Potential of MgB2 Superconductors for Magnetically Aided Wastewater Treatment: Feasibility and Future Prospects
Abstract
1. Introduction
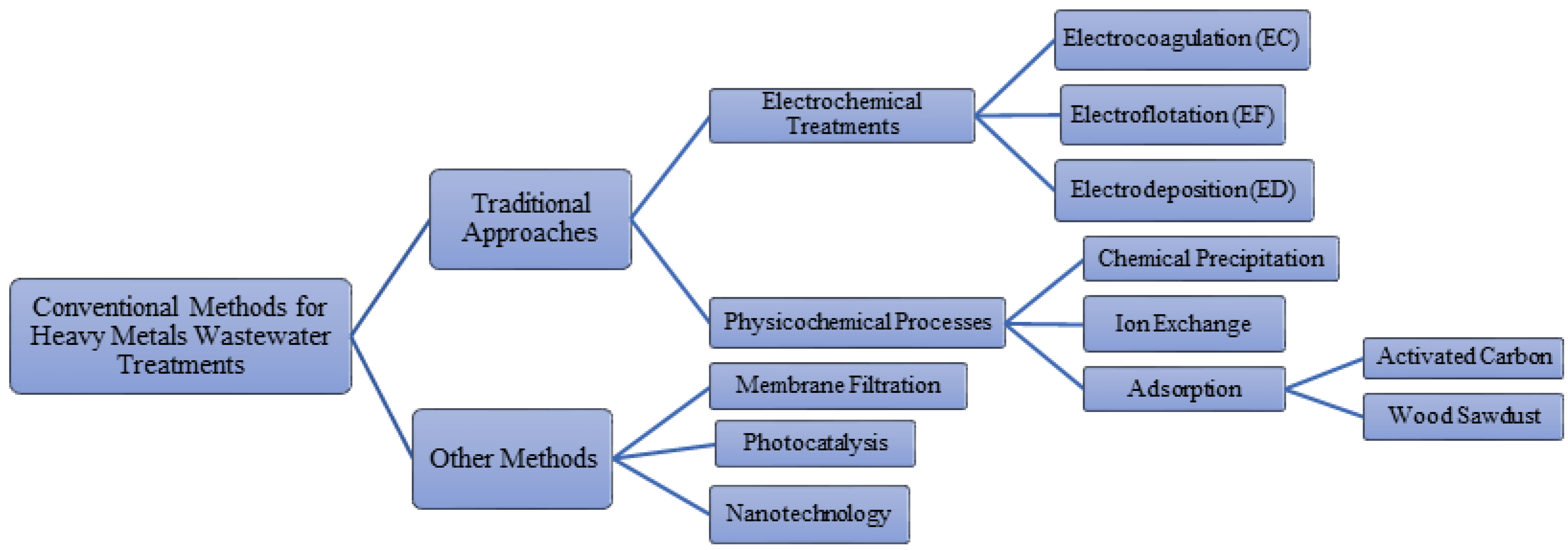
2. Heavy Metals
2.1. Electrocoagulation
2.2. Electroflotation
2.3. Electrodeposition
| Method | Advantages | Disadvantages |
| Electrocoagulation (EC) |
|
|
| Electroflotation (EF) |
|
|
| Electrodeposition (ED) |
|
|
| Method | Techniques Used | Investigation and Results | Reference |
|---|---|---|---|
| Adsorption on fly-ash-based substrates | Adsorption studies | Studied the adsorption of dyes and a hair conditioner to treat wastewater from a textile company; highlighted limitations of single-component studies for effective treatment. | [47] |
| Mesoporous alumina and calcium-doped alumina | Adsorption experiments | Investigated fluoride adsorption; found maximum removal capacities of 450 mg/g for fluoride and 200 L for arsenic at 100 ppb, treated effectively with just 1 g of mesoporous alumina. | [48] |
| Heat treatment of ordered mesoporous carbon | Surface modification | Modified surface chemistry via heat treatment in ammonia at 1173 K; significantly increased adsorption of three anionic dyes compared to commercial activated carbon. | [49] |
| Synthesis of magnetic iron oxide/silica | Characterization (SEM, TEM) | Developed a cost-effective method using vegetable oil; confirmed synthesis and characterization of nanocomposite particles through SEM and TEM. | [50] |
| N-butylimidazolium functionalized resin | Adsorption studies | Explored phenol adsorption; achieved maximum removal of 92.2 mg/g at pH 11.2, with effective regeneration using a 0.5 M NaOH and NaCl solution. | [51] |
| Humic acid-coated Fe3O4 nanoparticles | Adsorption experiments | Examined removal of methylene blue; achieved optimal removal efficiency at neutral pH, with easy regeneration of nanoparticles. | [52] |
| Untreated and modified Polyalthia longifolia | Comparative analysis | Evaluated Cr(VI) removal; found that acid-treated leaves performed best, enhancing removal efficiency significantly. | [53] |
| Green coconut shell powder | Adsorption modeling | Investigated trace metal removal; modeled adsorption characteristics with Langmuir and Freundlich isotherms, demonstrating effective metal uptake. | [54] |
| Diatomite modified with aluminum compounds | Sorbent development | Developed a fluoride-selective sorbent; achieved a 2.5-fold increase in specific surface area and enhanced fluoride removal capacity from 8.9 to 57.6 mg of F/g. | [55] |
| Comparison of sorbents | Performance evaluation | Studied removal of phenoxyalkanoic acid herbicides; identified a decreasing trend in herbicide uptake, with 2,4-DB being the most efficient sorbent. | [56] |
| Octadecyltrimethyl-ammonium micelle-montmorillonite | Comparative analysis | Focused on removing humic acid from water; found that composites outperformed activated carbon in HA removal, indicating higher efficacy. | [57] |
| Sorbent derived from coffee grounds | Adsorption studies | Evaluated fluoride removal; achieved the highest efficiency when calcined at 600 °C, indicating effective application for fluoride treatment. | [58] |
| UV irradiation with Fe(0)/air, ozone, and Fenton | Oxidation studies | Investigated humic acid oxidation; Fe(0)/air achieved over 99% oxidation of humic acid in 9 min, significantly reducing toxicity and THMFP. | [59] |
| Catalytic wet peroxide oxidation (CWPO) | Catalyst optimization | Stabilized landfill leachate with Al/Fe-pillared clay; achieved up to 50% removal of chemical oxygen demand (COD) and a biodegradability index of 0.3. | [60] |
| Hybrid process with beta-MnO2 nanowires | Oxidative removal studies | Studied oxidative removal of bisphenol A; removal efficiency varied with pH and was influenced by the presence of humic acid and metals. | [61] |
| Kinetics and oxidation products analysis | Kinetic studies, LC-MS/MS analysis | Investigated trimethoprim oxidation using ferrate(VI); revealed second-order kinetics and identified primary oxidation products. | [62] |
| Electrochemical reduction using titanium species | Electrochemical analysis | Studied perchlorate reduction; found that a high pitting potential of 12.77 V (SHE) was necessary for effective removal, with minimal impact from pH and electrode surface area. | [63] |
| BDD-ZVI electrochemical treatment | Electrochemical oxidation | Employed boron-doped diamond electrodes with zero-valent iron; achieved enhanced removal of p-nitrophenol through combined electrochemical oxidation and coagulation processes. | [51] |
3. Magnetic Field and Water Treatment
3.1. The Concepts of Magnetization in Wastewater
3.1.1. Magnetization and Magnetic Field
3.1.2. Magnetic Gradient
3.1.3. Lorentz Force
3.1.4. Magnetic Memory
3.2. CaCO3
3.3. Water Purification
3.4. Wastewater Treatment
4. Magnetic Water
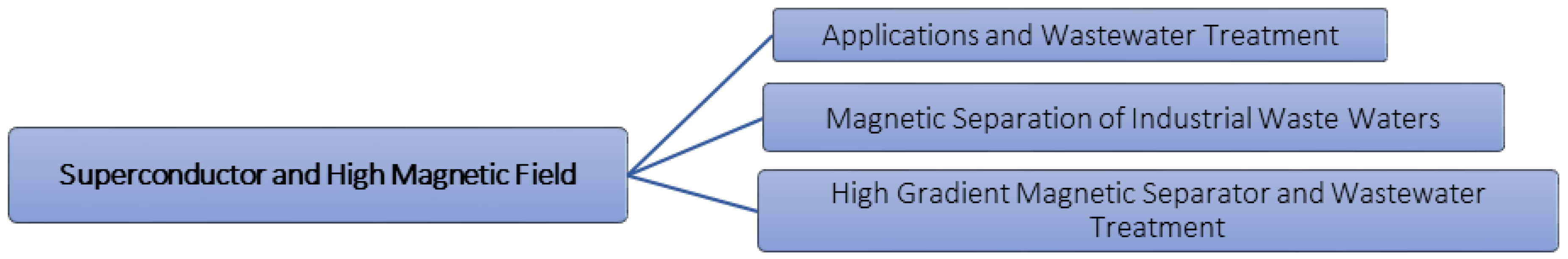
5. Superconductor and High Magnetic Field
5.1. Applications and Wastewater Treatment
5.2. Magnetic Separation of Industrial Waste Waters
5.3. High Gradient Magnetic Separator and Wastewater Treatment
6. MgB2 Superconductors Challenges
6.1. Challenges and Opportunities in WWT Applications
- Cryogenic Operational Efficiency:
- 2.
- Material Innovation:
