Nutrient Recovery from Dairy Processing Wastewater Using Biochar
Abstract
1. Introduction
2. Materials and Methods
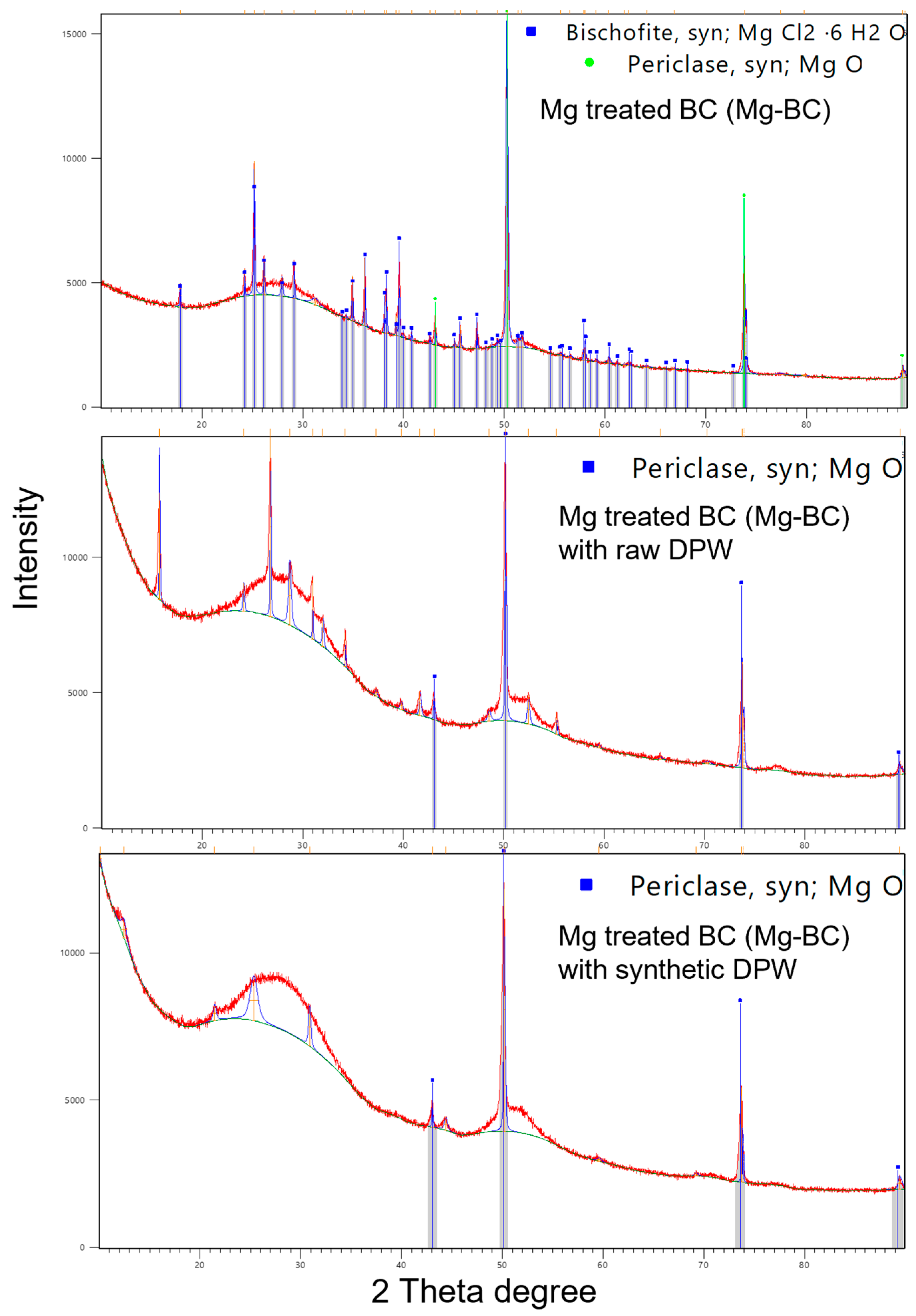
2.1. Biochar Synthesis
2.2. Wastewater Sample Collection
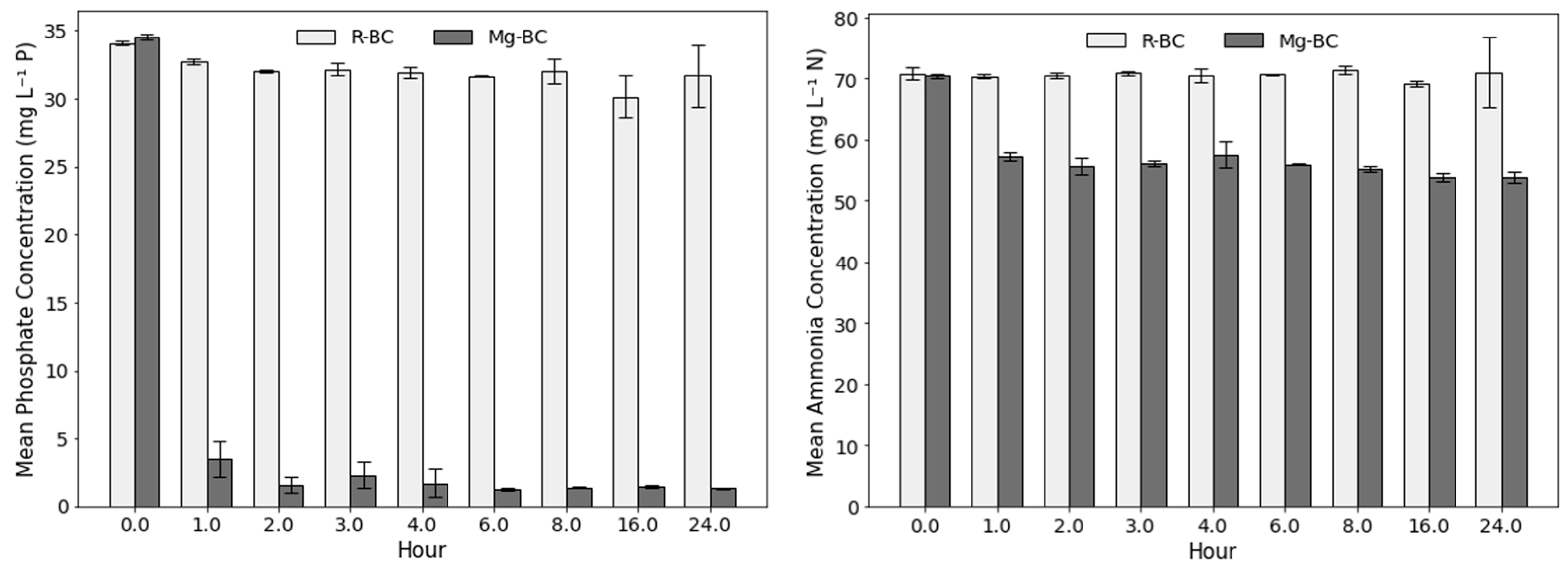
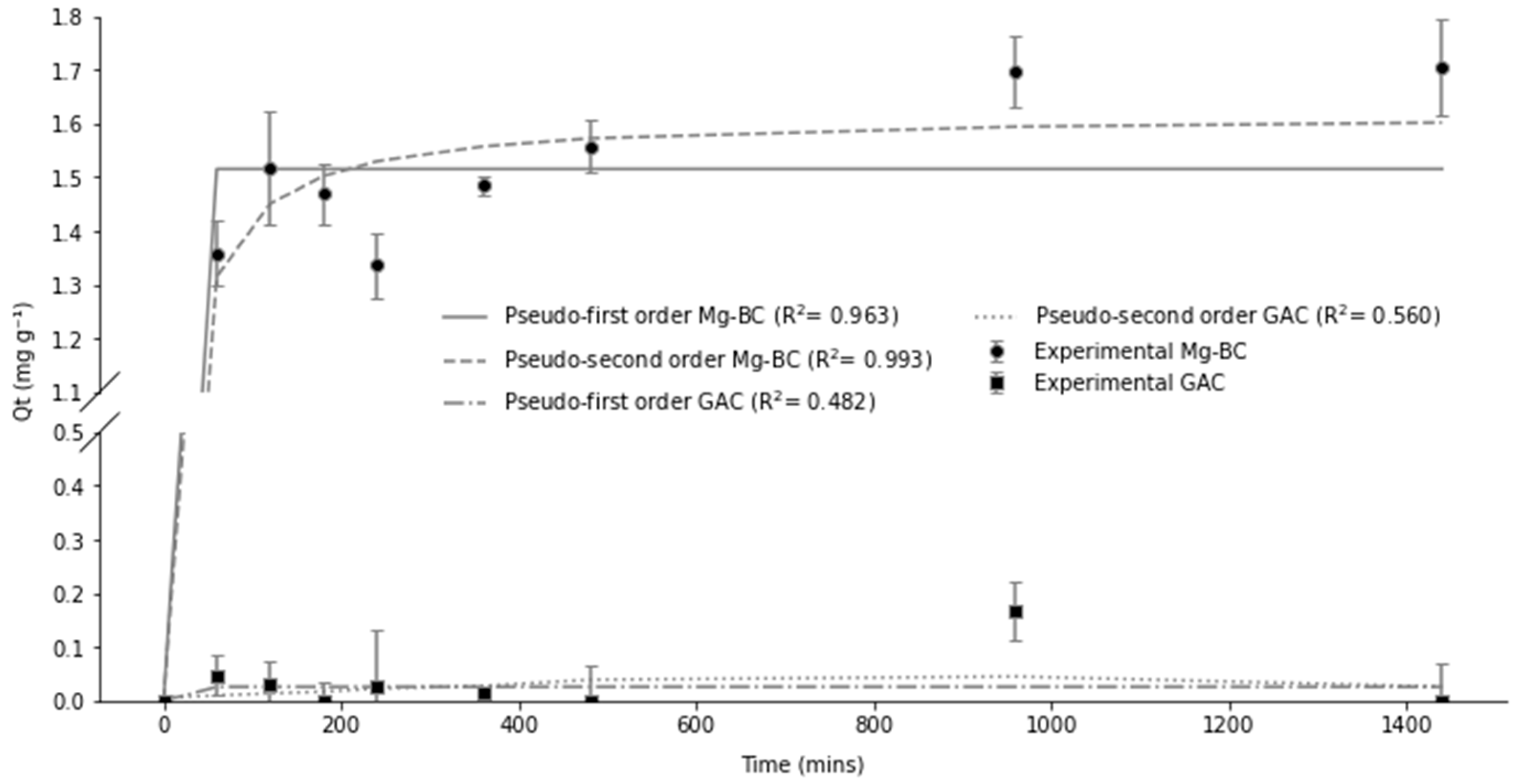
2.3. Adsorption and Kinetic Experiments
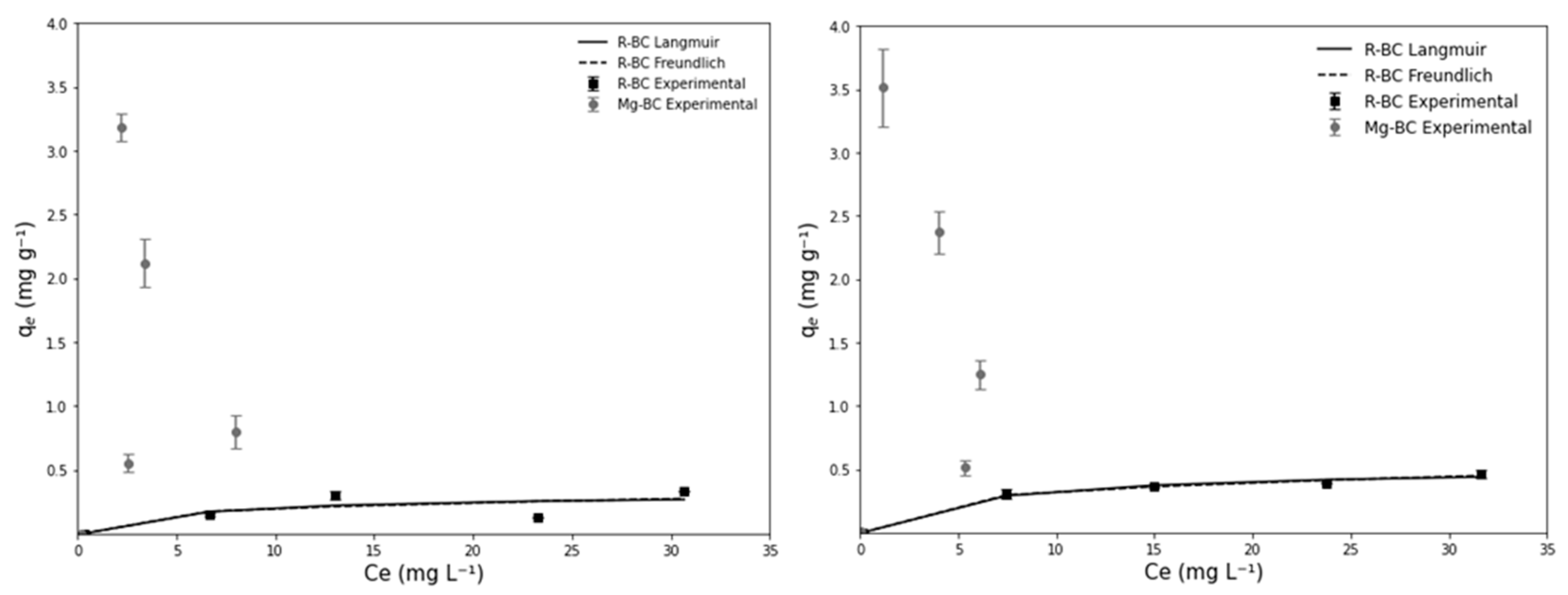
2.4. Adsorption Isotherms Experiments
2.5. Sampling and Analysis
3. Results and Discussion
3.1. Adsorption and Kinetics of Biochar
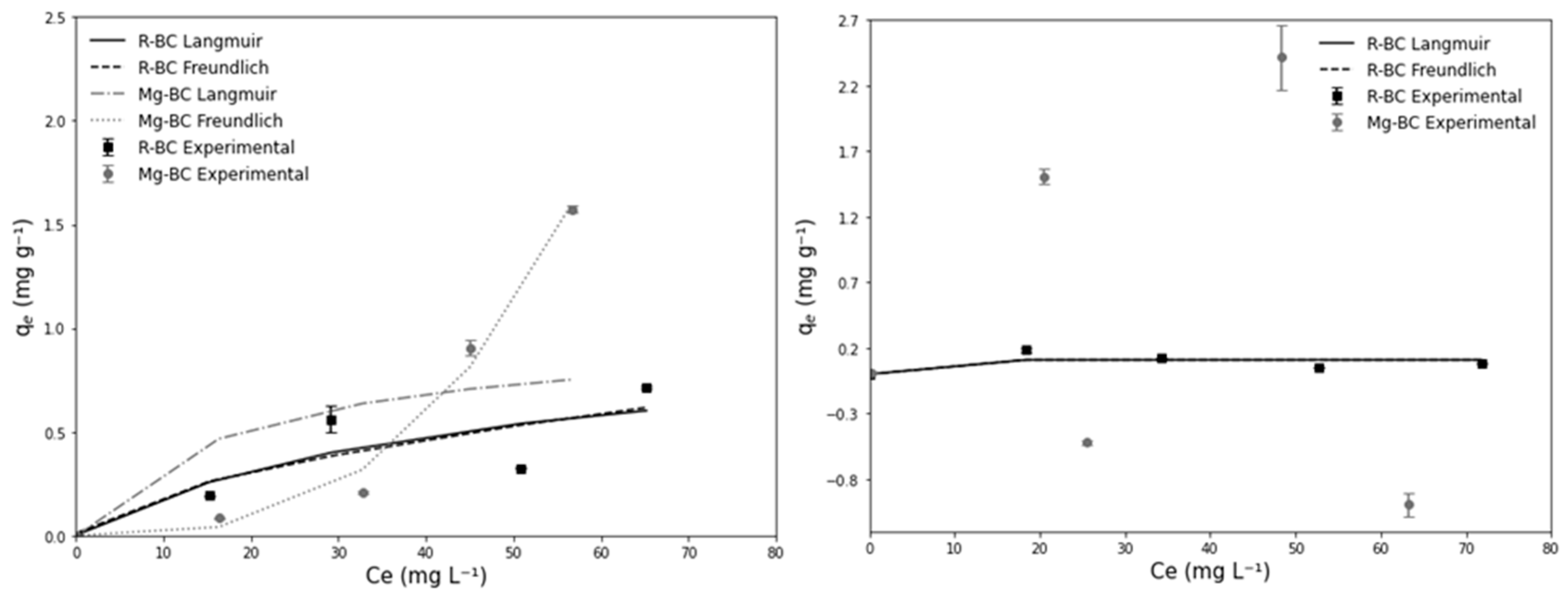
3.2. Adsorption Isotherms
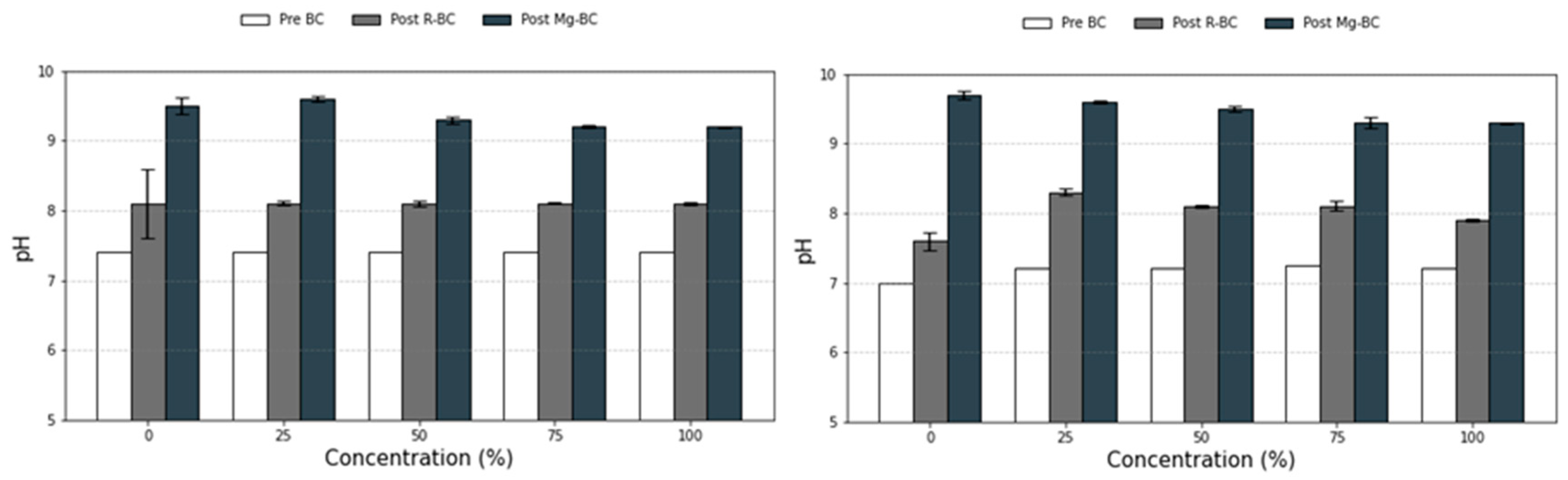
3.3. Effect of Solution pH on Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Mahoney, R.; Coughlan, N.E.; Walsh, É.; Jansen, M.A.K. Cultivation of lemna minor on industry-derived, anaerobically digested, dairy processing wastewater. Plants 2022, 11, 3027. [Google Scholar] [CrossRef]
- Leonard, P.; Clifford, E.; Finnegan, W.; Siggins, A.; Zhan, X. Deployment and optimisation of a pilot-scale IASBR system for treatment of dairy processing wastewater. Energies 2021, 14, 7365. [Google Scholar] [CrossRef]
- Cordell, D.; Naset, T.S.S. Phosphorus vulnerability: A qualitative framework for assessing the vulnerability of national and regional food systems to the multi-dimensional stressors of phosphorus scarcity. Glob. Environ. Change 2014, 24, 108–122. [Google Scholar] [CrossRef]
- Kumar, R.; Pal, P. Assessing the feasibility of N and P recovery by struvite precipitation from nutrient-rich wastewater: A review. Environ. Sci. Pollut. Res. 2015, 22, 17453–17464. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Shim, S.; Won, S.; Ra, C. Struvite recovered from various types of wastewaters: Characteristics, soil leaching behaviour, and plant growth. Land. Degrad. Dev. 2018, 29, 2864–2879. [Google Scholar] [CrossRef]
