Photocatalytic Degradation of Typical Fibrates by N and F Co-Doped TiO2 Nanotube Arrays Under Simulated Sunlight Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of the NF-TNAs
2.3. Fibrates Degradation Experiments
2.4. Analytical Methods
3. Results and Discussions
3.1. Characterization of NF-TNA
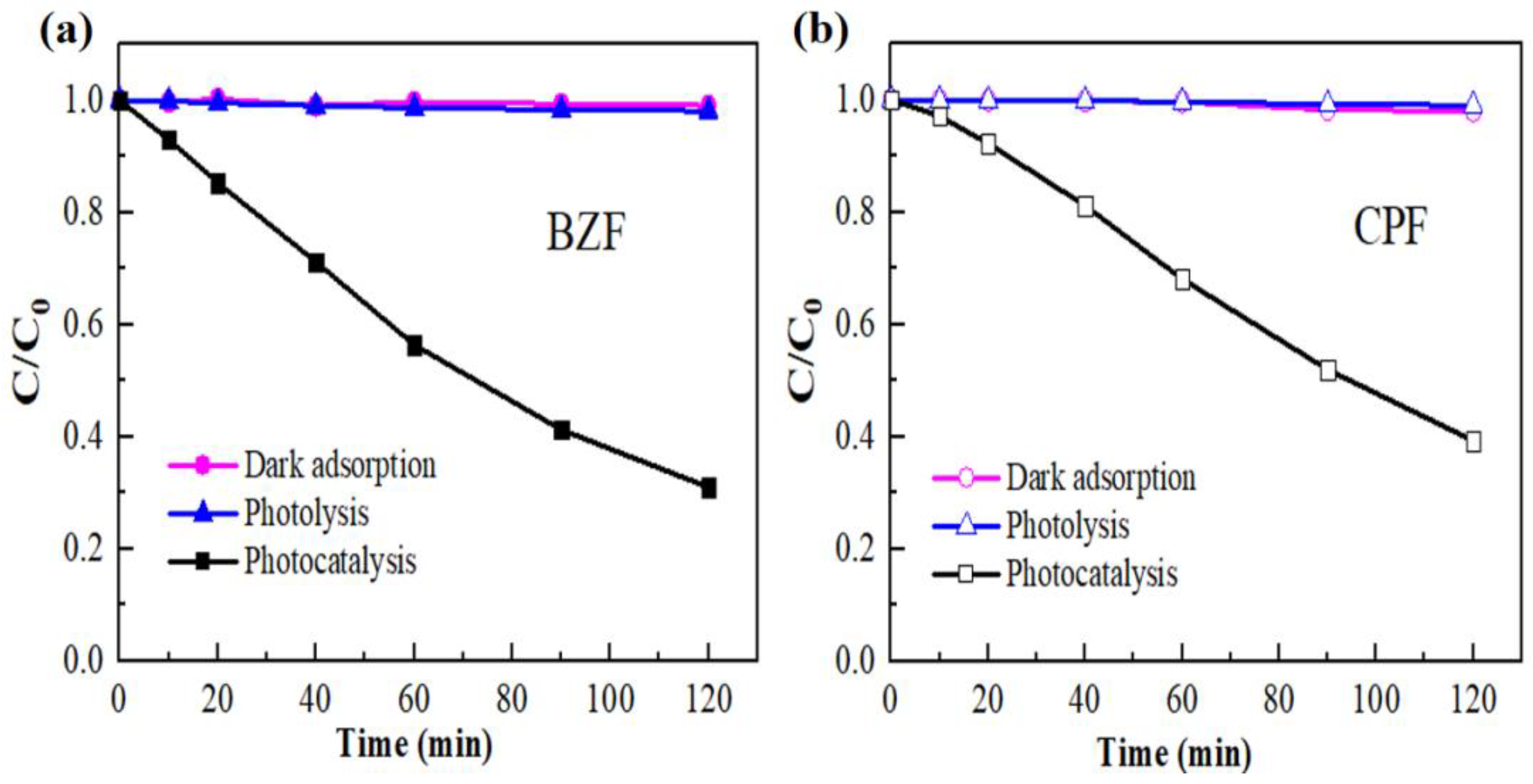
3.2. Photocatalytic Performance of NF-TNA Toward BZF and CPF Degradation
3.3. Insights into Reaction Mechanism
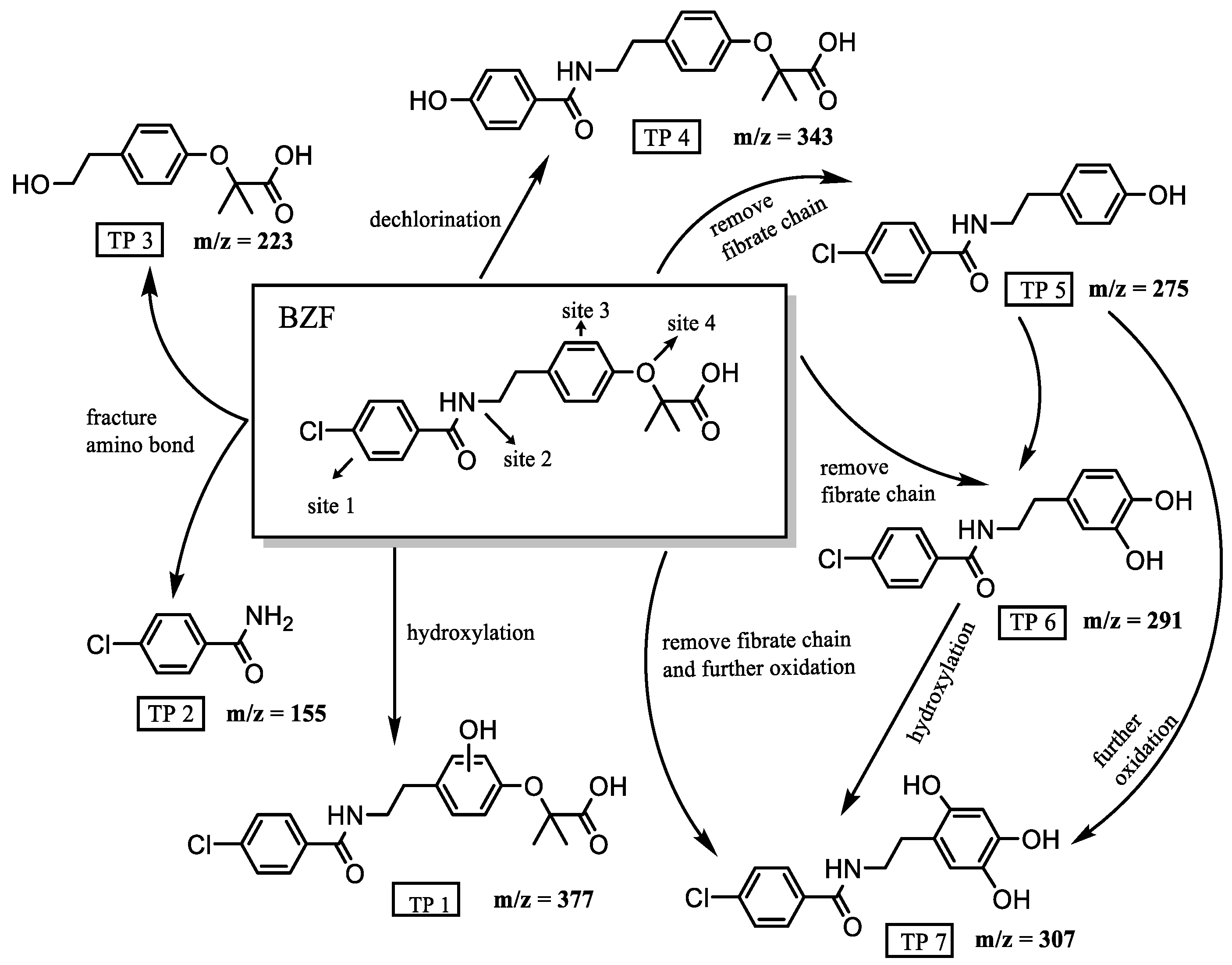
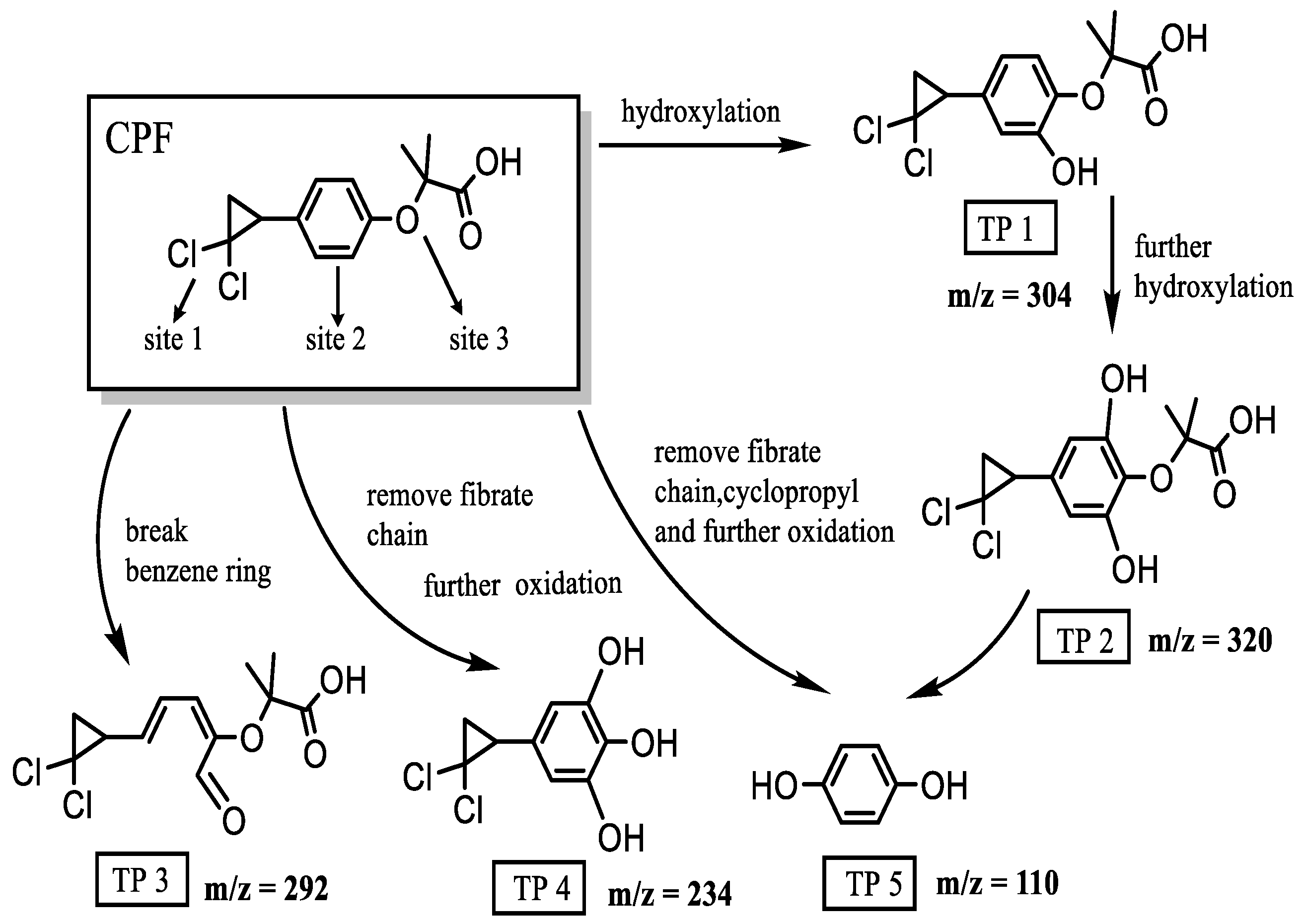
3.4. Possible Degradation Pathways of BZF/CPF During Photocatalysis
3.5. Influence of Environmental Factors and Stability Evaluation
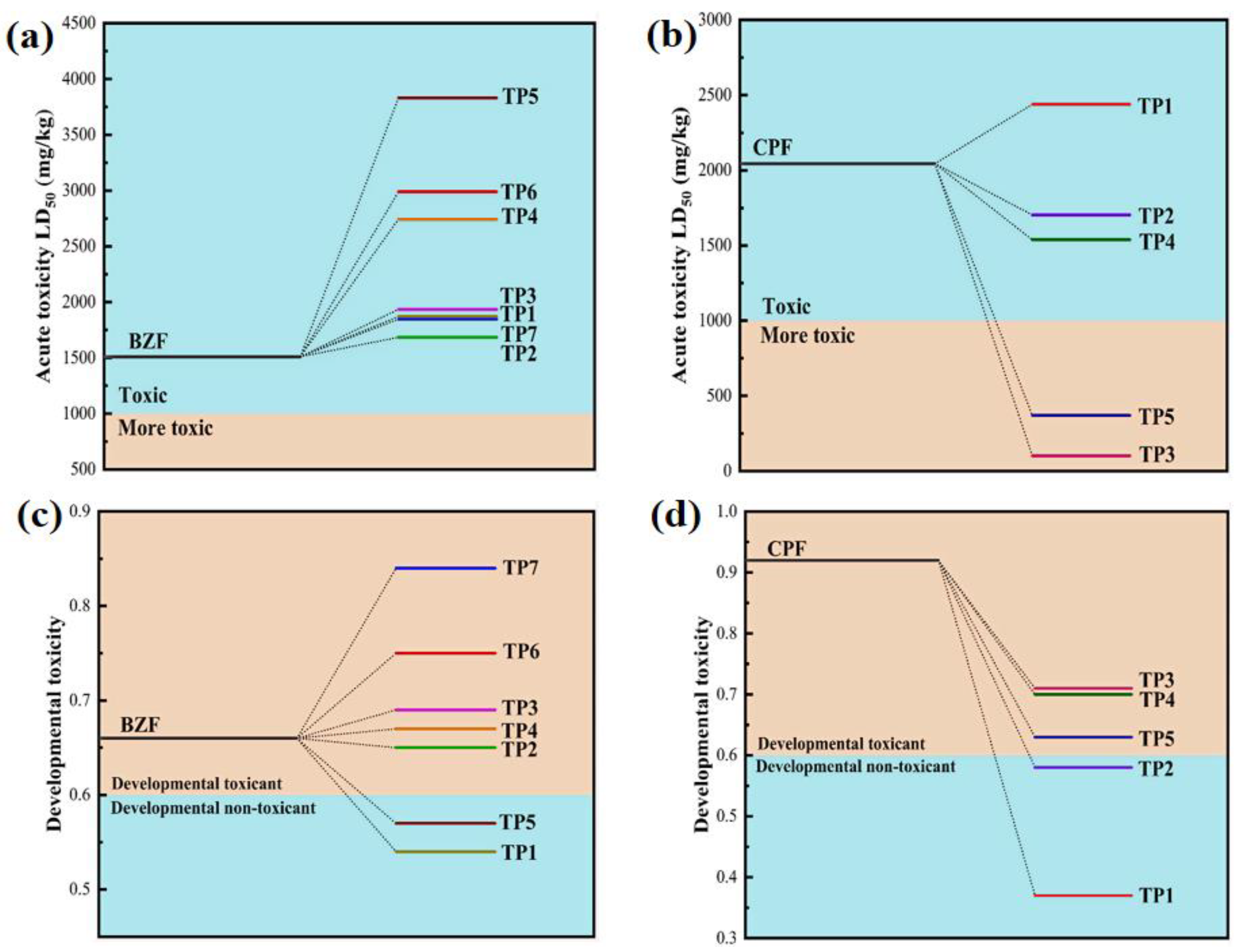
3.6. Toxicity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tete, V.S.; Nyoni, H.; Mamba, B.B.; Msagati, T.A.M. Occurrence and spatial distribution of statins, fibrates and their metabolites in aquatic environments. Arab. J. Chem. 2020, 13, 4358–4373. [Google Scholar] [CrossRef]
- Giebultowicz, J.; Stankiewicz, A.; Wroczynski, P.; Nalecz-Jawecki, G. Occurrence of cardiovascular drugs in the sewage-impacted Vistula River and in tap water in the Warsaw region (Poland). Environ. Sci. Pollut. Res. Int. 2016, 23, 24337–24349. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Occurrence and removal of PPCPs in municipal and hospital wastewaters in Greece. J. Hazard. Mater. 2010, 179, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Ido, A.; Hiromori, Y.H.; Meng, L.P.; Usuda, H.; Nagase, H.; Yang, M.; Hu, J.Y.; Nakanishi, T.S. Occurrence of fibrates and their metabolites in source and drinking water in Shanghai and Zhejiang, China. Sci. Rep. 2017, 7, 45931. [Google Scholar] [CrossRef]
