Investigating the Relationship Between Microcystin Concentrations and Water Quality Parameters in Three Agricultural Irrigation Ponds Using Random Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Monitoring of Agricultural Ponds
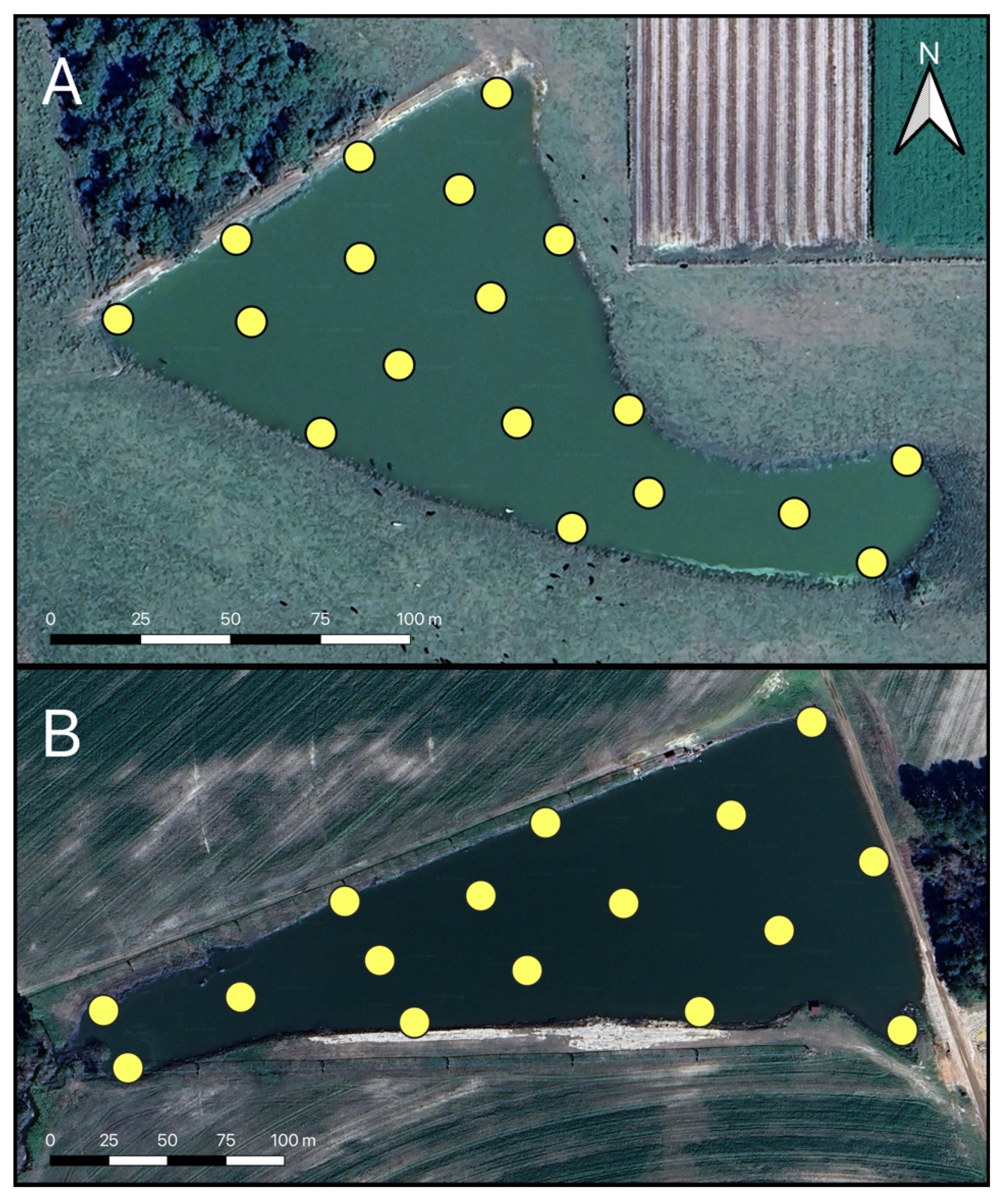
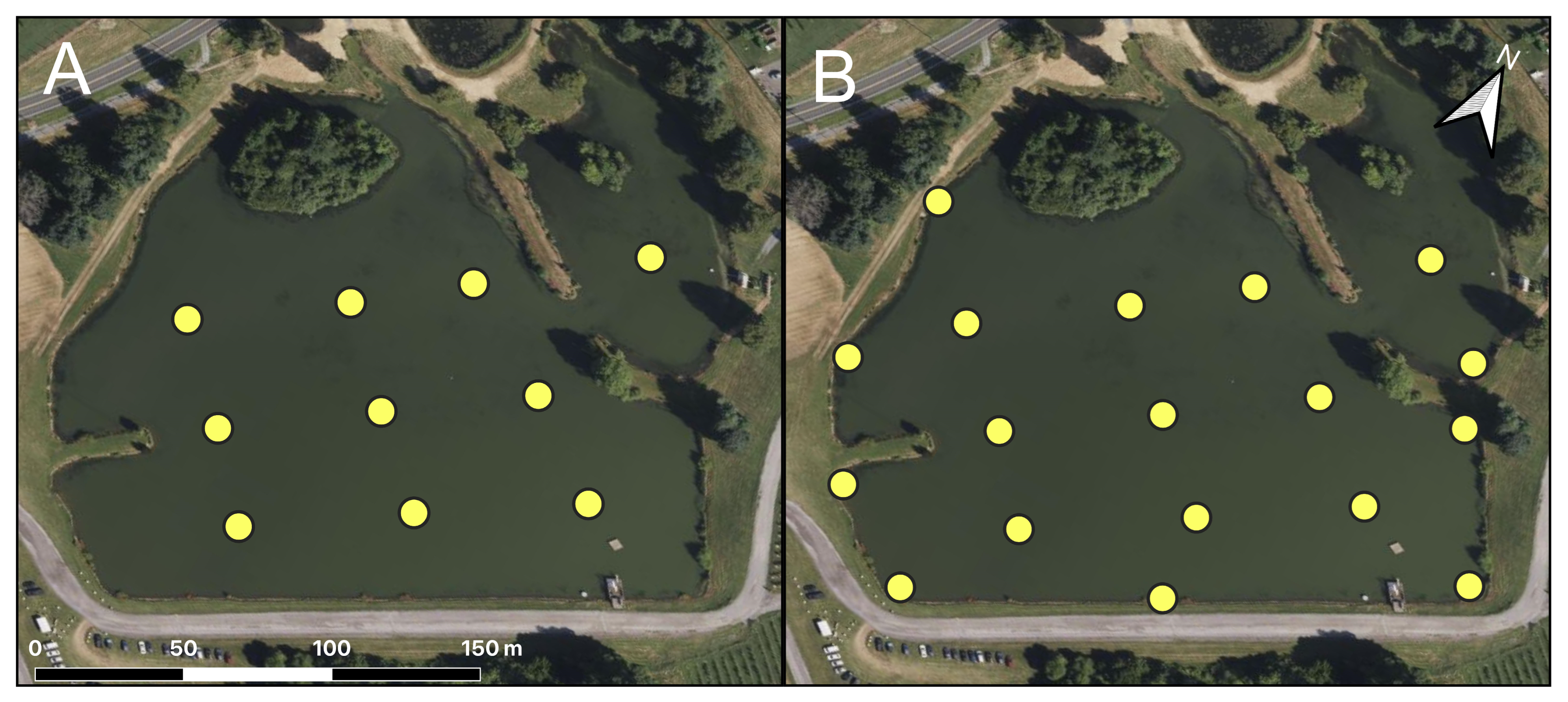
2.1.1. Georgia Field Sites
2.1.2. Maryland Field Sites
2.2. In Situ Measurements
2.3. Microcystin Measurements
2.4. Machine Learning Algorithms and Performance Metrics
2.5. Data Processing, Software, and Statistics
3. Results
3.1. Summary of Monitoring Data
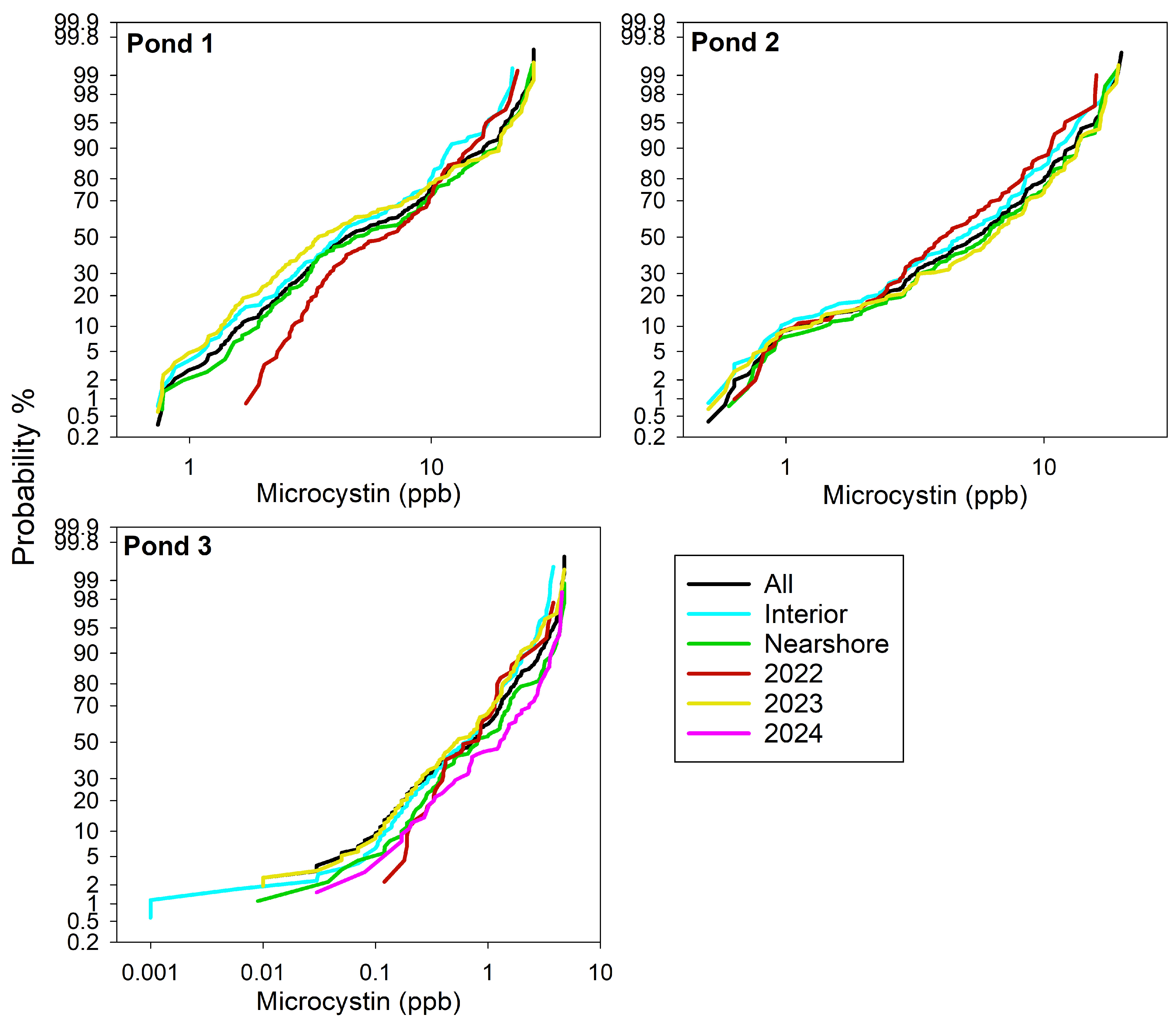
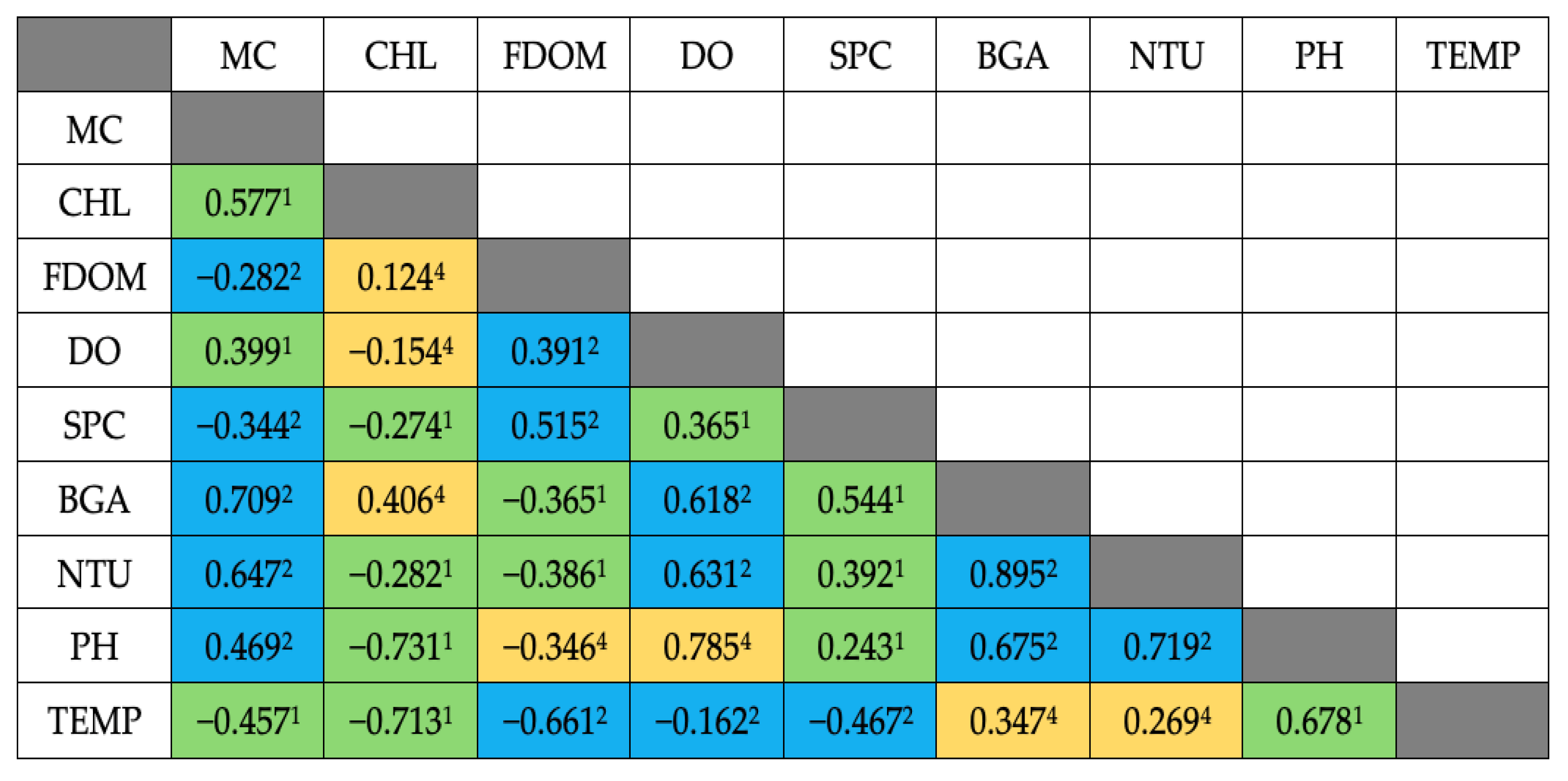
3.2. Spearman Correlations
3.3. Random Forest Applications
3.3.1. R2 Differences in Training and Testing Datasets
3.3.2. Similarities and Differences in Important Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press: London, UK, 2021; ISBN 978-1-003-08144-9. [Google Scholar]
- Martinez i Quer, A.; Larsson, Y.; Johansen, A.; Arias, C.A.; Carvalho, P.N. Cyanobacterial Blooms in Surface Waters—Nature-Based Solutions, Cyanotoxins and Their Biotransformation Products. Water Res. 2024, 251, 121122. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G.; Joyner, A.R. Moving towards Adaptive Management of Cyanotoxin-Impaired Water Bodies. Microb. Biotechnol. 2016, 9, 641–651. [Google Scholar] [CrossRef]
- Bensalem, M.; Amrani, A.; Zaidi, H.; Sedrati, F.; Laouar, O.; Wang, Z.; Nasri, H. Impact of Long-Term Cyanotoxin Exposure on Cattle: Biochemical, Histological, and Oxidative Stress Assessment. Vet. World 2025, 189–201. [Google Scholar] [CrossRef]
- Stewart, I.; Seawright, A.A.; Shaw, G.R. Cyanobacterial Poisoning in Livestock, Wild Mammals and Birds — An Overview. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 613–637. ISBN 978-0-387-75865-7. [Google Scholar]
- Mohamed, Z.A.; Mostafa, Y.; Alamri, S.; Hashem, M. Accumulation of Microcystin Toxin in Irrigation Water and Alfalfa (Medicago sativa) Forage Plant, and Assessing the Potential Risk to Animal Health. Chemosphere 2024, 364, 143248. [Google Scholar] [CrossRef] [PubMed]
