrobial Activity of Endophytic and Rhizosphere Fungi Agar Plug Tests
3.2. Antimicrobial Activity of Secondary Metabolites of Endophytic and Rhizospheric Fungi
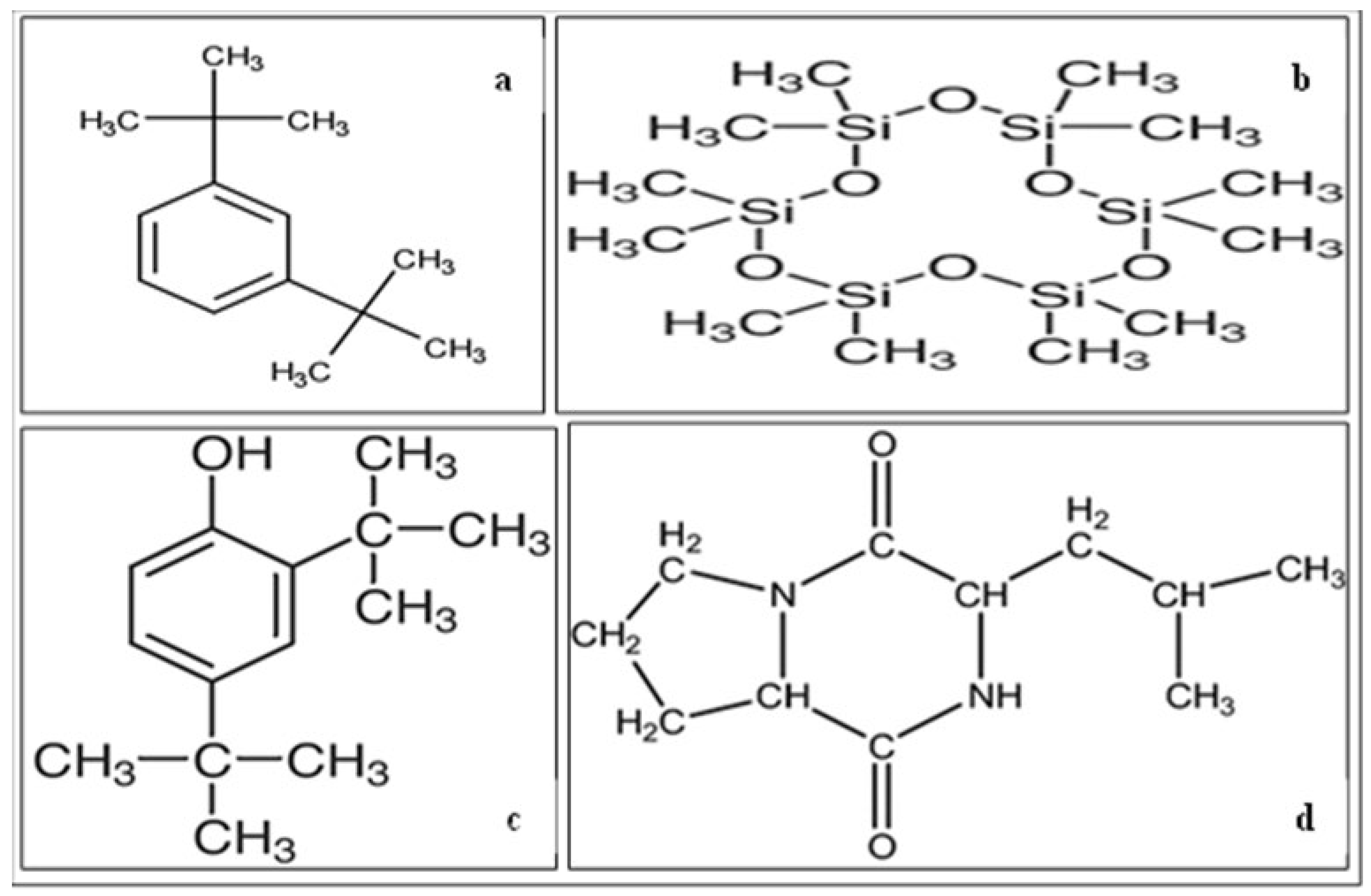
3.3. Identification of Secondary Metabolites Produced by Endophytic and Rhizosphere Fungi
4. Discussion of Results
5. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, A.; Nag, P. Global trends of population dynamics oncoming 2050. Popul. Geogr. 2024, 46, 1–26. [Google Scholar]
- David, G.; Rana, M.S.; Saxena, S.; Sharma, S.; Pant, D.; Prajapati, S.K. A review on design, operation, and maintenance of constructed wetlands for removal of nutrients and emerging contaminants. Int. J. Environ. Sci. Technol. 2023, 20, 9249–9270. [Google Scholar] [CrossRef]
- Tibebu, S.; Worku, A.; Angassa, K. Removal of Pathogens from Domestic Wastewater Using Small-Scale Gradual Hydroponics Planted with Duranta erecta, Addis Ababa, Ethiopia. J. Environ. Public Health 2022, 2022, 3182996. [Google Scholar] [CrossRef] [PubMed]
- Almuktar, S.A.A.A.N.; Abed, S.N.; Scholz, M. Wetlands for wastewater treatment and subsequent recycling of treated effluent: A review. Environ. Sci. Pollut. Res. 2018, 25, 23595–23623. [Google Scholar] [CrossRef]
- Hussain, I.; Lu, X.; Hussain, J. Nutrients Removal Efficiency Assessment of Constructed Wetland for the Rural Domestic Wastewater Growing Distinct Species of Vegetation. J. Environ. Anal. Toxicol. 2018, 8, 5. [Google Scholar] [CrossRef]
- Stefanakis, A.I.; Akratos, C.S. Removal of pathogenic bacteria in constructed wetlands: Mechanisms and efficiency. In Phytoremediation Management of Environmental Contaminants; Ansari, A.A., Gill, S.S., Gill, R., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 327–346. [Google Scholar]
- Garatsa, C.; Mohammadnezhad, M.; Kostrzynska, E.B.; Nwankwo, B.; Hagan, V.M. Risk Factors of Diarrhoea Among Under-Five Children in Zimbabwe: A Systematic Review. Am. J. Biomed. Sci. Res. 2023, 19, 334–344. [Google Scholar] [CrossRef]
- Badejo, A.A.; Omole, D.O.; Ndambuki, J.M. Municipal wastewater management using Vetiveria zizanioides planted in vertical flow constructed wetland. Appl. Water Sci. 2018, 8, 110. [Google Scholar] [CrossRef]
- Kumar, S.; Pratap, B.; Dubey, D.; Dutta, V. Microbial Communities in Constructed Wetland Microcosms and Their Role in Treatment of Domestic Wastewater. In Emerging Eco-Friendly Green Technologies for Wastewater Treatment, Microorganisms for Sustainability, 18th ed.; Bharagava, R.N., Ed.; Springer: Singapore, 2020; Volume 18, ISBN 978-981-15-1389-3. [Google Scholar]
- Rahman, E.; Izuan, M.; Halmi, B.; Yuso, M.; Samad, A.; Uddin, K.; Mahmud, K.; Yunus, M.; Shukor, A. Design, Operation and Optimization of Constructed Wetland for Removal of Pollutant. Int. J. Environ. Res. Public Health 2020, 17, 8339. [Google Scholar] [CrossRef]