- McIntosh, S.; Hunt, L.; Thompson Brewster, E.; Rose, A.; Thornton, A.; Erler, D. Struvite Production from Dairy Processing Waste. Sustainability 2022, 14, 15807. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Jang, H.M.; Kan, E.; Wallace, A.R.; Sun, W. Adsorption of phosphate in water on a novel calcium hydroxide-coated dairy manure-derived biochar. Environ. Eng. Res. 2019, 24, 434–442. [Google Scholar] [CrossRef]
- Yesigat, A.; Worku, A.; Mekonnen, A.; Bae, W.; Feyisa, G.L.; Gatew, S.; Han, J.-L.; Liu, W.; Wang, A.; Guadie, A. Phosphorus recovery as K-struvite from a waste stream: A review of influencing factors, advantages, disadvantages and challenges. Environ. Res. 2022, 214, 114086. [Google Scholar] [CrossRef] [PubMed]
- Prakongkep, N.; Gilkes, R.J.; Wiriyakitnateekul, W. Forms and solubility of plant nutrient elements in tropical plant waste biochars. J. Plant Nutr. Soil. Sci. 2015, 178, 732–740. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ye, Z.-L.; Chen, S. Engineered biochars for recovering phosphate and ammonium from wastewater: A review. Sci. Total Environ. 2021, 779, 146240. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Tait, S.; Astals, S.; del Valle-Zermeño, R.; Martínez, M.; Mata-Alvarez, J.; Chimenos, J.M. Reagent use efficiency with removal of nitrogen from pig slurry via struvite: A study on magnesium oxide and related by-products. Water Res. 2015, 84, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, C.-Y.; Zhou, H.-D.; Xue, D.-X.; Xiong, X.-L.; Zhu, G. Simultaneous adsorption of ammonia nitrogen and phosphate on electro-assisted magnesium/aluminum-loaded sludge-based biochar and its utilization as a plant fertilizer. PLoS ONE 2024, 19, e0311430. [Google Scholar] [CrossRef]