- Gworek, B.; Kijeńska, M.; Zaborowska, M.; Wrzosek, J.; Tokarz, L.; Chmielewski, J. Occurrence of pharmaceuticals in aquatic environment—A review. Desalination Water Treat. 2020, 184, 375–387. [Google Scholar] [CrossRef]
- Oliveira, M.A.d.; Silva, G.D.d.; Campos, M.S.T. Chemical degradation kinetics of fibrates: Bezafibrate, ciprofibrate and fenofibrate. Braz. J. Pharm. Sci. 2016, 52, 545–553. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Maldonado-Torres, S.; Gurung, R.; Rijal, H.; Chan, A.; Acharya, S.; Rogelj, S.; Piyasena, M.; Rubasinghege, G. Fate, Transformation, and Toxicological Impacts of Pharmaceutical and Personal Care Products in Surface Waters. Environ. Health Insights 2018, 12, 117863021879583. [Google Scholar] [CrossRef]
- Lee, D.-E.; Khan, A.; Husain, A.; Anwar, K.; Lee, H.-C.; Shin, S.-H.; Lee, J.-Y.; Danish, M.; Jo, W.-K. Synergistic performance of Z-scheme based g-CN/Zn0.5Cd0.5S/GO nanocomposite towards sustainable model organic pollutants detoxification: Mechanistic insights and practical implications. J. Alloys Compd. 2025, 1022, 179820. [Google Scholar] [CrossRef]
- Liang, X.; Yu, S.; Meng, B.; Wang, X.; Yang, C.; Shi, C.; Ding, J. Advanced TiO2-Based Photoelectrocatalysis: Material Modifications, Charge Dynamics, and Environmental–Energy Applications. Catalysts 2025, 15, 542. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, X.; Li, J.; Cheng, S.; Zhang, J.; Ma, Y. Recent progress in TiO2–biochar-based photocatalysts for water contaminants treatment: Strategies to improve photocatalytic performance. Rsc. Adv. 2024, 14, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Bruning, H.; Li, X.; Yntema, D.; Rijnaarts, H.H.M. Significant enhancement of micropollutant photocatalytic degradation using a TiO2 nanotube array photoanode based photocatalytic fuel cell. Chem. Eng. J. 2018, 354, 553–562. [Google Scholar] [CrossRef]
