Recent Progress in Selenium Remediation from Aqueous Systems: State-of-the-Art Technologies, Challenges, and Prospects
Abstract
1. Introduction
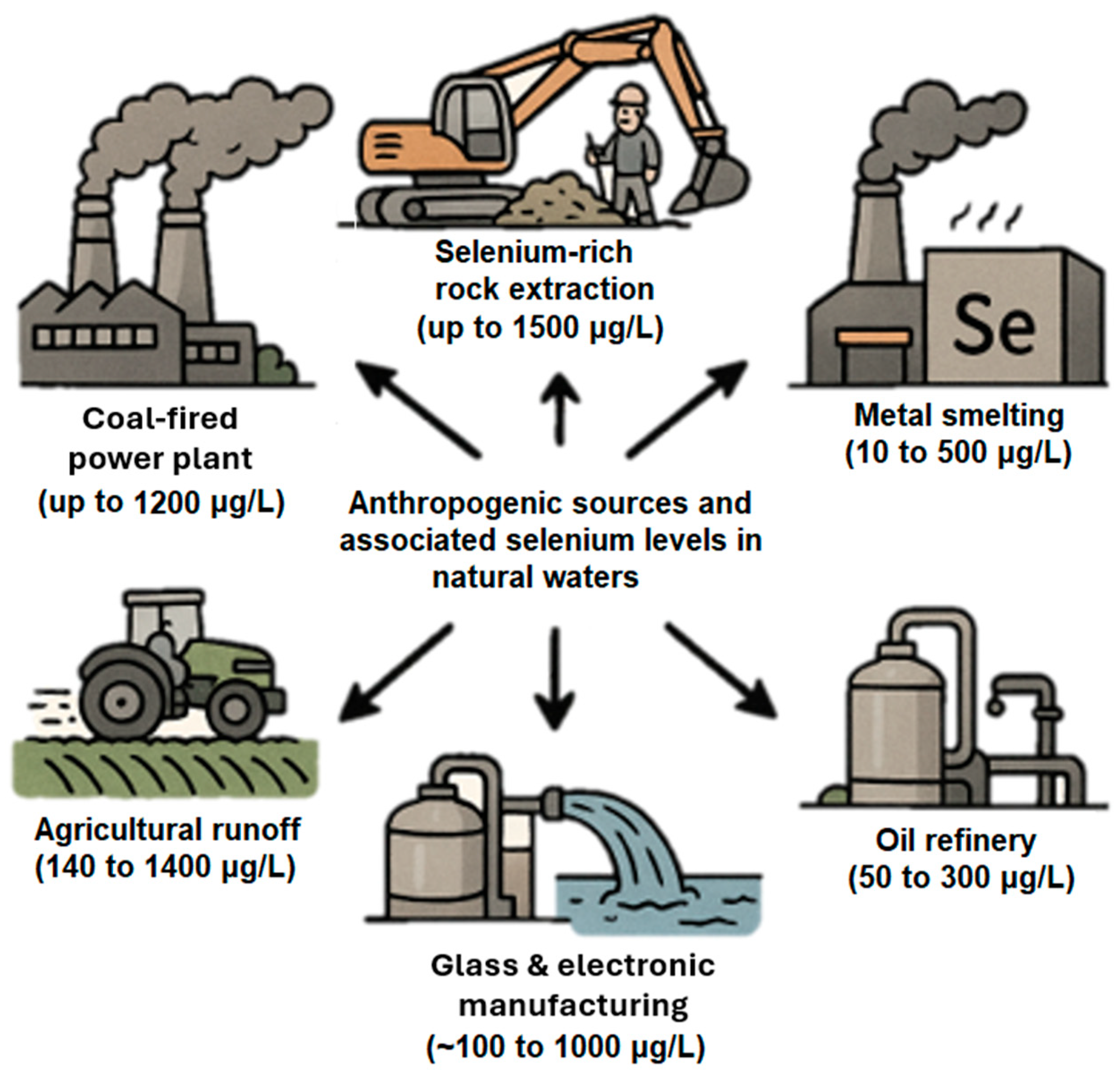
1.1. Sources of Selenium in Natural Waters
1.2. Distribution of Selenium in Natural Waters
1.2.1. Groundwater
1.2.2. Surface Waters
1.2.3. Seawater
1.3. Selenium Chemistry and Toxicity
1.3.1. Selenium Chemistry and Speciation
1.3.2. Toxicity and Health Implications
2. Conventional Selenium Removal Techniques
2.1. Physical Methods
2.2. Chemical Methods
2.3. Biological Methods
3. Recent Developments in Selenium Remediation Technologies
3.1. Adsorption
3.1.1. Batch Mode Adsorption
3.1.2. Continuous Mode Adsorption
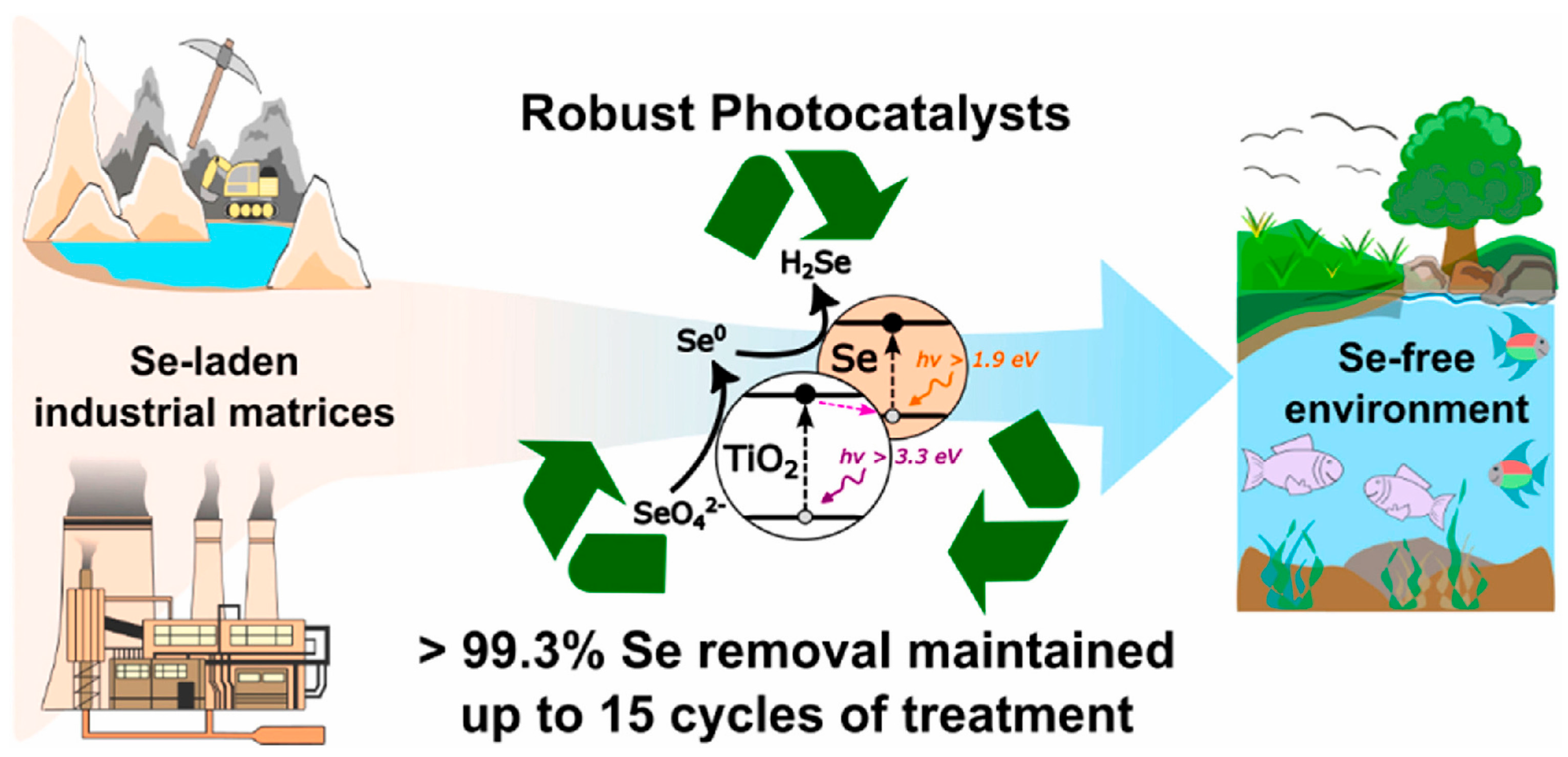
3.2. Photocatalysis
3.3. Membrane Technology
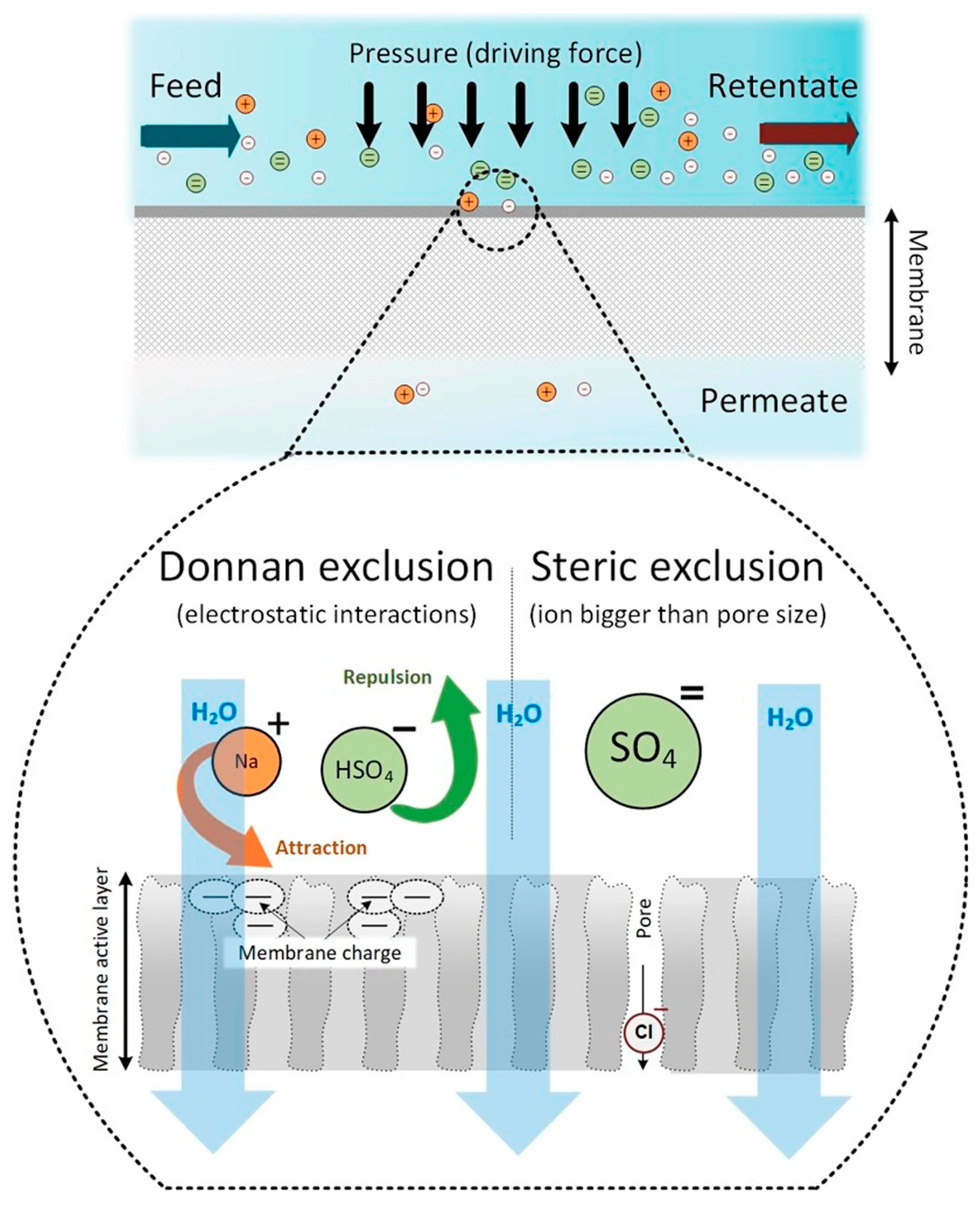
3.3.1. Se Removal by Pressure-Driven Membranes
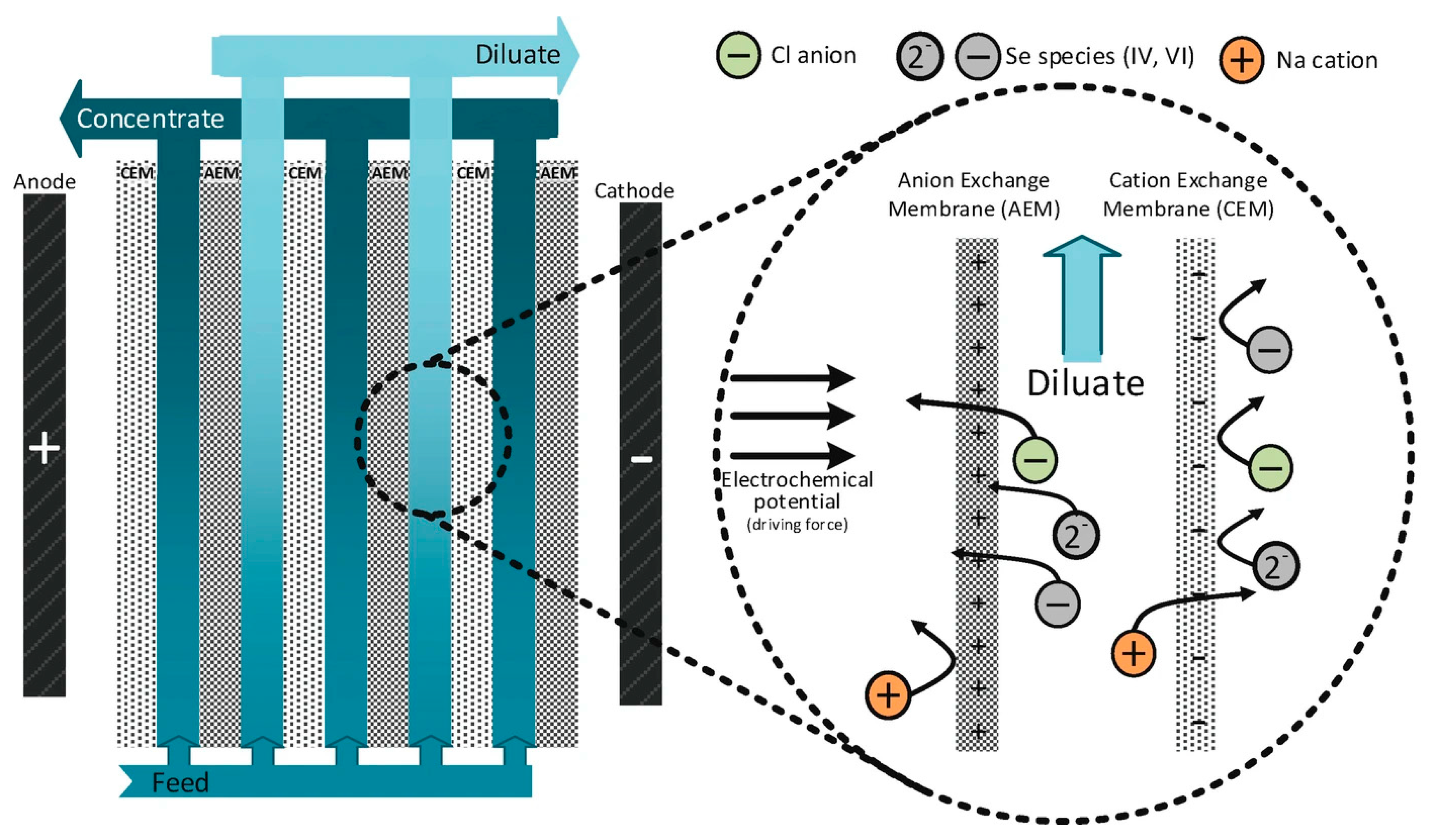
3.3.2. Se Removal by Electrodialysis
3.4. Electrochemical Methods
3.5. Biological Advancements
3.5.1. Anaerobic Biological Reduction of Selenium Oxyanions
3.5.2. Microbial Immobilization on Matrices for Selenium Removal
3.5.3. Microalgae-Based Selenium Accumulation
3.5.4. Phytoremediation: Plant-Based Selenium Removal
4. Challenges in Emerging Selenium Remediation Technologies
5. Prospects for Selenium Remediation
5.1. Integrated Approaches
5.1.1. Biologically Supported Adsorption Processes
5.1.2. Bio-Electrochemical Systems (BES)
5.1.3. Ion Exchange Membrane Bioreactors (IEMB)
5.1.4. Photocatalysis Coupled Adsorption Processes
5.2. Resource Recovery and Circular Economy Aspects
5.3. Research Trends and Emerging Areas:s
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Rudnick, R.L. Composition of the continental crust. Crust Treatise Geochem. 2005, 3, 17–18. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2000; ISBN 042919112X. [Google Scholar]