- Alufasi, R.; Parawira, W.; Stefanakis, A.I.; Lebea, P.; Chakauya, E.; Chingwaru, W. Internalisation of Salmonella spp. by Typha latifolia and Cyperus papyrus in vitro and implications for pathogen removal in Constructed Wetlands. Environ. Technol. 2020, 43, 949–961. [Google Scholar] [CrossRef]
- Dhir, B. Effective control of waterborne pathogens by aquatic plants. In Waterborne Pathogens; Prasad, M.V.N., Grobelak, A., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 339–361. [Google Scholar]
- Calheiros, C.S.C.; Ferreira, V.; Magalhães, R.; Teixeira, P.; Castro, P.M.L. Presence of microbial pathogens and genetic diversity of Listeria monocytogenes in a constructed wetland system. Ecol. Eng. 2017, 102, 344–351. [Google Scholar] [CrossRef]
- Mashe, T.; Leekitcharoenphon, P.; Mtapuri-Zinyowera, S.; Kingsley, R.A.; Robertson, V.; Tarupiwa, A.; Kock, M.M.; Makombe, E.P.; Chaibva, B.V.; Manangazira, P.; et al. Salmonella enterica serovar Typhi H58 clone has been endemic in Zimbabwe from 2012 to 2019. J. Antimicrob. Chemother. 2021, 76, 1160–1167. [Google Scholar] [CrossRef]
- Cuneo, C.N.; Sollom, R.; Beyrer, C. The cholera epidemic in Zimbabwe, 2008–2009: A review and critique of the evidence. Health Hum. Rights 2017, 19, 249–264. [Google Scholar]
- Dalu, T.; Barson, M.; Nhiwatiwa, T. Impact of intestinal microorganisms and protozoan parasites on drinking water quality in Harare, Zimbabwe. J. Water Sanit. Hyg. Dev. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Gufe, C.; Ndlovu, M.N.; Sibanda, Z.; Makuvara, Z.; Marumure, J. Prevalence and antimicrobial profile of potentially pathogenic bacteria isolated from abattoir effluents in Bulawayo, Zimbabwe. Sci. Afr. 2021, 14, e01059. [Google Scholar] [CrossRef]
- Cheung, S.; Zhou, N.A.; Ruhanya, V.; Jesser, K.J.; Nezomba, I.; Musvibe, J.; Manyisa, B.; Nyandoro, G.; Chibukira, P.; Mukaratirwa, A.; et al. Characterisation of enteric pathogens in Harare, Zimbabwe using environmental surveillance and metagenomics. J. Water Health 2025, 23, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Ugboko, H.U.; Nwinyi, O.C.; Oranusi, S.U.; Oyewale, J.O. Childhood diarrhoeal diseases in developing countries. Heliyon 2020, 6, e03690. [Google Scholar] [CrossRef]
- Norman, G.; Pedley, S.; Takkouche, B. Effects of sewerage on diarrhoea and enteric infections: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 536–544. [Google Scholar] [CrossRef]
- Wu, S.; Lyu, T.; Zhao, Y.; Vymazal, J.; Arias, C.A.; Brix, H. Rethinking intensification of con- structed wetlands as a green eco-technology for wastewater treatment. Environ. Sci. Technol. 2018, 52, 1693–1694. [Google Scholar] [CrossRef]