- 3.
- Magnetic Separation Superiority:
- 4.
- Economic and Hybrid Potential:
6.2. Pathways for Accelerated Adoption
- Material Optimization
- 2.
- Cryogenic System Innovation
- 3.
- Interdisciplinary Synergy
6.3. Limitations and Controversies in Applying MgB2 Superconductors to WWT
7. General Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Yahya, N.; Aziz, F.; Jamaludin, N.A.; Mutalib, M.A.; Ismail, A.F.; Salleh, W.N.W.; Jaafar, J.; Yusof, N.; Ludin, N.A. A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [PubMed]
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Farjadfard, S.; Esmaeili, H.; Saberi, M.; Sahebi, S.; Dobaradaran, S.; Ramavandi, B. Characteristics and performance of Cd, Ni, and Pb bio-adsorption using Callinectes sapidus biomass: Real wastewater treatment. Environ. Sci. Pollut. Res. 2019, 26, 6336–6347. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.H.; Tan, H.K.; Lau, S.Y.; Yap, P.-S.; Danquah, M.K. Potential and challenges of enzyme incorporated nanotechnology in dye wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103261. [Google Scholar] [CrossRef]
- Batstone, D.J.; Hülsen, T.; Mehta, C.M.; Keller, J. Platforms for energy and nutrient recovery from domestic wastewater: A review. Chemosphere 2015, 140, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Wilderer, P.A. Treatise on Water Science; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Baker, J.S.; Judd, S.J. Magnetic amelioration of scale formation. Water Res. 1996, 30, 247–260. [Google Scholar] [CrossRef]
- Vermeiren, T. Magnetic treatment of liquids for scale and corrosion prevention. Anti Corros. Methods Mater. 1958, 5, 215–219. [Google Scholar] [CrossRef]
- Zaidi, N.S.; Sohaili, J.; Muda, K.; Sillanpää, M. Magnetic field application and its potential in water and wastewater treatment systems. Sep. Purif. Rev. 2014, 43, 206–240. [Google Scholar] [CrossRef]
- Syamimi, Z.N.; Muda, K.; Sohaili, J.; Sillanpää, M. Optimization of activated sludge physical properties by magnetic field via response surface modeling. Appl. Mech. Mater. 2014, 567, 98–103. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, X.; Quan, J.; Xing, G.; Yang, L.; Zhao, C.; Wu, P.; Zhao, F.; Hu, B.; Hu, Y. Application of magnetic fields to wastewater treatment and its mechanisms: A review. Sci. Total Environ. 2021, 773, 145476. [Google Scholar] [CrossRef] [PubMed]
- Krzemieniewski, M.; Debowski, M.; Janczukowicz, W.; Pesta, J. Effect of the constant magnetic field on the composition of dairy wastewater and domestic sewage. Polish J. Environ. Stud. 2004, 13, 45–53. [Google Scholar]
- Ku, J.; Wang, K.; Wang, Q.; Lei, Z. Application of Magnetic Separation Technology in Resource Utilization and Environmental Treatment. Separations 2024, 11, 130. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Othmani, A.; Osagie, C.; Meskini, M.; AlKafaas, S.S.; Malloum, A.; Khanday, W.A.; Jacob, A.O.; et al. Recent advances on sustainable adsorbents for the remediation of noxious pollutants from water and wastewater: A critical review. Arab. J. Chem. 2023, 16, 105303. [Google Scholar] [CrossRef]
- Allahkarami, E.; Monfared, A.D.; Silva, L.F.O.; Dotto, G.L. Lead ferrite-activated carbon magnetic composite for efficient removal of phenol from aqueous solutions: Synthesis, characterization, and adsorption studies. Sci. Rep. 2022, 12, 10718. [Google Scholar] [CrossRef] [PubMed]
- Nakhlband, A.; Kholafazad-Kordasht, H.; Rahimi, M.; Mokhtarzadeh, A.; Soleymani, J. Applications of magnetic materials in the fabrication of microfluidic-based sensing systems: Recent advances. Microchem. J. 2022, 173, 107042. [Google Scholar] [CrossRef]
- Agasti, N.; Gautam, V.; Pandey, N.; Genwa, M.; Meena, P.L.; Tandon, S.; Samantaray, R. Carbon nanotube based magnetic composites for decontamination of organic chemical pollutants in water: A review. Appl. Surf. Sci. Adv. 2022, 10, 100270. [Google Scholar] [CrossRef]
- Dini, L.; Abbro, L. Bioeffects of moderate-intensity static magnetic fields on cell cultures. Micron 2005, 36, 195–217. [Google Scholar] [CrossRef] [PubMed]
- Janowski, T.; Glowacki, B.A.; Wojtasiewicz, G.; Kozak, S.; Kozak, J.; Kondratowicz-Kucewicz, B.; Majka, M.; Wozniak, M. Fault current limitation in power network by the superconducting transformers made of 2G HTS. IEEE Trans. Appl. Supercond. 2011, 21, 1413–1416. [Google Scholar] [CrossRef]
- Huck, W.; Maaß, J.; Sood, S.; Benmaghnia, T.; Schulte, A.; Heß, S.; Walter, M.A. The Right to Breathe Clean Air and Access to Justice-Legal State of Play in International, European and National Law. Eur. Natl. Law 2021. [Google Scholar] [CrossRef]
- Nishijima, S.; Takahata, K.; Saito, K.; Okada, T.; Nakagawa, S.; Yoshiwa, M. Applicability of superconducting magnet to high gradient magnetic separator. IEEE Trans. Magn. 1987, 23, 573–576. [Google Scholar] [CrossRef]
- Takeda, S.; Furuyoshi, T.; Tari, I.; Nakahira, A.; Kakehi, Y.; Kusaka, T.; Ogawa, S.; Katayama, J.; Inno, Y.; Nishijima, S.; et al. Separation of algae with magnetic iron (III) oxide particles using superconducting high gradient magnetic field. Nippon. Kagaku Kaishi 2000, 9, 661–663. [Google Scholar] [CrossRef]