- Xia, P.; Wang, X.; Wang, X.; Song, J.; Wang, H.; Zhang, J.; Zhao, J. Struvite crystallization combined adsorption of phosphate and ammonium from aqueous solutions by mesoporous MgO-loaded diatomite. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 220–227. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Liu, Y.; Zhang, X.; Guo, J.; Ni, B.-j.; Chang, S.W.; Nguyen, D.D. Insight into biological phosphate recovery from sewage. Bioresour. Technol. 2016, 218, 874–881. [Google Scholar] [CrossRef]
- Jung, K.-W.; Ahn, K.-H. Fabrication of porosity-enhanced MgO/biochar for removal of phosphate from aqueous solution: Application of a novel combined electrochemical modification method. Bioresour. Technol. 2016, 200, 1029–1032. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, J.J.; Zhou, B.; Zhang, Z.; Liu, S.; Lei, S.; Xiao, R. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J. Clean. Prod. 2017, 147, 96–107. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Zhang, M.; Inyang, M.; Li, Y.; Alva, A.; Yang, L. Engineered carbon (biochar) prepared by direct pyrolysis of Mg-accumulated tomato tissues: Characterization and phosphate removal potential. Bioresour. Technol. 2013, 138, 8–13. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, D.; Wu, Z.; Lv, Y.; Li, S. Magnesium-modified biochar was used to adsorb phosphorus from wastewater and used as a phosphorus source to be recycled to reduce the ammonia nitrogen of piggery digestive wastewater. J. Clean. Prod. 2022, 360, 132130. [Google Scholar] [CrossRef]