- Fordyce, F. Selenium geochemistry and health. Ambio 2007, 36, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhu, J.; Liang, L.; Wang, M.; Su, H. The bioavailability of selenium and risk assessment for human selenium poisoning in high-Se areas, China. Environ. Int. 2013, 52, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Huang, J.; Peng, Q.; Yu, D.; Wang, S.; Liang, D. Risk assessment for human health in a seleniferous area, Shuang’an, China. Environ. Sci. Pollut. Res. 2017, 24, 17701–17710. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Nahar, K.; Fujita, M. Selenium toxicity in plants and environment: Biogeochemistry and remediation possibilities. Plants 2020, 9, 1711. [Google Scholar] [CrossRef]
- Monsen, E.R. Dietary reference intakes for the antioxidant nutrients: Vitamin C, vitamin E, selenium, and carotenoids. J. Acad. Nutr. Diet. 2000, 100, 637. [Google Scholar]
- Li, T.; Xu, H.; Zhang, Y.; Zhang, H.; Hu, X.; Sun, Y.; Gu, X.; Luo, J.; Zhou, D.; Gao, B. Treatment technologies for selenium contaminated water: A critical review. Environ. Pollut. 2022, 299, 118858. [Google Scholar] [CrossRef]
- Nuttall, K.L. Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006, 36, 409–420. [Google Scholar]
- Aliaskari, M.; Ramos, R.L.; Schäfer, A.I. Removal of arsenic and selenium from brackish water using electrodialysis for drinking water production. Desalination 2023, 548, 116298. [Google Scholar] [CrossRef]
- He, Y.; Xiang, Y.; Zhou, Y.; Yang, Y.; Zhang, J.; Huang, H.; Shang, C.; Luo, L.; Gao, J.; Tang, L. Selenium contamination, consequences and remediation techniques in water and soils: A review. Environ. Res. 2018, 164, 288–301. [Google Scholar] [CrossRef]
- Perveen, S. Drinking water quality monitoring, assessment and management in Pakistan: A review. Heliyon 2023, 9, e13872. [Google Scholar] [CrossRef] [PubMed]
- Federal Ministry of Justice. Ordinance on the Quality of Water Intended for Human Consumption (Trinkwasserverordnung-TrinkwV). Available online: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/E/Englische_Dateien/Drinking_Water_Ordinance.pdf (accessed on 23 June 2025).