- Nishijima, S.; Izumi, Y.; Takeda, S.; Suemoto, H.; Nakahira, A.; Horie, S. Recycling of abrasives from wasted slurry by superconducting magnetic separation. IEEE Trans. Appl. Supercond. 2003, 13, 1596–1599. [Google Scholar] [CrossRef]
- Kakihara, Y.; Fukunishi, T.; Takeda, S.; Nishijima, S.; Nakahira, A. Superconducting high gradient magnetic separation for purification of wastewater from paper factory. IEEE Trans. Appl. Supercond. 2004, 14, 1565–1567. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Xu, X.; Li, L. Study on industrial wastewater treatment using superconducting magnetic separation. Cryogenics 2011, 51, 225–228. [Google Scholar] [CrossRef]
- Wang, J.; Qu, R.; Liu, Y.; He, J.; Zhu, Z.; Fang, H. Comparison study of superconducting wind generators with HTS and LTS field windings. IEEE Trans. Appl. Supercond. 2014, 25, 1–6. [Google Scholar] [CrossRef]
- Bovenkerk, H.P.; Bundy, F.P.; Hall, H.T.; Strong, H.M.; Wentorf, R.H. Preparation of diamond. Nature 1959, 184, 1094–1098. [Google Scholar] [CrossRef]
- Jones, M.E.; Marsh, R.E. The preparation and structure of magnesium boride, MgB2. J. Am. Chem. Soc. 1954, 76, 1434–1436. [Google Scholar] [CrossRef]
- Alecu, G.; Cosac, A.; Zamfir, S. Superconductivity in MgB2. Ann. Univ. Craiova. Electr. Eng. Ser. 2008, 30, 382. [Google Scholar]
- Bonura, M.; Senatore, C. Thermal conductivity and stability of commercial MgB2 conductors. Supercond. Sci. Technol. 2015, 28, 115014. [Google Scholar] [CrossRef]
- Busatto, S. Thermal Study of an Innovative Design for Superconducting Dipole Magnet in MgB2 for Energy Saving Purposes. Master’s Thesis, Polytechnic University of Milan, School of Industrial and Information Engineering, Milan, Italy, 2022. [Google Scholar]
- Bud’Ko, S.L.; Petrovic, C.; Lapertot, G.; Cunningham, C.E.; Canfield, P.C.; Jung, M.H.; Lacerda, A.H. Magnetoresistivity and H c 2 (T) in MgB2. Phys. Rev. B 2001, 63, 220503. [Google Scholar] [CrossRef]
- Hunsom, M.; Pruksathorn, K.; Damronglerd, S.; Vergnes, H.; Duverneuil, P. Electrochemical treatment of heavy metals (Cu2+, Cr6+, Ni2+) from industrial effluent and modeling of copper reduction. Water Res. 2005, 39, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.A.; Elimelech, M. Water and sanitation in developing countries: Including health in the equation. Environ. Sci. Technol. 2007, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.; Smaragdaki, E.; Vasilaki, G.; Gidarakos, E. Use of sediment quality guidelines and pollution indicators for the assessment of heavy metal and PAH contamination in Greek surficial sea and lake sediments. Environ. Monit. Assess. 2013, 185, 2843–2853. [Google Scholar] [CrossRef] [PubMed]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of heavy metals from industrial wastewaters: A review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- da Mota, I.D.O.; de Castro, J.A.; de Góes Casqueira, R.; de Oliveira Junior, A.G. Study of electroflotation method for treatment of wastewater from washing soil contaminated by heavy metals. J. Mater. Res. Technol. 2015, 4, 109–113. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J.; Sozhan, G. Studies on the Al–Zn–In-alloy as anode material for the removal of chromium from drinking water in electrocoagulation process. Desalination 2011, 275, 260–268. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)—science and applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Hanay, Ö.; Hasar, H. Effect of anions on removing Cu2+, Mn2+ and Zn2+ in electrocoagulation process using aluminum electrodes. J. Hazard. Mater. 2011, 189, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Wulan, D.R.; Hariyadi, H.R. Effect of electrodeposition reactor type on nickel recovery from electroplating wastewater. Procedia Chem. 2015, 16, 155–163. [Google Scholar] [CrossRef]
- Gode, F.; Pehlivan, E. Removal of chromium (III) from aqueous solutions using Lewatit S 100: The effect of pH, time, metal concentration and temperature. J. Hazard. Mater. 2006, 136, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Profeta, D.O.; da Silva, M.A.; Faria, D.N.; Cipriano, D.F.; Freitas, J.C.C.; dos Santos, F.S.; Lima, T.M.; Vasconcelos, S.C.; Pietre, M.K. Zeolite/calcium carbonate composite for a synergistic adsorption of cadmium in aqueous solution. Next Mater. 2025, 6, 100493. [Google Scholar] [CrossRef]
- Moraes, C.S.; Carneiro, P.A.; Faria, D.N.; Cipriano, D.F.; Freitas, J.C.C.; Amorim, R.G.; da Silva, R.S.; Pietre, M.K. High efficiency of myclobutanil adsorption by CTAB-zeolite structures: Experimental evidence meets theoretical investigation. Silicon 2024, 16, 3737–3753. [Google Scholar] [CrossRef]
- Visa, M.; Pricop, F.; Duta, A. Sustainable treatment of wastewaters resulted in the textile dyeing industry. Clean Technol. Environ. Policy 2011, 13, 855–861. [Google Scholar] [CrossRef]
- Lin, Q.S.; Chen, S.H.; Hu, M.Y.; Haq, M.R.U.; Yang, L.; Li, H. Biodegradation of cypermethrin by a newly isolated actinomycetes HU-S-01 from wastewater sludge. Int. J. Environ. Sci. Technol. 2011, 8, 45–56. [Google Scholar] [CrossRef]
- He, C.; Hu, X. Anionic dye adsorption on chemically modified ordered mesoporous carbons. Ind. Eng. Chem. Res. 2011, 50, 14070–14083. [Google Scholar] [CrossRef]