- Nardis, B.O.; Carneiro, J.S.D.S.; De Souza, I.M.G.; De Barros, R.G.; Melo, L.C.A. Phosphorus recovery using magnesium-enriched biochar and its potential use as fertilizer. Arch. Agron. Soil. Sci. 2021, 67, 1017–1033. [Google Scholar] [CrossRef]
- Zheng, Y.; Zimmerman, A.R.; Gao, B. Comparative investigation of characteristics and phosphate removal by engineered biochars with different loadings of magnesium, aluminum, or iron. Sci. Total Environ. 2020, 747, 141277. [Google Scholar] [CrossRef] [PubMed]
- Kizito, S.; Luo, H.; Wu, S.; Ajmal, Z.; Lv, T.; Dong, R. Phosphate recovery from liquid fraction of anaerobic digestate using four slow pyrolyzed biochars: Dynamics of adsorption, desorption and regeneration. J. Environ. Manag. 2017, 201, 260–267. [Google Scholar] [CrossRef]
- Samaraweera, H.; Palansooriya, K.N.; Dissanayake, P.D.; Khan, A.H.; Sillanpää, M.; Mlsna, T. Sustainable phosphate removal using Mg/Ca-modified biochar hybrids: Current trends and future outlooks. Case Stud. Chem. Environ. Eng. 2023, 8, 100528. [Google Scholar] [CrossRef]
- Luo, Z.; Wen, H.; Zhang, H.; Li, Y.; Mai, X.; Zhang, Y.; Wang, J.; Li, Y.; Zhang, Z. Biogas residue biochar integrated with phosphate from its ash for the effective recovery of nutrients from piggery biogas slurry. Biochar 2022, 4, 23. [Google Scholar] [CrossRef]
- Cai, G.; Ye, Z.-l. Concentration-dependent adsorption behaviors and mechanisms for ammonium and phosphate removal by optimized Mg-impregnated biochar. J. Clean. Prod. 2022, 349, 131453. [Google Scholar] [CrossRef]
