Persistent Pharmaceuticals in a South African Urban Estuary and Bioaccumulation in Endobenthic Sandprawns (Kraussillichirus kraussi)
Abstract
1. Introduction
2. Methods
2.1. Study Site
2.1.1. Field Sampling
2.1.2. Pharmaceutical Preparation and Extraction
2.1.3. Pharmaceutical Analysis, Quality Control and Assurance
2.1.4. Bioaccumulation Factor
2.2. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chia, M.A.; Lorenzi, A.S.; Ameh, I.; Dauda, S.; Cordeiro-Araújo, M.K.; Agee, J.T.; Okpanachi, I.Y.; Adesalu, A.T. Susceptibility of phytoplankton to the increasing presence of active pharmaceutical ingredients (APIs) in the aquatic environment: A review. Aquat. Toxicol. 2021, 234, 105809. [Google Scholar] [CrossRef]
- Gaw, S.; Thomas, K.V.; Hutchinson, T.H. Sources, impacts and trends of pharmaceuticals in the marine and coastal environment. Philos. Trans. R. Soc. 2014, 369, 20130572. [Google Scholar] [CrossRef]
- Ojemaye, C.Y.; Petrik, L. Pharmaceuticals and Personal Care Products in the Marine Environment Around False Bay, Cape Town, South Africa: Occurrence and Risk-Assessment Study. Environ. Toxicol. Chem. 2021, 41, 614–634. [Google Scholar] [CrossRef] [PubMed]
- Kock, A.; Glanville, H.C.; Law, A.C.; Stanton, T.; Carter, L.J.; Taylor, J.C. Emerging challenges of the impacts of pharmaceuticals on aquatic ecosystems: A diatom perspective. Sci. Total Environ. 2023, 878, 162939. [Google Scholar] [CrossRef]
- Fabbri, E.; Franzellitti, S. Human pharmaceuticals in the marine environment: Focus on exposure and biological effects in animal species. Environ. Toxicol. Chem. 2016, 35, 799–812. [Google Scholar] [CrossRef]
- Neuparth, T.; Martins, C.; Santos, C.B.; Costa, M.H.; Martins, I.; Costa, P.M.; Santos, M.M. Hypocholesterolaemic pharmaceutical simvastatin disrupts reproduction and population growth of the amphipod Gammarus locusta at the ng/L range. Aquat. Toxicol. 2014, 155, 337–347. [Google Scholar] [CrossRef]
- Srain, H.S.; Beazley, K.F.; Walker, T.R. Pharmaceuticals and personal care products and their sublethal and lethal effects in aquatic organisms. Environ. Rev. 2021, 29, 142–181. [Google Scholar] [CrossRef]
- Perissinotto, R.; Stretch, D.D.; Whitfield, A.K.; Adams, J.B.; Forbes, A.T.; Demetriades, N.T. Ecosystem functioning of temporarily open/closed estuaries in South Africa. In Estuaries: Types, Movement Patterns; Crane, J.R., Solomon, A.E., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; pp. 1–69. [Google Scholar]
- Adams, J.B.; Taljaard, S.; van Niekerk, L.; Lemley, D.A. Nutrient enrichment as a threat to the ecological resilience and health of South African microtidal estuaries. Afr. J. Aquat. Sci. 2020, 45, 23–40. [Google Scholar] [CrossRef]
- Branch, G.M.; Griffiths, C.L.; Branch, M.; Beckley, L.E. Two Oceans: A Guide to the Marine Life of Southern Africa; Struik Nature: Cape Town, South Africa, 2016. [Google Scholar]
- Pillay, D.; Branch, G.M.; Forbes, A.T. Effects of Callianassa kraussi on microbial biofilms and recruitment of macrofauna: A novel hypothesis for adult-juvenile interactions. Mar. Ecol. Prog. Ser. 2007, 347, 1–14. [Google Scholar] [CrossRef]
- Pillay, D.; Branch, G.M.; Forbes, A.T. The influence of bioturbation by the sandprawn Callianassa kraussi on feeding and survival of the bivalve Eumarcia paupercula and the gastropod Nassarius kraussianus. J. Exp. Mar. Biol. Ecol. 2007, 344, 1–9. [Google Scholar] [CrossRef]