- Abramson, S.; Safraou, W.; Malezieux, B.; Dupuis, V.; Borensztajn, S.; Briot, E.; Bée, A. An eco-friendly route to magnetic silica microspheres and nanospheres. J. Colloid Interface Sci. 2011, 364, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zheng, H.; Zhang, Z.; Tshukudu, T.; Zhang, P.; Xiang, X. Characterization and coagulation–flocculation behavior of polymeric aluminum ferric sulfate (PAFS). Chem. Eng. J. 2011, 178, 50–59. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Z.; Zhang, W.; Xia, M.; Dai, G.; Zeng, G.; Zou, B.; Zhang, P. Facile synthesis of humic acid-coated iron oxide nanoparticles and their applications in wastewater treatment. Funct. Mater. Lett. 2011, 4, 373–376. [Google Scholar] [CrossRef]
- Rehman, R.; Anwar, J.; Mahmud, T.; Salman, M.; Saleem, M. Evaluation of batch biosorption of chromium (VI) from aqueous solution by chemically modified Polyalthia longifolia leaves. J. Chem. Soc. Pak 2011, 33, 846. [Google Scholar]
- Sousa, F.W.; Oliveira, A.G.; Ribeiro, J.P.; De Keukeleire, D.; Sousa, A.F.; Nascimento, R.F. Single and multielementary isotherms of toxic metals in aqueous solution using treated coconut shell powder. Desalin. Water Treat. 2011, 36, 289–296. [Google Scholar] [CrossRef]
- Datsko, T.Y.; Zelentsov, V.I.; Dvornikova, E.E. Physicochemical and adsorption-structural properties of diatomite modified with aluminum compounds. Surf. Eng. Appl. Electrochem. 2011, 47, 530–539. [Google Scholar] [CrossRef]
- Nalcaci, O.O.; Böke, N.; Ovez, B. Comparative study on the removal of various phenoxyalkanoic acid herbicides from aqueous solutions on polycaprolactone and activated carbon. J. Environ. Eng. 2011, 137, 1136–1144. [Google Scholar] [CrossRef]
- Radian, A.; Carmeli, M.; Zadaka-Amir, D.; Nir, S.; Wakshal, E.; Mishael, Y.G. Enhanced removal of humic acid from water by micelle-montmorillonite composites: Comparison to granulated activated carbon. Appl. Clay Sci. 2011, 54, 258–263. [Google Scholar] [CrossRef]
- Ogata, F.; Tominaga, H.; Yabutani, H.; Kawasaki, N. Removal of fluoride ions from water by adsorption onto carbonaceous materials produced from coffee grounds. J. Oleo Sci. 2011, 60, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-C.; Wang, K.-S.; Hsiao, T.-E.; Lin, I.-C.; Wu, H.-J.; Wu, Y.-L.; Liu, P.-H.; Chang, S.-H. Effects of UV irradiation on humic acid removal by ozonation, Fenton and Fe0/air treatment: THMFP and biotoxicity evaluation. J. Hazard. Mater. 2011, 195, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Galeano, L.A.; Vicente, M.Á.; Gil, A. Treatment of municipal leachate of landfill by Fenton-like heterogeneous catalytic wet peroxide oxidation using an Al/Fe-pillared montmorillonite as active catalyst. Chem. Eng. J. 2011, 178, 146–153. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Yan, X.; Ng, J.; Wang, Y.; Sun, D.D. Removal of bisphenol A via a hybrid process combining oxidation on β-MnO2 nanowires with microfiltration. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 198–204. [Google Scholar] [CrossRef]
- Anquandah, G.A.K.; Sharma, V.K.; Knight, D.A.; Batchu, S.R.; Gardinali, P.R. Oxidation of trimethoprim by ferrate (VI): Kinetics, products, and antibacterial activity. Environ. Sci. Technol. 2011, 45, 10575–10581. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-J.; Tai, J.; Adav, S.S.; Su, A. Harvesting biohydrogen from toxic wastewater using isolated strain. Int. J. Hydrog. Energy 2011, 36, 13907–13913. [Google Scholar] [CrossRef]
- Ambashta, R.D.; Sillanpää, M. Water purification using magnetic assistance: A review. J. Hazard. Mater. 2010, 180, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, K. Experimental evidence for effects of magnetic fields on moving water. IEEE Trans. Magn. 1985, 21, 2059–2061. [Google Scholar] [CrossRef]
- Spiegel, M.S. Method and Apparatus for Applying Magnetic Fields to Fluids. U.S. Patent Application No. 08/417,143, 23 June 1998. [Google Scholar]
- Tuutijärvi, T.; Lu, J.; Sillanpää, M.; Chen, G. As (V) adsorption on maghemite nanoparticles. J. Hazard. Mater. 2009, 166, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Alimi, F.; Tlili, M.M.; Amor, M.B.; Maurin, G.; Gabrielli, C. Effect of magnetic water treatment on calcium carbonate precipitation: Influence of the pipe material. Chem. Eng. Process. Process Intensif. 2009, 48, 1327–1332. [Google Scholar] [CrossRef]
- Iwasaka, M.; Ueno, S. Effects of gradient magnetic fields on diffusion process of glycine in water. IEEE Trans. Magn. 1997, 33, 4254–4256. [Google Scholar] [CrossRef]
- Franzreb, M.; Holl, W.H. Phosphate removal by high-gradient magnetic filtration using permanent magnets. IEEE Trans. Appl. Supercond. 2000, 10, 923–926. [Google Scholar] [CrossRef]
- Srebrenik, S.; Nadiv, S.; Lin, I.J. Magnetic treatment of water—A theoretical quantum model. Phys. Sep. Sci. Eng. 1993, 5, 71–91. [Google Scholar] [CrossRef]
- Colic, M.; Morse, D. Effects of amplitude of the radiofrequency electromagnetic radiation on aqueous suspensions and solutions. J. Colloid Interface Sci. 1998, 200, 265–272. [Google Scholar] [CrossRef]
- Higashitani, K.; Kage, A.; Katamura, S.; Imai, K.; Hatade, S. Effects of a magnetic field on the formation of CaCO3 particles. J. Colloid Interface Sci. 1993, 156, 90–95. [Google Scholar] [CrossRef]
