Abstract
Nematodes are among the organisms found in treated water. While generally considered harmless to human health, under certain conditions, they may serve as vectors for pathogenic viruses and bacteria, posing potential risks. Conventional disinfection processes in water treatment can contribute to the inactivation or removal of nematodes, but their effectiveness varies. This study, conducted at a water treatment plant (WTP) in Mashhad, Iran, aimed to determine the optimal dose and contact time of sodium hypochlorite and ozone for enhancing nematode inactivation in the affected surface water. This research combined primary disinfection using sodium hypochlorite at the existing WTP with a pilot ozone injection system to evaluate their individual and combined effectiveness. The results show that sodium hypochlorite at a concentration of 2 mg/L achieved 68% nematode inactivation. At 2.0 mg/L, with a 20 min contact time, ozone disinfection resulted in 39% inactivation. However, the combined application of sodium hypochlorite and ozone significantly improved efficiency, reaching 92% nematode inactivation when sodium hypochlorite and ozone were applied at 2 mg/L and 3 mg/L, respectively, with a 20 min ozone contact time. These findings indicate that, among the disinfection methods examined, the combined use of sodium hypochlorite and ozone is the most effective approach for nematode inactivation in drinking water, offering a promising strategy for improving water quality and safety.
1. Introduction
Drinking water quality is crucial for human health, necessitating continuous improvements in safety. Advances in science have identified contaminants, including pathogens, as key contributors to poisoning and gastrointestinal diseases. Effective disinfection is crucial for mitigating these risks. Chlorine and its derivatives remain the most widely used disinfectants due to their efficacy at low concentrations, affordability, availability, and residual presence [1].
Nematodes are microscopic, thread-like, unsegmented worms with a chitinous cuticle and no flagella, commonly found in soil and aquatic environments, including drinking water sources. While many nematode species are harmless, some can be pathogenic or act as vectors (biological agents) for other harmful microorganisms. Their presence in drinking water raises concerns due to potential health risks and operational challenges in water treatment systems. Their significance in water treatment lies in their ability to ingest and transfer pathogenic microorganisms, which can potentially cause disease outbreaks. A single nematode can harbor between 10 and 4000 bacteria, facilitating the transmission of pathogens to humans [2]. Nematodes’ mobility enhances their resistance to disinfection [3]. Filters can serve as breeding grounds, making pre-filter inactivation and proper washing crucial for effective removal [4]. Their presence in water contributes to taste and odor issues, compromising drinking water quality, and can also cause damage to treatment facilities and distribution pipelines [5]. There is no mandatory control standard for invertebrates in drinking water in any country or region [6]. Research by Cui et al. [7] suggests that prolonged warming has a significant impact on nematode community composition, underscoring its effect on aquatic biodiversity. This finding is also supported by Locas et al. and Wu et al. [8,9]. Therefore, escalating climate warming also poses a growing threat to native ecosystems, including aquatic microbial communities.
One of the primary issues with nematodes in drinking water is their ability to harbor and protect pathogenic bacteria, including Legionella and Escherichia coli, by providing a safe environment within their bodies [8]. This can lead to increased microbial contamination and potential outbreaks of waterborne diseases. Additionally, certain nematodes, such as Angiostrongyluscantonensis, have been linked to human infections, resulting in neurological diseases like eosinophilic meningitis [10]. Nematodes were found to have a sheltering effect on indigenous bacteria and pathogenic bacteria, allowing them to resist chlorine disinfection and UV (ultraviolet) disinfection. When subjected to a UV dose of 40 mJ/cm2, the inactivation rates of indigenous bacteria and three pathogenic bacteria decreased by 85% and 39–50% when the living nematodes sheltered bacteria, while they decreased by 66% and 15–41% when they were sheltered by nematode residue [6].
Another concern is their resilience to conventional water treatment methods. Nematodes can survive in treated water due to their protective cuticle and resistance to disinfectants. Sodium hypochlorite and other chlorine-based disinfectants are commonly used in water treatment due to their availability, ease of application, and cost-effectiveness. While chlorine is effective against many microorganisms, nematodes can exhibit specific resistance due to their protective cuticle, requiring higher concentrations or prolonged exposure times for effective inactivation. Ozone is a strong oxidant that can damage nematode cell structures, leading to inactivation. Research has shown that chlorine dioxide and ozone treatment at sufficient dosages and contact times can significantly reduce nematode populations [11].
Various methods can be used to remove nematodes from water including the following: 1—Physical filtration methods, such as slow sand filtration, micro-filtration (MF), and ultra-filtration; (UF) 2—Chemical disinfection methods, such as the use of sodium hypochlorite, chlorine dioxide, and ozone; 3—Physical disinfection methods, such as UV radiation; 4—Combined approaches using two or more of the methods above [12,13]. If not effectively removed, nematodes can proliferate in distribution systems, leading to the formation of biofilms and a decline in water quality. Given these concerns, controlling nematode contamination in drinking water is crucial for maintaining public health and ensuring regulatory compliance. Effective removal strategies, including chemical disinfectants, such as sodium hypochlorite, and advanced oxidation processes, like ozone treatment, are essential for mitigating the risks associated with nematode presence in water supply systems [11].
Although some human pathogens resist chlorination, pre-disinfection for nematode removal can be an effective strategy [14]. In drinking water treatment, ozonation is widely used to control microorganisms and taste- and odor-causing compounds by promoting organic matter removal through pre-oxidation and adsorption [15]. Hoveydi et al. [16] investigated the effect of ozone application on the removal of nematodes in drinking water. They examined different concentrations of ozone and concluded that the use of ozone at a concentration of 3.25 ppm and a contact time of 5 min was capable of completely removing nematodes in laboratory samples.
