Abstract
Nitrogen-metabolizing microbes are the keystone drivers of reducing nitrogen pollutants in wastewater and natural waters, but the one-way experiment with fixed screening factors fails to discover the optimal scope of nitrogen-metabolizing microbes performing nitrogen reduction. This study novelly combines the one-way experiment and response surface methodology (RSM) modeling to synthesize an effective nitrogen reduction microbial community, with the RSM model showing high goodness-of-fit (R2 = 0.83, p = 0.01) for optimizing the strain combination. Eight bacterial strains were isolated from contaminated sediment and activated sludge. Three efficient strains, arranged to Ignatzschieria indica, Staphylococcus epidermidis, and Acinetobacter baumannii by 16S rDNA sequencing, were screened using the above combination method to synthesize a nitrogen reduction microbial community. Within the synthetic microbial community, Ignatzschieria indica and Staphylococcus epidermidis possessed denitrification abilities, and Acinetobacter baumannii contributed to nitrification with 99% of ammonium oxidation. This synthesis microbial community displayed synchronous nitrification and denitrification under interval aeration and possessed wide pH tolerance from 6 to 10, with a steady >80% total inorganic nitrogen reduction. This research managed to synthesize a tolerant nitrogen reduction microbial community and provides novel insight for constructing synthetic microbial consortia.
1. Introduction
Water eutrophication, primarily driven by the over-enrichment of nitrogen and phosphorus, is a pervasive global issue that has detrimental effects on aquatic ecosystems [1,2]. Nitrogen, in the forms of NH3-N and NO3-N, is one of the major pollutants contributing to this process [3]. According to the United Nations Environment Programme (UNEP), approximately 40% of the world’s water bodies are affected by eutrophication, with nitrogen being a significant factor in the degradation of water quality [4]. Excess nitrogen can lead to oxygen depletion in water bodies, increased frequency of harmful algal blooms, and a loss of biodiversity [5,6]. Effective nitrogen removal technologies are, therefore, essential to mitigate these environmental impacts and to improve water quality [7,8].
Traditional nitrogen removal methods, such as nitrification and denitrification, are widely used in wastewater treatment processes [9,10]. Nitrification involves the aerobic conversion of ammonia to nitrate, while denitrification reduces nitrate to nitrogen gas under anaerobic conditions [11,12]. However, these processes are typically performed in separate reactors, which increases the complexity and energy consumption of the treatment system [13,14,15]. According to the U.S. Environmental Protection Agency (EPA), energy consumption for nitrification–denitrification processes can account for over 50% of the total operational cost in conventional wastewater treatment plants [16,17]. Moreover, the requirement for external carbon sources, such as methanol, in denitrification, further elevates operational costs [18,19]. The inefficiency of these conventional methods, particularly under high nitrogen loads, emphasizes the need for more cost-effective and integrated solutions [20,21].
In recent years, new technologies, such as short-cut nitrification and anaerobic ammonia oxidation (anammox), have emerged as promising alternatives [22]. Short-cut nitrification limits the nitrification process to the conversion of ammonia to nitrite, reducing oxygen demand and carbon usage [22]. However, this method requires strict control over the process conditions, making it difficult to apply at large scales [23]. On the other hand, anammox, which occurs in anaerobic environments and involves the direct conversion of ammonia and nitrite to nitrogen gas, has gained attention due to its low carbon requirements and higher nitrogen removal efficiency [24,25]. Despite these advances, both methods face challenges in achieving long-term stability, high removal efficiency, and ease of scaling for real-world applications [26,27]. According to a study by Li et al. (2021), while anammox can remove up to 80% of nitrogen, its application remains limited due to the slow growth of the bacteria and sensitivity to environmental conditions [28]. The short-range denitrification coupled anammox (PD-A) process can reduce the carbon source by 40% and the aeration rate by 25%, but it also has the problems of weak low-temperature activity of ammonia-oxidizing bacteria, the interference of complex organic matter in microbial metabolism, and the restriction of engineering application [22,29]. Although aerobic denitrification coupled with short-range nitrification processes can shorten the reaction time, the efficiency of aerobic denitrifying bacteria is unstable, and it is difficult to screen the bacterial strains [24,25].
This study proposes a composite microbial system that combines heterotrophic nitrification and denitrification bacteria to overcome the limitations of the existing methods [30,31]. Heterotrophic nitrification bacteria, which can utilize organic carbon as an electron donor, are capable of simultaneously performing nitrification and denitrification, thus eliminating the need for separate reactors and external carbon sources [32,33,34]. Recent studies, such as those by Gupta et al. (2022) and Qin et al. (2024), have demonstrated that heterotrophic nitrifiers can effectively remove ammonia nitrogen and nitrate nitrogen in a single system under aerobic or micro-aerobic conditions [35,36]. The integrated system not only reduces the overall energy consumption, but also improves nitrogen removal efficiency by coupling nitrification with denitrification, achieving nitrogen removal rates as high as 90% in laboratory settings [37]. This combined approach is particularly beneficial for high-load nitrogen wastewater, where traditional methods are less effective [38,39].