- Hassan, I.; Chowdhury, S.R.; Prihartato, P.K.; Razzak, S.A. Wastewater Treatment Using Constructed Wetland: Current Trends and Future Potential. Processes 2021, 9, 1917. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef]
- Vymazal, J. The Historical Development of Constructed Wetlands for Wastewater Treatment. Land 2022, 11, 174. [Google Scholar] [CrossRef]
- Ramírez-vargas, C.A.; Arias, C.A.; Carvalho, P.; Zhang, L.; Esteve-núñez, A.; Brix, H. Electroactive biofilm-based constructed wetland (EABB-CW): A mesocosm-scale test of an innovative setup for wastewater treatment. Sci. Total Environ. 2019, 659, 796–806. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed wetlands for treatment of industrial wastewaters: A review. Ecol. Eng. 2014, 73, 724–751. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Ahmad, A.; Said, N.S.M.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Purwanti, I.F.; Hasan, H.A. Macrophytes as wastewater treatment agents: Nutrient uptake and potential of produced biomass utilization toward circular economy initiatives. Sci. Total Environ. 2021, 790, 148219. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Stottmeister, U.; Wießner, A.; Kuschk, P.; Kappelmeyer, U.; Kästner, M.; Bederski, O.; Müller, R.A.; Moormann, H. Effects of Plants and Microorganisms in Constructed Wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Alufasi, R.; Parawira, W.; Zvidzai, C.J.; Stefanakis, A.I.; Musili, N.; Lebea, P.; Chakauya, E.; Chingwaru, W. Performance Evaluation of a Pilot-Scale Constructed Wetland with Typha latifolia for Remediation of Domestic Wastewater in Zimbabwe. Water 2024, 16, 2843. [Google Scholar] [CrossRef]
- Hamad, M.T.M.H. Comparative study on the performance of Typha latifolia and Cyperus Papyrus on the removal of heavy metals and enteric bacteria from wastewater by surface constructed wetlands. Chemosphere 2020, 260, 127551. [Google Scholar] [CrossRef]
- Giácoman-Vallejos, G.; Ponce-Caballero, C.; Champagne, P. Pathogen removal from domestic and swine wastewater by experimental constructed wetlands. Water Sci. Technol. 2015, 71, 1263–1270. [Google Scholar] [CrossRef]
- Kipasika, H.J.; Buza, J.; Smith, W.A.; Njau, K.N. Removal capacity of faecal pathogens from wastewater by four wetland vegetation: Typha latifolia, Cyperus papyrus, Cyperus alternifolius and Phragmites australis. Afr. J. Microbiol. Res. 2016, 10, 654–661. [Google Scholar] [CrossRef]
- Hamad, M.T.M.H. Comparing the performance of Cyperus papyrus and Typha domingensis for the removal of heavy metals, roxithromycin, levofloxacin and pathogenic bacteria from wastewater. Environ. Sci. Eur. 2023, 35, 61. [Google Scholar] [CrossRef]