Matsumoto et al. [17] investigated the effect of simultaneous use of physical and chemical methods for the removal of nematodes. In their study, they investigated the effects of chlorine dioxide, ozone, and UV, as well as sand filtration and membrane filtration. According to their results, filtration alone was not able to completely remove nematodes. However, the combination of UV and sand filtration was very effective in removing nematodes from drinking water. The efficacy of ozone and ultrasound was studied by Steel et al. [18] to eliminate Angiostrongyluscantonensis and its larvae. According to the results of the study, irradiation of ultrasound waves with a frequency of 40 kHz for 60 s caused 46% mortality of larvae within 2 h, while exposing the larvae to different concentrations and durations of ozone gas caused100%mortality of the larvae. Based on the properties of ozone as a potent disinfectant, the inactivation or degradation of Helminth (Ascaris suum) eggs in water was studied by Velásquez et al. [19]. In the study, the ozone concentration was generally between 3.5 and 4.7 mg/L. The results show that the contact time required to inactivate 90%of the eggs (t90) was 1 h, and the remaining 10% were inactivated in 2 h. The study confirms that ozone effectively induces structural/biological changes in the eggs of parasitic worms and demonstrates its potential for use in water treatment systems. Ibañez et al. showed that simultaneous application of ozone and peroxone could only cause damage to the outer layer of eggs at high alkalinity [20]. The use of ozone in the urban wastewater treatment process has also shown that under optimal conditions, ozone can eliminate all nematodes and their larvae [21].
In 2019, Dong et al. [22] developed a biological activated carbon pilot system to assess bacterial elimination within invertebrates over a year, comparing it with other removal methods. The system featured a 20 cm diameter pipe containing 1.5 m of granular activated carbon, 20 cm of sand, and 10 cm of gravel, with a contact time of 12 min. The study identified seven invertebrate groups, primarily from the phyla Rotifera and Crustacea. The results show that internal bacterial counts ranged from 160 to 6000, following an exponential relationship with invertebrate length. No direct human pathogens were found, but opportunistic pathogenic bacteria were detected. The study also presented a bacterial quantification model, revealing bacterial loads between 800 and 100,000 CFU/m3 within invertebrates, which persisted in the water supply even after chlorination at 50 mg/L·min. Li et al. [23] investigated the effects of chlorine, ozone, and UV radiation on reducing eukaryotic microorganisms in drinking water. Their findings highlight the persistent risk of eukaryotic microbial contamination despite the use of disinfection. Wu et al. [24] found that sand filtration following a biologically activated carbon filter had limited effectiveness in removing nematodes. Even with chlorination, permissible chlorine levels in drinking water treatment plants (WTPs) were insufficient to inactivate nematodes, limiting their overall removal efficiency. Therefore, further research is needed to develop advanced technologies to prevent nematode intrusion into product water reservoirs.
The presence of nematodes in water used for agriculture is also important. Nematodes such as Meloidogyne or Pratylenchus are transported through surface irrigation systems, wells, and recycled water, causing substantial economic losses to agriculture. The most effective strategy for managing nematodes in agricultural water is to use a reliable, uncontaminated water source, followed by physical filtration of the water [25]. MacDonald’s research also showed that the use of ozone in agricultural water eliminates Phytophthora capsici in recirculated irrigation systems. In his research, ozone was used at different concentrations, and it was shown that at a concentration of 1.5 mg/L could completely remove Phytophthora capsica. Additionally, water turbidity up to 2 NTU had no effect on ozone activity [26]. Kanfra et al. [27] electrolytically ozonized water using diamond-coated electrodes and applied this water to soils infested with Pratylenchus penetrans nematodes. The results show that the number of active nematodes was reduced by 81% for about 6–8 weeks after treatment.
Cao et al. [28] investigated the synergistic effect of ozone and chlorine disinfection processes and showed that the use of these two disinfectants can lead to the production of hydroxyl radicals, which have a higher oxidation power than ozone or chlorine alone. According to the results of the study, the simultaneous use of ozone and chlorine improved disinfection efficiency by up to 18.5%.
In 2024, Cai et al. [29] reviewed the mechanisms of ozone disinfection and its associated challenges. They stated that ozone primarily affects organic compounds and cell membranes; by oxidizing lipids and proteins of the cell membrane, it disruptsthe microorganisms’ defense structure. This damage increases cell permeability. Shi et al. [30] demonstrated the positive effects of simultaneous use of ozone, UV, and chlorine in significantly reducing microbial populations. According to the study, ozone increases membrane permeability, while chlorine reacts with proteins, enzymes, and internal cell structures. This combination attacks multiple parts of the nematode structure, greatly reducing their chances of survival. Their results show that the injection of 3 mg/L ozone and 2.5 mg/L chlorine with 5 mJ/cm2 UV radiation provided the most cost-effective microbial removal efficiency for areal water reclamation plant (WRP).
At Mashhad WTP No. 1, water from the Kardeh Dam is treated using a pulsator clarifier and sodium hypochlorite as a disinfectant. Reduced rainfall in recent years has lowered the reservoir levels, leading to increased concentrations of organic matter, microbes, and nematodes in the incoming water. This study investigates the effectiveness of the combined use of sodium hypochlorite and ozone for nematode removal, aiming to determine the optimal dosage and contact time for the influent water at Mashhad WTP No. 1. This research is based on experiments conducted using the existing treatment system, along with a pilot ozonation unit.