- Feng, C.; Raziq, F.; Huang, H.; Wu, Z.P.; Alqahtani, H.S.; Alqahtani, R.; Rahman, M.Z.; Chang, B.; Gascon, J.; Zhang, H. Shining Light on Hydrogen: Solar-Powered Catalysis with Transition Metals. Adv. Mater. 2025, 37, e2410387. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.; Fu, P.; Shah, L.A.; Khan, H.M.; Nisar, J.; Ismail, M.; Zhang, P. VUV-Photocatalytic Degradation of Bezafibrate by Hydrothermally Synthesized Enhanced {001} Facets TiO2/Ti Film. J. Phys. Chem. A 2016, 120, 118–127. [Google Scholar] [CrossRef]
- Lambropoulou, D.A.; Hemando, M.D.; Konstantinou, I.K.; Thurman, E.M.; Ferrer, I.; Albanis, T.A.; Fernandez-Alba, A.R. Identification of photocatalytic degradation products of bezafibrate in TiO2 aqueous suspensions by liquid and gas chromatography. J. Chromatogr. A 2008, 1183, 38–48. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Yamada, T.; Gao, Y.F.; Nagai, M. Hydrothermal synthesis and evaluation of-visible-light-active photocatalyst of (N, F)-codoped anatase TiO2 from an F-containing titanium chemical. J. Ceram. Soc. Jpn. 2008, 116, 614–618. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, Z.; Xu, Y.; Ghazzal, M.N.; Colbeau-Justin, C.; Pan, D.; Wu, W. A Facile Strategy for the Preparation of N-Doped TiO2 with Oxygen Vacancy via the Annealing Treatment with Urea. Nanomaterials 2024, 14, 818. [Google Scholar] [CrossRef]
