The Hydrochemical Dynamics and Water Quality Evolution of the Rizhao Reservoir and Its Tributary Systems
Abstract
1. Introduction
2. Study Area
3. Materials and Methods
3.1. Collection and Methodology
3.2. Surface Water Quality Assessment
3.3. Evaluation of Agricultural Irrigation Water Quality
4. Results and Discussion
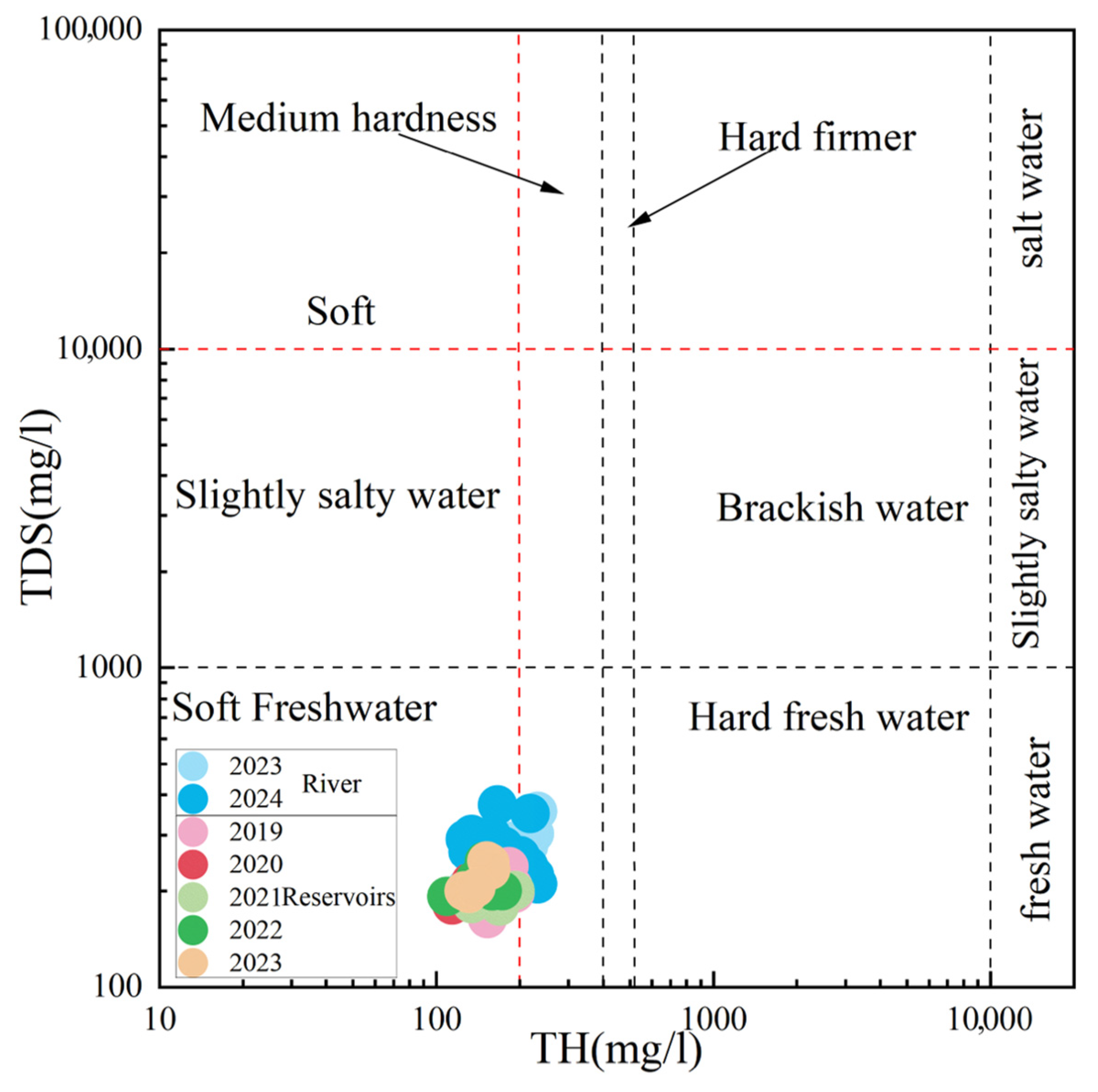
4.1. Statistical Characterization of Surface Water Chemistry
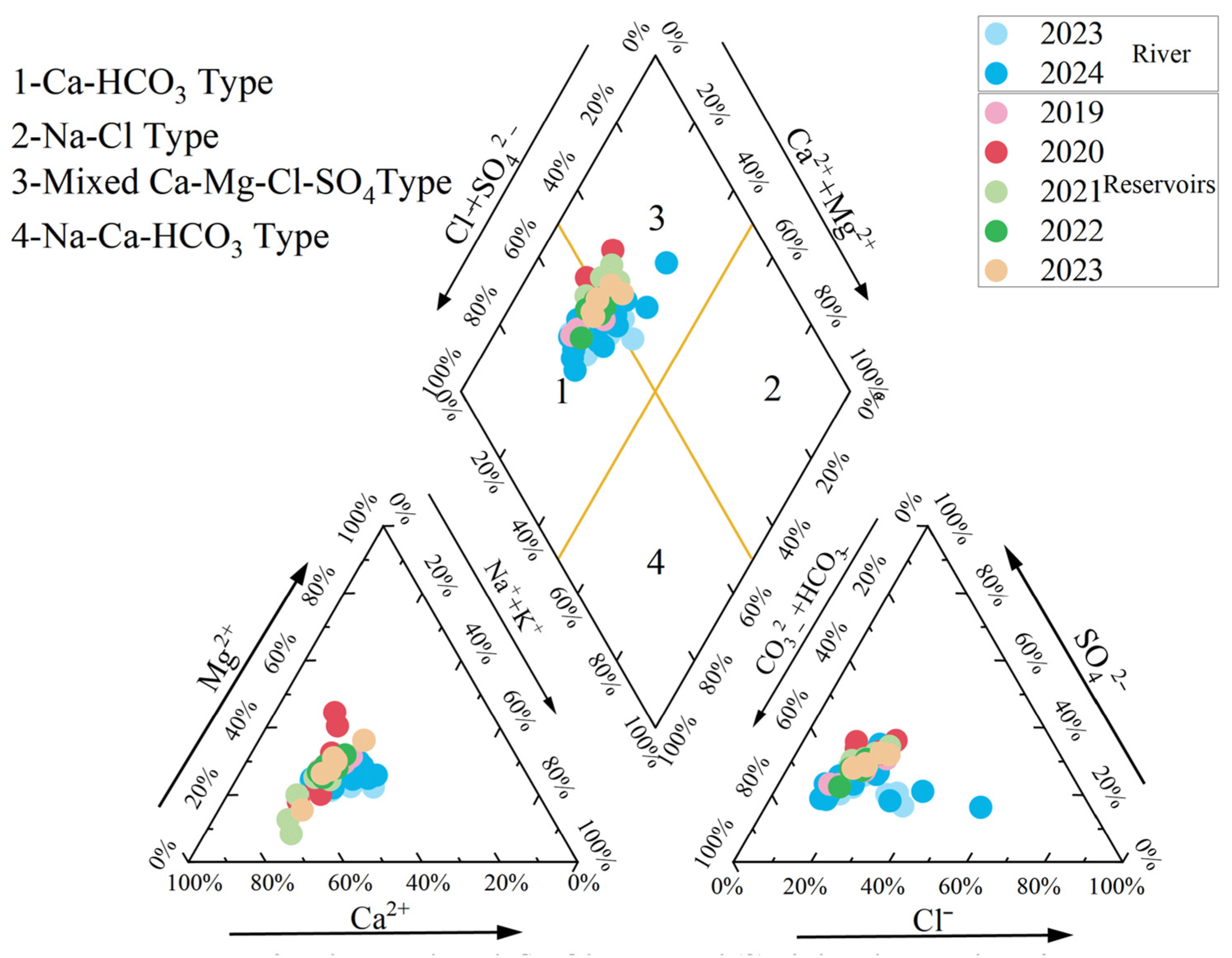
4.2. Types of Water Chemistry
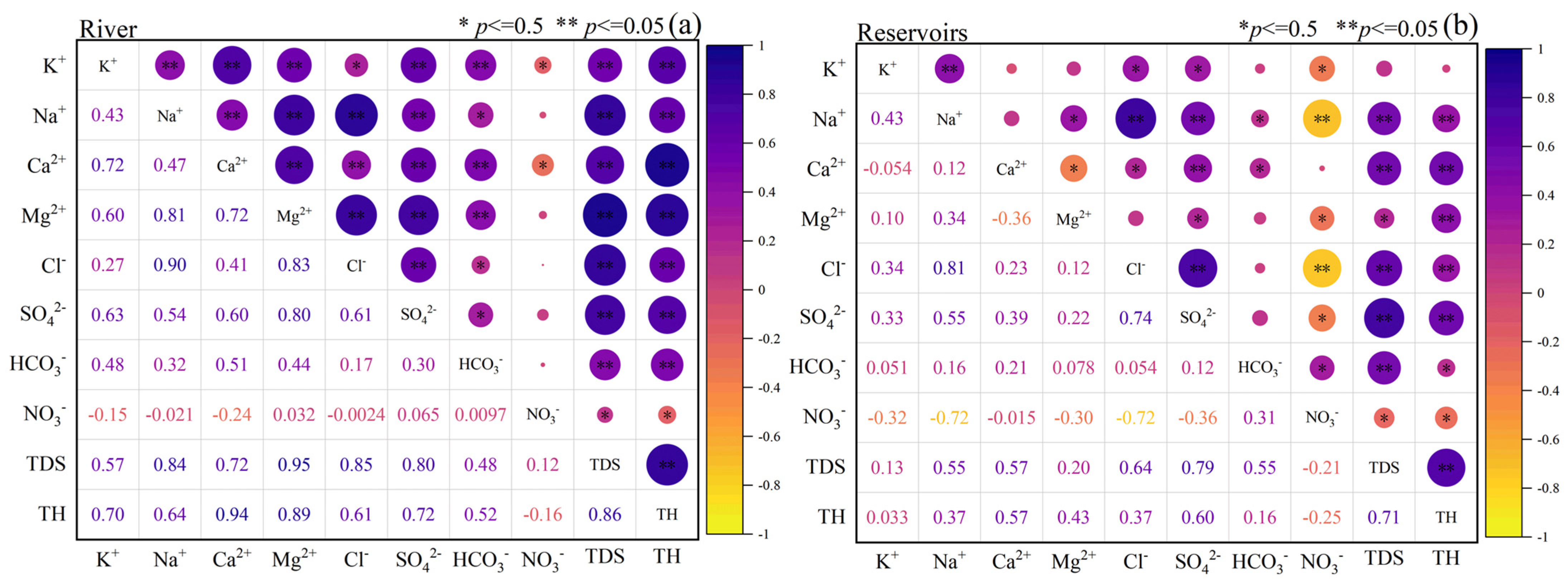
4.3. Correlation Analysis of Key Indicators of Water Chemistry
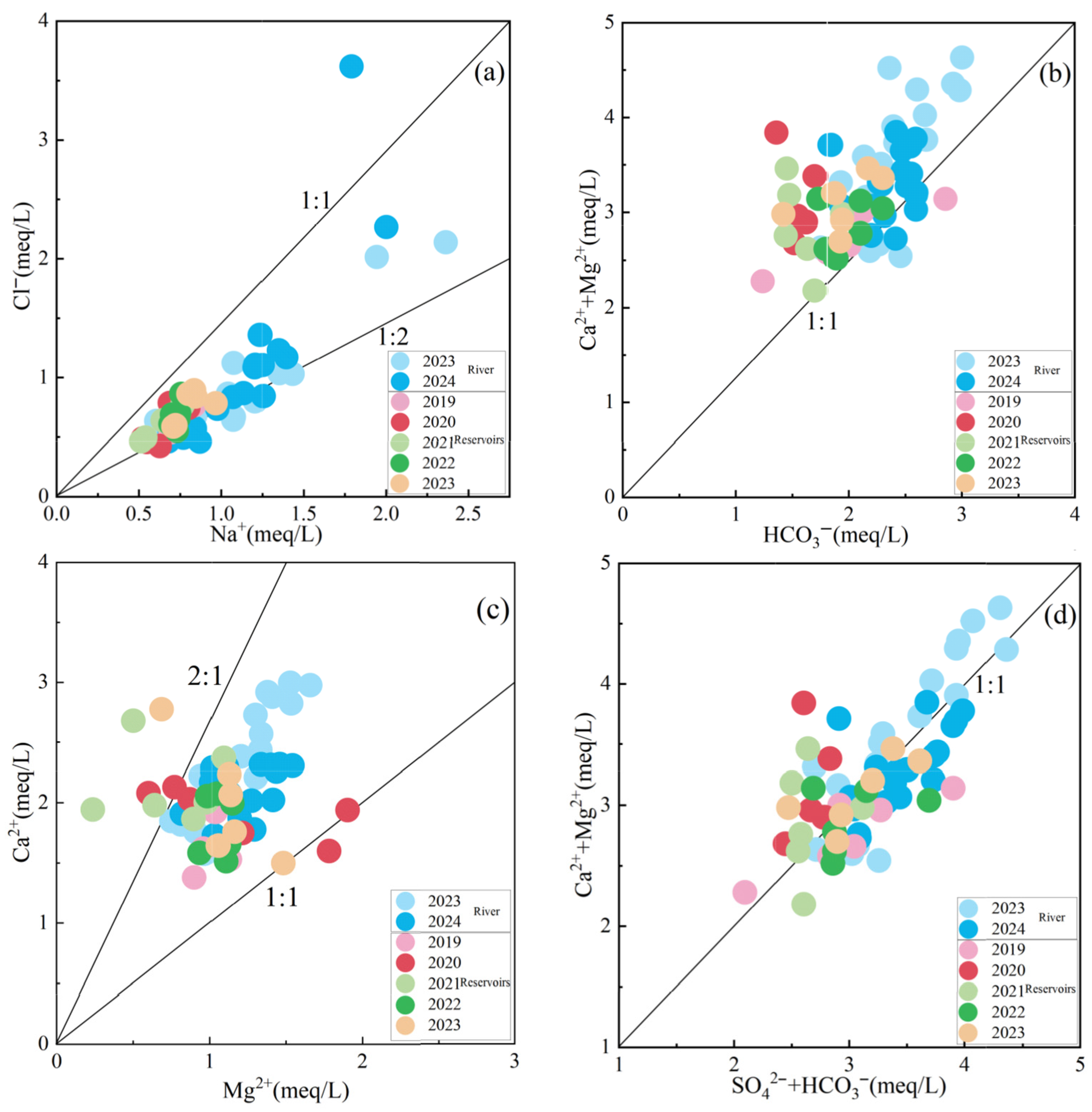
4.4. Analysis of the Hydrochemical Causes of Surface Water
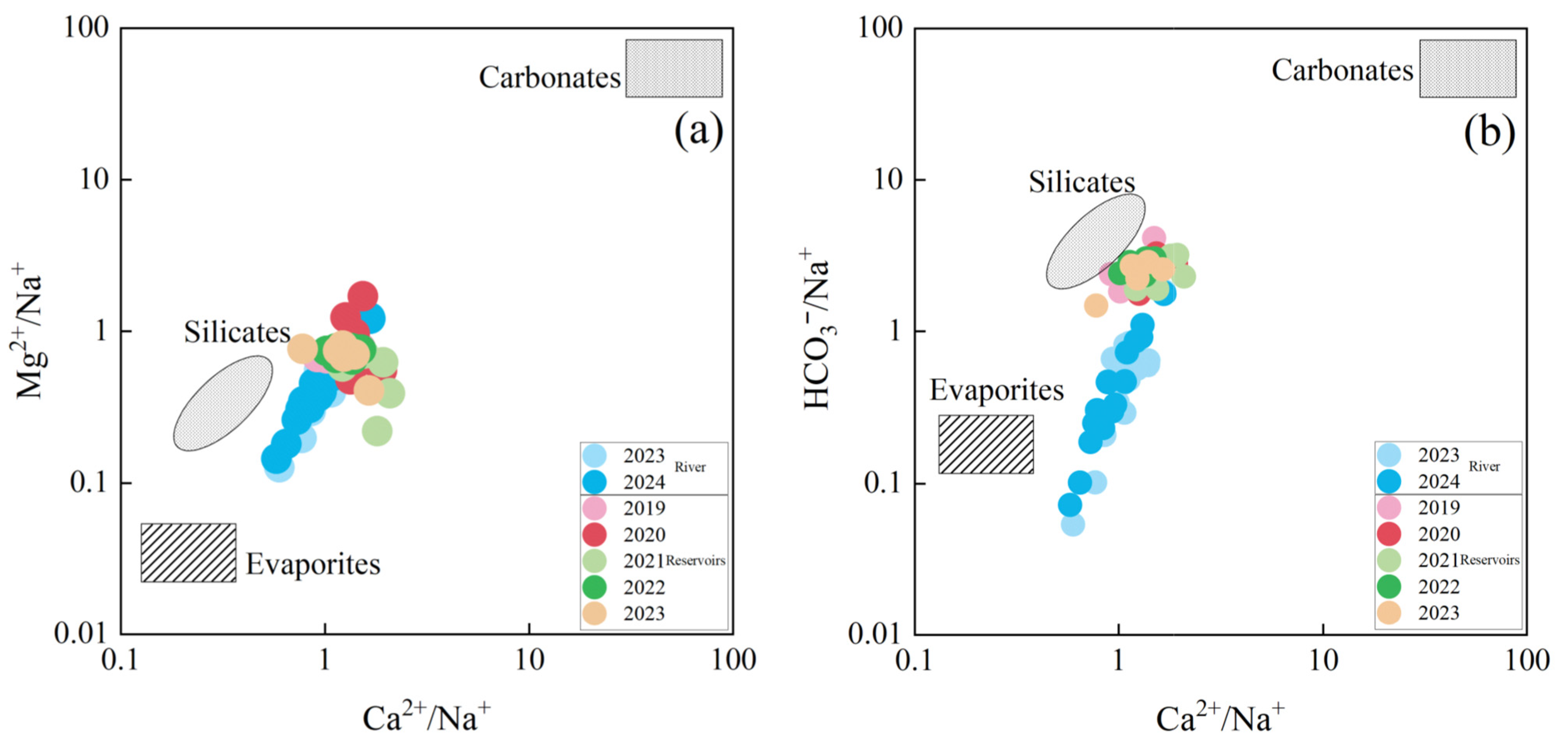