2. Materials and Methods
2.1. Description of the Existing WTP and the Implemented Ozonation Pilot
Mashhad WTP No. 1 was built in the west of Mashhad in 1992. It has a nominal capacity of 96,000 cubic meters per day and sources its water from the Kardeh Dam, located 40 km northeast of Mashhad. Water is transported to the plant by gravity through 46 km of 800 mm ductile iron pipes. The treatment process utilizes a pulsator system and includes the following stages: aeration at the plant inlet, pre-disinfection, chemical addition, rapid mixing, slow mixing, coagulation and sedimentation in pulsators, filtration, and final disinfection. Figure 1 presents a schematic diagram of the purification process at Mashhad WTP No. 1.
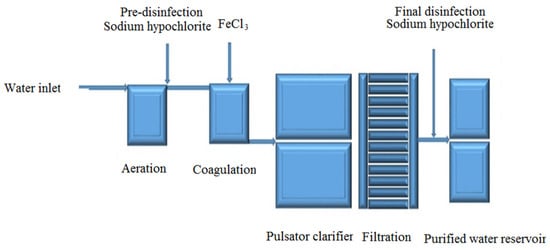
Figure 1.
Schematic diagram of the purification process at Mashhad WTP No. 1.
Initial and final disinfection are carried out using sodium hypochlorite. Given the treatment plant’s design flow rate and the availability of injection facilities and mixing basins, the contact time between raw water and sodium hypochlorite is 12 h, or 720 min. Therefore, in this study, only the sodium hypochlorite injection dose is considered variable. To assess the effect of ozone on nematode inactivation, a pilot ozone injection plant was constructed at the site of a full-scale WTP. The pilot consists of a PVC pipe with a 300 mm diameter and a length of 4.5 m. The ozone injector, made of ceramic, has a diameter of 180 mm. Water is delivered to the ozone injection unit through polyethylene pipes with a 25 mm diameter. It enters the ozone contact cell from the highest point of the pilot at 4.5 m and exits from the bottom. Figure 2 presents a view of the constructed pilot. The contact time between water and ozone can be adjusted by regulating the inlet water flow using a rotameter with an accuracy of 1 L per minute, within a range of 0 to 35 L per minute.
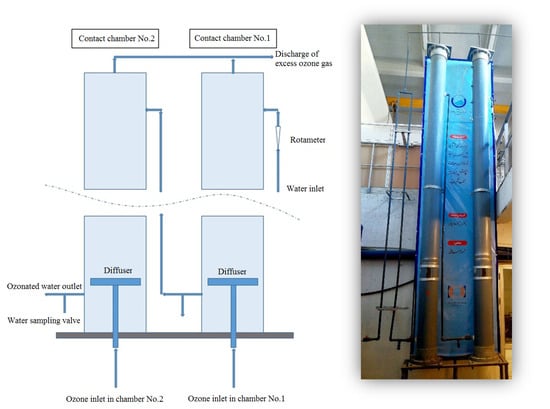
Figure 2.
Schematic representation (left) and photograph of the implemented pilot ozone injection system (right).
2.2. Materials Used
In this study, sodium hypochlorite, with a specific gravity of 1.12 g/cm3, is used in water treatment at WTP No. 1 in Mashhad. Based on the inflow rate to the ozonation pilot (Q0), the concentration of sodium hypochlorite (C0h), and the injection flow rate of the dosing pump (Q2), the concentration of pure chlorine (C2h) in the water passing through the system is calculated using Equation (1):
Q0 × C0h= Q2 × C2h
This research utilizes pure oxygen gas, produced by Khorakian Oxygen Company (Mashhad, Iran) and stored in 100 kg cylinders. The oxygen gas serves as the raw material for ozone generation and as the feed for the ozone generator.
Ozone gas is produced using an ozone generator manufactured by Ozoneab Company (Tehran, Iran) with a capacity of 25 g of ozone per hour. The concentration of ozone gas in the oxygen–ozone mixture injected into the pilot is measured and displayed in real-time by a BMT ozone meter manufactured by Ozonia (Zürich, Switzerland). The ozone generator is also equipped with a calibrated rotameter for pure oxygen. The concentration of injected ozone (C2O) in the water passing through the pilot cell is determined using Equations (2) and (3).
C0O = CBMT × Q0O
Q0O × C0O = Q2O × C2O
In these equations, C0O represents the concentration of ozone gas produced by the ozone generator in the oxygen–ozone gas mixture; CBMT denotes the value measured and is displayed on the BMT screen; Q0O is the oxygen flow rate passing through the rotameter; and Q2O is the water flow rate through the pilot.
2.3. Research Methodology
To qualitatively assess the inflow and outflow of water in the ozonation pilot cell, water samples were collected at 15-day intervals. Sampling and testing were conducted according to the 2017 standard method [31,32] at the WTP No. 1 laboratory unit in Mashhad, following guidelines issued by the NWWE (National Water and Wastewater Engineering) company. In each test, three samples were taken. Two samples were measured initially; if the difference between the two measurements exceeded 10%, the third sample was also measured, and the average was calculated [33]. The tests included the following parameters: turbidity, measured using a HACH N 2100 turbidity meter (Hach Company, Loveland, CO, USA); color, analyzed with a HACH DR3900 spectrophotometer; pH, determined using a Metrohm827 device (Metrohm, Herisau, Switzerland).; and electrical conductivity (EC), measured with a portable WTW Cond 3110 device (Xylem Analytics, Weilheim, Germany). To count nematodes, Sedgewick-Rafter counting chamber manufactured by Avantor Sciences used (Radnor Township, PA, USA).