- Yadav, V.; Saini, V.K.; Sharma, H. How different dopants leads to difference in photocatalytic activity in doped TiO2? Ceram. Int. 2020, 46, 27308–27317. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of Titanium Dioxide (TiO2) Modification on Its Application to Pollution Treatment—A Review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Ganguli, S.; Sabur, M.A. Nitrogen doped titanium dioxide (N-TiO2): Electronic band structure, visible light harvesting and photocatalytic applications. J. Water Process Eng. 2023, 55, 104183. [Google Scholar] [CrossRef]
- Xia, B.; Yao, J.-j.; Han, C.-x.; Zhang, Z.; Chen, X.-y.; Fang, Y.-j. Degradation of ofloxacin by UVA-LED/TiO2 nanotube arrays photocatalytic fuel cells. Chem. Pap. 2017, 72, 359–368. [Google Scholar] [CrossRef]
- Chen, X.; Yao, J.; Dong, H.; Hong, M.; Gao, N.; Zhang, Z.; Jiang, W. Enhanced bezafibrate degradation and power generation via the simultaneous PMS activation in visible light photocatalytic fuel cell. Water Res. 2021, 207, 117800. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.M.; Marquez, G.; Tena, M.; Alvarez, P.M.; Beltran, F.J. Determination of main species involved in the first steps of TiO2 photocatalytic degradation of organics with the use of scavengers: The case of ofloxacin. Appl. Catal. B-Environ. 2015, 178, 44–53. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, W.; Zhang, Q.; Yu, H.; Liu, M.; Ni, S.; Gao, B.; Xu, S. Evaluation of practical application potential of a photocatalyst: Ultimate apparent photocatalytic activity. Chemosphere 2021, 285, 131323. [Google Scholar] [CrossRef]
- Lee, D.-E.; Danish, M.; Jo, W.-K. Two in one is really better: NiAl-LDH/[CoNi(μ3-tp)2(μ2-py)2] nanocomposite for enhanced antibiotic norfloxacin degradation and H2 evolution reaction. Sep. Purif. Technol. 2024, 332, 125748. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09; Gaussian Inc: Wallingford, CT, USA, 2009. [Google Scholar]