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Mercury (Update); ATSDR/TP-93/10; Prepared by Clement International Corp. under contract 205-88-0608 for U.S. Department of Health and Human Services; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1994.
- Vinceti, M.; Filippini, T.; Wise, L.A. Environmental selenium and human health: An update. Curr. Environ. Health Rep. 2018, 5, 464–485. [Google Scholar] [CrossRef] [PubMed]
- Abejón, R. A bibliometric analysis of research on selenium in drinking water during the 1990–2021 period: Treatment options for selenium removal. Int. J. Environ. Res. Public Health 2022, 19, 5834. [Google Scholar] [CrossRef]
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review: Selenium interactions and toxicity. Cell Biol. Toxicol. 2012, 28, 31–46. [Google Scholar] [CrossRef]
- Gilliom, R.J. Preliminary Assessment of Sources, Distribution, and Mobility of Selenium in the San Joaquin Valley, California; Department of the Interior, US Geological Survey: Reston, VA, USA, 1989.
- Vance, G.F. Problems associated with selenium leaching from waste shale. A New Era Land Reclam. 2000, 17, 71–82. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Lee, T.R.; Beak, D.G.; Anderson, R.; Burns, B. Groundwater co-contaminant behavior of arsenic and selenium at a lead and zinc smelting facility. Appl. Geochem. 2018, 89, 255–264. [Google Scholar] [CrossRef]
- Guo, K.; Li, Y.; Wang, J.; Sui, Z.; Wang, T.; Pan, W.-P. A review on selenium in coal-fired power plants: Content and forms in coal, determination methods, migration, transformation, and control technologies. J. Environ. Chem. Eng. 2024, 12, 113579. [Google Scholar] [CrossRef]
- Lawson, S.; Macy, J.M. Bioremediation of selenite in oil refinery wastewater. Appl. Microbiol. Biotechnol. 1995, 43, 762–765. [Google Scholar] [CrossRef]
- Benis, K.Z.; McPhedran, K.N.; Soltan, J. Selenium removal from water using adsorbents: A critical review. J. Hazard. Mater. 2022, 424, 127603. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Al Kuisi, M.; Abdel-Fattah, A. Groundwater vulnerability to selenium in semi-arid environments: Amman Zarqa Basin, Jordan. Environ. Geochem. Health 2010, 32, 107–128. [Google Scholar] [CrossRef]
- Leybourne, M.I.; Cameron, E.M. Source, transport, and fate of rhenium, selenium, molybdenum, arsenic, and copper in groundwater associated with porphyry–Cu deposits, Atacama Desert, Chile. Chem. Geol. 2008, 247, 208–228. [Google Scholar] [CrossRef]
- Afzal, S.; Younas, M.; Ali, K. Selenium speciation studies from Soan-Sakesar Valley, salt range, Pakistan. Water Int. 2000, 25, 425–436. [Google Scholar] [CrossRef]
- Virk, H.S. Groundwater contamination in Punjab due to arsenic, selenium and uranium heavy metals. Res. Rev. J. Toxicol. 2020, 10, 1–6. [Google Scholar]
- Lemly, A.D. Aquatic selenium pollution is a global environmental safety issue. Ecotoxicol. Environ. Saf. 2004, 59, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Presser, T.S.; Luoma, S.N. Forecasting Selenium Discharges to the San Francisco Bay-Delta Estuary: Ecological Effects of a Proposed San Luis Drain Extension; US Geological Survey: Reston, VA, USA, 2006.
- Cutter, G.A.; Cutter, L.S. Selenium biogeochemistry in the San Francisco Bay estuary: Changes in water column behavior. Estuar. Coast. Shelf Sci. 2004, 61, 463–476. [Google Scholar] [CrossRef]
- Lei, X.G.; Combs, G.F., Jr.; Sunde, R.A.; Caton, J.S.; Arthington, J.D.; Vatamaniuk, M.Z. Dietary selenium across species. Annu. Rev. Nutr. 2022, 42, 337–375. [Google Scholar] [CrossRef]
- Kumkrong, P.; LeBlanc, K.L.; Mercier, P.H.J.; Mester, Z. Selenium analysis in waters. Part 1: Regulations and standard methods. Sci. Total Environ. 2018, 640, 1611–1634. [Google Scholar] [CrossRef]
- Vlaev, L.T.; Genieva, S.D. Electron transport properties of ions in aqueous solutions of sodium selenite. J. Struct. Chem. 2004, 45, 825–831. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Morin-Crini, N.; Bradu, C.; Boussouga, Y.-A.; Aliaskari, M.; Schäfer, A.I.; Das, S.; Wilson, L.D.; Ike, M.; Inoue, D. Methods for selenium removal from contaminated waters: A review. Environ. Chem. Lett. 2022, 20, 2019–2041. [Google Scholar] [CrossRef]
- Séby, F.; Potin-Gautier, M.; Giffaut, E.; Borge, G.; Donard, O.F.X. A critical review of thermodynamic data for selenium species at 25 C. Chem. Geol. 2001, 171, 173–194. [Google Scholar] [CrossRef]
- Ostovar, M.; Saberi, N.; Ghiassi, R. Selenium contamination in water; analytical and removal methods: A comprehensive review. Sep. Sci. Technol. 2022, 57, 2500–2520. [Google Scholar] [CrossRef]
- Vinceti, M.; Crespi, C.M.; Bonvicini, F.; Malagoli, C.; Ferrante, M.; Marmiroli, S.; Stranges, S. The need for a reassessment of the safe upper limit of selenium in drinking water. Sci. Total Environ. 2013, 443, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Su, L.; Li, L.; Wu, J.; He, X.; Mao, D.; Cheng, Y.; Liu, J.; Chen, C.; Jin, Y. Higher selenium was associated with higher risk of diabetes: Consistent evidence from longitudinal and cross-sectional studies based on nail and serum selenium measures. Sci. Total Environ. 2022, 840, 156618. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-J.; Rathinasabapathi, B.; Wu, B.; Luo, J.; Pu, L.-P.; Ma, L.Q. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 2014, 69, 148–158. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Khaleque, M.A. Efficient selenium (IV) detection and removal from water by tailor-made novel conjugate adsorbent. Sensors Actuators B Chem. 2015, 209, 194–202. [Google Scholar] [CrossRef]
- Fernández-Martínez, A.; Charlet, L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Environ. Sci. Biotechnol. 2009, 8, 81–110. [Google Scholar] [CrossRef]
- Jadhav, A.S.; Amrani, M.A.; Singh, S.K.; Al-Fatesh, A.S.; Bansiwal, A.; Srikanth, V.V.S.S.; Labhasetwar, N.K. γ-FeOOH and γ-FeOOH decorated multi-layer graphene: Potential materials for selenium (VI) removal from water. J. Water Process Eng. 2020, 37, 101396. [Google Scholar] [CrossRef]
- Su, C. Environmental implications and applications of engineered nanoscale magnetite and its hybrid nanocomposites: A review of recent literature. J. Hazard. Mater. 2017, 322, 48–84. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhao, Y.; Li, W.; Yang, Y.; Liu, R.; Morgen, D. Global profile of heavy metals and semimetals adsorption using drinking water treatment residual. Chem. Eng. J. 2019, 372, 1019–1027. [Google Scholar] [CrossRef]
- Gong, Y.; Tang, J.; Zhao, D. Application of iron sulfide particles for groundwater and soil remediation: A review. Water Res. 2016, 89, 309–320. [Google Scholar] [CrossRef]
- Sharrad, M.O.M.; Liu, H.; Fan, M. Evaluation of FeOOH performance on selenium reduction. Sep. Purif. Technol. 2012, 84, 29–34. [Google Scholar] [CrossRef]
- Kapoor, A.; Tanjore, S.; Viraraghavan, T. Removal of selenium from water and wastewater. Int. J. Environ. Stud. 1995, 49, 137–147. [Google Scholar] [CrossRef]
- Sen, M.; Manna, A.; Pal, P. Removal of arsenic from contaminated groundwater by membrane-integrated hybrid treatment system. J. Memb. Sci. 2010, 354, 108–113. [Google Scholar] [CrossRef]