All the devices used in this study had valid calibration certificates. To measure the number of live and inactive nematodes in the water, samples were first collected in 3 L plastic containers. Nematode counting was conducted within a maximum time frame of 12 h. For improved accuracy, the samples were concentrated using a Sartorius vacuum system (Sartorius, Göttingen, Germany). In this method, a 3 L water sample passed through a Sartorius membrane filter (pore size 3 μm) using vacuum pressure generated by a pump. The filter paper was then carefully removed with tweezers and placed into a 100 mL beaker containing 2 mL of water. To ensure complete transfer of nematodes, the filter paper was rinsed 10 times using a narrow pipette. For nematode counting, 1 mL of the concentrated solution was transferred to a Sedgewick-Rafter counting chamber. All one thousand slides’ chambers were examined under a binocular microscope at 100× magnification.
If the number of nematodes in each mL of the concentrated solution exceeded 10, the total nematode count was calculated by multiplying the observed number by the volume of the wash water (in milliliters) and dividing by the sample volume (in milliliters), yielding the number of nematodes per liter. If the number of nematodes in each concentrated solution was less than 10 per milliliter, an additional 1 mL sample from the remaining concentrated solution was analyzed. The final nematode count was determined by averaging the two measurements. Figure 3 presents two nematodes observed and counted under a microscope at 400× magnification.
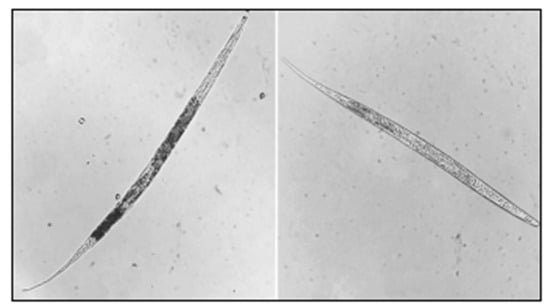
Figure 3.
Example of a counted nematode sample.
The effect of different sodium hypochlorite doses (0.5–2.5 mg/L) on the inactivation of nematodes in raw water was examined to determine the optimal dose. Based on the volume of the incoming water reservoir at WTP No. 1 in Mashhad and the flow rate of the incoming pipeline, the water retention time in this reservoir is approximately 12 h, or 720 min. Consequently, the contact time for sodium hypochlorite in this study was 720 min. Next, the impact of different ozone doses (0.5–2.5 mg/L) on nematode inactivation was examined at contact times ranging from 10 to 20 min. Finally, to assess the combined (hybrid) effect of sodium hypochlorite and ozone, sodium hypochlorite was first applied at the optimal dose determined in the initial phase, followed by ozone treatment (0–2.5 mg/L) at contact times of 10, 15, and 20 min. The results of nematode inactivation were then analyzed.
In the analysis of the results, the standard deviation (SD) of the efficiency of nematode removal under different disinfectants and contact times was calculated using Equations (4) and (5). In these equations, n is the number of data (in this study, n = 3), = is the efficiency of nematode removal in the i-th test, and NIE is the average efficiency of nematode removal.
3. Results
The following are the results of the impact of variables such as contact time, disinfectant type, and concentration on nematode inactivation efficiency. Based on the experiments conducted, the range of water quality parameters for the incoming water to the WTP was measured and is presented in Table 1.

Table 1.
Range of analyzed water quality parameters of incoming water to the WTP during the experiments.
Injection of different doses of ozone and sodium hypochlorite was carried out at 15-day intervals. Due to variations in the quality of the water entering the treatment plant, the number of live nematodes fluctuated significantly on different days.
3.1. Effect of Sodium Hypochlorite on Nematode Removal
Figure 4 illustrates the variation in the number of active and inactivated nematodes at different sodium hypochlorite concentrations, along with their corresponding removal efficiencies. Table 2 presents the SD of nematode removal efficiency for different sodium hypochlorite doses. The results indicate that increasing the sodium hypochlorite dose improves nematode removal efficiency. However, complete removal was not achieved in any case. The maximum removal efficiency was observed at a dose of 2.5 mg/L, reaching 71%, while a dose of 2 mg/L achieved 68% removal efficiency. Considering that this study simulates operational conditions at WTP No. 1 in Mashhad and that the increase in removal efficiency at 2.5 mg/L is negligible compared to 2 mg/L, a sodium hypochlorite dose of 2 mg/L is considered optimal for further research.
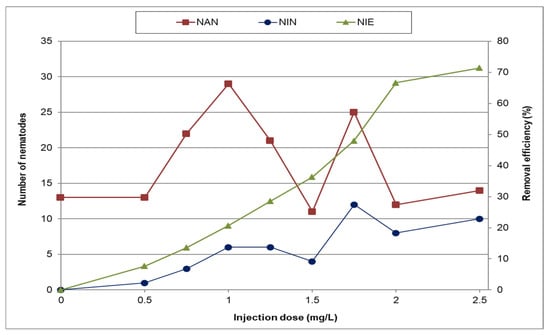
Figure 4.
Changes in the number of active and inactive nematodes at different sodium hypochlorite doses (NIE—nematode inactivation efficiency; NAN—no. of active nematodes; NIN—no. of inactive nematodes).

Table 2.
Standard deviation of nematode inactivation efficiency at different sodium hypochlorite doses.