- Lu, T.; Chen, F.W. Calculation of Molecular Orbital Composition. Acta Chim. Sin. 2011, 69, 2393–2406. [Google Scholar]
- Kumar, N.; Maitra, U.; Hegde, V.I.; Waghmare, U.V.; Sundaresan, A.; Rao, C.N. Synthesis, characterization, photocatalysis, and varied properties of TiO2 cosubstituted with nitrogen and fluorine. Inorg. Chem. 2013, 52, 10512–10519. [Google Scholar] [CrossRef]
- Mittal, A.; Mari, B.; Sharma, S.; Kumari, V.; Maken, S.; Kumari, K.; Kumar, N. Non-metal modified TiO2: A step towards visible light photocatalysis. J. Mater. Sci.-Mater. El. 2019, 30, 3186–3207. [Google Scholar] [CrossRef]
- Wu, G.; Wen, J.; Nigro, S.; Chen, A. One-step synthesis of N- and F-codoped mesoporous TiO2 photocatalysts with high visible light activity. Nanotechnology 2010, 21, 85701. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.L.; Dai, Z.Y.; Bai, X.; Ye, Z.F.; Gu, H.X.; Huang, X. A facile one-step electrochemical strategy of doping iron, nitrogen, and fluorine into titania nanotube arrays with enhanced visible light photoactivity. J. Hazard. Mater. 2015, 293, 112–121. [Google Scholar] [CrossRef]
- Li, Q.; Shang, J.K. Self-organized nitrogen and fluorine co-doped titanium oxide nanotube arrays with enhanced visible light photocatalytic performance. Environ. Sci. Technol. 2009, 43, 8923–8929. [Google Scholar] [CrossRef]
- Orge, C.A.; Fernando, R.; Pereira, M.; Faria, J.L. Bezafibrate removal by coupling ozonation and photocatalysis: Effect of experimental conditions. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100610. [Google Scholar] [CrossRef]