- Zeeshan, M.H.; Khan, R.U.; Shafiq, M.; Sabir, A. Polyamide intercalated nanofiltration membrane modified with biofunctionalized core shell composite for efficient removal of Arsenic and Selenium from wastewater. J. Water Process Eng. 2020, 34, 101175. [Google Scholar] [CrossRef]
- Gude, V.G. Desalination and sustainability—An appraisal and current perspective. Water Res. 2016, 89, 87–106. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Q.; Chen, G.; Liu, H.; Qu, J. Removal of Se (IV) and Se (VI) from drinking water by coagulation. Sep. Purif. Technol. 2015, 142, 65–70. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Shan, C.; Zhang, W.; Pan, B. A novel combined process for efficient removal of Se (VI) from sulfate-rich water: Sulfite/UV/Fe (III) coagulation. Chemosphere 2018, 211, 867–874. [Google Scholar] [CrossRef]
- Viktor, Z.; Wang, L.; Ma, J. Promotional effect of Mn (II)/K2FeO4 applying onto Se (IV) removal. J. Hazard. Mater. 2020, 384, 121264. [Google Scholar] [CrossRef]
- Santos, S.; Ungureanu, G.; Boaventura, R.; Botelho, C. Selenium contaminated waters: An overview of analytical methods, treatment options and recent advances in sorption methods. Sci. Total Environ. 2015, 521, 246–260. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Nikoletopoulos, A.-A.; Tsiftsakis, N.; Pinakidou, F.; Mitrakas, M. Adsorption of Se (IV) and Se (VI) species by iron oxy-hydroxides: Effect of positive surface charge density. Sci. Total Environ. 2019, 687, 1197–1206. [Google Scholar] [CrossRef]
- Oremland, R.S.; Newman, D.K.; Kail, B.W.; Stolz, J.F. Bacterial Respiration of Arsenate and Its Significance in the Environment. Chem. Arsen. 2001, 273–295. [Google Scholar]
- Stolz, J.F.; Oremland, R.S. Bacterial respiration of arsenic and selenium. FEMS Microbiol. Rev. 1999, 23, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, O.; Yamashita, M. Selenium recovery from wastewater using the selenate-reducing bacterium Pseudomonas stutzeri NT-I. Hydrometallurgy 2020, 197, 105470. [Google Scholar] [CrossRef]
- Wessels, C.E. Reduction of Selenium by Pseudomonas Stutzeri NT-l; Growth Reduction and Kinetics; University of Pretoria (South Africa): Pretoria, South Africa, 2017; ISBN 9798380989930. [Google Scholar]
- Banuelos, G.S.; Lin, Z.Q.; Wu, L.; Terry, N. Phytoremediation of selenium-contaminated soils and waters: Fundamentals and future prospects. Rev. Environ. Health 2002, 17, 291–306. [Google Scholar] [CrossRef]
- Etteieb, S.; Zolfaghari, M.; Magdouli, S.; Brar, K.K.; Brar, S.K. Performance of constructed wetland for selenium, nutrient and heavy metals removal from mine effluents. Chemosphere 2021, 281, 130921. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, J.-C.; He, S.; Zhou, W. Enhancement of a constructed wetland water treatment system for selenium removal. Sci. Total Environ. 2020, 714, 136741. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Akinnawo, S.O.; Ajala, O.A.; Adebusuyi, T.A.; Maxakato, N.W.; Bello, O.S. Progress and challenges in batch and optimization studies on the adsorptive removal of heavy metals using modified biomass-based adsorbents. Bioresour. Technol. Rep. 2022, 19, 101115. [Google Scholar] [CrossRef]
- John, M.M.; Benettayeb, A.; Belkacem, M.; Mitchel, C.R.; Brahim, M.H.; Benettayeb, I.; Haddou, B.; Al-Farraj, S.; Alkahtane, A.A.; Ghosh, S. An overview on the key advantages and limitations of batch and dynamic modes of biosorption of metal ions. Chemosphere 2024, 357, 142051. [Google Scholar] [CrossRef]
- He, B.; Zhang, W.; Diao, Y.; Sun, S.; Zhang, Y.; Zhao, W.; Wen, F.; Yang, G. Mechanistic study of the adsorption capabilities of heavy metals on the surface of ferrihydrite: Batch sorption, modeling, and density functional theory. RSC Adv. 2025, 15, 1072–1080. [Google Scholar] [CrossRef]
- Gheibi, M.; Masoomi, S.R.; Magala, M.U.; Fathollahi-Fard, A.M.; Ghazikhani, A.; Behzadian, K. The Application of Artificial Intelligence (AI) in Adsorption Process of Heavy Metals: A Systematic Review. Environ. Ind. Lett. 2024, 2, 57–78. [Google Scholar]
- Behera, P.; Sahu, H.B.; Behera, S.; Das, S. Bioremediation of toxic selenium from aqueous solution using Bacillus selenatarsenatis 9470T and machine learning approach. J. Water Process Eng. 2025, 72, 107449. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, S.; Chen, B.; Shahab, A.; Riaz, L.; Lun, L.; Wu, N. Machine learning approach to predict adsorption capacity of Fe-modified biochar for selenium. Carbon Res. 2023, 2, 29. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Hu, X.; Zhang, Y.; Zhang, D.; Zhang, H.; Xu, H.; Sun, Y.; Gu, X.; Luo, J. Reclaiming selenium from water using aluminum-modified biochar: Adsorption behaviors, mechanisms, and effects on growth of wheat seedlings. Environ. Pollut. 2024, 361, 124835. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Rizvi, O.S.; Khan, E.; Abbas, T. Selenium removal from water using modified biochar: A critical review and insights to adsorption mechanisms through computational analyses. J. Water Process Eng. 2025, 69, 106668. [Google Scholar] [CrossRef]
- Priya, V.N.; Rajkumar, M.; Rajendran, V.; Mobika, J.; Sibi, S.P.L.; Veena, B.; Vijayalakshmi, V.; Ahila, P. Sustainable selenium ions adsorption of cyclodextrin and cellulose functionalized layered double hydroxide/reduced graphene oxide nanocomposites. J. Water Process Eng. 2025, 69, 106580. [Google Scholar] [CrossRef]
- Jiménez-López, B.A.; Leyva-Ramos, R.; Salazar-Rábago, J.J.; Jacobo-Azuara, A.; Aragón-Piña, A. Adsorption of selenium (iv) oxoanions on calcined layered double hydroxides of Mg-Al-CO3 from aqueous solution. Effect of calcination and reconstruction of lamellar structure. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100580. [Google Scholar] [CrossRef]
- Modak, S.; Kar, B.; Esfahani, M.R. Selective Removal of Selenium from Produced Water Using MOF-808 and MIL-100 (Fe): Performance and Mechanism Assessment. ACS Appl. Eng. Mater. 2025, 3, 1718–1735. [Google Scholar] [CrossRef]
- Sharma, S.; Let, S.; Desai, A.V.; Dutta, S.; Karuppasamy, G.; Shirolkar, M.M.; Babarao, R.; Ghosh, S.K. Rapid, selective capture of toxic oxo-anions of Se (iv), Se (vi) and As (v) from water by an ionic metal–organic framework (iMOF). J. Mater. Chem. A 2021, 9, 6499–6507. [Google Scholar] [CrossRef]
- López-Toyos, L.; Rodríguez, E.; García, R.; Martínez-Tarazona, M.R.; López-Antón, M.A. Sorption of Selenium (IV) and Selenium (VI) onto iron oxide/hydroxide-based carbon materials: Activated carbon and carbon foam. Water 2023, 15, 3499. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, T.; Lei, W.; Feng, Y.; Tang, X.; Ding, Z.; Liu, X.; Qi, P.; Wang, Y. A mechanically robust chitosan-based macroporous foam for sustainable Se (IV) elimination from wastewater. Carbohydr. Polym. 2025, 352, 123238. [Google Scholar] [CrossRef]
- Qureshi, S.S.; Memon, S.A.; Ram, N.; Saeed, S.; Mubarak, N.M.; Karri, R.R. Rapid adsorption of selenium removal using iron manganese-based micro adsorbent. Sci. Rep. 2022, 12, 17207. [Google Scholar] [CrossRef]