3.2. Effect of Ozone on Nematode Removal
In this part of the study, the effect of ozone at different doses and contact times on nematode inactivation in water was investigated. Ozone was injected at doses up to 3.0 mg/L, with contact times ranging from 10 to 20 min. Figure 5 shows the variations in nematode removal efficiency at different contact times and ozone doses. The results indicate that increasing the ozone dose improved nematode removal efficiency. Similarly, longer contact times further enhanced removal efficiency. At a contact time of 20 min, increasing the ozone dose from 0.5 to 0.75 mg/L raised the removal efficiency from 5.2% to 9.1%, representing a 69% increase. However, as the injection dose continued to rise, the rate of improvement diminished. For example, increasing the ozone dose from 1.75 to 2 mg/L resulted in a more modest efficiency increase, from 34.5% to 39.3%, equivalent to a 14% improvement. This trend is consistent across all the contact times. The findings also show that at a contact time of 20 min, the gradient of removal efficiency increased more significantly with higher injection doses than at shorter contact times. In other words, the impact of extended contact time became more pronounced as the ozone dose increased. Despite these trends, with an injection dose of 2 mg/L and a 20 min contact time, the removal efficiency reached 39.3%, which remains significantly lower than the efficiency achieved with sodium hypochlorite. The maximum observed removal efficiency at a 20 min contact time and an injection dose of 3.0 mg/L was 41.2%, showing only a negligible improvement compared to the injection dose of 2.0 mg/L.
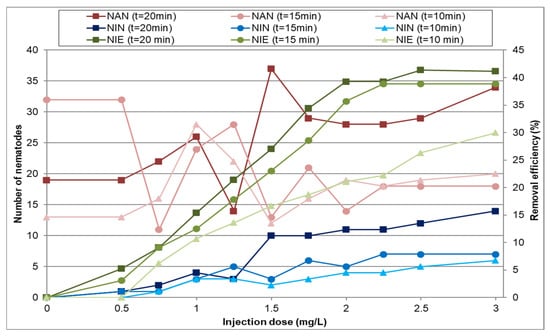
Figure 5.
Changes in the number of active and inactive nematodes at different ozone doses for various contact times (NIE—nematode inactivation efficiency; NAN—no. of active nematodes; NIN—no. of inactive nematodes).
Table 3 presents the SD of the nematode removal efficiency results at different contact times and different ozone injection doses. It can be seen that the dispersion of the results is limited.

Table 3.
Standard deviation of nematode inactivation efficiency at different ozone doses and contact times.
3.3. Effect of Combined Application of Sodium Hypochlorite and Ozone onNematode Removal
In the following research phase, a sodium hypochlorite concentration of 2 mg/L was used to investigate nematode inactivation. Subsequently, ozone was injected at varying doses, with contact times ranging from 10 to 20 min. Figure 6 and Table 4 show the inactivation efficiency of nematodes at different ozone doses for various contact times and the corresponding SD of inactivation efficiency, respectively. The results indicate that nematodes were not completely eliminated in any of the tested cases. However, the findings show that the combined application of ozone and sodium hypochlorite significantly enhanced nematode removal efficiency compared to their individual use. The maximum removal efficiency, achieved through the combined use of sodium hypochlorite and an ozone injection dose of 3 mg/L with a contact time of 20 min, reached 92%. This represents a substantial improvement compared to the maximum efficiencies observed with sodium hypochlorite alone (71%) or ozone alone (41%). The results also indicate that when sodium hypochlorite and ozone were combined, increasing the ozone dose beyond 2 mg/L led to only a marginal improvement in nematode removal efficiency.
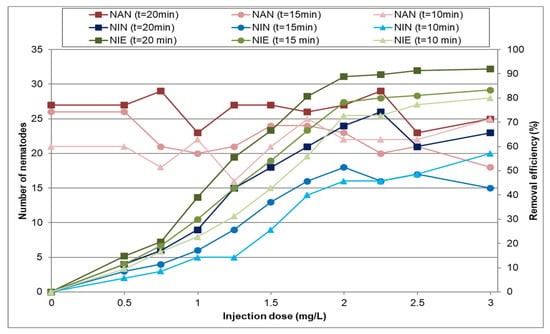
Figure 6.
Changes in the number of active and inactive nematodes for a sodium hypochlorite concentration of 2 mg/L and contact time of 720 min combined with different ozone doses and various contact times (NIE—nematode inactivation efficiency; NAN—no. of active nematodes; NIN—no. of inactive nematodes).

Table 4.
Standard deviation of nematode inactivation efficiency at different ozone doses and contact times during combined application with sodium hypochlorite (2 mg/L, 720 min contact time).
4. Discussion
The results of this study highlight the significant potential of combining sodium hypochlorite and ozone for the inactivation of nematodes in drinking water treatment. If nematodes carrying pathogenic bacteria enter the water supply network, they may pose risks to drinking water safety. Once the residual chlorine concentration in the water drops below a certain level, some bacteria will utilize invertebrate residues as a reproduction matrix, while others will be excreted from invertebrates and discharged into the water supply, where they can propagate [6]. Although limited in number, some previous studies have focused on removing nematodes in general, or specific organisms from this group, from drinking water using various treatment methods. Dehghani et al. [34] investigated the effect of ultraviolet (UV) light on the inactivation of nematodes, particularly Rhabditidae species. Their study utilized a 1 L reactor equipped with an 11W UV lamp emitting 24 µW/cm2, initially housed in a quartz sleeve before being placed in the reactor. Conventional urban water treatment systems often fail to obliterate nematodes due to their chlorination resistance and active motility. The study assessed the effects of exposure time, turbidity, pH, and temperature on the inactivation of nematodes. Complete removal of larvae and adult nematodes required 9 and 10 min of UV exposure, respectively. Increased turbidity up to 25 NTU reduced inactivation efficiency by 34% for larvae and 36% for adults. pH variations between 6 and 9 did not significantly affect nematode removal, whereas higher temperatures enhanced inactivation. The study concluded that UV radiation was more effective against larvae than adult nematodes due to their simpler structure, though the overall efficiency remained limited. Chen et al. [35] investigated the use of peroxymonosulfate (PMS)/UV-C for eliminating Caenorhabditis elegans (a type of nematode) in drinking water, achieving 100% inactivation under optimal conditions. Their method involved UV light interacting with , generating and radicals, which induce cellular apoptosis and nematode inactivation. The study concluded that PMS/UV-C is a viable alternative to chlorination in WTPs, effectively removing chlorine-resistant invertebrates from secondary storage sources. Yan et al. [36] conducted an inactivation experiment using sodium hypochlorite, selecting the nematode Plectussp. as the test organism. Their findings indicate that at a chlorine dosage of 12.0 mg/L and a contact time of 30 min, the inactivation rate was only approximately 3.3%. Msayleb and Ibrahim [37] demonstrated that ozone was highly effective in causing nematode mortality through gas fumigation and direct ozonation of samples. They observed that increasing the ozone dosage applied to nematode-infected soil resulted in significantly higher mortality rates among the nematodes.