- Wang, S.E.; Sun, K.C.; Xiang, H.M.; Zhao, Z.Q.; Shi, Y.; Su, L.H.; Tan, C.Q.; Zhang, L.J. Biochar-seeded struvite precipitation for simultaneous nutrient recovery and chemical oxygen demand removal in leachate: From laboratory to pilot scale. Front. Chem. 2022, 10, 990321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Lalley, J.; Han, C.; Li, X.; Dionysiou, D.D.; Nadagouda, M.N. Phosphate adsorption using modified iron oxide-based sorbents in lake water: Kinetics, equilibrium, and column tests. Chem. Eng. J. 2016, 284, 1386–1396. [Google Scholar] [CrossRef]
- Xu, K.; Lin, F.; Dou, X.; Zheng, M.; Tan, W.; Wang, C. Recovery of ammonium and phosphate from urine as value-added fertilizer using wood waste biochar loaded with magnesium oxides. J. Clean. Prod. 2018, 187, 205–214. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Visual MINTEQ 4 User Guide; KTH, Department of Land and Water Resources: Stockholm, Sweden, 2011. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2017. [Google Scholar]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef]
- Finn, M.; Rodriguez, R.; Contrino, D.; Swenson, J.; Mazyck, D.W.; Suau, S. Impact of inherent magnesium in biochar for phosphate removal from reclaimed water streams. J. Environ. Eng. 2022, 148, 04021085. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, P.; Zhang, Z.; Liu, J.; Xiao, J.; Gao, F. Simultaneous removal of ammonia nitrogen and recovery of phosphate from swine wastewater by struvite electrochemical precipitation and recycling technology. J. Clean. Prod. 2016, 127, 302–310. [Google Scholar] [CrossRef]