- Higashitani, K.; Iseri, H.; Okuhara, K.; Kage, A.; Hatade, S. Magnetic effects on zeta potential and diffusivity of nonmagnetic colloidal particles. J. Colloid Interface Sci. 1995, 172, 383–388. [Google Scholar] [CrossRef]
- Ha, D.W.; Kwon, J.M.; Baik, S.K.; Lee, Y.J.; Han, K.S.; Ko, R.K.; Sohn, M.H.; Seong, K.C. Purification of condenser water in thermal power station by superconducting magnetic separation. Phys. C Supercond. Its Appl. 2011, 471, 1530–1532. [Google Scholar] [CrossRef]
- Sarıkaya, M.; Abbasov, T.; Erdemoğlu, M. Some aspects of magnetic filtration theory for removal of fine particles from aqueous suspensions. J. Dispers. Sci. Technol. 2006, 27, 193–198. [Google Scholar] [CrossRef]
- Ma, J.; Ma, Y.; Yu, F. A novel one-pot route for large-scale synthesis of novel magnetic CNTs/Fe@ C hybrids and their applications for binary dye removal. ACS Sustain. Chem. Eng. 2018, 6, 8178–8191. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Gong, Z.; Gao, K.; Ou, Y.; Zhang, J. The effect of a static magnetic field on the hydrogen bonding in water using frictional experiments. J. Mol. Struct. 2013, 1052, 102–104. [Google Scholar] [CrossRef]
- Cai, R.; Yang, H.; He, J.; Zhu, W. The effects of magnetic fields on water molecular hydrogen bonds. J. Mol. Struct. 2009, 938, 15–19. [Google Scholar] [CrossRef]
- Inaba, H.; Saitou, T.; Tozaki, K.; Hayashi, H. Effect of the magnetic field on the melting transition of H2O and D2O measured by a high resolution and supersensitive differential scanning calorimeter. J. Appl. Phys. 2004, 96, 6127–6132. [Google Scholar] [CrossRef]
- Chang, K.-T.; Weng, C.-I. An investigation into the structure of aqueous NaCl electrolyte solutions under magnetic fields. Comput. Mater. Sci. 2008, 43, 1048–1055. [Google Scholar] [CrossRef]
- Akopian, S.N.; Aĭrapetian, S.N. A study of specific electrical conductivity of water by the action of constant magnetic field, electromagnetic field, and low-frequency mechanical vibrations. Biofizika 2005, 50, 265–270. [Google Scholar] [PubMed]
- Holysz, L.; Szczes, A.; Chibowski, E. Effects of a static magnetic field on water and electrolyte solutions. J. Colloid Interface Sci. 2007, 316, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Iwasaka, M.; Ueno, S. Structure of water molecules under 14 T magnetic field. J. Appl. Phys. 1998, 83, 6459–6461. [Google Scholar] [CrossRef]
- Toledo, E.J.L.; Ramalho, T.C.; Magriotis, Z.M. Influence of magnetic field on physical–chemical properties of the liquid water: Insights from experimental and theoretical models. J. Mol. Struct. 2008, 888, 409–415. [Google Scholar] [CrossRef]
- Fan, P.; Jiang, X.; Qiao, J.; Li, L. Enhanced removal of heavy metals by zerovalent iron in designed magnetic reactors. Environ. Technol. 2018, 39, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- López, S.L.F.; Virgen, M.R.M.; Montoya, V.H.; Morán, M.A.M.; Gómez, R.T.; Vázquez, N.A.R.; Cruz, M.A.P.; González, M.S.E. Effect of an external magnetic field applied in batch adsorption systems: Removal of dyes and heavy metals in binary solutions. J. Mol. Liq. 2018, 269, 450–460. [Google Scholar] [CrossRef]
- Szatylowicz, E.; Skoczko, I. Magnetic Field Usage for the Removal of Iron by Filtration-Assisted Different Filter Materials. Proceedings 2019, 16, 6. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, L.; Li, L.; Xu, Z.; Su, J.; Li, B.; Huang, J. Effects of magnetic fields on the enzymatic synthesis of naringin palmitate. RSC Adv. 2018, 8, 13364–13369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, X.; Liu, Y.; Jia, Y.; Yu, G.; Ouyang, S. Copper (II) adsorption on Ca-rectorite, and effect of static magnetic field on the adsorption. J. Colloid Interface Sci. 2004, 278, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Duangduen, C.; Nathaporn, A.; Kitiphatmontree, M. The effects of magnetic field on the removal of organic compounds and metals by coagulation and flocculation. Phys. Status Solidi C 2006, 3, 3201–3205. [Google Scholar] [CrossRef]
- Rajczykowski, K.; Loska, K. Stimulation of heavy metal adsorption process by using a strong magnetic field. Water Air Soil Pollut. 2018, 229, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gonza, O.F.; Moreno Virgen, M.D.R.; Hernandez Montoya, V.; Tovar Gomez, R.; Alcantara Flores, J.L.; Pérez Cruz, M.A.; Montes Morán, M.A. Adsorption of heavy metals in the presence of a magnetic field on adsorbents with different magnetic properties. Ind. Eng. Chem. Res. 2016, 55, 9323–9331. [Google Scholar] [CrossRef]
- Jung, J.; Sanji, B.; Godbole, S.; Sofer, S. Biodegradation of phenol: A comparative study with and without applying magnetic fields. J. Chem. Technol. Biotechnol. 1993, 56, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Nitta, Y.; Takahashi, F. A submerged filter system consisting of magnetic tubular support media covered with a biofilm fixed by magnetic force. Water Res. 1994, 28, 1175–1179. [Google Scholar] [CrossRef]
- Szcześ, A.; Chibowski, E.; Hołysz, L.; Rafalski, P. Effects of static magnetic field on water at kinetic condition. Chem. Eng. Process. Process Intensif. 2011, 50, 124–127. [Google Scholar] [CrossRef]
- Pingping, Z.; Ruochun, Y.I.N.; Zhiyou, C.; Lifang, W.; Zengliang, Y. Genotoxic effects of superconducting static magnetic fields (SMFs) on wheat (Triticum aestivum) pollen mother cells (PMCs). Plasma Sci. Technol. 2007, 9, 241. [Google Scholar] [CrossRef]