- Redouane, E.M.; Tazart, Z.; Lahrouni, M.; Mugani, R.; Elgadi, S.; Zine, H.; Zerrifi, S.E.A.; Haida, M.; Martins, J.C.; Campos, A.; et al. Health Risk Assessment of Lake Water Contaminated with Microcystins for Fruit Crop Irrigation and Farm Animal Drinking. Env. Sci. Pollut. Res. 2023, 30, 80234–80244. [Google Scholar] [CrossRef]
- Faulkner, S.; Sweetman, C.; Hutson, J.; Soole, K.; Hobson, P.; Fallowfield, H. Uptake of the Cyanobacterial Toxin Microcystin by Crop Plants Irrigated with Contaminated Wastewater: A Review. Rev. Env. Sci. Biotechnol. 2025, 24, 217–238. [Google Scholar] [CrossRef]
- Shingai, Q.K.; Wilkinson, G.M. Microcystin as a Biogeochemical Cycle: Pools, Fluxes, and Fates of the Cyanotoxin in Inland Waters. Limnol. Oceanogr. Lett. 2023, 8, 406–418. [Google Scholar] [CrossRef]
- Zhang, Y.; Husk, B.R.; Duy, S.V.; Dinh, Q.T.; Sanchez, J.S.; Sauvé, S.; Whalen, J.K. Quantitative Screening for Cyanotoxins in Soil and Groundwater of Agricultural Watersheds in Quebec, Canada. Chemosphere 2021, 274, 129781. [Google Scholar] [CrossRef]
- Haida, M.; El Khalloufi, F.; Mugani, R.; Essadki, Y.; Campos, A.; Vasconcelos, V.; Oudra, B. Microcystin Contamination in Irrigation Water and Health Risk. Toxins 2024, 16, 196. [Google Scholar] [CrossRef]
- Van Hassel, W.H.R.; Tardy, E.; Cottyn, B.; Andjelkovic, M.; Decombel, A.; Van Wichelen, J.; Masquelier, J.; Rajkovic, A. Irrigation-Dependent Accumulation of Microcystin in Different Crops under Mid-Scale Greenhouse Conditions. J. Agric. Food Res. 2025, 20, 101753. [Google Scholar] [CrossRef]
- Chaffin, J.D.; Westrick, J.A.; Reitz, L.A.; Bridgeman, T.B. Microcystin Congeners in Lake Erie Follow the Seasonal Pattern of Nitrogen Availability. Harmful Algae 2023, 127, 102466. [Google Scholar] [CrossRef]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. CyanoMetDB, a Comprehensive Public Database of Secondary Metabolites from Cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef] [PubMed]