4.5. Major Ion Sources
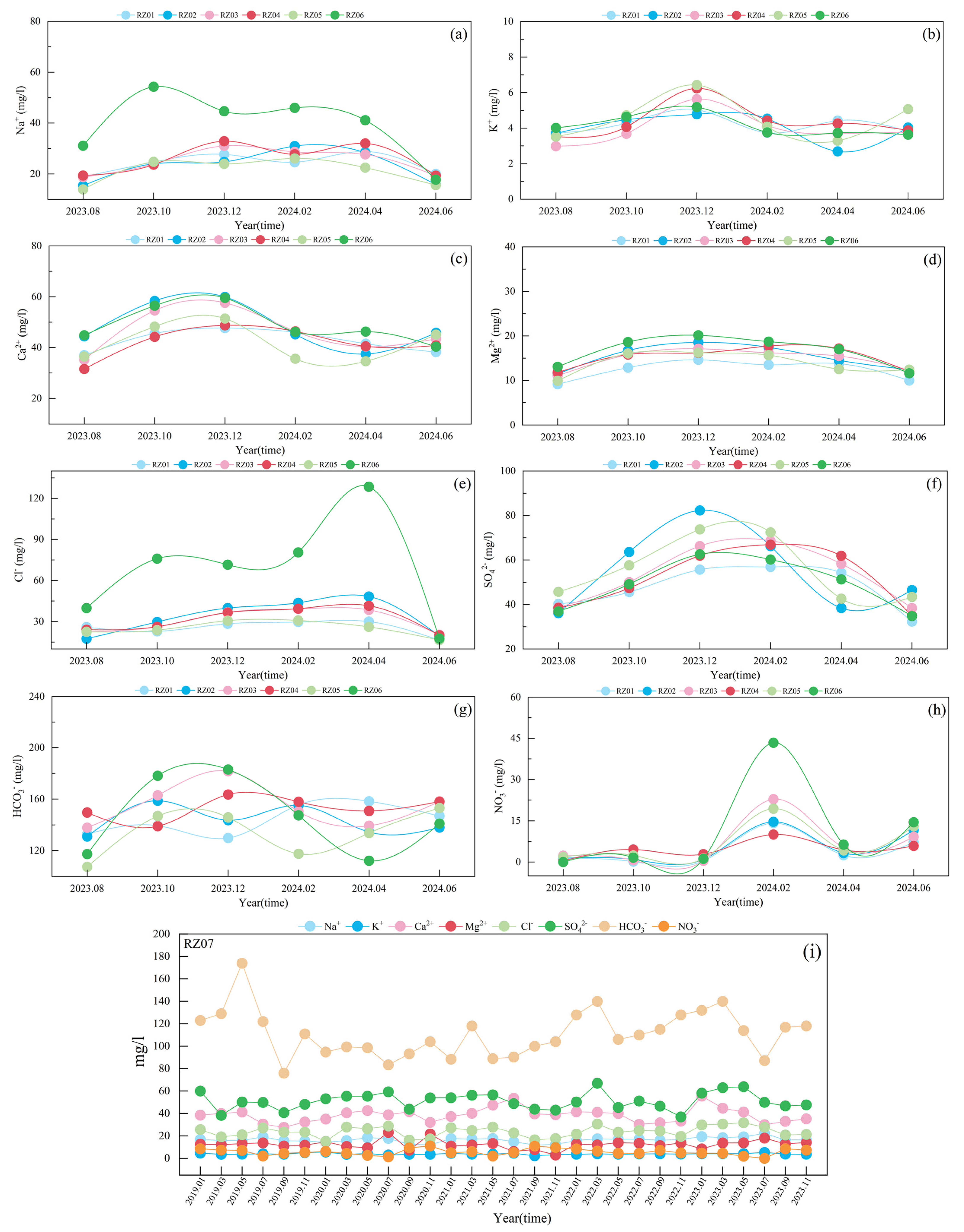
4.6. Characterization of Changes in the Content of Major Ions in Surface Water
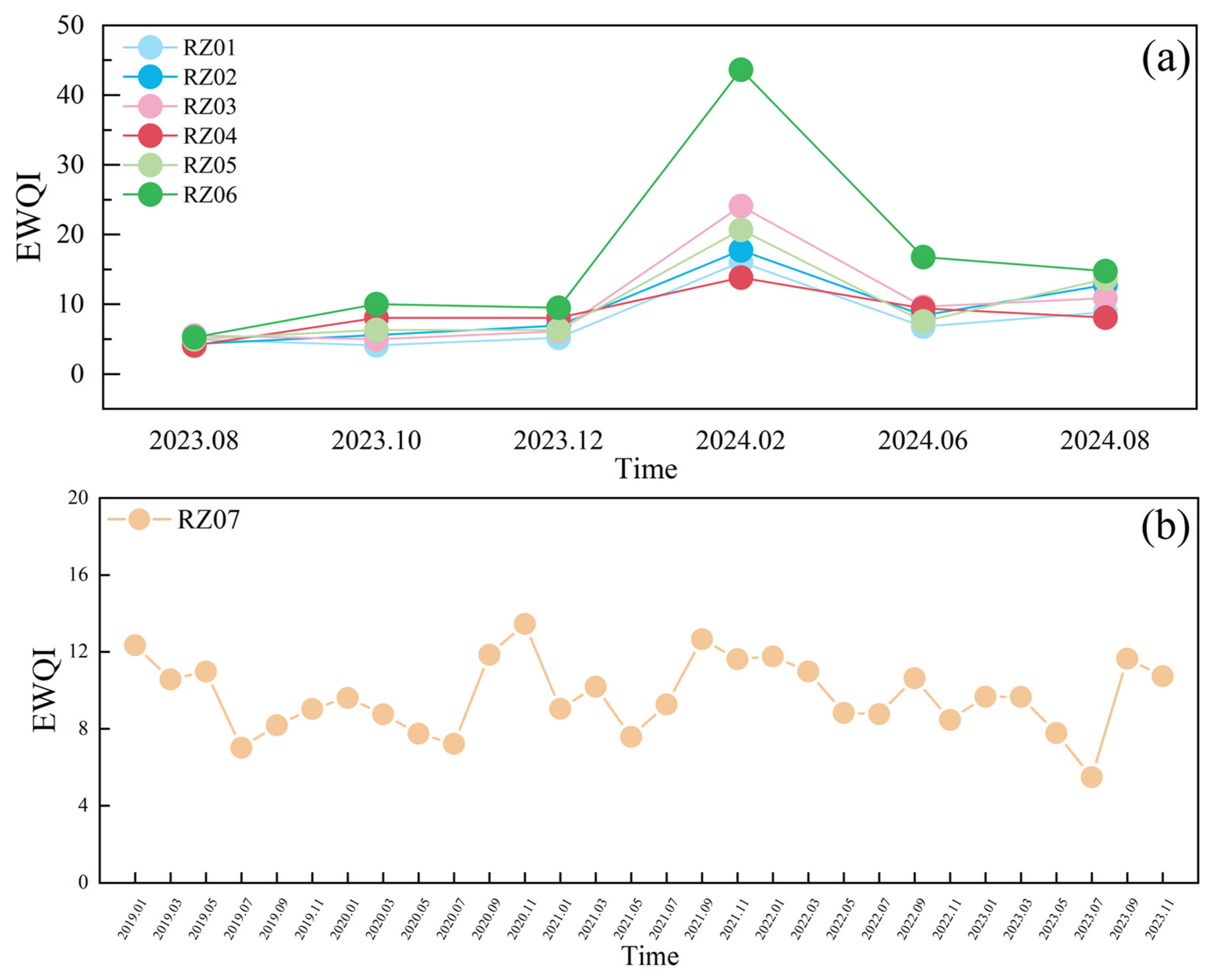
4.7. Evaluation of Drinking Water Quality
4.8. Evaluation of Irrigation Water Quality
4.9. Optimized Management Strategies for Surface Water Resources as Urban Water Supply Sources
- (1)
- Monitoring indicates weakly alkaline surface water (pH7.3–9.78; mean 8.29–8.6). To prevent pipeline corrosion and scaling, implement an automated pH adjustment system using food-grade CO2 or dilute H2SO4 to maintain effluent pH at 7.0–8.5.
- (2)