- Vasanthkumar, K.; Porkodi, K.; Selvaganapathi, A. Constrain in solving Langmuir–Hinshelwood kinetic expression for the photocatalytic degradation of Auramine O aqueous solutions by ZnO catalyst. Dye. Pigment. 2007, 75, 246–249. [Google Scholar] [CrossRef]
- Khezrianjoo, S. Photodestruction of Direct Yellow 11 in aqueous TiO2 suspension: Effect of operational parameters on detoxification, Langmuir–Hinshelwood kinetic expression and biodegradability. Desalination Water Treat. 2019, 153, 264–278. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, J.; Zhang, Y.N.; Qu, J.; Li, C.; Qin, W.; Zhao, Y.; Chen, J.; Peijnenburg, W. Trace amounts of fenofibrate acid sensitize the photodegradation of bezafibrate in effluents: Mechanisms, degradation pathways, and toxicity evaluation. Chemosphere 2019, 235, 900–907. [Google Scholar] [CrossRef]
- Ge, M.Z.; Cao, C.Y.; Huang, J.Y.; Li, S.H.; Zhang, S.N.; Deng, S.; Li, Q.S.; Zhang, K.Q.; Lai, Y.K. Synthesis, modification, and photo/photoelectrocatalytic degradation applications of TiO2 nanotube arrays: A review. Nanotechnol. Rev. 2016, 5, 75–112. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.; Song, W.; Cooper, W.J.; Greaves, J.; Jeong, J. Free-radical-induced oxidative and reductive degradation of fibrate pharmaceuticals: Kinetic studies and degradation mechanisms. J. Phys. Chem. A 2009, 113, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Tian, Y.; Gao, P.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. Photodegradation mechanism and genetic toxicity of bezafibrate by Pd/g-C3N4 catalysts under simulated solar light irradiation: The role of active species. Chem. Eng. J. 2020, 379, 122294. [Google Scholar] [CrossRef]