- Martin, L.; Théophile, F.; Etienne, P.T.; Akoa, A. Removal of faecal bacteria and nutrients from domestic wastewater in a horizontal surface flow wetland vegetated with Echinochloa pyramidalis. Afr. J. Environ. Sci. Technol. 2012, 6, 337–345. [Google Scholar] [CrossRef]
- Kaevska, M.; Lvoncik, S.; Slana, I.; Kulich, P.; Kralik, P. Microscopy, Culture, and Quantitative Real-Time PCR Examination Confirm Internalization of Mycobacteria in Plants. Appl. Environ. Microbiol. 2014, 80, 3888–3894. [Google Scholar] [CrossRef]
- Hirneisen, K.A.; Sharma, M.; Kniel, K.E. Human Enteric Pathogen Internalization by Root Uptake into Food Crops. Foodborne Pathog. Dis. 2012, 9, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; van Iersel, M.W.; Chen, J.; Brackett, R.E.; Beuchat, L.R. Evidence of Association of Salmonellae with Tomato Plants Grown Hydroponically in Inoculated Nutrient Solution. Appl. Environ. Microbiol. 2002, 68, 3639–3643. [Google Scholar] [CrossRef] [PubMed]
- Haichar, Z.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions below ground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Jayatilake, P.L.; Munasinghe, H. Antimicrobial Activity of Cultivable Endophytic and Rhizosphere Fungi Associated with “Mile-a-Minute,” Mikania cordata (Asteraceae). BioMed Res. Int. 2020, 2020, 5292571. [Google Scholar] [CrossRef]
- Liang, H.; Xing, Y.; Chen, J.; Zhang, D.; Guo, S.; Wang, C. Antimicrobial activities of endophytic fungi isolated from Ophiopogon japonicus (Liliaceae). BMC Complement. Altern. Med. 2012, 12, 238. [Google Scholar] [CrossRef]
- Aljuraifani, A.; Aldosary, S.; Ababutain, I. In Vitro Antimicrobial Activity of Endophytes, Isolated from Moringa peregrina Growing in Eastern Region of Saudi Arabia. Natl. Acad. Sci. Lett. 2019, 42, 75–80. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Mao, Z.; Ling, J.; Yang, Y.; Li, Y.; Xie, B. Bioactive secondary metabolites from Trichoderma spp. Against phytopathogenic bacteria and root-knot nematode. Microorganisms 2020, 8, 401. [Google Scholar] [CrossRef]
- Sundar, R.D.V.; Arunachalam, S. 2,4-Di-tert-butylphenol from Endophytic Fungi Fusarium oxysporum attenuates the growth of multidrug-resistant pathogens. Front. Microbiol. 2025, 16, 1575021. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.-M.; Chen, J.; Cui, J.-L.; Chen, X.-M.; Guo, S.-X. Antimicrobial Activity and Biodiversity of Endophytic Fungi in Dendrobium devonianum and Dendrobium thyrsiflorum from Vietman. Curr. Microbiol. 2011, 62, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Marcellano, J.P.; Collanto, A.S.; Fuentes, R.G. Antibacterial Activity of Endophytic Fungi Isolated from the Bark of Cinnamomum mercadoi. Pharmacogn. J. 2017, 9, 405–409. [Google Scholar] [CrossRef]
- Beiranvand, M.; Amin, M.; Hashemi-shahraki, A.; Romani, B.; Yaghoubi, S.; Sadeghi, P. Antimicrobial activity of endophytic bacterial populations isolated from medical plants of Iran. Iran. J. Microbiol. 2017, 9, 11–18. [Google Scholar]