- El-Tantawy, A.; Abu Elgoud, E.M.; Sharaf El-Deen, S.E.A. Evaluation of anion exchange resin for sorption of selenium (IV) from aqueous solutions. BMC Chem. 2025, 19, 10. [Google Scholar] [CrossRef]
- Rovira, M.; Giménez, J.; Martínez, M.; Martínez-Lladó, X.; de Pablo, J.; Martí, V.; Duro, L. Sorption of selenium (IV) and selenium (VI) onto natural iron oxides: Goethite and hematite. J. Hazard. Mater. 2008, 150, 279–284. [Google Scholar] [CrossRef]
- Ji, Y.; Li, L.; Wang, Y. Selenium removal by activated alumina in batch and continuous-flow reactors. Water Environ. Res. 2020, 92, 51–59. [Google Scholar] [CrossRef]
- Jadhav, A.S.; Ramteke, P.; Singh, S.K.; Labhasetwar, N.K. Sustainable selenium remediation from water using aluminium–iron mixed oxide: Batch and column adsorption studies. J. Water Process Eng. 2022, 48, 102824. [Google Scholar] [CrossRef]
- Wei, J.; Shen, B.; Ye, G.; Wen, X.; Song, Y.; Wang, J.; Meng, X. Selenium and arsenic removal from water using amine sorbent, competitive adsorption and regeneration. Environ. Pollut. 2021, 274, 115866. [Google Scholar] [CrossRef]
- Usman, M.; Zarebanadkouki, M.; Waseem, M.; Katsoyiannis, I.A.; Ernst, M. Mathematical modeling of arsenic (V) adsorption onto iron oxyhydroxides in an adsorption-submerged membrane hybrid system. J. Hazard. Mater. 2020, 400, 123221. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Vourlias, G.; Stavropoulos, G.; Mitrakas, M. Kilogram-scale synthesis of iron oxy-hydroxides with improved arsenic removal capacity: Study of Fe (II) oxidation–precipitation parameters. Water Res. 2012, 46, 5255–5267. [Google Scholar] [CrossRef] [PubMed]
- Westerhoff, P.; Highfield, D.; Badruzzaman, M.; Yoon, Y. Rapid small-scale column tests for arsenate removal in iron oxide packed bed columns. J. Environ. Eng. 2005, 131, 262–271. [Google Scholar] [CrossRef]
- Yoon, I.-H.; Kim, K.-W.; Bang, S.; Kim, M.G. Reduction and adsorption mechanisms of selenate by zero-valent iron and related iron corrosion. Appl. Catal. B Environ. 2011, 104, 185–192. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Manquián-Cerda, K.; de la Luz Mora, M.; Molina-Roco, M.; Rubio, M.A.; Sarkar, B.; Bolan, N.; Arancibia-Miranda, N. Efficient and selective removal of SeVI and AsV mixed contaminants from aqueous media by montmorillonite-nanoscale zero valent iron nanocomposite. J. Hazard. Mater. 2021, 403, 123639. [Google Scholar] [CrossRef]
- Phanthasri, J.; Yan, D.Y.-S.; Wantala, K.; Khunphonoi, R.; Khamdahsag, P.; Tanboonchuy, V. Selenate removal via continuous fixed-bed column with nanoscale zero-valent iron supported on bentonite-zeolite pellets. J. Water Process Eng. 2023, 53, 103843. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Q.; Wang, F.; Fang, M.; Li, L.; Niu, H.; Wang, B.; Wang, N.; Wang, K.; Mo, Z. Selenium-contaminated water: Recent advances in material function and adsorption performance. J. Environ. Chem. Eng. 2023, 11, 110468. [Google Scholar] [CrossRef]
- Zoroufchi Benis, K.; Shakouri, M.; McPhedran, K.; Soltan, J. Enhanced arsenate removal by Fe-impregnated canola straw: Assessment of XANES solid-phase speciation, impacts of solution properties, sorption mechanisms, and evolutionary polynomial regression (EPR) models. Environ. Sci. Pollut. Res. 2021, 28, 12659–12676. [Google Scholar] [CrossRef]
- Kwon, J.H.; Wilson, L.D.; Sammynaiken, R. Sorptive uptake of selenium with magnetite and its supported materials onto activated carbon. J. Colloid Interface Sci. 2015, 457, 388–397. [Google Scholar] [CrossRef]
- Jegadeesan, G.B.; Mondal, K.; Lalvani, S.B. Adsorption of Se (IV) and Se (VI) using copper-impregnated activated carbon and fly ash-extracted char carbon. Water Air Soil Pollut. 2015, 226, 234. [Google Scholar] [CrossRef]
- Yan, D.; Gang, D.D.; Zhang, N.; Lin, L. Adsorptive selenite removal using iron-coated GAC: Modeling selenite breakthrough with the pore surface diffusion model. J. Environ. Eng. 2013, 139, 213–219. [Google Scholar] [CrossRef]
- Bleiman, N.; Mishael, Y.G. Selenium removal from drinking water by adsorption to chitosan–clay composites and oxides: Batch and columns tests. J. Hazard. Mater. 2010, 183, 590–595. [Google Scholar] [CrossRef]
- Pan, B.; Xiao, L.; Nie, G.; Pan, B.; Wu, J.; Lv, L.; Zhang, W.; Zheng, S. Adsorptive selenite removal from water using a nano-hydrated ferric oxides (HFOs)/polymer hybrid adsorbent. J. Environ. Monit. 2010, 12, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Losev, V.; Didukh-Shadrina, S.; Orobyeva, A.; Borodina, E.; Elsuf’ev, E.; Metelitsa, S.; Ondar, U. Speciation of inorganic selenium in natural water by in situ solid-phase extraction using functionalized silica. Anal. Methods 2022, 14, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Benis, K.Z.; Sokhansanj, A.; Hughes, K.A.; McPhedran, K.N.; Soltan, J. An engineered biochar for treatment of selenite contaminated water: Mass transfer characteristics in fixed bed adsorption. Chem. Eng. J. 2023, 469, 143946. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Mahari, W.A.W.; Lee, X.Y.; Han, C.S.; Vo, D.-V.N.; Van Le, Q. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Lee, C.-G.; Alvarez, P.J.J.; Nam, A.; Park, S.-J.; Do, T.; Choi, U.-S.; Lee, S.-H. Arsenic (V) removal using an amine-doped acrylic ion exchange fiber: Kinetic, equilibrium, and regeneration studies. J. Hazard. Mater. 2017, 325, 223–229. [Google Scholar] [CrossRef]
- Benhamou, A.; Baudu, M.; Derriche, Z.; Basly, J.-P. Aqueous heavy metals removal on amine-functionalized Si-MCM-41 and Si-MCM-48. J. Hazard. Mater. 2009, 171, 1001–1008. [Google Scholar] [CrossRef]
- Holmes, A.B.; Khan, D.; de Oliveira Livera, D.; Gu, F. Enhanced photocatalytic selectivity of noble metallized TiO2 nanoparticles for the reduction of selenate in water: Tunable Se reduction product H 2 Se (g) vs. Se (s). Environ. Sci. Nano 2020, 7, 1841–1852. [Google Scholar] [CrossRef]
- Ngan, A.; Milan, E.; Chen, Z.Q.; Chan, C.C.; Iman, A.; Gu, F. In situ formed Se–TiO2 as a highly reusable photocatalyst for selenium reduction and removal from industrial wastewater. Chemosphere 2025, 370, 143959. [Google Scholar] [CrossRef]
- Ahmed, S.; Vohra, M.S. Selenocyanate removal using combined system of TiO2-photocatalysis and Fe (III)/SiO2 adsorption {UV-TiO2/[Fe (III)/SiO2]}: Complex destruction and simultaneous selenium species adsorption. Chem. Eng. J. 2024, 500, 156525. [Google Scholar] [CrossRef]
- Holmes, A.B.; Ngan, A.; Ye, J.; Gu, F. Selective photocatalytic reduction of selenate over TiO2 in the presence of nitrate and sulfate in mine-impacted water. Chemosphere 2022, 287, 131951. [Google Scholar] [CrossRef]
- Chalastara, K.; Demopoulos, G.P. Selenate Se (VI) reduction to elemental selenium on heterojunctioned rutile/brookite nano-photocatalysts with enhanced charge utilization. Chem. Eng. J. 2022, 437, 135470. [Google Scholar] [CrossRef]
- Malhotra, M.; Pal, M.; Pal, P. A response surface optimized nanofiltration-based system for efficient removal of selenium from drinking Water. J. Water Process Eng. 2020, 33, 101007. [Google Scholar] [CrossRef]