- MacKeigan, P.W.; Zastepa, A.; Taranu, Z.E.; Westrick, J.A.; Liang, A.; Pick, F.R.; Beisner, B.E.; Gregory-Eaves, I. Microcystin Concentrations and Congener Composition in Relation to Environmental Variables across 440 North-Temperate and Boreal Lakes. Sci. Total Environ. 2023, 884, 163811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Gu, Y.; Cheng, C.; Xue, Q.; Xie, L. Changes in Microcystin Concentration in Lake Taihu, 13 Years (2007–2020) after the 2007 Drinking Water Crisis. Environ. Res. 2024, 241, 117597. [Google Scholar] [CrossRef]
- Golshan, A.; Evans, C.; Geary, P.; Morrow, A.; Maeder, M.; Tauler, R. Patterns of Cyanobacterial Abundance in a Major Drinking Water Reservoir: What 3 Years of Comprehensive Monitoring Data Reveals? Env. Monit. Assess. 2020, 192, 113. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Xie, L.; Yang, J.R.; Yang, J.; Su, X. The Prevalence and Persistence of Microcystin in Seven Subtropical Reservoirs in China and Associated Potential Risks for Human Health. Environ. Technol. Innov. 2024, 33, 103476. [Google Scholar] [CrossRef]
- Chernova, E.; Sidelev, S.; Russkikh, I.; Korneva, L.; Solovyova, V.; Mineeva, N.; Stepanova, I.; Zhakovskaya, Z. Spatial Distribution of Cyanotoxins and Ratios of Microcystin to Biomass Indicators in the Reservoirs of the Volga, Kama and Don Rivers, the European Part of Russia. Limnologica 2020, 84, 125819. [Google Scholar] [CrossRef]
- Graham, J.L.; Dubrovsky, N.M.; Foster, G.M.; King, L.R.; Loftin, K.A.; Rosen, B.H.; Stelzer, E.A. Cyanotoxin Occurrence in Large Rivers of the United States. Inland. Waters 2020, 10, 109–117. [Google Scholar] [CrossRef]
- Burch, M.D. Effective Doses, Guidelines & Regulations. Adv. Exp. Med. Biol. 2008, 619, 831–853. [Google Scholar] [CrossRef]
- World Health Organization Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2002; ISBN 978-92-4-154535-8.