- dos Reis, J.B.A.; Lorenzi, A.S.; do Vale, H.M.M. Methods used for the study of endophytic fungi: A review on methodologies and challenges, and associated tips. Arch. Microbiol. 2022, 204, 675. [Google Scholar] [CrossRef]
- Fan, S.; Miao, L.; Li, H.; Lin, A.; Song, F.; Zhang, P. Illumina-based analysis yields new insights into the diversity and composition of endophytic fungi in cultivated Huperzia serrata. PLoS ONE 2020, 15, e0242258. [Google Scholar] [CrossRef]
- Wang, L.; Ren, L.; Li, C.; Gao, C.; Liu, X.; Wang, M.; Luo, Y. Effects of endophytic fungi diversity in different coniferous species on the colonization of Sirex noctilio (Hymenoptera: Siricidae). Sci. Rep. 2019, 9, 5077. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptability test protocol. Am. Soc. Microbiol. 2009, 15, 1–23. [Google Scholar]
- Biemer, J.J. Antimicrobial susceptibility testing by the Kirby-Bauer Disc Diffusion Method. Ann. Clin. Lab. Sci. 1973, 3, 135–140. [Google Scholar]
- Kumari, N.; Menghani, E. Evaluation of antibacterial activity and identification of bioactive metabolites by GCMS technique from rhizospheric Actinomycetes. Indian J. Nat. Prod. Resour. 2020, 11, 287–294. [Google Scholar] [CrossRef]
- Roma, K.; Kiran, S.; Sahoo, D. Extraction and screening of bioactive compounds of some common hydrophytic and wetland plants from East Singbhum, Jharkhand, India. IOSR J. Pharm. 2017, 7, 23–29. [Google Scholar]
- Sharma, D.; Pramanik, A.; Agrawal, P.K. Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D.Don. 3 Biotech 2016, 6, 210. [Google Scholar] [CrossRef]
- Malhadas, C.; Malheiro, R.; Pereira, J.A.; de Pinho, P.G.; Baptista, P. Antimicrobial activity of endophytic fungi from olive tree leaves. World J. Microbiol. Biotechnol. 2017, 33, 46. [Google Scholar] [CrossRef] [PubMed]
- Techaoei, S.; Jirayuthcharoenkul, C.; Jarmkom, K.; Dumrongphuttidecha, T.; Khobjai, W. Chemical evaluation and antibacterial activity of novel bioactive compounds from endophytic fungi in Nelumbo nucifera. Saudi J. Biol. Sci. 2020, 27, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Gagan, S.L.; Shivanna, M.B. Diversity and Antibacterial Activity of Endophytic Fungi In Memecylon umbellatum Burm. A Medicinal Plant in the Western Ghats of Karnataka, India. Indian J. Ecol. 2020, 47, 171–180. [Google Scholar]
- dos Santos, I.P.; da Silva, L.C.N.; da Silva, M.V.; de Araújo, J.M.; da Silva Cavalcanti, C.M.; de Menezes Lima, V.L. Antibacterial activity of endophytic fungi from leaves of Indigofera suffruticosa Miller (Fabaceae). Front. Microbiol 2015, 6, 350. [Google Scholar] [CrossRef]