- Solis, R.R.; Rivas, F.J.; Chavez, A.M.; Dionysiou, D.D. Simulated solar photo-assisted decomposition of peroxymonosulfate. Radiation filtering and operational variables influence on the oxidation of aqueous bezafibrate. Water Res. 2019, 162, 383–393. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, J.; Zhao, Q.; Xie, Q.; Wei, X. Unveiling self-sensitized photodegradation pathways by DFT calculations: A case of sunscreen p-aminobenzoic acid. Chemosphere 2016, 163, 227–233. [Google Scholar] [CrossRef]
- Rodrigues, A.; Brito, A.; Janknecht, P.; Proenca, M.F.; Nogueira, R. Quantification of humic acids in surface water: Effects of divalent cations, pH, and filtration. J. Environ. Monit. 2009, 11, 377–382. [Google Scholar] [CrossRef]
- Mostafa, E.; Reinsberg, P.; Garcia-Segura, S.; Baltruschat, H. Chlorine species evolution during electrochlorination on boron-doped diamond anodes: In-situ electrogeneration of Cl2, Cl2O and ClO2. Electrochim. Acta 2018, 281, 831–840. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Mártire, D.O.; Gonzalez, M.C. Aqueous Phase Kinetic Studies Involving Intermediates of Environmental Interest: Phosphate Radicals and Their Reactions with Substituted Benzenes. Prog. React. Kinet. Mech. 2001, 26, 201–218. [Google Scholar] [CrossRef]
- Liu, T.; Yin, K.; Liu, C.; Luo, J.; Crittenden, J.; Zhang, W.; Luo, S.; He, Q.; Deng, Y.; Liu, H.; et al. The role of reactive oxygen species and carbonate radical in oxcarbazepine degradation via UV, UV/H2O2: Kinetics, mechanisms and toxicity evaluation. Water Res. 2018, 147, 204–213. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziolek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B-Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Goncalves, A.; Orfao, J.J.M.; Pereira, M.F.R. Ozonation of bezafibrate promoted by carbon materials. Appl. Catal. B-Environ. 2013, 140, 82–91. [Google Scholar] [CrossRef]