- Yeager, N.; Carpenter, A. State Approaches to Addressing Cyanotoxins in Drinking Water. AWWA Water Sci. 2019, 1, e1121. [Google Scholar] [CrossRef]
- US Environmental Protection Agency Drinking Water Health Advisories for Two Cyanobacterial Toxins; EPA: Washington, DC, USA, 2015; p. 3.
- FDA (US Food and Drug Administration) The New FDA Food Modernization Act (FSMA). Preventative Controls and Produce Safety Rules. Available online: https://www.fda.gov/food/guidance-regulation-food-and-dietary-supplements/food-safety-modernization-act-fsma (accessed on 4 June 2025).
- Loftin, K.A.; Dietze, J.E.; Meyer, M.T.; Graham, J.L.; Maksimowicz, M.M.; Toyne, K.D. Total Cylindrospermopsins, Microcystins/Nodularins, and Saxitoxins Data for the 2007 United States Environmental Protection Agency National Lake Assessment; U.S. Geological Survey: Reston, VA, USA, 2016.
- Pollard, A.I.; Hampton, S.E.; Leech, D.M. The Promise and Potential of Continental-Scale Limnology Using the U.S. Environmental Protection Agency’s National Lakes Assessment. Limnol. Oceanogr. Bull. 2018, 27, 36–41. [Google Scholar] [CrossRef]
- Guan, M.; Zhang, Y.; Li, W.; Li, N.; Qi, L.; Shi, K.; Zhang, Y.; Qin, B.; Huang, C. Quantifying the Effects of Wind Wave on Cyanobacterial Blooms in Large Shallow Lake from 10 Years High Frequency Satellite Observation. Hydrobiologia 2025, 852, 891–908. [Google Scholar] [CrossRef]
- Zastepa, A.; Taranu, Z.E.; Kimpe, L.E.; Blais, J.M.; Gregory-Eaves, I.; Zurawell, R.W.; Pick, F.R. Reconstructing a Long-Term Record of Microcystins from the Analysis of Lake Sediments. Sci. Total Environ. 2017, 579, 893–901. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, W.; Wang, R.; Feng, G.; Xue, Z. Rapid Horizontal Accumulation and Bloom Formation of the Cyanobacterium Microcystis under Wind Stress. Hydrobiologia 2023, 850, 123–135. [Google Scholar] [CrossRef]
- Smith, J.E.; Widmer, J.A.; Wolny, J.L.; Dunn, L.L.; Stocker, M.D.; Hill, R.L.; Pisani, O.; Coffin, A.W.; Pachepsky, Y. Persistence of Microcystin in Three Agricultural Ponds in Georgia, USA. Toxins 2024, 16, 482. [Google Scholar] [CrossRef]
- Chaffin, J.D.; Westrick, J.A.; Furr, E.; Birbeck, J.A.; Reitz, L.A.; Stanislawczyk, K.; Li, W.; Weber, P.K.; Bridgeman, T.B.; Davis, T.W.; et al. Quantification of Microcystin Production and Biodegradation Rates in the Western Basin of Lake Erie. Limnol. Oceanogr. 2022, 67, 1470–1483. [Google Scholar] [CrossRef]
- Palagama, D.S.W.; Baliu-Rodriguez, D.; Snyder, B.K.; Thornburg, J.A.; Bridgeman, T.B.; Isailovic, D. Identification and Quantification of Microcystins in Western Lake Erie during 2016 and 2017 Harmful Algal Blooms. J. Great Lakes Res. 2020, 46, 289–301. [Google Scholar] [CrossRef]
- Scherer, P.I.; Raeder, U.; Geist, J.; Zwirglmaier, K. Influence of Temperature, Mixing, and Addition of Microcystin-LR on Microcystin Gene Expression in Microcystis Aeruginosa. MicrobiologyOpen 2017, 6, e00393. [Google Scholar] [CrossRef]
- Lind, O.; Dávalos-Lind, L.; López, C.; López, M.; Dyble Bressie, J. Seasonal Morphological Variability in an in Situ Cyanobacteria Monoculture: Example from a Persistent Cylindrospermopsis Bloom in Lake Catemaco, Veracruz, Mexico. J. Limnol. 2016, 75, 66–80. [Google Scholar] [CrossRef]
- Hayes, N.M.; Vanni, M.J. Microcystin Concentrations Can Be Predicted with Phytoplankton Biomass and Watershed Morphology. Inland. Waters 2018, 8, 273–283. [Google Scholar] [CrossRef]
- Smith, J.E. Multiyear Spatial and Temporal Variation of Microcystin Concentrations in an Irrigation Pond in Maryland, USA. In Proceedings of the European Geosciences Union General Assembly 2024 (EGU24), Vienna, Austria, 14–19 April 2014. [Google Scholar]
- Guo, C.; Zhu, G.; Paerl, H.W.; Zhu, M.; Yu, L.; Zhang, Y.; Liu, M.; Zhang, Y.; Qin, B. Extreme Weather Event May Induce Microcystis Blooms in the Qiantang River, Southeast China. Env. Sci. Pollut. Res. 2018, 25, 22273–22284. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Borges, H.; Puddick, J.; Biessy, L.; Atalah, J.; Hawes, I.; Dietrich, D.R.; Hamilton, D.P. Contrasting Cyanobacterial Communities and Microcystin Concentrations in Summers with Extreme Weather Events: Insights into Potential Effects of Climate Change. Hydrobiologia 2017, 785, 71–89. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom Dynamics of Cyanobacteria and Their Toxins: Environmental Health Impacts and Mitigation Strategies. Front. Microbiol. 2015, 6, 1254. [Google Scholar] [CrossRef]
- Gobler, C.J.; Davis, T.W.; Coyne, K.J.; Boyer, G.L. Interactive Influences of Nutrient Loading, Zooplankton Grazing, and Microcystin Synthetase Gene Expression on Cyanobacterial Bloom Dynamics in a Eutrophic New York Lake. Harmful Algae 2007, 6, 119–133. [Google Scholar] [CrossRef]
- Hayes, N.M.; Haig, H.A.; Simpson, G.L.; Leavitt, P.R. Effects of Lake Warming on the Seasonal Risk of Toxic Cyanobacteria Exposure. Limnol. Oceanogr. Lett. 2020, 5, 393–402. [Google Scholar] [CrossRef]
- Jacquemin, S.J.; Doll, J.C.; Johnson, L.T.; Newell, S.E. Exploring Long-Term Trends in Microcystin Toxin Values Associated with Persistent Harmful Algal Blooms in Grand Lake St Marys. Harmful Algae 2023, 122, 102374. [Google Scholar] [CrossRef] [PubMed]