- Pillay, D.; Branch, G.M. Bioengineering effects of burrowing thalassinidean shrimps on marine soft-bottom ecosystems. Oceanogr. Mar. Biol. 2011, 49, 137–192. [Google Scholar]
- Pillay, D. Ecosystem engineering by thalassinidean crustaceans: Response variability, contextual dependencies and perspectives on future research. Diversity 2019, 11, 64. [Google Scholar] [CrossRef]
- Venter, O.; Pillay, D.; Prayag, K. Water filtration by burrowing sandprawns provides novel insights on endobenthic engineering and solutions for eutrophication. Sci. Rep. 2020, 10, 1913. [Google Scholar] [CrossRef]
- Thomas, C.M.; de Cerff, C.; Maniel, G.A.V.; Oyatoye, A.E.; Rocke, E.; Marco, H.G.; Pillay, D. Water filtration by endobenthic sandprawns enhances resilience agaisnnt eutrophication under experimental global change conditions. Sci. Rep. 2023, 13, 19067. [Google Scholar] [CrossRef]
- de Cerff, C.; Rocke, E.; Oyatoye, A.E.; Maniel, G.; Pillay, D. Mesocosm evidence for sandprawn-mediated shifts in pelagic resource ratios and phytoplankton traits. Estuar. Coast. Shelf Sci. 2024, 305, 108874. [Google Scholar] [CrossRef]
- Van Niekerk, L.; Adams, J.B.; Lamberth, S.J.; MacKay, C.F.; Taljaard, S.; Turpie, J.K.; Weerts, S.P.; Raimondo, D.C. South African National Biodiversity Assessment 2018: Technical Report. CSIR Rep. 2019, 3, 1–30. [Google Scholar]
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Gqomfa, B.; Maphanga, T.; Shale, K. The impact of informal settlement on water quality of Diep River in Dunoon. Sustain. Water Resour. Manag. 2021, 8, 27. [Google Scholar] [CrossRef]
- Swartz, C.; Genthe, B.; Chamier, J.; Petrik, L.F.; Tijani, J.O.; Adeleye, A.; Coomans, C.J.; Ohlin, A.; Falk, D.; Menge, J.G. Emerging Contaminants in Wastewater Treated for Direct Potable Re-Use: The Human Health Risk; Water Research Commission: Pretoria, South Africa, 2016.
- Swartz, C.D.; Genthe, B.; Chamier, J.; Petrik, L.F.; Tijani, J.O.; Adeleye, A.; Coomans, C.J.; Ohlin, A.; Falk, D.; Menge, J.G. Emerging Contaminants in Wastewater Treated for Direct Potable Re-Use: The Human Health Risk Priorities in South Africa. In VOLUME III: Occurrence, Fate, Removal and Health Risk Assessment of Chemicals of Emerging Concern in Reclaimed Water for Potable Reuse; Water Research Commission: Pretoria, South Africa, 2018. [Google Scholar]
- Harding, W.R. Water quality trends and the influence of salinity in a highly regulated estuary near Cape Town, South Africa. S. Afr. J. Sci. 1994, 90, 240–246. [Google Scholar]
- Na, G.; Fang, X.; Cai, Y.; Ge, L.; Zong, H.; Yuan, X.; Yao, Z.; Zhang, Z. Occurance, distribution, and bioaccumulation of antibiotics in coastal environment of Dalian, China. Mar. Pollut. Bull. 2013, 69, 233–237. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Luo, X.; Wang, J.; Chen, S.; Guan, Y.; Mai, B. Isomer-specific bioaccumulation and trophic transfer of dechlorane plus in the freshwater food web from a highly contaminated site, south China. Environ. Sci. Technol. 2010, 44, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Schlacher, T.A.; Wooldridge, T.H. Origin and trophic importance of detritus-evidence from stable isotopes in the benthos of a small, temperate estuary. Oecologia 1996, 106, 382–388. [Google Scholar] [CrossRef]
- Osunmakinde, C.S.; Tshabalala, O.S.; Dube, S.; Nindi, M.M. Verification and Validation of Analytical Methods for Testing the Levels of PPHCPs (Pharmaceutical and Personal Health Care Products) in Treated Drinking Water and Sewage; Water Research Commission: Pretoria, South Africa, 2013.