- Johan, S. Effect of Magnetic Field on the Sedimentation of Suspended Solids of Sewage. Ph.D. Thesis, Universiti Teknologi Malaysia, Skudai, Malaysia, 2003. [Google Scholar]
- Baker, J.S.; Judd, S.J.; Parsons, S.A. Antiscale magnetic pretreatment of reverse osmosis feedwater. Desalination 1997, 110, 151–165. [Google Scholar] [CrossRef]
- Kobe, S.; Dražić, G.; Cefalas, A.C.; Sarantopoulou, E.; Stražišar, J. Nucleation and crystallization of CaCO3 in applied magnetic fields. Cryst. Eng. 2002, 5, 243–253. [Google Scholar] [CrossRef]
- Kuo, W.C.; Lee, Y.P. Application of magnetic field in industrial wastewater treatment processes. J. Uni. Sci. Technol. Beijing 2006, 28, 74–78. [Google Scholar]
- Ali, Y.; Samaneh, R.; Zohre, R.; Mostafa, J. Magnetic water treatment in environmental management: A review of the recent advances and future perspectives. Curr. World Environ. 2014, 9, 1008–1016. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Arabshahi, H.; Saeidi, M.R.; Mahdavi, B. The effect of magnetic water on growth and quality improvement of poultry. Middle East J. Sci. Res. 2008, 3, 140–144. [Google Scholar]
- Kronenberg, K.J. Magnetized II: More alluring facts about treating water with magnets. Aqua Magazine, September 1993; pp. 20–24. [Google Scholar]
- Cuartero, J.; Fernández-Muñoz, R. Tomato and salinity. Sci. Hortic. 1998, 78, 83–125. [Google Scholar] [CrossRef]
- Bolto, B.A. Magnetic particle technology for wastewater treatment. Waste Manag. 1990, 10, 11–21. [Google Scholar] [CrossRef]
- Baik, S.K.; Ha, D.W.; Ko, R.K.; Kwon, J.M. Magnetic field analysis of high gradient magnetic separator via finite element analysis. Phys. C Supercond. 2012, 480, 111–117. [Google Scholar] [CrossRef]
- Seo, K.; Morita, M. Guidelines for LTS magnet design based on transient stability. Cryogenics 2006, 46, 354–361. [Google Scholar] [CrossRef]
- Ma, W.; Dai, Y.; Huang, H.; Song, S.; Zhang, B.; Kim, K.; Oh, S. Development of a 6-T conduction-cooled superconducting magnet. IEEE Trans. Appl. Supercond. 2012, 22, 4905605. [Google Scholar] [CrossRef]
- Ohara, T.; Kumakura, H.; Wada, H. Magnetic separation using superconducting magnets. Phys. C Supercond. 2001, 357, 1272–1280. [Google Scholar] [CrossRef]
- Oka, T.; Kanayama, H.; Fukui, S.; Ogawa, J.; Sato, T.; Ooizumi, M.; Terasawa, T.; Itoh, Y.; Yabuno, R. Application of HTS bulk magnet system to the magnetic separation techniques for water purification. Phys. C Supercond. 2008, 468, 2128–2132. [Google Scholar] [CrossRef]
- Okada, H.; Kudo, Y.; Nakazawa, H.; Chiba, A.; Mitsuhashi, K.; Ohara, T.; Wada, H. Removal system of arsenic from geothermal water by high gradient magnetic separation-HGMS reciprocal filter. IEEE Trans. Appl. Supercond. 2004, 14, 1576–1579. [Google Scholar] [CrossRef]
- Liu, H.; Xu, D.; Shen, F.; Zhang, H.; Li, L. Development of large bore superconducting magnet for wastewater treatment application. Prog. Supercond. Cryog. 2017, 19, 13–16. [Google Scholar] [CrossRef][Green Version]
- Di Costanzo, N.; Di Capua, F.; Cesaro, A.; Mascolo, M.C.; Pirozzi, F.; Esposito, G. Impact of high-intensity static magnetic field on chemical properties and anaerobic digestion of sewage sludge. Waste Biomass Valorization 2023, 14, 2469–2479. [Google Scholar] [CrossRef]
- Gillet, G.; Diot, F.; Lenoir, M. Removal of heavy metal ions by superconducting magnetic separation. Sep. Sci. Technol. 1999, 34, 2023–2037. [Google Scholar] [CrossRef]
- Hartikainen, T.; Nikkanen, J.-P.; Mikkonen, R. Magnetic separation of industrial waste waters as an environmental application of superconductivity. IEEE Trans. Appl. Supercond. 2005, 15, 2336–2339. [Google Scholar] [CrossRef]
- He, C.; Fang, K.; Gong, H.; Liu, J.; Song, X.; Liang, R.; He, Q.; Yuan, Q.; Wang, K. Advanced organic recovery from municipal wastewater with an enhanced magnetic separation (EMS) system: Pilot-scale verification. Water Res. 2022, 217, 118449. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.K.; Sung, T.I.; Cho, E.S.; Namkung, K.C.; Bae, D.J.; Park, I.H. Magnetic separation for contaminants in wastewater using magnetic micro bead. IEEE Trans. Magn. 2012, 48, 3768–3771. [Google Scholar] [CrossRef]
- Vincent-Viry, O.; Mailfert, A.; Gillet, G.; Diot, F. Magnetic percolation phenomenon in high-field high-gradient separators. IEEE Trans. Magn. 2000, 36, 3947–3952. [Google Scholar] [CrossRef]
- Newns, A.; Pascoe, R.D. Influence of path length and slurry velocity on the removal of iron from kaolin using a high gradient magnetic separator. Miner. Eng. 2002, 15, 465–467. [Google Scholar] [CrossRef]
- Karaboğa, F.; Ulgen, A.T.; Yetiş, H.; Akdoğan, M.; Pakdil, M.; Belenli, I. Mechanical properties and uniformity of Fe-MgB2 wires upon various wire drawing steps. Mater. Sci. Eng. A 2018, 721, 89–95. [Google Scholar] [CrossRef]
- Yetiş, H.; Karaboğa, F.; Avcı, D.; Belenli, İ. Nano-sized amorphous/semi-crystalline boron ratio and external Mg effects on transport and structural properties in MgB2 IMD wires. Phys. C Supercond. Its Appl. 2021, 581, 1353807. [Google Scholar] [CrossRef]
- Fair, R.; Stautner, W.; Douglass, M.; Rajput-Ghoshal, R.; Moscinski, M.; Riley, P.; Wagner, D.; Kim, J.; Hou, S.; Lopez, F.; et al. Superconductivity for Large Scale Wind Turbines; General Electric-Global Research: Niskayuna, NY, USA, 2012. [Google Scholar]