- Begum, I.F.; Mohankumar, R.; Jeevan, M.; Ramani, K. GC MS Analysis of Bio-active Molecules derived from Paracoccus pantotrophus FMR19 and the Antimicrobial Activity Against Bacterial Pathogens and MDROs. Indian J. Microbiol. 2016, 56, 426–432. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, P.; Lucardi, R. Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef]
- Seenivasan, A.; Manikkam, R.; Kaari, M.; Sahu, A.K.; Said, M.; Dastager, S.G. 2,4-Di-tert-butylphenol (2,4-DTBP) purified from Streptomyces sp. KCA1 from Phyllanthus niruri: Isolation, characterization, antibacterial and anticancer properties. J. King Saud Univ.-Sci. 2022, 34, 102088. [Google Scholar] [CrossRef]
- Rouvier, F.; Abou, L.; Wafo, E.; Andre, P.; Cheyrol, J.; Khacef, M.; Nappez, C.; Lepidi, H.; Brunel, J.M. Identification of 2,4-Di-tert-Butylphenol as an Antimicrobial Agent Against Cutibacterium acnes Bacteria from Rwandan Propolis. Antibiotics 2024, 13, 1080. [Google Scholar] [CrossRef]
- Mishra, R.; Kushveer, J.S.; Khan, M.I.K.; Pagal, S.; Meena, C.K.; Murali, A.; Dhayalan, A.; Sarma, V.V. 2,4-Di-Tert-Butylphenol Isolated from an Endophytic Fungus, Daldinia eschscholtzii, Reduces Virulence and Quorum Sensing in Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 1668. [Google Scholar] [CrossRef]
- Putra, M.Y.; Karim, F. Antibacterial and antioxidant activity-guided isolation studies on Fusarium sp. associated with the ascidian Botryllus schlosseri. In Proceedings of the 8th International Conference of the Indonesian Chemical Society (ICICS 2019), Jakarta, Indonesia, 6–13 June 2019. [Google Scholar]
- Singh, V.K.; Mishra, A.; Jha, B. 3-Benzyl-Hexahydro-Pyrrolo[1,2-a]Pyrazine-1,4-Dione Extracted From Exiguobacterium indicum Showed Antibiofilm Activity Against Pseudomonas aeruginosa by Attenuating Quorum Sensing. Front. Microbiol. 2019, 10, 1269. [Google Scholar] [CrossRef]
- Yang, L.; Wang, R.; Lin, W.; Li, B.; Jin, T.; Weng, Q.; Zhang, M. Efficacy of 2,4-Di-tert-butylphenol in Reducing Ralstoniasolanacearum Vilulence: Insights into the Underlying Mechanismisms. ACS Omega 2024, 9, 4647–4655. [Google Scholar] [CrossRef]

| Bacterial Species | Fungal Species | Mean ± Standard Deviation |
|---|---|---|
| E. coli | RTGS | 9.1667 ± 1.6021 |
| RTGRS | 8.3333 ± 0.8165 | |
| LB | 9.8333 ± 1.6021 | |
| RHCP | 9.8333 ± 0.9832 | |
| Shigella spp. | RTGS | 10.3333 ± 1.3663 |
| RTGRS | 5.0000 ± 0.0000 | |
| LB | 11.8333 ± 0.9832 | |
| RHCP | 9.1667 ± 2.2286 | |
| Salmonella spp. | RTGS | 9.3333 ± 0.5164 |
| RTGRS | 21.1667 ± 0.7528 | |
| LB | 18.3333 ± 1.3663 | |
| RHCP | 9.5000 ± 0.5477 | |
| Vibrio spp. | RTGS | 19.0000 ± 1.8708 |
| RTGRS | 5.0000 ± 0.0000 | |
| LB | 10.8333 ± 0.7528 | |
| RHCP | 18.8333 ± 2.1370 |
| Degree of Freedom | Sum of Squares | Mean Sum of Squares | F Value | p-Value | |
|---|---|---|---|---|---|