- Patiño, R.; Christensen, V.G.; Graham, J.L.; Rogosch, J.S.; Rosen, B.H. Toxic Algae in Inland Waters of the Conterminous United States—A Review and Synthesis. Water 2023, 15, 2808. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Chen, Y. The Effects of Temperature and Nutrient Ratios on Microcystis Blooms in Lake Taihu, China: An 11-Year Investigation. Harmful Algae 2011, 10, 337–343. [Google Scholar] [CrossRef]
- Leigh, C.; Burford, M.A.; Roberts, D.T.; Udy, J.W. Predicting the Vulnerability of Reservoirs to Poor Water Quality and Cyanobacterial Blooms. Water Res. 2010, 44, 4487–4496. [Google Scholar] [CrossRef]
- Baer, M.M.; Godwin, C.M.; Johengen, T.H. The Effect of Single versus Dual Nutrient Decreases on Phytoplankton Growth Rates, Community Composition, and Microcystin Concentration in the Western Basin of Lake Erie. Harmful Algae 2023, 123, 102382. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.D.; Quach, E.; Buscho, S.; Ricciardelli, A.; Kannan, A.; Naung, S.W.; Phillip, G.; Sheppard, B.; Ferguson, L.; Allen, A.; et al. Nitrogen Form, Concentration, and Micronutrient Availability Affect Microcystin Production in Cyanobacterial Blooms. Harmful Algae 2021, 103, 102002. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Yang, N.; Cui, H.; Yang, Q.; Wu, Z.; Shao, B.; Zhao, Y.; Tong, Y. Interspecific Competition Enhances Microcystin Production by Microcystis Aeruginosa under the Interactive Influences of Temperature and Nutrients. Water Res. 2024, 265, 122308. [Google Scholar] [CrossRef] [PubMed]
- Merder, J.; Harris, T.; Zhao, G.; Stasinopoulos, D.M.; Rigby, R.A.; Michalak, A.M. Geographic Redistribution of Microcystin Hotspots in Response to Climate Warming. Nat. Water 2023, 1, 844–854. [Google Scholar] [CrossRef]
- Cunha, D.G.F.; Dodds, W.K.; Loiselle, S.A. Factors Related to Water Quality and Thresholds for Microcystin Concentrations in Subtropical Brazilian Reservoirs. Inland. Waters 2018, 8, 368–380. [Google Scholar] [CrossRef]
- Te, S.H.; Gin, K.Y.-H. The Dynamics of Cyanobacteria and Microcystin Production in a Tropical Reservoir of Singapore. Harmful Algae 2011, 10, 319–329. [Google Scholar] [CrossRef]
- Fickas, K.C.; O’Shea, R.E.; Pahlevan, N.; Smith, B.; Bartlett, S.L.; Wolny, J.L. Leveraging Multimission Satellite Data for Spatiotemporally Coherent cyanoHAB Monitoring. Front. Remote Sens. 2023, 4, 1157609. [Google Scholar] [CrossRef]
- Hong, S.M.; Morgan, B.J.; Stocker, M.D.; Smith, J.E.; Kim, M.S.; Cho, K.H.; Pachepsky, Y.A. Using Machine Learning Models to Estimate Escherichia Coli Concentration in an Irrigation Pond from Water Quality and Drone-Based RGB Imagery Data. Water Res. 2024, 260, 121861. [Google Scholar] [CrossRef]
- Bunyon, C.L.; Fraser, B.T.; McQuaid, A.; Congalton, R.G. Using Imagery Collected by an Unmanned Aerial System to Monitor Cyanobacteria in New Hampshire, USA, Lakes. Remote Sens. 2023, 15, 2839. [Google Scholar] [CrossRef]
- Sharp, S.L.; Forrest, A.L.; Bouma-Gregson, K.; Jin, Y.; Cortés, A.; Schladow, S.G. Quantifying Scales of Spatial Variability of Cyanobacteria in a Large, Eutrophic Lake Using Multiplatform Remote Sensing Tools. Front. Environ. Sci. 2021, 9, 612934. [Google Scholar] [CrossRef]
- Ivey, J.E.; Wolny, J.L.; Heil, C.A.; Murasko, S.M.; Brame, J.A.; Parks, A.A. Urea Inputs Drive Picoplankton Blooms in Sarasota Bay, Florida, U.S.A. Water 2020, 12, 2755. [Google Scholar] [CrossRef]
- Hagh, S.F.; Amngostar, P.; Zylka, A.; Zimmerman, M.; Cresanti, L.; Karins, S.; O’Neil-Dunne, J.P.; Ritz, K.; Williams, C.J.; Morales-Williams, A.M.; et al. Autonomous UAV-Mounted LoRaWAN System for Real-Time Monitoring of Harmful Algal Blooms (HABs) and Water Quality. IEEE Sens. J. 2024, 24, 11414–11424. [Google Scholar] [CrossRef]
- Venkatachari, A.; Bourbonnais, A.; Salman, I.; Rekleitis, I.; Li, A.Q.; Cottingham, K.; Ewing, H.; Bruesewitz, D.; Arsenault, E.; Shingai, Q. Use of an Autonomous Surface Vehicle to Collect High Spatial Resolution Water Quality Data at Lake Wateree, SC. J. South Carol. Water Resour. 2024, 9, 10. [Google Scholar] [CrossRef]
- Bartelt, G.; You, J.; Hondzo, M. Remote Cyanobacteria Detection by Multispectral Drone Imagery. Lake Reserv. Manag. 2024, 40, 236–247. [Google Scholar] [CrossRef]
- Gómez, D.; Salvador, P.; Sanz, J.; Casanova, J.L. A New Approach to Monitor Water Quality in the Menor Sea (Spain) Using Satellite Data and Machine Learning Methods. Environ. Pollut. 2021, 286, 117489. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Pyo, J.; Kwon, Y.-H.; Duan, H.; Cho, K.H.; Park, Y. Drone-Based Hyperspectral Remote Sensing of Cyanobacteria Using Vertical Cumulative Pigment Concentration in a Deep Reservoir. Remote Sens. Environ. 2020, 236, 111517. [Google Scholar] [CrossRef]
- Copetti, D.; Matarrese, R.; Bresciani, M.; Guzzella, L. Integration of In Situ and Remote Sensing Measurements for the Management of Harmful Cyanobacteria Blooms. A Lesson from a Strategic Multiple-Uses Reservoir (Lake Occhito, South Italy). Water 2021, 13, 2162. [Google Scholar] [CrossRef]
- Wilkinson, A.A.; Hondzo, M.; Guala, M. Vertical Heterogeneities of Cyanobacteria and Microcystin Concentrations in Lakes Using a Seasonal In Situ Monitoring Station. Glob. Ecol. Conserv. 2020, 21, e00838. [Google Scholar] [CrossRef]