- Significant spatiotemporal variability occurs in river water quality (e.g., RZ06 exhibited Cl− and NO3−peaks in April/February 2024, respectively, potentially linked to agricultural runoff and domestic sewage). Recommended actions: Install automatic Cl−/NO3− monitoring upstream; implement soil testing and organic fertilizer programs; construct wastewater treatment facilities near RZ06.
- (3)
- Geochemical analysis confirms Rizhao Reservoir’s ionic sourcing from rock weathering. Despite minimal anthropogenic pollution, long-term contaminant accumulation requires vigilance. Mitigation measures: Establish vegetative buffers in upstream catchments; designate core protection zones; restrict quarrying/mining activities.
- (4)
- EWQI assessment verifies reservoir compliance with drinking standards, though seasonal and episodic pollution occurs. Establish: Multi-source water allocation framework; 2–3 backup reservoir systems; enhanced emergency response protocols.
- (5)
- Irrigation evaluations confirm surface water suitability without salinization risks. Optimization strategies: Substitute groundwater with surface sources; Implement crop-specific irrigation quotas; Prioritize water allocation for high-consumption crops; address groundwater depletion trends.
5. Conclusions
- (1)
- River water exhibited alkaline conditions (pH 7.63–9.78, mean = 8.6), with mean TDS (257.76 mg/L) and TH (172.54 mg/L) characteristic of freshwater systems. Dominant ions were Ca2+ (45.04 mg/L) and HCO3− (146 mg/L), confirming freshwater classification for most samples. Reservoir water (2019–2023) similarly showed alkalinity (pH 7.3–9.0, mean = 8.29), lower mean TDS (207.55 mg/L) and TH (147.17 mg/L), with identical dominant ions at reduced concentrations (Ca2 + =38.5 mg/L; HCO3− = 111.10 mg/L). Hydrochemical facies primarily comprised Ca-HCO3 and Ca-Mg-SO4-Cl types.
- (2)
- Rizhao Reservoir surface water hydrochemical characteristics of the main controlling factors for the weathering of rocks. The source of hydrochemical constituents is mainly related to the weathering and dissolution of silicate rocks, carbonate rocks, and evaporite salt rocks. The concentration of NO3− in surface water is relatively low (mean value), indicating that it is less affected by anthropogenic inputs.