- Madikizela, L.M.; Chimuka, L. Occurrence of naproxen, ibuprofen, and diclofenac residues in wastewater and river water of KwaZulu-Natal Province in South Africa. Environ. Monit. Assess. 2017, 189, 348. [Google Scholar] [CrossRef]
- Moslah, B.; Hapeshi, E.; Jrad, A.; Fatta-kassinos, D. Pharmaceuticals and illicit drugs in wastewater samples in north-eastern Tunisia. Environ. Sci. Pollut. Res. 2018, 25, 18226–18241. [Google Scholar] [CrossRef]
- Ohoro, C.R.; Adeniji, A.O.; Semerjian, L.; Okoh, O.O.; Okoh, A.I. Occurrence and distribution of pharmaceuticals in surface water and sediment of Buffalo and Sundays River estuaries, South Africa and their ecological risk assessment. Emerg. Contam. 2021, 7, 187–195. [Google Scholar] [CrossRef]
- Reis-Santos, P.; Pais, M.; Duarte, B.; Cacador, I.; Freitas, A.; Pouca, A.S.V.; Barbosa, J.; Leston, S.; Rosa, J.; Ramos, F.; et al. Screening of human and veterinary pharmaceuticals in estuarine waters: A baseline assessment for the Tejo estuary. Mar. Pollut. Bull. 2018, 135, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, Y.; Li, M.; Ashfaq, M.; Lv, M.; Wang, H.; Hu, A.; Yu, C.P. PCPs in Jiulong River estuary (China): Spatiotemporal distributions, fate, and their use as chemical markers of wastewater. Chemosphere 2016, 150, 596–604. [Google Scholar] [CrossRef]
- Lara-Martin, P.A.; González-Mazo, E.; Petrovic, M.; Barcelo, D. Occurrence, distribution and partitioning of nonionic surfactants and pharmaceuticals in the urbanized Long Island Sound Estuary (NY). Mar. Pollut. Bull. 2014, 85, 710–719. [Google Scholar] [CrossRef]
- Birch, G.F.; Drage, D.S.; Thompson, K.; Eaglesham, G.; Mueller, J.F. Emerging contaminants (pharmaceuticals, personal care products, a food additive and pesticides) in waters of Sydney estuary, Australia. Mar. Pollut. Bull. 2015, 97, 56–66. [Google Scholar] [CrossRef]
- Agunbiade, F.O.; Moodley, B. Pharmaceuticals as emerging organic contaminants in Umgeni River water system, KwaZulu-Natal, South Africa. Environ. Monit. Assess. 2014, 186, 7273–7291. [Google Scholar] [CrossRef] [PubMed]
- Sigonya, S.; Onwubu, S.C.; Mdluli, P.S. Method optimisation and application based on solid phase extraction of non steriodal anti-inflammatory drugs, antiretroviral drugs, and a lipid regulator from coastal areas of Durban, South Africa. SN Appl. Sci. 2022, 4, 231. [Google Scholar] [CrossRef]
- Wanjeri, V.O.W.; Okuku, E.; Gachanja, A.; Ngila, J.C.; Ndungu, P.G. Occurrence, distribution, and environmental risk of pharmaceutical residues in Mombasa peri-urban creeks, Kenya. Chemosphere 2023, 31, 137144. [Google Scholar] [CrossRef]
- Klosterhaus, S.L.; Grace, R.; Hamilton, M.C.; Yee, D. Method validation and reconnaissance of pharmaceuticals, personal care products, and alkylphenols in surface waters, sediments, and mussels in an urban estuary. Environ. Int. 2013, 54, 92–99. [Google Scholar] [CrossRef]