- Harris, T.D.; Graham, J.L. Predicting Cyanobacterial Abundance, Microcystin, and Geosmin in a Eutrophic Drinking Water Reservoir Using a 14-Year Dataset. Lake Reserv. Manag. 2017, 33, 32–48. [Google Scholar] [CrossRef]
- Mellios, N.; Moe, S.J.; Laspidou, C. Machine Learning Approaches for Predicting Health Risk of Cyanobacterial Blooms in Northern European Lakes. Water 2020, 12, 1191. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, Y.; Zhao, H.; Sun, M.; Li, X. Comparison of Models for Predicting the Changes in Phytoplankton Community Composition in the Receiving Water System of an Inter-Basin Water Transfer Project. Environ. Pollut. 2017, 223, 676–684. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, J.; Gu, Y.; Zhu, X.; Xia, J.; Wu, J.; You, G.; Yang, Z.; Ding, W.; Miao, L. Spatiotemporal Variation Assessment and Improved Prediction Of Cyanobacteria Blooms in Lakes Using Improved Machine Learning Model Based on Multivariate Data. Environ. Manag. 2025, 75, 694–709. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, D.; Lin, J.; Peng, Q.; Lei, X.; Jin, T.; Wang, J.; Yuan, R. Prediction of Phytoplankton Biomass and Identification of Key Influencing Factors Using Interpretable Machine Learning Models. Ecol. Indic. 2024, 158, 111320. [Google Scholar] [CrossRef]
- Hwang, S.-Y.; Choi, B.-W.; Park, J.-H.; Shin, D.-S.; Chung, H.-S.; Son, M.-S.; Lim, C.-H.; Chae, H.-M.; Ha, D.-W.; Jung, K.-Y. Evaluating Statistical Machine Learning Algorithms for Classifying Dominant Algae in Juam Lake and Tamjin Lake, Republic of Korea. Water 2023, 15, 1738. [Google Scholar] [CrossRef]
- Drobnič, F.; Kos, A.; Pustišek, M. On the Interpretability of Machine Learning Models and Experimental Feature Selection in Case of Multicollinear Data. Electronics 2020, 9, 761. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R Using the Caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Couronné, R.; Probst, P.; Boulesteix, A.-L. Random Forest versus Logistic Regression: A Large-Scale Benchmark Experiment. BMC Bioinform. 2018, 19, 270. [Google Scholar] [CrossRef]
- Probst, P.; Wright, M.N.; Boulesteix, A.-L. Hyperparameters and Tuning Strategies for Random Forest. WIREs Data Min. Knowl. Discov. 2019, 9, e1301. [Google Scholar] [CrossRef]
- Francy, D.S.; Brady, A.M.G.; Ecker, C.D.; Graham, J.L.; Stelzer, E.A.; Struffolino, P.; Dwyer, D.F.; Loftin, K.A. Estimating Microcystin Levels at Recreational Sites in Western Lake Erie and Ohio. Harmful Algae 2016, 58, 23–34. [Google Scholar] [CrossRef]
- Fu, X.; Zheng, M.; Su, J.; Xi, B.; Wei, D.; Wang, X. Spatiotemporal Patterns and Threshold of Chlorophyll-a in Lake Taihu Based on Microcystins. Env. Sci. Pollut. Res. 2023, 30, 49327–49338. [Google Scholar] [CrossRef]
- Koreivienė, J.; Anne, O.; Kasperovičienė, J.; Burškytė, V. Cyanotoxin Management and Human Health Risk Mitigation in Recreational Waters. Env. Monit. Assess. 2014, 186, 4443–4459. [Google Scholar] [CrossRef]
- Sakai, H.; Hao, A.; Iseri, Y.; Wang, S.; Kuba, T.; Zhang, Z.; Katayama, H. Occurrence and Distribution of Microcystins in Lake Taihu, China. Sci. World J. 2013, 2013, 838176. [Google Scholar] [CrossRef]
- Yu, L.; Kong, F.; Zhang, M.; Yang, Z.; Shi, X.; Du, M. The Dynamics of Microcystis Genotypes and Microcystin Production and Associations with Environmental Factors during Blooms in Lake Chaohu, China. Toxins 2014, 6, 3238–3257. [Google Scholar] [CrossRef]
- Sinang, S.C.; Reichwaldt, E.S.; Ghadouani, A. Spatial and Temporal Variability in the Relationship between Cyanobacterial Biomass and Microcystins. Env. Monit. Assess. 2013, 185, 6379–6395. [Google Scholar] [CrossRef]
- Duong, T.T.; Le, T.P.Q.; Dao, T.-S.; Pflugmacher, S.; Rochelle-Newall, E.; Hoang, T.K.; Vu, T.N.; Ho, C.T.; Dang, D.K. Seasonal Variation of Cyanobacteria and Microcystins in the Nui Coc Reservoir, Northern Vietnam. J. Appl. Phycol. 2013, 25, 1065–1075. [Google Scholar] [CrossRef]
- Singh, S.; Rai, P.K.; Chau, R.; Ravi, A.K.; Neilan, B.A.; Asthana, R.K. Temporal Variations in Microcystin-Producing Cells and Microcystin Concentrations in Two Fresh Water Ponds. Water Res. 2015, 69, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, H.K.; Shin, J.-K.; Chon, K.; Kim, S.; Cho, K.H.; Kim, J.H.; Baek, S.-S. A Machine Learning Approach for Early Warning of Cyanobacterial Bloom Outbreaks in a Freshwater Reservoir. J. Environ. Manag. 2021, 288, 112415. [Google Scholar] [CrossRef] [PubMed]
- Swartz, T.M.; Miller, J.R. The American Pond Belt: An Untold Story of Conservation Challenges and Opportunities. Front. Ecol. Environ. 2021, 19, 501–509. [Google Scholar] [CrossRef]
- Ni, J.; Liu, R.; Li, Y.; Tang, G.; Shi, P. An Improved Transfer Learning Model for Cyanobacterial Bloom Concentration Prediction. Water 2022, 14, 1300. [Google Scholar] [CrossRef]
- Mu, W.; Kleter, G.A.; Bouzembrak, Y.; Dupouy, E.; Frewer, L.J.; Radwan Al Natour, F.N.; Marvin, H.J.P. Making Food Systems More Resilient to Food Safety Risks by Including Artificial Intelligence, Big Data, and Internet of Things into Food Safety Early Warning and Emerging Risk Identification Tools. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13296. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis 2001. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- George, D.; Mallery, P. SPSS for Windows Step by Step: A Simple Guide and Reference 18.0 Update, 11th ed.; Prentice Hall Press: Upper Saddle River, NJ, USA, 2010; ISBN 978-0-205-01124-7. [Google Scholar]