- Boussouga, Y.-A.; Than, H.; Schäfer, A.I. Selenium species removal by nanofiltration: Determination of retention mechanisms. Sci. Total Environ. 2022, 829, 154287. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Wickramasinghe, S.R. Produced water treatment by nanofiltration and reverse osmosis membranes. J. Memb. Sci. 2008, 322, 162–170. [Google Scholar] [CrossRef]
- Malhotra, M.; Roy, M.; Pal, P. A membrane-based green and low-cost system for ensuring safe drinking water in a selenium-affected region. J. Environ. Manag. 2022, 324, 116361. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, J.; Han, G.; Chung, T.-S. Novel thin-film composite nanofiltration membranes consisting of a zwitterionic co-polymer for selenium and arsenic removal. J. Memb. Sci. 2018, 555, 299–306. [Google Scholar] [CrossRef]
- Richards, L.A.; Richards, B.S.; Schäfer, A.I. Renewable energy powered membrane technology: Salt and inorganic contaminant removal by nanofiltration/reverse osmosis. J. Memb. Sci. 2011, 369, 188–195. [Google Scholar] [CrossRef]
- Owusu-Agyeman, I.; Jeihanipour, A.; Luxbacher, T.; Schäfer, A.I. Implications of humic acid, inorganic carbon and speciation on fluoride retention mechanisms in nanofiltration and reverse osmosis. J. Memb. Sci. 2017, 528, 82–94. [Google Scholar] [CrossRef]
- He, Y.; Tang, Y.P.; Chung, T.S. Concurrent removal of selenium and arsenic from water using polyhedral oligomeric silsesquioxane (POSS)–polyamide thin-film nanocomposite nanofiltration membranes. Ind. Eng. Chem. Res. 2016, 55, 12929–12938. [Google Scholar] [CrossRef]
- Figoli, A.; Cassano, A.; Criscuoli, A.; Mozumder, M.S.I.; Uddin, M.T.; Islam, M.A.; Drioli, E. Influence of operating parameters on the arsenic removal by nanofiltration. Water Res. 2010, 44, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, P.S.; Lugo, A.; Ahmed, M.F.; Stoll, Z.; Moe, N.E.; Barber, J.; Walker, W.S.; Xu, P.; Wang, H. Electrodialysis modeling for desalination and resource recovery. Curr. Opin. Chem. Eng. 2025, 47, 101081. [Google Scholar] [CrossRef]
- Onorato, C.; Banasiak, L.J.; Schäfer, A.I. Inorganic trace contaminant removal from real brackish groundwater using electrodialysis. Sep. Purif. Technol. 2017, 187, 426–435. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Morin-Crini, N.; Bradu, C.; Boussouga, Y.-A.; Aliaskari, M.; Schäfer, A.I.; Das, S.; Wilson, L.D.; Ike, M.; Inoue, D. Technologies to remove selenium from water and wastewater. In Emerging Contaminants Volume 2: Remediation; Springer: Berlin/Heidelberg, Germany, 2021; pp. 207–304. [Google Scholar]
- Staicu, L.C.; Van Hullebusch, E.D.; Lens, P.N.L.; Pilon-Smits, E.A.H.; Oturan, M.A. Electrocoagulation of colloidal biogenic selenium. Environ. Sci. Pollut. Res. 2015, 22, 3127–3137. [Google Scholar] [CrossRef]
- Baek, K.; Ciblak, A.; Mao, X.; Kim, E.-J.; Alshawabkeh, A. Iron anode mediated transformation of selenate in sand columns. Water Res. 2013, 47, 6538–6545. [Google Scholar] [CrossRef]
- Baek, K.; Kasem, N.; Ciblak, A.; Vesper, D.; Padilla, I.; Alshawabkeh, A.N. Electrochemical removal of selenate from aqueous solutions. Chem. Eng. J. 2013, 215, 678–684. [Google Scholar] [CrossRef]
- Licht, K.; Halkijević, I.; Posavčić, H.; Nakić, D. Simultaneous Electrochemical Removal of Selenium and Strontium from Aqueous Solution. Appl. Sci. 2025, 15, 2786. [Google Scholar] [CrossRef]
- Hao, S.; Feng, Y.; Wang, D.; Cho, J.; Qiu, C.; Wi, T.-U.; Xu, Z.; Yu, Z.; Sellers, C.; Zou, S. Electrochemical Removal of Se (IV) from Wastewater Using RuO2-Based Catalysts. Nano Lett. 2025, 25, 2547–2553. [Google Scholar] [CrossRef]
- Tan, L.C.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N.L. Selenium: Environmental significance, pollution, and biological treatment technologies. Biotechnol. Adv. 2016, 34, 886–907. [Google Scholar] [CrossRef]
- Nie, X.; Yang, X.; He, J.; Liu, P.; Shi, H.; Wang, T.; Zhang, D. Bioconversion of inorganic selenium to less toxic selenium forms by microbes: A review. Front. Bioeng. Biotechnol. 2023, 11, 1167123. [Google Scholar] [CrossRef]
- Wang, D.; Rensing, C.; Zheng, S. Microbial reduction and resistance to selenium: Mechanisms, applications and prospects. J. Hazard. Mater. 2022, 421, 126684. [Google Scholar] [CrossRef] [PubMed]
- Werkneh, A.A.; Gebretsadik, G.G.; Gebru, S.B. Review on environmental selenium: Occurrence, public health implications and biological treatment strategies. Environ. Chall. 2023, 11, 100698. [Google Scholar] [CrossRef]
- Sinharoy, A.; Lens, P.N.L. Biological selenate and selenite reduction by waste activated sludge using hydrogen as electron donor. J. Environ. Manag. 2022, 319, 115745. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Gao, J.; Chen, X.; Ru, Y.; Zhang, D.; Pan, X. Efficient recovery of heavy metals and selenium from wastewater using granular sludge: The crucial role of glutathione (GSH). Water Res. 2025, 270, 122826. [Google Scholar] [CrossRef]
- Mal, J.; Sinharoy, A.; Lens, P.N.L. Simultaneous removal of lead and selenium through biomineralization as lead selenide by anaerobic granular sludge. J. Hazard. Mater. 2021, 420, 126663. [Google Scholar] [CrossRef]
- He, Z.; Shen, J.; Zhao, Y.; Ru, Y.; Zhang, D.; Pan, X. Efficient and synergistic treatment of selenium (IV)-contaminated wastewater and mercury (II)-contaminated soil by anaerobic granular sludge: Performance and mechanisms. Chemosphere 2024, 350, 141038. [Google Scholar] [CrossRef]
- Koepnick, H.R.; Peyton, B.M.; Lauchnor, E.G. Factors influencing the efficacy of microbial remediation of selenium in groundwater near a coal-fired power plant. Geo-Bio Interfaces 2025, 2, e7. [Google Scholar] [CrossRef]
- Logan, M.; Tan, L.C.; Lens, P.N.L. Anaerobic co-digestion of dissolved air floatation slurry and selenium rich wastewater for simultaneous methane production and selenium bioremediation. Int. Biodeterior. Biodegrad. 2022, 172, 105425. [Google Scholar] [CrossRef]
- Gullett, K.L.; Ford, C.L.; Garvey, I.J.; Miller, T.J.; Leahy, C.A.; Awaitey, L.N.; Hofmann, D.M.; Woods, T.J.; Fout, A.R. Formation of Red Elemental Selenium from Seleniferous Oxyanions: Deoxygenation by a Homogeneous Iron Catalyst. J. Am. Chem. Soc. 2023, 145, 20868–20873. [Google Scholar] [CrossRef]
- Chi, Z.; Li, W.; Zhang, P.; Li, H. Hydraulic retention time (HRT) extension and nitrate addition mitigate Se (VI) inhibition and enhance selenium removal in constructed wetlands: Potential role of nitrate and Fe/Mn pumps. Bioresour. Technol. 2025, 425, 132328. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Weijma, J.; Buisman, C.J.N.; Van Der Weijden, R.D. How sulfur species can accelerate the biological immobilization of the toxic selenium oxyanions and promote stable hexagonal Se0 formation. J. Hazard. Mater. 2022, 437, 129367. [Google Scholar] [CrossRef] [PubMed]
- Lenz, M.; Enright, A.M.; O’Flaherty, V.; van Aelst, A.C.; Lens, P.N.L. Bioaugmentation of UASB reactors with immobilized Sulfurospirillum barnesii for simultaneous selenate and nitrate removal. Appl. Microbiol. Biotechnol. 2009, 83, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.C.; Espinosa-Ortiz, E.J.; Nancharaiah, Y.V.; van Hullebusch, E.; Gerlach, R.; Lens, P.N.L. Selenate Removal in Biofilm Systems: Effect of Nitrate and Sulfate on Selenium Removal Efficiency, Biofilm Structure, and Microbial Community. In Anaerobic Treatment of Mine Wastewater for the Removal of Selenate and Its Co-Contaminants; CRC Press: Boca Raton, FL, USA, 2018; pp. 101–125. [Google Scholar]