- Jensen, B.B.; Mijatovic, N.; Abrahamsen, A.B. Development of superconducting wind turbine generators. J. Renew. Sustain. Energy 2013, 5, 023137. [Google Scholar] [CrossRef]
- Abrahamsen, A.B.; Magnusson, N.; Jensen, B.B.; Liu, D.; Polinder, H. Design of an MgB2 race track coil for a wind generator pole demonstration. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2014; p. 32001. [Google Scholar]
- Kováč, P.; Melišek, T.; Hušek, I. Ic anisotropy of in situ made MgB2 tapes. Supercond. Sci. Technol. 2005, 18, L45. [Google Scholar] [CrossRef]
- Häßler, W.; Kovac, P.; Eisterer, M.; Abrahamsen, A.B.; Herrmann, M.; Rodig, C.; Nenkov, K.; Holzapfel, B.; Melisek, T.; Kulich, M.; et al. Anisotropy of the critical current in MgB2 tapes made of high energy milled precursor powder. Supercond. Sci. Technol. 2010, 23, 65011. [Google Scholar] [CrossRef]
- Zhuang, C.; Liu, X.; Guo, T.; Wang, B.; Li, X.; Chen, C.; Feng, Q. The size effect of raw materials on the phase formation of polycrystalline MgB2. Supercond. Sci. Technol. 2007, 20, 1125. [Google Scholar] [CrossRef]
- Larbalestier, D.C.; Cooley, L.D.; Rikel, M.O.; Polyanskii, A.A.; Jiang, J.; Patnaik, S.; Cai, X.Y.; Feldmann, D.M.; Gurevich, A.; Squitieri, A.A. Strongly linked current flow in polycrystalline forms of the superconductor MgB2. Nature 2001, 410, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Takeya, H.; Fujii, H.; Kumakura, H.; Hatano, T.; Togano, K.; Kito, H.; Ihara, H. Superconducting properties of MgB2 bulk materials prepared by high-pressure sintering. Appl. Phys. Lett. 2001, 78, 2914–2916. [Google Scholar] [CrossRef]
- Narozhnyi, V.N.; Fuchs, G.; Handstein, A.; Gümbel, A.; Eckert, J.; Nenkov, K.; Hinz, D.; Gutfleisch, O.; Wälte, A.; Bogacheva, L.N. Comparative study of dense bulk MgB 2 materials prepared by different methods. J. Supercond. 2002, 15, 599–601. [Google Scholar] [CrossRef]
- Lee, S.; Yamamoto, A.; Mori, H.; Eltsev, Y.; Masui, T.; Tajima, S. Single crystals of MgB2 superconductor grown under high-pressure in Mg–B–N system. Phys. C Supercond. 2002, 378, 33–37. [Google Scholar] [CrossRef]
- Karpinski, J.; Angst, M.; Jun, J.; Kazakov, S.M.; Puzniak, R.; Wisniewski, A.; Roos, J.; Keller, H.; Perucchi, A.; Degiorgi, L. MgB2 single crystals: High pressure growth and physical properties. Supercond. Sci. Technol. 2003, 16, 221. [Google Scholar] [CrossRef]
- Blank, D.H.A.; Hilgenkamp, H.; Brinkman, A.; Mijatovic, D.; Rijnders, G.; Rogalla, H. Superconducting Mg–B films by pulsed-laser deposition in an in situ two-step process using multicomponent targets. Appl. Phys. Lett. 2001, 79, 394–396. [Google Scholar] [CrossRef]
- Kang, W.N.; Kim, H.-J.; Choi, E.-M.; Jung, C.U.; Lee, S.-I. MgB2 superconducting thin films with a transition temperature of 39 Kelvin. Science 2001, 292, 1521–1523. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Mavoori, H.; Bower, C.; Van Dover, R.B. High critical currents in iron-clad superconducting MgB2 wires. Nature 2001, 411, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.K.; Feng, Y.; Zhao, Y.; Koshizuka, N.; Zhou, L.; Zhang, P.X.; Liu, X.H.; Ji, P.; Du, S.J.; Liu, C.F. Transport behavior and critical current densities in MgB2 wires. Appl. Phys. Lett. 2001, 79, 1649–1651. [Google Scholar] [CrossRef]
- Grasso, G.; Malagoli, A.; Ferdeghini, C.; Roncallo, S.; Braccini, V.; Siri, A.S.; Cimberle, M.R. Large transport critical currents in unsintered MgB2 superconducting tapes. Appl. Phys. Lett. 2001, 79, 230–232. [Google Scholar] [CrossRef]
- Fang, H.; Padmanabhan, S.; Zhou, Y.X.; Salama, K. High critical current density in iron-clad MgB2 tapes. Appl. Phys. Lett. 2003, 82, 4113–4115. [Google Scholar] [CrossRef]
- Masui, T.; Lee, S.; Tajima, S. Effect of the growing process on the electronic properties of MgB2 single crystals. Phys. C Supercond. 2003, 392, 281–285. [Google Scholar] [CrossRef]
- Eltsev, Y.; Lee, S.; Nakao, K.; Chikumoto, N.; Tajima, S.; Koshizuka, N.; Murakami, M. Anisotropic superconducting properties of MgB2 single crystals. Phys. C Supercond. 2002, 378, 61–64. [Google Scholar] [CrossRef]
- Buzea, C.; Yamashita, T. Review of the superconducting properties of MgB2. Supercond. Sci. Technol. 2001, 14, R115. [Google Scholar] [CrossRef]
- Thompson, J.R.; Paranthaman, M.; Christen, D.K.; Sorge, K.D.; Kim, H.J.; Ossandon, J.G. High temporal stability of supercurrents in MgB2 materials. Supercond. Sci. Technol. 2001, 14, L17. [Google Scholar] [CrossRef]
- Dhallé, M.; Toulemonde, P.; Beneduce, C.; Musolino, N.; Decroux, M.; Flükiger, R. Transport and inductive critical current densities in superconducting MgB2. Phys. C Supercond. 2001, 363, 155–165. [Google Scholar] [CrossRef]
- Vinod, K.; Upendran, S. Studies on development of MgB2 superconductor with improved in-field critical current density. Adv. Mater. Res. 2010, 117, 63–68. [Google Scholar]
- Gajda, D.; Zaleski, A.J.; Morawski, A.; Czujko, T.; Avci, D.; Karaboga, F.; Akdogan, M.; Yetis, H.; Cetner, T.; Belenli, I. The significant influence of packing density of unreacted Mg+ 2B mixture and heat treatment conditions on some of critical parameters for MgB2/Fe wires. J. Alloys Compd. 2021, 889, 161665. [Google Scholar] [CrossRef]