- Kosmulski, M. Surface Charging and Points of Zero Charge; CRC press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater. 2011, 190, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Biochar derived from anaerobically digested sugar beet tailings: Characterization and phosphate removal potential. J. Bioresour. Technol. 2011, 102, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. J. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef]
- Huggins, T.M.; Haeger, A.; Biffinger, J.C.; Ren, Z.J. Granular biochar compared with activated carbon for wastewater treatment and resource recovery. Water Res. 2016, 94, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Tan, X.; Zhu, H.; Sun, T.; Wang, X. Effects of Commonly Occurring Metal Ions on Hydroxyapatite Crystallization for Phosphorus Recovery from Wastewater. Water 2018, 10, 1619. [Google Scholar] [CrossRef]
- Ramdani, A.; Kadeche, A.; Adjdir, M.; Taleb, Z.; Ikhou, D.; Taleb, S.; Deratani, A. Lead and cadmium removal by adsorption process using hydroxyapatite porous materials. Water Pract. Technol. 2020, 15, 130–141. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, T.; Song, W.; Xu, L.; Dong, J. Biochar of distillers’ grains anaerobic digestion residue: Influence of pyrolysis conditions on its characteristics and ammonium adsorptive optimization. Waste Manag. Res. J. A Sustain. Circ. Econ. 2019, 38, 86–97. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Biochar-supported nanomaterials for environmental applications. J. Ind. Eng. Chem. 2019, 78, 21–33. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Netshidzivhe, T.; Mamakoa, E.; Masindi, V.; Neomagus, H.W.J.P. Impact of magnesium source on the yield and structure of struvite. In Proceedings of the International Conference on Science, Engineering, Technology and Waste Management 2020, Johannesburg, South Africa, 16–17 November 2020. [Google Scholar]