Kos et al. [11] investigated the effects of ozone, chlorine, and chlorine dioxide concentrations on nematode removal from drinking water over varying exposure times. Their study aimed to identify the most accurate predictive model among Chick’s, Hom’s, and Probit models. The Probit model proved to be the best fit. At an LC (90) molar concentration, chlorine dioxide demonstrated the highest nematode removal efficiency, followed by ozone and chlorine [11]. They reported that utilizing ozone alone at a concentration of 4.88 mg/L with a contact time of 30 min achieved a 90% nematode removal rate. However, high ozone dosages are required for complete removal, making cost and implementation factors key considerations. In contrast, the findings of this study indicate that the combined application of sodium hypochlorite and ozone significantly enhances the efficiency of nematode removal, directly contributing to cost optimization and streamlining of the process.
The combination of ozone and sodium hypochlorite to remove nematodes from water creates a synergistic effect that increases disinfection efficiency compared to when each of these disinfectants is used alone. The reason for this synergy maybe the increased production of hydroxyl radicals (). These radicals are much stronger and more reactive than ozone and hypochlorite alone. On the other hand, the pH of water in the presence of hypochlorite tends to be alkaline. Ozone in an alkaline environment tends to produce more free radicals, which, in turn, help to strengthen the oxidative mechanism.
In the research by Velásquez et al. [19], the minimum dose of ozone and the minimum contact time required to remove 90% of nematodes were 3.5 mg/Land 1 h, respectively. In contrast, this study achieved 92% inactivation at 2.5–3.0 mg/L ozone in just 20 min but, critically, only when preceded by sodium hypochlorite pre-disinfection. This suggests an apparent synergistic effect, likely due to enhanced oxidative mechanisms resulting from the formation of hydroxyl radicals (), as also observed in studies by Cao et al. [28] and Shi et al. [30]. This synergy not only enhances disinfection performance but also provides operational benefits, including reduced ozone requirements and shorter contact times, ultimately leading to lower energy consumption and chemical usage.
From a practical and economic standpoint, this combined approach is particularly relevant for utilities facing increased biological loads due to climate-induced changes in water quality, such as those observed at WTP No. 1 in Mashhad. The feasibility of integrating ozone into existing chlorination infrastructure, as explored in this pilot-scale study, underscores its potential scalability. Moreover, this dual-disinfectant strategy provides resilience against nematode-associated biofilm formation and microbial regrowth in distribution systems, which is a growing concern in global water safety.
Despite these promising outcomes, further research is needed to address certain limitations. For instance, while this study focused on nematode inactivation, the formation of disinfection byproducts (DBPs), especially under high ozone and chlorine conditions, warrants attention. Studies, such as those by Zhang et al. [1], have shown that disinfection sequences can influence the persistence of antibiotic resistance genes, which is an emerging public health issue. Additionally, long-term impacts on microbial ecology and nematode adaptation potential remain poorly understood.
5. Conclusions
This study investigated the efficiency of nematode removal using sodium hypochlorite, ozone, and their combination as disinfectants, contributing valuable operational insights, and represents one of the few field-based investigations integrating ozone and sodium hypochlorite under realistic WTP conditions. The comparison with single-disinfectant applications highlights the practical and performance-related advantages of the combined method, which may serve as a reference point for future research and utility-level implementation. Specifically conducted for application at WTP No. 1 in Mashhad, this research considered the facility’s operational capabilities, setting the contact time for sodium hypochlorite at 720 min. The results indicate that increasing the dosage of sodium hypochlorite enhances nematode removal efficiency, achieving a removal rate of 68% at a concentration of 2 mg/L. However, further increases in the dosage beyond 2 mg/L resulted in only negligible improvements. For ozone disinfection alone, the highest nematode inactivation efficiency was 39%, achieved with a 2.5 mg/L ozone dose over a 20 min contact time. In contrast, the combined application of sodium hypochlorite and ozone demonstrated superior effectiveness, achieving a nematode removal efficiency of 92% at a 3 mg/L ozone dose with a 20 min contact time. This clearly highlights that the combined use of sodium hypochlorite and ozone significantly enhances nematode removal. However, it was observed that increasing the ozone dose from 2.0 mg/L to 3 mg/L with a contact time of 20 min did not produce a significant additional improvement in nematode inactivation. Moreover, the findings suggest that such a dual-disinfection strategy allows for optimization of operational conditions, namely, reducing required ozone concentrations and shortening contact times, thereby contributing to more sustainable, cost-effective, and energy-efficient water treatment processes. This approach could be particularly valuable in regions facing challenges from increased microbial loads, climate variability, or limited treatment infrastructure flexibility. The integration of this combined method into existing water treatment frameworks may offer a promising pathway to strengthen microbial control, mitigate biofilm-related risks, and enhance overall water safety. However, comprehensive evaluation of the environmental and economic feasibility of large-scale implementation is still needed. Future research should also explore the effectiveness of this approach across varying water qualities and broader classes of invertebrates, as well as its compatibility with other advanced oxidation processes and filtration technologies. With proper optimization and validation, the sodium hypochlorite–ozone combination could emerge as a reliable and scalable solution for enhanced biological risk management in drinking water systems.