- (3)
- River water displays greater parameter variability than reservoir water. Most monitoring points exhibited ion concentration peaks followed by declines, particularly at RZ06, where Cl− (April 2024 peak) and NO3− (February 2024 peak) showed significant fluctuations. In contrast, RZ07 maintained relatively stable ion concentrations.
- (4)
- EWQI assessment confirms excellent reservoir water quality meeting drinking standards (seasonal variations notwithstanding). Irrigation suitability evaluation indicates all surface waters pose negligible salinization risk and require no pretreatment for agricultural use.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khatri, N.; Tyagi, S. Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front. Life Sci. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Akhtar, N.; Syakir, M.; Rai, S.; Saini, R.; Pant, N.; Anees, M.; Qadir, A.; Khan, U. Multivariate investigation of heavy metals in the groundwater for irrigation and drinking in Garautha Tehsil, Jhansi District, India. Anal. Lett. 2020, 53, 774–794. [Google Scholar] [CrossRef]
- Manjunatha, S.; Bobade, K.; Kudale, M. Pre-cooling technique for a thermal discharge from the coastal thermal power plant. Anal. Lett. 2015, 116, 358–365. [Google Scholar] [CrossRef][Green Version]
- Parris, K. Impact of agriculture on water pollution in OECD countries: Recent trends and future prospects. Int. J. Water Resour. Dev. 2014, 27, 33–52. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Coelho, L.M.; Rezende, H.C.; Coelho, L.M.; De Sousa, P.; Melo, D.; Coelho, N. Bioremediation of polluted waters using microorganisms. Adv. Bioremediat. Wastewater Polluted Soil 2015, 10, 60770. [Google Scholar]
- Li, H.; Yu, X.; Zhang, W.; Huan, Y.; Yu, J.; Zhang, Y. Risk assessment of groundwater organic pollution using hazard, intrinsic vulnerability, and groundwater value, Suzhou City in China. Expo. Health 2018, 10, 99–115. [Google Scholar] [CrossRef]
- Rasheed, H.; Altaf, F.; Anwaar, K.; Ashraf, M. Drinking Water Quality in Pakistan: Current Status and Challenges; Pakistan Council of research in water resources (PCRWR): Islamabad, Pakistan, 2021; p. 141. [Google Scholar]
- Taloor, A.K.; Pir, R.A.; Adimalla, N.; Ali, S.; Manhas, D.S.; Roy, S.; Singh, A.K. Spring water quality and discharge assessment in the Basantar watershed of Jammu Himalaya using geographic information system (GIS) and water quality Index (WQI). Groundw. Sustain. Dev. 2020, 10, 100364. [Google Scholar] [CrossRef]
- Dippong, T.; Resz, M.A. Heavy metal contamination assessment and potential human health risk of water quality of lakes situated in the protected area of Tisa, Romania. Heliyon 2024, 10, e28860. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.E.; Meybeck, M.; Chapman, D.V. Effects of human activities on water quality. In Encyclopedia of Hydrological Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Zhang, L.; Li, P.; He, X. Interactions between surface water and groundwater in selected tributaries of the Wei River (China) revealed by hydrochemistry and stable isotopes. Hum. Ecol. Risk Assess. Int. J. 2022, 28, 79–99. [Google Scholar] [CrossRef]
- Vatanpour, N.; Malvandi, A.M.; Hedayati Talouki, H.; Gattinoni, P.; Scesi, L.; Research, P. Impact of rapid urbanization on the surface water’s quality: A long-term environmental and physicochemical investigation of Tajan river, Iran (2007–2017). Environ. Sci. Pollut. Res. 2020, 27, 8439–8450. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, C.; Jiang, X.; Zhao, T.; Huang, H. Research on Surface Water Quality Assessment and Its Driving Factors: A Case Study in Taizhou City, China. Water 2022, 15, 26. [Google Scholar] [CrossRef]
- Yan, T.; Shen, S.-L.; Zhou, A. Indices and models of surface water quality assessment: Review and perspectives. Environ. Pollut. 2022, 308, 119611. [Google Scholar] [CrossRef] [PubMed]
- Ndondo, G.R.N.; Boum, S.N.; Song, F.; Eyong, G.E.T.; Komba, D.E.; Nlend, B.; Etame, J.; Protection, E. Hydrogeochemical Characteristics and Quality Assessment of Surface Water in an Agricultural Area in Equatorial Africa: The Mungo River Basin, South West Cameroon, Central Africa. J. Geosci. Environ. Prot. 2021, 9, 164–181. [Google Scholar] [CrossRef]
- Ubuoh, E.; Nwogu, F.; Ossai-Abeh, E.; Ikwuemesi, J.; Oke, A.; Umoh, J. Evaluation of hydro-chemical facies and surface water quality dynamics using multivariate statistical approaches in Southern Nigeria. Sci. Rep. 2024, 14, 31600. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Wang, Z.Z.; Cheng, L.; Zhang, L.L.; Du, H.H.; Tan, L.Y. Optimal operation of interbasin water transfer multireservoir systems: An empirical analysis from China. Environ. Earth Sci. 2019, 78, 238. [Google Scholar] [CrossRef]
- Song, H.Y.; Liu, J.Q.; Yin, P.; Zhang, Y. Distribution, enrichment and source of heavy metals in Rizhao offshore area, southeast Shandong Province. Mar. Pollut. Bull. 2017, 119, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Li, X.; Yuan, J.; Tian, H.; Wei, T.; Wang, M.; Dai, Y.; Feng, J.; Zhang, Y.; Yang, P. Hydrogeochemical Signatures and Spatiotemporal Variation of Groundwater Quality in the Upper and Lower Reaches of Rizhao Reservoir. Water 2025, 17, 1659. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland; International Electrotechnical Commission: Paris, France, 2017.