- Kulasekara, H.M.I.P.; Zhang, Y.; Papelis, C. Microbial enhancement of selenium removal in chemically modified Zeolite columns. Water 2023, 15, 1837. [Google Scholar] [CrossRef]
- Ullah, H.; Chen, B.; Rashid, A.; Zhao, R.; Shahab, A.; Yu, G.; Wong, M.H.; Khan, S. A critical review on selenium removal capacity from water using emerging non-conventional biosorbents. Environ. Pollut. 2023, 339, 122644. [Google Scholar] [CrossRef]
- Razaviarani, V.; Arab, G.; Lerdwanawattana, N.; Gadia, Y. Algal biomass dual roles in phycoremediation of wastewater and production of bioenergy and value-added products. Int. J. Environ. Sci. Technol. 2023, 20, 8199–8216. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Parrasia, S.; Dalla Vecchia, F. Selenium accumulation and metabolism in algae. Aquat. Toxicol. 2017, 189, 1–8. [Google Scholar] [CrossRef]
- Saleem, S.; Iftikhar, R.; Arshad, M.; Zeeshan, M.; Hassan, M. Operation of microalgal horizontal twin layer system for treatment of real wastewater and production of lipids. J. Water Process Eng. 2022, 48, 102932. [Google Scholar] [CrossRef]
- De Morais, E.G.; Murillo, A.M.; Lens, P.N.L.; Ferrer, I.; Uggetti, E. Selenium recovery from wastewater by the green microalgae Chlorella vulgaris and Scenedesmus sp. Sci. Total Environ. 2022, 851, 158337. [Google Scholar] [CrossRef]
- Sohail, N.F.; Iftikhar, R.; Saleem, S. Microalgal treatment of high-nutrient wastewater using twin layer cultivation system. J. Environ. Chem. Eng. 2023, 11, 109248. [Google Scholar] [CrossRef]
- Penloglou, G.; Pavlou, A.; Kiparissides, C. Recent advancements in photo-bioreactors for microalgae cultivation: A brief overview. Processes 2024, 12, 1104. [Google Scholar] [CrossRef]
- Siddharthan, S.; Thangaraj, S.; Paulraj, S.; RajaMohmed, B.; Rakkamuthu, K.; Dharmaraj, V.; Renganathan, M.; Umadevi, P. Myco-remediation of selenium contaminated environment and future prospects: An overview. Environ. Qual. Manag. 2024, 33, 869–877. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Hua, Y.; Chen, G.; Fu, P.; Liu, J. Heterotrophic selenium incorporation into Chlorella vulgaris K-01: Selenium tolerance, assimilation, and removal through microalgal cells. Foods 2024, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Huang, J.-C.; Zhou, C.; Gao, W.; Xia, S.; He, S.; Zhou, W. Development of an algal treatment system for selenium removal: Effects of environmental factors and post-treatment processing of Se-laden algae. J. Hazard. Mater. 2019, 365, 546–554. [Google Scholar] [CrossRef]
- Khanna, K.; Kumar, P.; Ohri, P.; Bhardwaj, R. Harnessing the role of selenium in soil–plant-microbe ecosystem: Ecophysiological mechanisms and future prospects. Plant Growth Regul. 2023, 100, 197–217. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A.H. Selenium biofortification and phytoremediation phytotechnologies: A review. J. Environ. Qual. 2017, 46, 10–19. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, S. Effect of Paenibacillus favisporus CHP14 inoculation on selenium accumulation and tolerance of Pakchoi (Brassica chinensis L.) under exogenous selenite treatments. Int. J. Phytoremediat. 2025, 27, 271–286. [Google Scholar] [CrossRef]
- Morris, S.; Quispe-Arpasi, D.; Lens, P.N.L. Effect of Rhodococcus opacus PD630 on selenium phytoremediation by Brassica oleracea. Int. J. Phytoremediat. 2024, 26, 1280–1290. [Google Scholar] [CrossRef]
- Deepali, D.; Prakash, N.T.; Reddy, M.S. Plant Growth Promotion and Selenium Accumulation in Zea Mays in Seleniferous Soils by Selenium Tolerant Bacteria. Water Air Soil Pollut. 2025, 236, 1–17. [Google Scholar] [CrossRef]
- Somagattu, P.; Chinnannan, K.; Yammanuru, H.; Reddy, U.K.; Nimmakayala, P. Selenium dynamics in plants: Uptake, transport, toxicity, and sustainable management strategies. Sci. Total Environ. 2024, 949, 175033. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, W.; Li, D.; Wang, H.; Yang, Y.; You, J.; Liu, H.; Ai, L.; Zhang, M. Combined transcriptome and metabolome analyses reveal the effects of selenium on the growth and quality of Lilium lancifolium. Front. Plant Sci. 2024, 15, 1399152. [Google Scholar] [CrossRef]
- Li, Z.; Tian, Y.; Wang, B.; Peng, R.; Xu, J.; Fu, X.; Han, H.; Wang, L.; Zhang, W.; Deng, Y. Enhanced phytoremediation of selenium using genetically engineered rice plants. J. Plant Physiol. 2022, 271, 153665. [Google Scholar] [CrossRef]
- Okonji, S.O.; Achari, G.; Pernitsky, D. Environmental impacts of selenium contamination: A review on current-issues and remediation strategies in an aqueous system. Water 2021, 13, 1473. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Xu, L.; Ma, A.; Zhuang, G.; Huo, S.; Zou, B.; Qian, J.; Cui, Y. Selenium volatilization in plants, microalgae, and microorganisms. Heliyon 2024, 10, e26023. [Google Scholar] [CrossRef] [PubMed]
- Piacenza, E.; Presentato, A.; Zonaro, E.; Lampis, S.; Vallini, G.; Turner, R.J. Microbial-Based Bioremediation of Selenium and Tellurium Compounds. In Biosorption; InTechOpen Limited: London, UK, 2018; pp. 117–147. ISBN 1789234735. [Google Scholar]
- Tang, Y.; Zhu, J.; Yu, H.; Zhang, F.; Hu, S.; Chen, H.; Zhang, C.; Wu, H.; Yu, L.; Wang, X. Novel PA/PVDF hollow fiber nanofiltration membrane with high permeability and Ca2+/antibiotics selectivity for drinking water purification. Adv. Membr. 2024, 4, 100102. [Google Scholar] [CrossRef]
- Sravan, J.S.; Nancharaiah, Y.V.; Lens, P.N.L.; Mohan, S.V. Cathodic selenium recovery in bioelectrochemical system: Regulatory influence on anodic electrogenic activity. J. Hazard. Mater. 2020, 399, 122843. [Google Scholar] [CrossRef] [PubMed]
- Riveros, A.; Asefaw, B.K.; Wang, Q.; Maqbool, T.; Tang, Y.; Jiang, D. Selenium treatment via integrating flow electrode capacitive deionization (FCDI) and bio-electrochemical systems (BES). Water Res. 2025, 271, 122844. [Google Scholar] [CrossRef]
- Ricardo, A.R.; Carvalho, G.; Velizarov, S.; Crespo, J.G.; Reis, M.A.M. Kinetics of nitrate and perchlorate removal and biofilm stratification in an ion exchange membrane bioreactor. Water Res. 2012, 46, 4556–4568. [Google Scholar] [CrossRef]
- Oehmen, A.; Vergel, D.; Fradinho, J.; Reis, M.A.M.; Crespo, J.G.; Velizarov, S. Mercury removal from water streams through the ion exchange membrane bioreactor concept. J. Hazard. Mater. 2014, 264, 65–70. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Velizarov, S. New insights into the definition of membrane cleaning strategies to diminish the fouling impact in ion exchange membrane separation processes. Sep. Purif. Technol. 2021, 277, 119445. [Google Scholar] [CrossRef]
- Lopes, M.P.; Galinha, C.F.; Crespo, J.G.; Velizarov, S. Optimisation of arsenate removal from water by an integrated ion-exchange membrane process coupled with Fe co-precipitation. Sep. Purif. Technol. 2020, 246, 116894. [Google Scholar] [CrossRef]