- Gajda, D.; Zaleski, A.J.; Morawski, A.; Małecka, M.; Akdoğan, M.; Karaboğa, F.; Avcı, D.; Yetiş, H.; Belenli, I.; Czujko, T. Influence of amorphous boron grain size, high isostatic pressure, annealing temperature, and filling density of unreacted material on structure, critical parameters, n-value, and engineering critical current density in MgB2 wires. Materials 2021, 14, 3600. [Google Scholar] [CrossRef] [PubMed]

| The Impact of Magnetic Fields on Water Purification | References |
|---|---|
| Exposure to a SMF of 0.4–0.6 T accelerated precipitation and improved sludge coagulation by reducing electrokinetic potential. | [14] |
| SMF of 0.0025 T lowered the conductivity of deionized water, attributed to changes in the ionic hydration shell. | [82,83] |
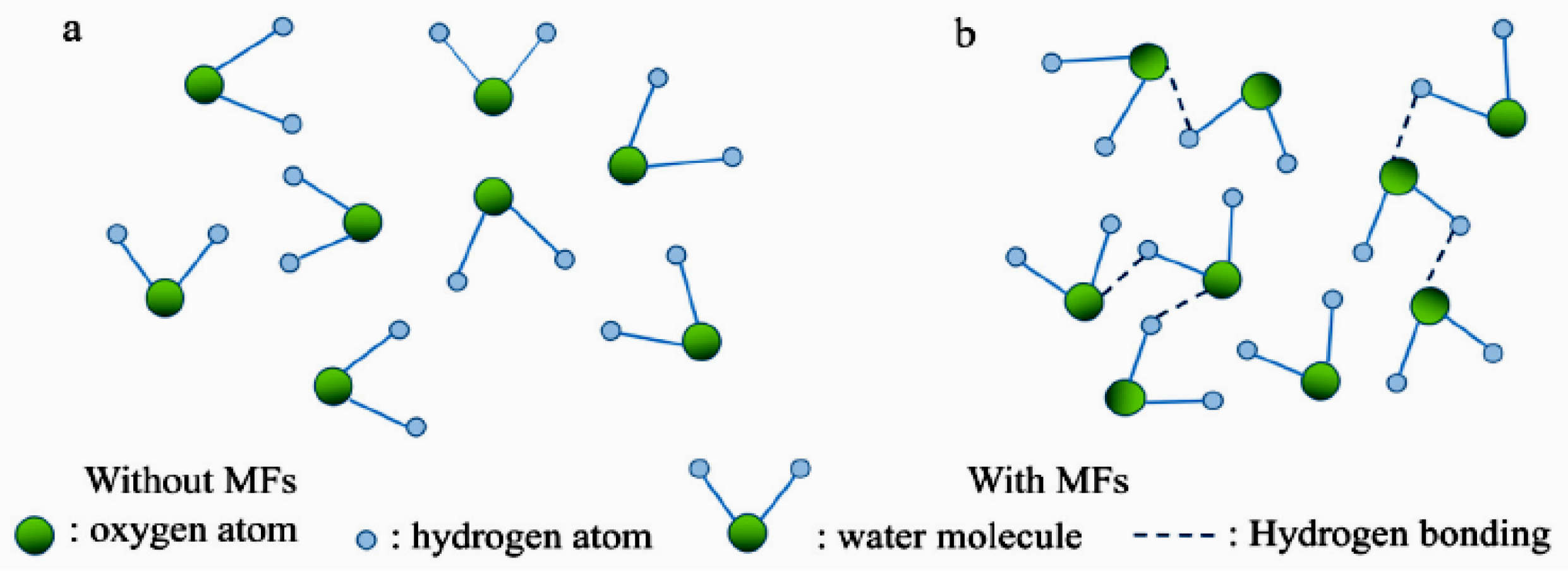
| Water's structural stability and viscosity are closely tied to its hydrogen bonding. | [84] |
| SMF exposure from 0 to 10 T increased hydrogen bonds by up to 0.34%, decreasing the self-diffusion coefficient while enhancing viscosity and stability. | [81] |
| A 6 T magnetic field strengthened hydrogen bonds in H2O and D2O by inhibiting thermal motion through the Lorentz force, termed “enhanced-dynamic magnetic susceptibility.” | [80] |
| Moderate MFs (less than 1 T) can also promote hydrogen bond synthesis. | [79] |
| MFs may weaken hydrogen bonds between water clusters but enhance bonding within the clusters. | [85] |
| Magnetized water has a lower friction coefficient than unmagnetized water, indicating reduced hydrogen bond strength. | [78] |
| MFs alter hydrogen bonding dynamics in water, enhancing reactions like improved adsorption. | [77] |
| Removed Species | Adsorbents | MFI (T) | Observed Effects | References |
|---|---|---|---|---|
| Cu2+ | Zerovalent iron | <0.001 | Removal efficiency increased by 88% | [86] |
| Cd2+, Zn2+ | Zeolite and carbon modified with calcium and iron | 0.086 | Total molar removal increased by 10%–20% | [87] |
| Cu2+, Pb2+, Cd2+ | Granular activated alumina | 0.118 | Removal efficiency increased by 1.9%–8.2% | [88] |
| Zn2+ | Na-rectorite | 0.32 | Adsorption capacity increased by 10% | [89] |
| Cu2+ | Ca-rectorite | 0.34 | Adsorption capacity increased by 12% | [90] |
| As5+ | Ferric chloride | 0.35 | Removal efficiency increased by 6%–50% at different coagulant levels | [91] |
| Cu2+, Ni2+, Cd2+ | Activated carbon | 0.517 | Total molar removal increased by 11% | [92] |
| Cu2+, Ni2+ | Vermiculite and halloysite | 0.518 | Removal efficiency decreased by 5.2% and 20.5%, respectively | [92] |
| Cd2+, Zn2+ | Activated carbon | 1 | Adsorption capacity increased by 63% and 15%, respectively | [93] |
| Heavy Metals Concentrations in Wastewater Before Separation | Concentration (mg/L) |
|---|---|
| Solid matter | 8.8 |
| Dissolved Cr | 0.13 |
| Dissolved Ni | <0.01 |
| Dissolved Fe | 0.05 |
| Dissolved Mo | 9.8 |
| Total Cr | 0.25 |
| Total Ni | 0.34 |
| Total Fe | 0.39 |
| Total Mo | 9.8 |
| Magnetic Flux Density (T) | Cr Concentration After Separation |
| 0.7 | 6 mg/L |
| 1.3 | 6 mg/L |
| 1.8 | 5.5 mg/L |
| 2.4 | 5.5 mg/L |
| 3.0 | 5.5 mg/L |
| Magnetic Flux Density (T) | Mo Concentration After Separation |
| 1.0 | 6.2 mg/L |
| 1.5 | 6.0 mg/L |
| 2.0 | 5.9 mg/L |
| 2.5 | 5.6 mg/L |
| 3.0 | 5.5 mg/L |
| Parameter | NbTi | Nb3Sn | MgB2 | YBCO | Bi-2223 |
|---|---|---|---|---|---|
| Tc (K) | 9 | 18 | 39 | 92 | 110 |
| Anisotropy | Negligible | Negligible | 1.5~5 | 5~7 | 50~200 |
| Jc at 4.2 K (A/cm2) | ~106 | ~106 | ~106 | ~106 | ~107 |
| Hc2 at 4.2 K (T) | 11–12 | 25–29 | 15–20 | >100 | >100 |
| Hirr at 4.2 K (T) | 10~11 | 21~24 | 6~12 | 5~7 (77 K) | 0.2 (77 K) |
| Coherence length ξ(0) (nm) | 4~5 | 3 | 4~5 | 1.5 | 1.5 |
| Penetration depth λ(0) (nm) | 240 | 65 | 100~140 | 150 | 150 |
| Resistivity ρ(Tc) (μΩcm) | 60 | 5 | 0.4 | 150~800 | 40~60 |
| Fabrication Route | Description | Key Process Parameters | Advantages | Disadvantages |
|---|---|---|---|---|
| In Situ PIT | Precursor powders (Mg + B) are packed, cold-worked, and then reacted within a metallic sheath to form the MgB2 conductor. |
|
|
|
| Ex Situ PIT | Pre-reacted MgB2 powder is packed, cold-worked, and then sintered within a metallic sheath to form the final conductor. |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahadeh, M.; Belenli, I.; van Lier, J.B.; Mahmoud, N. Potential of MgB2 Superconductors for Magnetically Aided Wastewater Treatment: Feasibility and Future Prospects. Water 2025, 17, 2129. https://doi.org/10.3390/w17142129
Shahadeh M, Belenli I, van Lier JB, Mahmoud N. Potential of MgB2 Superconductors for Magnetically Aided Wastewater Treatment: Feasibility and Future Prospects. Water. 2025; 17(14):2129. https://doi.org/10.3390/w17142129
Chicago/Turabian StyleShahadeh, Mahran, Ibrahim Belenli, Jules B. van Lier, and Nidal Mahmoud. 2025. "Potential of MgB2 Superconductors for Magnetically Aided Wastewater Treatment: Feasibility and Future Prospects" Water 17, no. 14: 2129. https://doi.org/10.3390/w17142129
APA StyleShahadeh, M., Belenli, I., van Lier, J. B., & Mahmoud, N. (2025). Potential of MgB2 Superconductors for Magnetically Aided Wastewater Treatment: Feasibility and Future Prospects. Water, 17(14), 2129. https://doi.org/10.3390/w17142129