| Fungal species | 3 | 105.3 | 35.09 | 22.73 | <0.0001 |
| Bacterial species | 3 | 572.7 | 190.90 | 123.66 | <0.0001 |
| Fungal species/bacterial species ratio | 9 | 1551.7 | 172.41 | 111.68 | <0.0001 |
| Residuals | 80 | 123.5 | 1.54 |
| Compound | Component Retention Time | Area % | Chemical Formula |
|---|---|---|---|
| 2,5-Hexanediol, 2,5-dimethyl | 3.9830 | 90.5–92.2 | C8H18O2 |
| Undecane | 4.2214 | 91.6–96.7 | C11H24 |
| Dodecane | 4.9290 | 95.5–96.7 | C12H26 |
| Benzene, 1, 3-bis(1,1-dimethylethyl) | 5.4270 | 91.3–95.9 | C14H22 |
| Cyclohexasiloxane, dodecamethyl | 5.9361 | 92.1–94.7 | C12H36O6Si6 |
| Cyclopentasiloxane, decamethyl- | 5.5945 | 92.1–94.7 | C10H30O5Si5 |
| Tetradecane | 6.7384 | 90.1–93.9 | C14H30 |
| 2,4-Di-tert-butylphenol | 8.1923 | 95.7–99.0 | C14H22O |
| Hexadecane | 9.5273 | 90.6–92.7 | C16H34 |
| Octadecane | 13.2007 | 90.1–90,2 | C18H38 |
| Decane, 3,7-dimethyl | 13.2008 | 60.2 | |
| 1-Iodo-2-methylundecane | 13.2008 | 90.7 | C12H25I |
| Undecane, 3,8-dimethyl- | 13.2008 | 91.4 | C13H28 |
| Pyrrolo[1,2-a]pyrazine-1,4-dione, hexhyro-3-(2-methylpropyl)- | 15.9242 | 91.0–93.5 | C11H18N2O2 |
| Hexadecanoic acid, methyl ester | 5.8292 | 90.5 | |
| Methyl stearate | 20.3415 | 94.5–96.1 | C19H38O2 |
| Isopropyl Alcohol | 21.3250 | 90.2 | C3H8O |
| Benzyl butyl phthalate | 23.3805 | 92.0–95.3 | C19H20O4 |
| Hexadecanamide | 21.2174 | 92.5–92.8 | C16H33NO |
| Benzyl butyl phthalate | 23.3561 | 91.1–92.7 | C19H20O4 |
| 9-Octadecenamide, (Z) | 23.4420 | 90.8–96.8 | C18H35NO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alufasi, R.; Chingwaru, W.; Zvidzai, C.J.; Musili, N.; Chakauya, E.; Lebea, P.; Goredema, M.; Zhou, R.; Stefanakis, A.I.; Parawira, W. Bioremediation of Bacteria in Constructed Wetlands: Role of Endophytic and Rhizosphere Fungi. Water 2025, 17, 2468. https://doi.org/10.3390/w17162468
Alufasi R, Chingwaru W, Zvidzai CJ, Musili N, Chakauya E, Lebea P, Goredema M, Zhou R, Stefanakis AI, Parawira W. Bioremediation of Bacteria in Constructed Wetlands: Role of Endophytic and Rhizosphere Fungi. Water. 2025; 17(16):2468. https://doi.org/10.3390/w17162468
Chicago/Turabian StyleAlufasi, Richwell, Walter Chingwaru, Cuthbert J. Zvidzai, Nancy Musili, Ereck Chakauya, Phiyani Lebea, Marvelous Goredema, Rudo Zhou, Alexandros I. Stefanakis, and Wilson Parawira. 2025. "Bioremediation of Bacteria in Constructed Wetlands: Role of Endophytic and Rhizosphere Fungi" Water 17, no. 16: 2468. https://doi.org/10.3390/w17162468
APA StyleAlufasi, R., Chingwaru, W., Zvidzai, C. J., Musili, N., Chakauya, E., Lebea, P., Goredema, M., Zhou, R., Stefanakis, A. I., & Parawira, W. (2025). Bioremediation of Bacteria in Constructed Wetlands: Role of Endophytic and Rhizosphere Fungi. Water, 17(16), 2468. https://doi.org/10.3390/w17162468