- Kozik, A.; Hutnik, N.; Piotrowski, K.; Mazieńczuk, A.; Matynia, A. Precipitation and crystallization of struvite from synthetic wastewater under stoichiometric conditions. Adv. Chem. Eng. Sci. 2013, 3, 20–26. [Google Scholar] [CrossRef]
- Tasis, D.; Kastanis, D.; Galiotis, C.; Bouropoulos, N. Growth of calcium phosphate mineral on carbon nanotube buckypapers. Phys. Status Solidi 2006, 243, 3230–3233. [Google Scholar] [CrossRef]
- Vu, T.M.; Trinh, V.T.; Doan, D.P.; Van, H.T.; Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H. Removing ammonium from water using modified corncob-biochar. Sci. Total Environ. 2017, 579, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Voroney, R.P.; Price, G.W. Effects of temperature and activation on biochar chemical properties and their impact on ammonium, nitrate, and phosphate sorption. J. Environ. Qual. 2017, 46, 889–896. [Google Scholar] [CrossRef]
- Parasana, N.; Shah, M.; Unnarkat, A. Recent advances in developing innovative sorbents for phosphorus removal—Perspective and opportunities. Environ. Sci. Pollut. Res. 2022, 29, 38985–39016. [Google Scholar] [CrossRef]

| Parameters | Values |
|---|---|
| Particle size | 1.70–0.43 mm |
| Total Surface Area | 1150–1200 m2/g |
| Apparent Density | 0.50–0.52 g/cm3 |
| pH | 7–8 |
| Ash content (maximum) | 0.5% |
| Moisture content | 2% |
| Iodine Number | 1150–1200 mg/g |
| CTC No (Carbon Tetrachloride No) (minimum) | 60 |
| Elements | Concentration (mg/L) |
|---|---|
| Phosphorus | 31.8 |
| Ammonium | 72.4 |
| Iron | 1.12 |
| Silicon | 11.8 |
| Magnesium | 11.1 |
| Potassium | 28.8 |
| Sodium | 390 |
| Chloride | 37.4 |