- Barreñada, L.; Dhiman, P.; Timmerman, D.; Boulesteix, A.-L.; Van Calster, B. Understanding Overfitting in Random Forest for Probability Estimation: A Visualization and Simulation Study. Diagn. Progn. Res. 2024, 8, 14. [Google Scholar] [CrossRef]
- Ying, X. An Overview of Overfitting and Its Solutions. J. Phys. Conf. Ser. 2019, 1168, 022022. [Google Scholar] [CrossRef]
- Allen Akselrud, C.I. Random Forest Regression Models in Ecology: Accounting for Messy Biological Data and Producing Predictions with Uncertainty. Fish. Res. 2024, 280, 107161. [Google Scholar] [CrossRef]
- Suikkanen, S.; Uusitalo, L.; Lehtinen, S.; Lehtiniemi, M.; Kauppila, P.; Mäkinen, K.; Kuosa, H. Diazotrophic Cyanobacteria in Planktonic Food Webs. Food Webs 2021, 28, e00202. [Google Scholar] [CrossRef]
- Smith, J.E.; Wolny, J.L.; Hill, R.L.; Stocker, M.D.; Pachepsky, Y. Examining the Relationship between Phytoplankton Community Structure and Water Quality Measurements in Agricultural Waters: A Machine Learning Application. Environments 2022, 9, 142. [Google Scholar] [CrossRef]
- Moldaenke, C.; Fang, Y.; Yang, F.; Dahlhaus, A. Early Warning Method for Cyanobacteria Toxin, Taste and Odor Problems by the Evaluation of Fluorescence Signals. Sci. Total Environ. 2019, 667, 681–690. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, N.; Zamyadi, A.; Prévost, M.; Bird, D.F.; Dorner, S. Use of in Vivo Phycocyanin Fluorescence to Monitor Potential Microcystin -Producing Cyanobacterial Biovolume in a Drinking Water Source. J. Environ. Monit. 2011, 13, 455–463. [Google Scholar] [CrossRef]
- Mchau, G.J.; Makule, E.; Machunda, R.; Gong, Y.Y.; Kimanya, M. Phycocyanin as a Proxy for Algal Blooms in Surface Waters: Case Study of Ukerewe Island, Tanzania. Water Pract. Technol. 2019, 14, 229–239. [Google Scholar] [CrossRef]
- Schürmann, Q.J.F.; Visser, P.M.; Sollie, S.; Kardinaal, W.E.A.; Faassen, E.J.; Lokmani, R.; van der Oost, R.; Van de Waal, D.B. Risk Assessment of Toxic Cyanobacterial Blooms in Recreational Waters: A Comparative Study of Monitoring Methods. Harmful Algae 2024, 138, 102683. [Google Scholar] [CrossRef]
- Li, J.; Persson, K.M.; Pekar, H.; Jansson, D. Evaluation of Indicators for Cyanobacterial Risk in 108 Temperate Lakes Using 23 Years of Environmental Monitoring Data. Env. Sci. Eur. 2021, 33, 54. [Google Scholar] [CrossRef]
- Griffith, A.W.; Gobler, C.J. Harmful Algal Blooms: A Climate Change Co-Stressor in Marine and Freshwater Ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms Like It Hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef]
- Mowe, M.A.D.; Porojan, C.; Abbas, F.; Mitrovic, S.M.; Lim, R.P.; Furey, A.; Yeo, D.C.J. Rising Temperatures May Increase Growth Rates and Microcystin Production in Tropical Microcystis Species. Harmful Algae 2015, 50, 88–98. [Google Scholar] [CrossRef]
- Walls, J.T.; Wyatt, K.H.; Doll, J.C.; Rubenstein, E.M.; Rober, A.R. Hot and Toxic: Temperature Regulates Microcystin Release from Cyanobacteria. Sci. Total Environ. 2018, 610–611, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Qin, B.; Paerl, H.W.; Brookes, J.D.; Hall, N.S.; Shi, K.; Zhou, Y.; Guo, J.; Li, Z.; Xu, H.; et al. The Persistence of Cyanobacterial (Icrocystis spp.) Blooms throughout Winter in Lake Taihu, China. Limnol. Oceanogr. 2016, 61, 711–722. [Google Scholar] [CrossRef]
- Šeparović, L.; Alexandru, A.; Laprise, R.; Martynov, A.; Sushama, L.; Winger, K.; Tete, K.; Valin, M. Present Climate and Climate Change over North America as Simulated by the Fifth-Generation Canadian Regional Climate Model. Clim. Dyn. 2013, 41, 3167–3201. [Google Scholar] [CrossRef]
- Zibordi, G.; Mélin, F.; Berthon, J.-F.; Holben, B.; Slutsker, I.; Giles, D.; D’Alimonte, D.; Vandemark, D.; Feng, H.; Schuster, G.; et al. AERONET-OC: A Network for the Validation of Ocean Color Primary Products. J. Atmos. Ocean. Technol. 2009, 26, 1634–1651. [Google Scholar] [CrossRef]
- Jiang, M.; Brereton, A.; Beckler, J.; Moore, T.; Brewton, R.A.; Hu, C.; Lapointe, B.E.; McFarland, M.N. Modeling Water Quality and Cyanobacteria Blooms in Lake Okeechobee: I. Model Descriptions, Seasonal Cycles, and Spatial Patterns. Ecol. Model. 2025, 502, 111018. [Google Scholar] [CrossRef]
- Lofton, M.E.; Brentrup, J.A.; Beck, W.S.; Zwart, J.A.; Bhattacharya, R.; Brighenti, L.S.; Burnet, S.H.; McCullough, I.M.; Steele, B.G.; Carey, C.C.; et al. Using Near-Term Forecasts and Uncertainty Partitioning to Inform Prediction of Oligotrophic Lake Cyanobacterial Density. Ecol. Appl. 2022, 32, e2590. [Google Scholar] [CrossRef] [PubMed]
- Beversdorf, L.J.; Miller, T.R.; McMahon, K.D. Long-Term Monitoring Reveals Carbon–Nitrogen Metabolism Key to Microcystin Production in Eutrophic Lakes. Front. Microbiol. 2015, 6, 456. [Google Scholar] [CrossRef] [PubMed]
- Garland, D.; Koehler, H.; McGirr, S.; Parkes, R.; E Lucy, F.; Touzet, N. Seasonal Community Dynamics and Toxicity Potential of Cyanobacteria in Lough Arrow, an Oligo-Mesotrophic Lake in the North-West of Ireland. Limnologica 2023, 103, 126124. [Google Scholar] [CrossRef]