- Singh, K.R.; Dutta, R.; Kalamdhad, A.S.; Kumar, B. Information entropy as a tool in surface water quality assessment. Environ. Earth Sci. 2019, 78, 15. [Google Scholar] [CrossRef]
- Nguyen, A.; Pham, N.; Tat, V.; Truong, H.; Vo, P. Application of Entropy weight in groundwater quality index (EWQI) and GIS for groundwater quality zoning in the Southeastern Coastal region, Vietnam. In Proceedings of the IOP Conference Series: Earth and Environmental Science, International Conference on Environment, Resources and Earth Sciences, Ho Chi Minh City, Vietnam, 1–5 December 2020; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Zhang, Y.; Gao, S.; Hu, C.; Zhao, Z.; Gao, Z.; Liu, J. Hydrochemical assessment of groundwater utilizing statistical analysis, integrated geochemical methods, and EWQI: A case study of Laiwu region, North China. Environ. Monit. Assess. 2024, 196, 1222. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Augustine, C. Entropy-weighted water quality index (EWQI) modeling of groundwater quality and spatial mapping in Uppar Odai Sub-Basin, South India. Model. Earth Syst. Environ. 2022, 8, 911–924. [Google Scholar] [CrossRef]
- Latha, P. Evaluation of groundwater quality for domestic and irrigation purposes in a coastal alluvial aquifer using multivariate statistics and entropy water quality index approach: A case study from West Godavari Delta, Andhra Pradesh (India). Environ. Earth Sci. 2022, 81, 275. [Google Scholar] [CrossRef]
- GB 3838-2002; China National Standardization Administration. Environmental Quality Standards for Surface Water. China Environmental Science Press: Beijing, China, 2002.
- Misaghi, F.; Delgosha, F.; Razzaghmanesh, M.; Myers, B. Introducing a water quality index for assessing water for irrigation purposes: A case study of the Ghezel Ozan River. Sci. Total Environ. 2017, 589, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M. Groundwater geochemistry in the Alisadr, Hamadan, western Iran. Environ. Monit. Assess. 2010, 166, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, A.Y.; Khan, M.Z.A. Deterministic modeling of Al-Madinah Al-Munawarah groundwater quality using lumped parameter approach. J. King Abdul Aziz Univ. Earth Sci. 1992, 5, 89–107. [Google Scholar]
- Rawat, K.S.; Singh, S.K.; Gautam, S. Assessment of groundwater quality for irrigation use: A peninsular case study. Appl. Water Sci. 2018, 8, 233. [Google Scholar] [CrossRef]
- GB 5749-2022; Standards for Drinking Water Quality. State Administration for Market Regulation, Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar]
- Singh, K.K.; Tewari, G.; Kumar, S. Evaluation of groundwater quality for suitability of irrigation purposes: A case study in the Udham Singh Nagar, Uttarakhand. J. Chem. 2020, 2020, 6924026. [Google Scholar] [CrossRef]
- Marghade, D.; Malpe, D.B.; Subba Rao, N. Applications of geochemical and multivariate statistical approaches for the evaluation of groundwater quality and human health risks in a semi-arid region of eastern Maharashtra, India. Environ. Geochem. Health 2021, 43, 683–703. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Lin, J.; Wang, J.; Yan, T.; Ren, K.; Zhou, J.; Li, Y. Analysis of the evolution and causes of groundwater chemistry after ecological water replenishment of the Jialu River, China. Sci. Rep. 2024, 14, 18759. [Google Scholar] [CrossRef] [PubMed]
- Eid, M.H.; Eissa, M.; Mohamed, E.A.; Ramadan, H.S.; Tamás, M.; Kovács, A.; Szucs, P. New approach into human health risk assessment associated with heavy metals in surface water and groundwater using Monte Carlo Method. Sci. Rep. 2024, 14, 1008. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, H.; Zhang, Q.; Health, P. Assessment of the evolution of groundwater chemistry and its controlling factors in the Huangshui River Basin of northwestern China, using hydrochemistry and multivariate statistical techniques. Int. J. Environ. Res. Public Health 2021, 18, 7551. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, J.; Zhang, S.; Bai, Y.; Zhang, X.; Chen, D.; Hu, J. Groundwater hydrochemical signatures, nitrate sources, and potential health risks in a typical karst catchment of North China using hydrochemistry and multiple stable isotopes. Environ. Geochem. Health 2024, 46, 173. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Feng, S.B. Hydrochemical Characteristics and Water Quality Evaluation of Surface Water in Chating Mining Area, Xuancheng, Anhui, China. Pol. J. Environ. Stud. 2022, 31, 5611–5620. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Guo, H.; Yin, J.; Hu, H.; Cui, D.; Gao, B. Genesis of high hexavalent chromium groundwater in deep aquifers from loess plateau of Northern Shaanxi, China. Water Res. 2022, 216, 118323. [Google Scholar] [CrossRef] [PubMed]
- Yidana, S.M.; Bawoyobie, P.; Sakyi, P.; Fynn, O. Evolutionary analysis of groundwater flow: Application of multivariate statistical analysis to hydrochemical data in the Densu Basin, Ghana. J. Afr. Earth Sci. 2018, 138, 167–176. [Google Scholar] [CrossRef]