| Fibrate Drug | Quencher | K20 (min−1) | K120 (min−1) | R2120 | Total Removal (120 min) |
|---|---|---|---|---|---|
| BZF | Control group | 0.008 | 0.01 | 0.9982 | 69.01% |
| Tiron | 0.0017 | 0.003 | 0.9975 | 21.79% | |
| TBA | 0.0048 | 0.0041 | 0.9951 | 38.47% | |
| L-His | 0.0033 | 0.003 | 0.9984 | 30.26% | |
| EDTA·2Na | 0.0058 | 0.0055 | 0.9965 | 47.93% | |
| CPF | Control group | 0.004 | 0.008 | 0.9879 | 60.72% |
| Tiron | 0.0012 | 0.0013 | 0.999 | 14.68% | |
| TBA | 0.0025 | 0.0039 | 0.9934 | 36.70% | |
| L-His | 0.0023 | 0.0032 | 0.9756 | 31.91% | |
| EDTA·2Na | 0.003 | 0.0046 | 0.995 | 42.15% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhong, H.; Yao, J.; Gan, J.; Cong, H.; Zhu, T. Photocatalytic Degradation of Typical Fibrates by N and F Co-Doped TiO2 Nanotube Arrays Under Simulated Sunlight Irradiation. Water 2025, 17, 2261. https://doi.org/10.3390/w17152261
Chen X, Zhong H, Yao J, Gan J, Cong H, Zhu T. Photocatalytic Degradation of Typical Fibrates by N and F Co-Doped TiO2 Nanotube Arrays Under Simulated Sunlight Irradiation. Water. 2025; 17(15):2261. https://doi.org/10.3390/w17152261
Chicago/Turabian StyleChen, Xiangyu, Hao Zhong, Juanjuan Yao, Jingye Gan, Haibing Cong, and Tengyi Zhu. 2025. "Photocatalytic Degradation of Typical Fibrates by N and F Co-Doped TiO2 Nanotube Arrays Under Simulated Sunlight Irradiation" Water 17, no. 15: 2261. https://doi.org/10.3390/w17152261
APA StyleChen, X., Zhong, H., Yao, J., Gan, J., Cong, H., & Zhu, T. (2025). Photocatalytic Degradation of Typical Fibrates by N and F Co-Doped TiO2 Nanotube Arrays Under Simulated Sunlight Irradiation. Water, 17(15), 2261. https://doi.org/10.3390/w17152261