- Du, B.; Haddad, S.P.; Scott, W.C.; Chambliss, C.K.; Brooks, B.W. Pharmaceutical bioaccumulation by periphyton and snails in an effluent-dependent stream during an extreme drought. Chemosphere 2015, 119, 927–934. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Hooda, P.S.; Swinden, J.; Barker, J.; Barton, S. Spatial (bio) accumulation of pharmaceuticals, illicit drugs, plasticisers, perfluorinated compounds and metabolites in river sediment, aquatic plants and benthic organisms. Environ. Pollut. 2018, 234, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Meredith-Williams, M.; Carter, L.J.; Fussell, R.; Raffaelli, D.; Ashauer, R.; Boxall, A.B.A. Uptake and depuration of pharmaceuticals in aquatic invertebrates. Environ. Pollut. 2012, 165, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Grabicová, K.; Stañová, A.V.; Švecová, H.; Nováková, P.; Kodeš, V.; Leontovyčová, D.; Brooks, B.W.; Grabic, R. Invertebrates differentially bioaccumulate pharmaceuticals: Implications for routine biomonitoring. Environ. Pollut. 2022, 309, 119715. [Google Scholar] [CrossRef] [PubMed]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.N.; Singh, G.G. Meta-analysis reveals negative yet variable effects of ocean acidification. Ecol. Lett. 2010, 13, 1419–1434. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Gaylord, B.; Hill, T.M.; Hosfelt, J.D.; Miller, S.H.; Sanford, E. The role of temperature in determining species’ vulnerability to ocean acidification: A case study using Mytilus galloprovincialis. PLoS ONE 2014, 9, e100353. [Google Scholar] [CrossRef]
- Serra-Compte, A.; Maulvault, A.L.; Camacho, C.; Álvarez-Muñoz, D.; Barceló, D.; Rodríguez-Mozaz, S.; Margues, A. Effects of water warming and acidification on bioconcentration, metabolization and depuration of pharmaceuticals and endocrine disrupting compounds in marine mussels (Mytilis galloprovincialis). Environ. Pollut. 2018, 236, 824–834. [Google Scholar] [CrossRef]
- Bethke, K.; Kropidłowska, K.; Stepnowski, P.; Caban, M. Review of warming and acidification effects to the ecotoxicity of pharmaceuticals on aquatic organisms in the era of climate change. Sci. Total Environ. 2023, 877, 162829. [Google Scholar] [CrossRef] [PubMed]

| Pharmaceutical | Therapeutic Class | Molecular Structure | Molecular Weight (g/mol) | Log Kow | RT (min) | Ion Transition (m/z) | CE (eV) | LOD | LOQ | Recovery (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sandprawn (µg/g) | Water (µg/L) | Water (µg/L) | Sandprawn (µg/g) | Sandprawn | Water | |||||||||||
| Acetaminophen | Analgesics and antipyretics |  | 151.16 | 1.10 | 3.22 | 152 > 93 | 152 > 110 | 24 | 0.43 | 0.01 | 1.30 | 0.03 | 98.5 | 98.1 | ||
| Amitriptyline | Tricyclic antidepressants |  | 277.40 | 4.81 | 5.45 | 278 > 117 | 278 > 233 | 20 | 0.07 | 0.005 | 0.22 | 0.017 | 98.5 | 98.9 | ||
| Bezafibrate | Fibrates |  | 361.82 | 3.81 | 9.06 | 360 > 274 | 360 > 274 | 20 | 0.80 | 0.02 | 2.50 | 0.05 | 98.9 | 99.0 | ||
| Carbamazepine | Anticonvulsant | 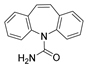 | 236.27 | 2.67 | 6.43 | 237 > 135 237 > 179 | 237 > 194 | 10 | 0.20 | 0.01 | 0.50 | 0.02 | 99.2 | 99.7 | ||
| Diclofenac | Nonsteroidal anti-inflammatory | 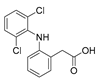 | 296.15 | 4.06 | 9.12 | 296 > 215 | 296 > 250 | 15 | 0.70 | 0.02 | 2.00 | 0.05 | 99.1 | 99.9 | ||