- Xiao, J.; Jin, Z.; Zhang, F.; Wang, J. Major ion geochemistry of shallow groundwater in the Qinghai Lake catchment, NE Qinghai-Tibet Plateau. Environ. Earth Sci. 2012, 67, 1331–1344. [Google Scholar] [CrossRef]
- Akshitha, V.; Balakrishna, K.; Udayashankar, H. Assessment of hydrogeochemical characteristics and saltwater intrusion in selected coastal aquifers of southwestern India. Mar. Pollut. Bull. 2021, 173, 112989. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Improved Estimates of the Spatial Distribution and Temporal Trends of Water Quality Parameters Using Geostatistical Data Fusion Methods; University of Michigan: Ann Arbor, MI, USA, 2013. [Google Scholar]
- Wei, S.M.; Zhang, Y.X.; Cai, Z.Z.; Bi, D.W.; Wei, H.H.; Zheng, X.F.; Man, X.B. Evaluation of groundwater quality and health risk assessment in Dawen River Basin, North China. Environ. Res. 2025, 264, 120292. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Loganathan, V.A.; Bhatt, V. Development of entropy and deviation-based water quality index: Case of River Ganga, India. Ecol. Indic. 2022, 143, 109319. [Google Scholar] [CrossRef]
- Baï, R.; Kouame, I.K.; Kouassı, L.K.; Konan, S.K.; N’Cho, H. Assessment of the physicochemical quality of irrigation water and soil for sustainable irrigated rice cultivation: Case of irrigated perimeter of M’Bahiakro (central-east of Côte d’Ivoire). J. Environ. Prot. 2019, 10, 1536. [Google Scholar] [CrossRef]
- Wilcox, L. Classification and Use of Irrigation Waters; US Department of Agriculture: Washington, DC, USA, 1955. [Google Scholar]
- Etteieb, S.; Cherif, S.; Tarhouni, J. Hydrochemical assessment of water quality for irrigation: A case study of the Medjerda River in Tunisia. Appl. Water Sci. 2017, 7, 469–480. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; US Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Srivastava, A.K.; Parimal, P. Source rock weathering and groundwater suitability for irrigation in Purna alluvial basin, Maharashtra, central India. J. Earth Syst. Sci. 2020, 129, 1. [Google Scholar] [CrossRef]




| Evaluation Parameters | Classification Reference Values | Applicable Grade | Evaluation Parameters | Classification Reference Values | Applicable Grade |
|---|---|---|---|---|---|
| %Na | <20 | well suited | SAR | <10 | well suited |
| 20–40 | better suited | 10–18 | better suited | ||
| 40–60 | suitability | 18–26 | suitability | ||
| 60–80 | inconclusive | >26 | unsuitable | ||
| >80 | unsuitable | ||||
| RSC | <1.25 | well suited | PI% | >75 | I (well suited) |
| 1.25–2.50 | suitability | 25–75 | II (suitability) | ||
| >2.50 | unsuitable | <25 | III (unsuitable) | ||
| Norm (Unit) | Square Sum | Mean Square | F | Significance |
|---|---|---|---|---|
| Ca2+ (mg/L) | 698.953 | 698.953 | 14.322 | <0.0003 |
| Mg2+ (mg/L) | 79.284 | 79.284 | 6.815 | 0.0112 |
| K+ (mg/L) | 1.332 | 1.332 | 2.438 | 0.1234 |
| Na+ (mg/L) | 1611.784 | 1611.784 | 34.828 | <0.0000 |
| HCO3− (mg/L) | 19,930.701 | 19,930.701 | 54.704 | <0.0000 |
| SO42− (mg/L) | 16.322 | 16.322 | 0.138 | 0.7116 |
| Cl− (mg/L) | 2330.208 | 2330.208 | 8.290 | 0.0054 |
| NO3− (mg/L) | 12.714 | 12.714 | 0.288 | 0.5935 |
| pH (/) | 1.573 | 1.573 | 5.919 | 0.0178 |
| TDS (mg/L) | 41,253.239 | 41,253.239 | 27.332 | <0.0000 |
| TH (mg/L) | 10,533.744 | 10,533.744 | 18.547 | <0.0001 |
| EWQI | Level | Category |
|---|---|---|
| <25 | I | Excellent |
| 25~50 | II | Good |
| 51~100 | III | Medium |
| 101~150 | IV | Poor |
| >150 | V | Very poor |
| Parameters | Rank Value | Water Classification | Number of Samples | |
|---|---|---|---|---|
| River | Reservoirs | |||
| %Na | <20 | Excellent | 6 | 20 |
| 20–40 | Good | 30 | 10 | |
| 40–60 | Permissible | 0 | 0 | |
| 60–80 | Doubtful | 0 | 0 | |
| >80 | Unsuitable | 0 | 0 | |
| RSC | <1.25 | Good | 36 | 30 |
| 1.25–2.50 | Doubtful | 0 | 0 | |
| >2.50 | Unsuitable | 0 | 0 | |
| SAR | 0–6 | Good | 36 | 30 |
| 6–9 | Doubtful | 0 | 0 | |
| >9 | unsuitable | 0 | 0 | |
| PI% | >75 | Good | 0 | 0 |
| 25–75 | Suitable | 36 | 30 | |
| <25 | Unsuitable | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Q.; Lv, Y.; Feng, J.; Lei, W.; Zhang, Y.; Gao, M.; Zhang, L.; Zhao, B.; Zhao, D.; Lou, K. The Hydrochemical Dynamics and Water Quality Evolution of the Rizhao Reservoir and Its Tributary Systems. Water 2025, 17, 2224. https://doi.org/10.3390/w17152224
Feng Q, Lv Y, Feng J, Lei W, Zhang Y, Gao M, Zhang L, Zhao B, Zhao D, Lou K. The Hydrochemical Dynamics and Water Quality Evolution of the Rizhao Reservoir and Its Tributary Systems. Water. 2025; 17(15):2224. https://doi.org/10.3390/w17152224
Chicago/Turabian StyleFeng, Qiyuan, Youcheng Lv, Jianguo Feng, Weidong Lei, Yuqi Zhang, Mingyu Gao, Linghui Zhang, Baoqing Zhao, Dongliang Zhao, and Kexin Lou. 2025. "The Hydrochemical Dynamics and Water Quality Evolution of the Rizhao Reservoir and Its Tributary Systems" Water 17, no. 15: 2224. https://doi.org/10.3390/w17152224
APA StyleFeng, Q., Lv, Y., Feng, J., Lei, W., Zhang, Y., Gao, M., Zhang, L., Zhao, B., Zhao, D., & Lou, K. (2025). The Hydrochemical Dynamics and Water Quality Evolution of the Rizhao Reservoir and Its Tributary Systems. Water, 17(15), 2224. https://doi.org/10.3390/w17152224