Author Contributions
Conceptualization, E.A. and M.R.; methodology, E.A. and B.Đ.; software, E.A., S.D., D.N. and B.Đ.; validation, E.A., B.Đ., S.D. and D.N.; formal analysis, E.A.; investigation, E.A. and M.R.; resources, E.A.; data curation, E.A. and M.R.; writing—original draft preparation, E.A., S.D. and M.R.; writing—review and editing, B.Đ. and D.N.; visualization, E.A. and D.N.; supervision, E.A.; project administration, E.A. and M.R.; funding acquisition, E.A. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors appreciate Mashhad Water and Wastewater Company’s support in providing the required equipment. They would also like to express their gratitude for support from University North, Croatia.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| WTP | Water treatment plant |
| NIE | Nematode inactivation efficiency |
| NAN | No. of active nematodes |
| WRP | Water reclamation plant |
| NIN | No. of inactive nematodes |
| SD | Standard deviation |
| MF | Micro-filtration |
| UF | Ultra-filtration |
References
- Zhang, J.; Li, W.; Guw, X.; Zhang, X.; Wang, X.; Lv, L. Chlorine and UV combination sequence: Effects on antibiotic resistance control and health risks of ARGs. Environ. Manag. 2025, 373, 123780. [Google Scholar] [CrossRef]
- Basyoni, M.M.; Enas, M.R. Nematodes ultrastructure: Complex systems and processes. J. Parasit. Dis. 2016, 40, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Mc Carlie, S.; Charlotte, E.B.; Robert, B. Molecular basis of bacterial disinfectant resistance. Drug Resist. Updat. 2020, 48, 100672. [Google Scholar] [CrossRef] [PubMed]
- Faridirad, F.; Gholinezhad, M.; Tabrizi, S.; Salabarzi, N. Investigation of nematode removal by units of pardis drinking water treatment plant. J. Water Wastewater Sci. Eng. 2021, 6, 27–37. [Google Scholar]
- Diemert, D.J. 365-Intestinal nematode infections. In Goldman’s Cecil Medicine, 24th ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2. [Google Scholar]
- Ren, X.; Li, J.; Zhou, Z.; Zhang, Y.; Wang, Z.; Zhang, D.; Tang, X.; Chen, H. Impact of invertebrates on water quality safety and their sheltering effect on bacteria in water supply systems. Environ. Pollut. 2023, 330, 121750. [Google Scholar] [CrossRef]
- Cui, H.; Liu, X.; Chen, S.; Liu, Z.; Chen, J.; Zhou, H.; Nielsen, U.N. Contrasting responses of nematode composition, richness and biomass to long-term warming. Sci. Total Environ. 2023, 894, 165074. [Google Scholar] [CrossRef]
- Locas, A.; Barbeau, B.; Gauthier, V. Nematodes as a source of total coliforms in a distribution system. Can. J. Microbiol. 2007, 53, 580–582. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, X.; Chen, H. Seasonal and treatment-process variations in invertebrates in drinking water treatment plants. Front. Environ. Sci. Eng. 2021, 15, 62. [Google Scholar] [CrossRef]
- Barratt, J.; Chan, D.; Sandaradura, I.; Malik, R.; Spielman, D.; Lee, R.; Marriott, D.; Harkness, J.; Ellis, J.; Stark, D. Angiostrongyluscantonensis: A review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology 2016, 143, 1087–1118. [Google Scholar] [CrossRef]
- Kos, J.; Brmež, M.; Markić, M.; Sipos, L. The mortality of nematodes in drinking water in the presence of ozone, chlorine dioxide, and chlorine. Ozone Sci. Eng. 2020, 42, 120–127. [Google Scholar] [CrossRef]
- Tian, N.; Nie, Y.; Tian, X.; Wang, Y. Current Water Treatment Technologies: An Introduction, Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer Nature: Cham, Switzerland, 2021; pp. 2033–2066. [Google Scholar]
- Trifirò, F.; Zanirato, T. Water Purification: Physical, Mechanical, Chemical and Biological Treatments. Mathews J. Pharm. Sci. 2024, 8, 3. [Google Scholar]
- Hotte, H.; Neveux, M.S.; Ollivier, F.; Mariette, N.; Folcher, L.; Le Roux, A.C. Can quarantine plant-parasitic nematodes within wastes be managed by useful tools in a circular economy approach? J. Environ. Manag. 2022, 323, 116184. [Google Scholar] [CrossRef]
- Lim, S.; Shi, J.L.; von Gunten, U.; McCurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef]
- Hoveydi, H.; Nabi, B.G.R.; Jafari, H.R.; Nasrabadi, T.; Shahriari, T. Evaluating the Use of Ozone for Disinfection of Drinking Water, Case Study: Tehran Pars Water Treatment Plant (Iran). Environ. Sci. 2008, 5, 31–38. [Google Scholar]
- Matsumoto, N.; Aizawa, T.; Ohgaki, S.; Hirata, T.; Toyooka, K.; Kanbayashi, T.; Tsutsumi, T.; Hasegawa, T. Removal methods of nematoda contained in the effluent of activated carbon. Water Supply 2002, 2, 183–190. [Google Scholar] [CrossRef]