- Tekanova, E.; Sidelev, S.; Kalinkina, N.; Chernova, E.; Barinova, S.; Sharov, A.; Smirnova, V. Toxigenic Cyanobacteria and Microcystins in a Large Northern Oligotrophic Lake Onego, Russia. Toxins 2024, 16, 457. [Google Scholar] [CrossRef]
- Mariani, M.A.; Padedda, B.M.; Kaštovský, J.; Buscarinu, P.; Sechi, N.; Virdis, T.; Lugliè, A. Effects of Trophic Status on Microcystin Production and the Dominance of Cyanobacteria in the Phytoplankton Assemblage of Mediterranean Reservoirs. Sci. Rep. 2015, 5, 17964. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.d.A.B.; Rodrigues, R.d.A.M.; Podduturi, R.; Jørgensen, N.O.G.; Calijuri, M.d.C. Prediction of Cyanotoxin Episodes in Freshwater: A Case Study on Microcystin and Saxitoxin in the Lobo Reservoir, São Paulo State, Brazil. Environments 2023, 10, 143. [Google Scholar] [CrossRef]
- Wilkinson, G.M.; Walter, J.A.; Albright, E.A.; King, R.F.; Moody, E.K.; Ortiz, D.A. An Evaluation of Statistical Models of Microcystin Detection in Lakes Applied Forward under Varying Climate Conditions. Harmful Algae 2024, 137, 102679. [Google Scholar] [CrossRef] [PubMed]
- Taranu, Z.E.; Pick, F.R.; Creed, I.F.; Zastepa, A.; Watson, S.B. Meteorological and Nutrient Conditions Influence Microcystin Congeners in Freshwaters. Toxins 2019, 11, 620. [Google Scholar] [CrossRef]

| Pond 1 | Min | Max | Med | Mean | Std Dev | Std Err | Skew | Kurt |
| MC | 0.74 | 27.51 | 4.45 | 7.09 | 6.06 | 0.36 | 1.38 | 1.28 |
| CHL | 0.94 | 40.95 | 8.41 | 11.67 | 8.34 | 0.49 | 1.11 | 0.77 |
| FDOM | 2.97 | 67.87 | 50.27 | 50.50 | 8.67 | 0.51 | −0.51 | 1.97 |
| DO | 2.96 | 13.95 | 9.43 | 9.34 | 1.60 | 0.09 | −0.40 | 0.90 |
| SPC | 7.10 | 151.50 | 120.70 | 125.08 | 15.25 | 0.90 | −1.59 | 10.80 |
| BGA | 1.18 | 21.84 | 8.84 | 8.53 | 4.20 | 0.25 | 0.27 | −0.32 |
| NTU | 3.70 | 182.22 | 54.93 | 61.73 | 31.38 | 1.85 | 1.20 | 1.79 |
| pH | 5.49 | 9.70 | 8.40 | 8.20 | 0.96 | 0.06 | −0.23 | −1.21 |
| TEMP | 13.59 | 33.28 | 27.06 | 23.86 | 6.12 | 0.36 | −0.31 | −1.51 |
| Pond 2 | Min | Max | Med | Mean | Std Dev | Std Err | Skew | Kurt |
| MC | 0.50 | 20.03 | 5.46 | 6.31 | 4.46 | 0.28 | 0.91 | 0.34 |
| CHL | 0.22 | 10.70 | 3.81 | 4.17 | 1.97 | 0.12 | 0.78 | 0.39 |
| FDOM | 0.78 | 70.39 | 33.74 | 34.93 | 13.60 | 0.86 | 0.88 | 0.79 |
| DO | 6.67 | 19.39 | 11.06 | 11.45 | 2.89 | 0.18 | 0.87 | 0.04 |
| SPC | 2.20 | 282.70 | 216.45 | 215.72 | 31.50 | 1.98 | −1.13 | 7.78 |
| BGA | 0.37 | 34.18 | 4.54 | 5.83 | 4.59 | 0.29 | 1.88 | 6.05 |
| NTU | 4.02 | 118.05 | 27.39 | 31.59 | 19.21 | 1.21 | 1.26 | 2.05 |
| pH | 4.02 | 10.40 | 9.12 | 8.94 | 0.94 | 0.06 | −1.37 | 4.60 |
| TEMP | 13.43 | 31.66 | 26.04 | 24.89 | 4.94 | 0.31 | −0.55 | −0.72 |
| Pond 3 | Min | Max | Med | Mean | Std Dev | Std Err | Skew | Kurt |
| MC | 0.00 | 5.96 | 0.72 | 1.12 | 1.16 | 0.07 | 1.61 | 2.19 |
| CHL | 0.01 | 115.56 | 1.96 | 4.33 | 8.96 | 0.55 | 8.05 | 90.68 |
| FDOM | 6.70 | 42.88 | 24.17 | 25.22 | 5.61 | 0.34 | 0.42 | 1.09 |
| DO | 4.74 | 14.07 | 9.36 | 9.35 | 1.93 | 0.12 | 0.07 | −0.33 |
| SPC | 85.60 | 197.40 | 165.00 | 168.18 | 12.48 | 0.77 | −1.48 | 9.59 |
| BGA | 0.01 | 51.88 | 1.13 | 1.95 | 3.54 | 0.22 | 11.12 | 151.91 |
| NTU | 0.01 | 143.79 | 9.45 | 12.30 | 13.39 | 0.82 | 5.62 | 44.16 |
| pH | 6.25 | 10.13 | 9.06 | 8.80 | 0.83 | 0.05 | −0.86 | −0.28 |
| TEMP | 17.14 | 32.92 | 26.49 | 26.23 | 3.28 | 0.20 | −0.57 | 0.24 |
| Pond | Model | Training R2 | Testing R2 | Imp Pred #1 | Imp Pred #2 | Imp Pred #3 |
|---|---|---|---|---|---|---|
| Pond 1 | All | 0.642 | 0.589 | CHL | TEMP | FDOM |
| Interior | 0.669 | 0.676 | CHL | TEMP | FDOM | |
| Nearshore | 0.576 | 0.596 | TEMP | CHL | FDOM | |
| Pond 2 | All | 0.631 | 0.642 | BGA | TEMP | SPC |
| Interior | 0.727 | 0.695 | TEMP | NTU | BGA | |
| Nearshore | 0.622 | 0.614 | BGA | TEMP | NTU | |
| Pond 3 | All | 0.462 | 0.474 | TEMP | NTU | PH |
| Interior | 0.545 | 0.570 | BGA | NTU | TEMP | |
| Nearshore | 0.358 | 0.388 | NTU | PH | BGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, J.E.; Widmer, J.A.; Stocker, M.D.; Wolny, J.L.; Hill, R.L.; Pachepsky, Y. Investigating the Relationship Between Microcystin Concentrations and Water Quality Parameters in Three Agricultural Irrigation Ponds Using Random Forest. Water 2025, 17, 2361. https://doi.org/10.3390/w17162361
Smith JE, Widmer JA, Stocker MD, Wolny JL, Hill RL, Pachepsky Y. Investigating the Relationship Between Microcystin Concentrations and Water Quality Parameters in Three Agricultural Irrigation Ponds Using Random Forest. Water. 2025; 17(16):2361. https://doi.org/10.3390/w17162361
Chicago/Turabian StyleSmith, Jaclyn E., James A. Widmer, Matthew D. Stocker, Jennifer L. Wolny, Robert L. Hill, and Yakov Pachepsky. 2025. "Investigating the Relationship Between Microcystin Concentrations and Water Quality Parameters in Three Agricultural Irrigation Ponds Using Random Forest" Water 17, no. 16: 2361. https://doi.org/10.3390/w17162361
APA StyleSmith, J. E., Widmer, J. A., Stocker, M. D., Wolny, J. L., Hill, R. L., & Pachepsky, Y. (2025). Investigating the Relationship Between Microcystin Concentrations and Water Quality Parameters in Three Agricultural Irrigation Ponds Using Random Forest. Water, 17(16), 2361. https://doi.org/10.3390/w17162361