- Oehmen, A.; Valerio, R.; Llanos, J.; Fradinho, J.; Serra, S.; Reis, M.A.M.; Crespo, J.G.; Velizarov, S. Arsenic removal from drinking water through a hybrid ion exchange membrane–coagulation process. Sep. Purif. Technol. 2011, 83, 137–143. [Google Scholar] [CrossRef]
- Badoni, A.; Thakur, S.; Vijayan, N.; Swart, H.C.; Bechelany, M.; Chen, Z.; Sun, S.; Cai, Q.; Chen, Y.; Prakash, J. Recent progress in understanding the role of graphene oxide, TiO2 and graphene oxide-TiO2 nanocomposites as multidisciplinary photocatalysts in energy and environmental applications. Catal. Sci. Technol. 2025, 15, 1702–1770. [Google Scholar] [CrossRef]
- Chen, R.; Fan, Y.; Song, J.; Zhou, W.; Peng, C.; Li, M.; Wu, J.; Wang, S.; Xu, J.; Sun, X. Enhanced photocatalytic removal of mercury by Z scheme F-doped g-C3N4/TiO2 photocatalysts with rich nitrogen vacancies. Chem. Phys. Lett. 2025, 876, 142220. [Google Scholar] [CrossRef]
- Sewnet, A.; Abebe, M.; Asaithambi, P.; Alemayehu, E. Visible-light-driven g-C3N4/TiO2 based heterojunction nanocomposites for photocatalytic degradation of organic dyes in wastewater: A review. Air Soil Water Res. 2022, 15, 11786221221117266. [Google Scholar] [CrossRef]
- Ekwonna, T.; Akindeju, O.; Amos, B.; Lin, Z.-Q. Selenium Removal from Wastewater by Microbial Transformation and Volatilization. In Mixed Cultures in Industrial Bioprocesses; Springer: Berlin/Heidelberg, Germany, 2024; pp. 125–136. [Google Scholar]

| Adsorbent | Se Form | Initial Concentration (mg/L) | Experimental Conditions | Adsorption Capacity (mg/g) | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|
| Aluminum-modified bamboo biochar | Se(VI) | 50 | 120 min, pH 4.0, 1 g/L | 37.6 | 99.6% | [72] |
| Nano-zerovalent zinc-functionalized biochar | Se(IV), Se(VI) | 1–130 | 1440 min, pH 4.0–10.0, 2 g/L | 23.83 (IV), 27.16 (VI) | 93–94% | [73] |
| β-Cyclodextrin-functionalized LDH/rGO | Se(IV), Se(VI) | 100 | 60–360 min, pH ≤ 7.0, 145.3 m2/g, 0.01–0.1 g/L | 169.6, 275.4 | ≥80% | [74] |
| Calcined layered double hydroxide of Mg/Al-CO3 | Se(IV) | 1000 | pH 5.0, 210 m2/g | 134.4 | - | [75] |
| Zirconium-based metal organic framework | Se(IV), Se(VI) | 5–150 | 360 min, pH 2.0–8.0, 0.5 g/L | 107.1 (IV), 47.6 (VI) | 74% | [76] |
| Ionic metal organic framework | Se(IV), Se(VI) | 1–10 | 30 min, pH 7.0, 1 g/L | 140.5 (IV), 73 (VI) | 99.9%, 99.4% | [77] |
| Fe-oxide-impregnated activated carbon | Se(IV), Se(VI) | 25 | 1920 min, pH 4.0, 1183 m2/g, 10 g/L | - | 70–85% | [78] |
| composite foam, incorporating chitosan, FeOOH and CNF | Se(IV) | 0.2–5 | 180 min, pH 3.0–10.0, 0.16 g/L | 90 | - | [79] |
| Fe-Mn bimetallic micro composite | Se(IV), Se(VI) | 0.1–10 | 60 min, pH 8.5, 59.345 m2/g, 0.5 g/L | - | ~95% | [80] |
| Amberlite, anion exchange resin with tertiary amine group | Se(IV) | 100 | 10 min, pH 3.0, 2 g/L | 18.52 | 80.3% | [81] |
| Adsorbent | Water Matrix | Adsorption Performance for Se(IV) | Adsorption Performance for Se(VI) | Reference |
|---|---|---|---|---|
| Bayoxide (BET surface area = 135 m2/g, IEP = 7.4) | Artificial groundwater at pH 7. Initial Se(IV) conc. = 50 µg/L or Initial Se(VI) conc. = 50 µg/L. EBCT = 3.5 ± 0.5 min. | Q10 = 1.7 mg/g | Q10 = 0 mg/g | [58] |
| GFH (BET surface area = 237 m2/g, P = 7.2) | Q10 = 0.7 mg/g | Q10 = 4 µg/g | [58] | |
| FeOOH (synthesized through precipitation of FeSO4 at pH 2, BET surface area = 100 m2/g, IEP = 5.7) | Q10 = 3.5 mg/g | Q10 = 17 µg/g | [58] | |
| Activated Alumina | Deionized water at pH 7 (NaHCO3 was added as pH buffer). Initial Se(IV) conc. = 10 mg/L or Initial Se(VI) conc. = 10 mg/L. | Qmax = ~2.1 mg/g | Qmax = ~0.8 mg/g | [83] |
| Aluminum–iron (Al–Fe) mixed oxide | Deionized water at pH 7. Initial Se(VI) conc. = 400 µg/L. | - | Qmax = 26.6 mg/g | [84] |
| Acrylic amine fiber (AAF) | Tap water at pH 7.2. Initial Se(VI) = 100 µg/L. EBCT = 4 min | - | 900 BV *t | [85] |
| Membrane | Water Matrix | Pressure (Bar) | Flux (L/m2·h) | Se Removal | Reference |
|---|---|---|---|---|---|
| NF90 | Groundwater, Se = 15 µg/L, | 9 | 23.1 | ≥93% | [114] |
| NF1 NF2 | Groundwater, Se(VI) = 1600 µg/L, pH = 8 | ≈15 | 125 280 | 94% 67% | [109] |
| NF20 | Groundwater, Se(VI) = 1600 µg/L, pH = 8 | ≈15 | 164 | 74% | |
| BW30 | Groundwater, Se = 15 µg/L, | 15 | 15.5 | ≥94% | [114] |
| Method | Efficiency (%) | Advantages | Limitations |
|---|---|---|---|
| Anaerobic Microbial Reduction | High (>90%) | Effective in diverse environments | Requires controlled conditions |
| Phytoremediation | Moderate | Sustainable, low-cost, enhances soil quality | Limited by plant growth cycles |
| Microalgae-Based Accumulation | Moderate | Rapid growth, versatile applications | Requires large-scale cultivation |
| Technology | Removal Efficiency (%) | Selectivity | Operational Feasibility | Scalability | Key Advantages | Reference |
|---|---|---|---|---|---|---|
| Adsorption | 70–95 | Moderate–High | High | High | Simple, low cost, effective at low Se concentration | [24] |
| Photocatalysis | 80–98 | High | Moderate | Moderate | Light-driven, can degrade other pollutants | [105] |
| Membrane Filtration | 85–98 | High | Moderate–High | Moderate | High purity removal, compact system | [9,110] |
| Electrochemical Methods | 85–95 | High | Moderate | Moderate | On-site treatment, no added chemicals | [124] |
| Biological Advancements (Free/Immobilized) | 80–99 | High | Moderate | Moderate–High | Eco-friendly, Se recovery possible | [9,162] |
| Recovery Strategy | Recovery Yield | Recovered From | Potential Reuse | Reference |
|---|---|---|---|---|
| Chemical or microbial reduction | Up to 99% | Elemental selenium (Se0) | Glass, pigments, electronics | [126,131] |
| Precipitation as metal selenides | 73–88% | CdSe, Cu1.08Se | Photovoltaic cells, semiconductors | [131] |
| Biosorption into algae (e.g., Chlorella) | 43–52% uptake | Se-enriched biomass (up to 323 mg/kg dry weight) | Feed additives, biofertilizers | [146] |
| Bio-volatilization (e.g., Pseudomonas spp.) | ~79–88% | Dimethyl selenide (DMSe) | Refined Se compounds for industrial reuse | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inam, M.A.; Usman, M.; Iftikhar, R.; Velizarov, S.; Ernst, M. Recent Progress in Selenium Remediation from Aqueous Systems: State-of-the-Art Technologies, Challenges, and Prospects. Water 2025, 17, 2241. https://doi.org/10.3390/w17152241
Inam MA, Usman M, Iftikhar R, Velizarov S, Ernst M. Recent Progress in Selenium Remediation from Aqueous Systems: State-of-the-Art Technologies, Challenges, and Prospects. Water. 2025; 17(15):2241. https://doi.org/10.3390/w17152241
Chicago/Turabian StyleInam, Muhammad Ali, Muhammad Usman, Rashid Iftikhar, Svetlozar Velizarov, and Mathias Ernst. 2025. "Recent Progress in Selenium Remediation from Aqueous Systems: State-of-the-Art Technologies, Challenges, and Prospects" Water 17, no. 15: 2241. https://doi.org/10.3390/w17152241
APA StyleInam, M. A., Usman, M., Iftikhar, R., Velizarov, S., & Ernst, M. (2025). Recent Progress in Selenium Remediation from Aqueous Systems: State-of-the-Art Technologies, Challenges, and Prospects. Water, 17(15), 2241. https://doi.org/10.3390/w17152241