- Steel, S.; Platz, M.S.; Riglos, A.; Garcia, B.; Jacob, J.I.S. Larvicidal Efficacy of Ozone and Ultrasound on Angiostrongylus cantonensis (Rat Lungworm) Third-Stage Larvae. Foods 2022, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, M.T.O.; Martínez, J.L.; Monje–Ramírez, I.; Rojas-Valencia, M.N. Destruction of Helminth (Ascaris suum) Eggs by Ozone. Ozone Sci. Eng. 2004, 26, 359–366. [Google Scholar] [CrossRef]
- Ibañez-Cervantes, G.; Ramírez-Cortina, C.R.; Márquez-Navarro, A.; Alonso-Gutiérrez, M.S.; León-Ávila, G.; León-García, G.; Nogueda-Torres, B. Effect of Ozone and Peroxone on HelminthHymenolepis NanaEggs. Ozone Sci. Eng. 2013, 35, 201–207. [Google Scholar] [CrossRef]
- Ferral-Pérez, H.; Torres Bustillos, L.G.; Méndez, H.; Rodríguez-Santillan, J.L.; Chairez, I. Sequential Treatment of Tequila Industry Vinasses by Biopolymer-based Coagulation/Flocculation and Catalytic Ozonation. Ozone Sci. Eng. 2016, 38, 279–290. [Google Scholar] [CrossRef]
- Dong, Z.; Yin, W.; Yang, J.; Zhang, J.; Jiang, C. Risk assessment and inactivation of invertebrate-internalized bacteria in pilot-scale biological activated carbon filtration. Sci. Total Environ. 2019, 676, 321–332. [Google Scholar] [CrossRef]
- Li, H.; Feng, M.; Yu, X. Qualitative and quantitative analysis of the effects of drinking water disinfection processes on eukaryotic microorganisms: A meta-analysis. Chemosphere 2023, 332, 138839. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, J.; Tang, X.; Chen, H. Synergistic effect of chlorination and sand filtration for efficient elimination of invertebrate leakage in BAC filter. Desal. Water Treat. 2017, 79, 235–242. [Google Scholar] [CrossRef]
- James, A. Biology, Detection, and Management of Plant Pathogens in Irrigation Water, Chapter 9: Plant-Parasitic Nematodes in Irrigation Water; The American Phytopathological Society: St. Paul, MN, USA, 2017. [Google Scholar]
- McDonald, G.V. Ozone (O3) Efficacy on Reduction of Phytophthora Capsici in Recirculated Horticultural Irrigation Water. Ph.D. Dissertation, Texas A&M University, College Station, TX, USA, 2009. [Google Scholar]
- Kanfra, X.; Elhady, A.; Thiem, H.; Pleger, S.; Höfer, M.; Heuer, H. Ozonated water electrolytically generated by diamond-coated electrodes controlled phytonematodes in replanted soil. J. Plant Dis. Prot. 2021, 128, 1657–1665. [Google Scholar] [CrossRef]
- Cao, K.F.; Chen, Z.; Shi, Q.; Wu, Y.H.; Lu, Y.; Mao, Y.; Hu, H.Y. An insight to sequential ozone-chlorine process for synergistic disinfection on reclaimed water: Experimental and modelling studies. Sci. Total Environ. 2021, 793, 148563. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, Y.; Wang, C.; Yadav, A.K.; Wei, T.; Kang, P. Ozone disinfection of waterborne pathogens: A review of mechanisms, applications, and challenges. Environ. Sci. Pollut. Res. 2024, 31, 60709–60730. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, Z.; Liu, H.; Lu, Y.; Li, K.; Shi, Y.; Hu, H.Y. Efficient synergistic disinfection by ozone, ultraviolet irradiation and chlorine in secondary effluents. Sci. Total Environ. 2021, 758, 143641. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- AWWA Standard B100-01; American Water Works Association. NSF Standard 61, Standard 61 Approved for Drinking Water and NSF Standard 50 Approved for Swimming Pools. American National Standards Institute: New York, NY, USA, 2002.
- National Water and Wastewater Engineering Company. Instructions for Quality Control Methods for Water Chemistry Tests; Iran Water Resources Management Company: Tehran, Iran, 2015. [Google Scholar]
- Dehghani, M.H.; Jahed, G.R.; Zarei, A. Investigation of low-pressure ultraviolet radiation on inactivation of rhabitidae nematode from water. Iran. J. Public Health 2013, 42, 314. [Google Scholar] [PubMed]
- Chen, T.; Li, J.; Xu, L.; Zhang, D.; Wang, Z.; Chen, H. Deactivation of Caenorhabditis elegans nematodes in drinking water by PMS/UV-C: Efficiency and mechanisms. Environ. Sci. Pollut. Res. 2021, 28, 58606–58616. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Zhang, M.; Zhou, W.; Cai, Y.; Zou, L.; Ding, G. Inactivation Kinetics of Plectus sp. in application of disinfection with sodium hypochlorite. Water Purif. Technol. 2010, 6, 28–31. [Google Scholar]
- Msayleb, N.; Ibrahim, S. Treatment of nematodes with ozone gas: A sustainable alternative to nematicides. Phys. Procedia 2011, 21, 187–192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).