| Nevirapine | non-nucleoside reverse transcriptase inhibitors |  | 266.30 | 2.5 | 4.19 | 267 > 107 | 267 > 226 | 25 | 0.16 | 0.005 | 0.48 | 0.015 | 98.2 | 98.5 | ||
| Sulfamethoxazole | Antibiotic |  | 253.28 | 1.31 | 4.50 | 254 > 147 | 254 > 156 | 25 | 0.30 | 0.01 | 1.00 | 0.02 | 98.9 | 99.5 | ||
| 1 | 2 | 3 | 4 | 5 | ||
| Physico-chemical data | Temperature (°C) | 14.35 | 14.24 | 13.73 | 13.56 | 14.60 |
| Salinity (‰) | 16.76 | 16.84 | 15.39 | 15.92 | 18.19 | |
| Dissolved oxygen (mg/L) | 9.31 | 8.24 | 9.6 | 9.4 | 8.95 | |
| pH | 8.52 | 8.47 | 8.67 | 8.70 | 8.56 | |
| Depth (m) | 1.2 | 1.4 | 1.0 | 1.7 | 0.4 | |
| Chl-a (µg/L) | 81.6 | 54.5 | 54.4 | 51.2 | 20.7 | |
| Pharmaceuticals (µg/L) | Acetaminophen | 0.121 ± 0.0051 | 0.317 ± 0.0134 | 1.307 ± 0.0582 | 0.329 ± 0.0091 | 2.531 ± 0.0762 |
| Amitriptyline | 0.011 ± 0.0022 | 0.012 ± 0.0009 | 0.006 ± 0.0003 | 0.006 ± 0.0003 | 0.09 ± 0.0010 | |
| Bezafibrate | 0.022 ± 0.0055 | 0.027 ± 0.0021 | 0.019 ± 0.0031 | 0.015 ± 0.0033 | 0.028 ± 0.0013 | |
| Carbamazepine | 0.071 ± 0.0205 | 0.067 ± 0.0096 | 0.157 ± 0.0586 | 0.100 ± 0.0055 | 0.082 ± 0.0144 | |
| Diclofenac | 0.016 ± 0.0057 | 0.037 ± 0.0132 | 0.040 ± 0.0060 | 0.016 ± 0.0032 | 0.021 ± 0.0034 | |
| Nevirapine | 0.009 ± 0.0006 | 0.009 ± 0.0007 | 0.009 ± 0.0006 | 0.007 ± 0.0003 | 0.008 ± 0.0006 | |
| Sulfamethoxazole | 0.069 ± 0.0129 | 0.094 ± 0.0056 | 0.093 ± 0.0163 | 0.085 ± 0.0038 | 0.138 ± 0.0027 |
| Sample | Acetaminophen (µg/g) dw | Amitriptyline (µg/g) dw | Bezafibrate (µg/g) dw | Carbamazepine (µg/g) dw | Diclofenac (µg/g) dw | Nevirapine (µg/g) dw | Sulfamethoxazole (µg/g) dw |
|---|---|---|---|---|---|---|---|
| A | 14.398 | 0.003 | 0.005 | 2.612 | 0.018 | 0.359 | 2.094 |
| B | 7.194 | 0 | 0 | 5.695 | 0.040 | 0 | 0.520 |
| C | 12.334 | 0 | 0 | 1.879 | 0.013 | 0 | 0.480 |
| Bioaccumulation Factor (L/kg) | 4469 * | 92 | 60 | 41,257 ** | 1148 | 43,775 ** | 7450 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murgatroyd, O.; Petrik, L.; Ojemaye, C.Y.; Pillay, D. Persistent Pharmaceuticals in a South African Urban Estuary and Bioaccumulation in Endobenthic Sandprawns (Kraussillichirus kraussi). Water 2025, 17, 2289. https://doi.org/10.3390/w17152289
Murgatroyd O, Petrik L, Ojemaye CY, Pillay D. Persistent Pharmaceuticals in a South African Urban Estuary and Bioaccumulation in Endobenthic Sandprawns (Kraussillichirus kraussi). Water. 2025; 17(15):2289. https://doi.org/10.3390/w17152289
Chicago/Turabian StyleMurgatroyd, Olivia, Leslie Petrik, Cecilia Y. Ojemaye, and Deena Pillay. 2025. "Persistent Pharmaceuticals in a South African Urban Estuary and Bioaccumulation in Endobenthic Sandprawns (Kraussillichirus kraussi)" Water 17, no. 15: 2289. https://doi.org/10.3390/w17152289
APA StyleMurgatroyd, O., Petrik, L., Ojemaye, C. Y., & Pillay, D. (2025). Persistent Pharmaceuticals in a South African Urban Estuary and Bioaccumulation in Endobenthic Sandprawns (Kraussillichirus kraussi). Water, 17(15), 2289. https://doi.org/10.3390/w17152289