| Sulfur | 21.4 |
| Calcium | 35 |
| Adsorption Kinetics | Pseudo-First-Order | Pseudo-Second-Order | ||
|---|---|---|---|---|
| K1 (h−1) | qe (mg g−1) | K2 (g mg−1 h−1) | qe (mg g−1) | |
| PO43−-P Raw R-BC | 0.0083 | 0.27 | 0.038 | 0.31 |
| PO43−-P Raw Mg–BC | 1 | 3.2 | 0.074 | 3.3 |
| NH4+-N Raw R-BC | 1 | 0.03 | 0.0217 | 0.07 |
| NH4+-N Raw Mg–BC | 0.6 | 1.5 | 0.0448 | 1.6 |
| Adsorption Isotherm | Q (mg g−1) | R2 (Langmuir) | R2 (Freundlich) |
|---|---|---|---|
| PO43−-P Raw R-BC | 0.3 | 0.63 | 0.63 |
| PO43−-P Raw Mg–BC | 1.8 | 0.34 | 0.1 |
| PO43−-P Synthetic R-BC | 0.5 | 0.99 | 0.99 |
| PO43−-P Synthetic Mg–BC | 1.9 | 0.36 | 0.07 |
| NH4+-N Raw R-BC | 1.0 | 0.72 | 0.72 |
| NH4+-N Raw Mg–BC | 1.0 | 0.42 | 0.98 |
| NH4+-N Synthetic R-BC | 0.1 | 0.50 | 0.50 |
| NH4+-N Synthetic Mg–BC | 0.6 | 0.03 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shapiro Ellis, T.; Rahman, M.S.; Ingram, M.; McIntosh, S.; Erler, D. Nutrient Recovery from Dairy Processing Wastewater Using Biochar. Water 2025, 17, 2250. https://doi.org/10.3390/w17152250
Shapiro Ellis T, Rahman MS, Ingram M, McIntosh S, Erler D. Nutrient Recovery from Dairy Processing Wastewater Using Biochar. Water. 2025; 17(15):2250. https://doi.org/10.3390/w17152250
Chicago/Turabian StyleShapiro Ellis, Toby, Md Sydur Rahman, Michael Ingram, Shane McIntosh, and Dirk Erler. 2025. "Nutrient Recovery from Dairy Processing Wastewater Using Biochar" Water 17, no. 15: 2250. https://doi.org/10.3390/w17152250
APA StyleShapiro Ellis, T., Rahman, M. S., Ingram, M., McIntosh, S., & Erler, D. (2025). Nutrient Recovery from Dairy Processing Wastewater Using Biochar. Water, 17(15), 2250. https://doi.org/10.3390/w17152250