This study innovatively integrates one-way experiments with RSM modeling (with high goodness-of-fit). The screened functionally complementary strains can achieve simultaneous nitrification and denitrification and maintain a stable and efficient total inorganic nitrogen removal rate over a wide pH range. The main objective was to screen, isolate, and optimize the performance of heterotrophic nitrifying and denitrifying bacteria for efficient nitrogen removal through compounding between efficient strains. Specifically, we aim to evaluate their potential in treating inorganic nitrogen in synthetic wastewater. Additionally, this study seeks to optimize key operational parameters, such as temperature, pH, and inoculum concentration, using response surface methodology (RSM) to achieve maximum nitrogen removal efficiency. By improving these parameters, this research aims to demonstrate the feasibility of large-scale applications of this composite microbial system in wastewater treatment, offering a more sustainable and cost-effective solution for global nitrogen pollution.
2. Materials and Methods
2.1. Bacterial Strains and Culture Media
In this study, bacterial strains were isolated from contaminated sediments (from Wangshen Village, Lingbi County, Suzhou, Anhui) and activated sludge (from a sewage treatment plant in Suzhou). Contaminated sediments (obtained from Wangshen Village, Lingbi County, Suzhou, Anhui) have long been affected by nitrogen and other pollutants, containing functional microorganisms adapted to the polluted environment. Activated sludge (obtained from a sewage treatment plant in Suzhou) is rich in microbial communities involved in nitrogen cycling and other pollutant degradation and commercial bacterial agents. The heterotrophic nitrification and denitrification bacteria were cultured using specialized media. The Luria–Bertani medium was used for the general growth of bacteria and initial isolation. The heterotrophic nitrification medium consisted of ammonium sulfate (0.5 g/L) (Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China), sodium succinate (2.2 g/L) (Sinopharm Group Chemical Reagent Co., Ltd.), and a trace element solution. The denitrification medium included potassium nitrate (0.4 g/L) (Sinopharm Group Chemical Reagent Co., Ltd.), magnesium sulfate (0.1 g/L) (Shanghai Maclean Biochemical Technology Co., Ltd., Shanghai, China), sodium phosphate dibasic (7.9 g/L) (Sinopharm Group Chemical Reagent Co., Ltd.), potassium dihydrogen phosphate (1.5 g/L) (Sinopharm Group Chemical Reagent Co., Ltd.), and sodium succinate (2.8 g/L) (Sinopharm Group Chemical Reagent Co., Ltd.), along with a trace element solution. All media were prepared with distilled water and sterilized by autoclaving at 121 °C for 30 min.
2.2. Growth and Nitrogen Removal Experiments
The growth characteristics of the isolated bacterial strains were determined by measuring the optical density (OD) at 600 nm at various time intervals. The bacteria were inoculated into LB liquid medium and incubated at 30 °C with shaking at 120 rpm. Growth was monitored over a 72 h period, with OD600 measurements taken at 0, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 36, 48, 60, and 72 h. In parallel, the pH value of the culture medium was measured using a pH meter (Model AMXYQ-23, Anhui Junzhun Testing Technology Co., Ltd., Hefei, China) at the same time points to evaluate the pH changes induced by the bacterial metabolic activities. For the nitrogen removal experiments, the NH3-N and NO3-N removal rates were determined by incubating the bacterial strains in their respective media. The ammonia nitrogen concentration was analyzed using the Nessler reagent method. Nitrate nitrogen was measured by ultraviolet–visible spectrophotometry using a UV-Vis spectrophotometer (T6 New Century, Beijing Purkinje General Instrument Co., Ltd., Beijing, China). The nitrogen removal experiments were conducted under controlled conditions at 30 °C, with samples taken at 0, 12, 24, 36, 48, 60, and 72 h for analysis.
2.3. Isolation and Identification of Bacterial Strains
Bacterial strains with significant nitrogen removal potential were isolated from the sediment and activated sludge samples. A small amount of sample was inoculated into the respective enrichment media and incubated at 30 °C for 48 h. After incubation, the bacterial cultures were serially diluted and spread onto LB agar and heterotrophic nitrification media plates to obtain isolated colonies. The bacterial colonies were morphologically examined and characterized based on their color, size, and shape. Colonies exhibiting distinct morphological features were selected for further analysis. The selected strains were then identified through 16S rDNA sequencing. DNA was extracted using a standard extraction kit (Sigma-Aldrich, St. Louis, MO, USA), and the 16S rDNA gene was amplified with universal primers (27F and 1492R). The resulting sequences were compared with the GenBank database using BLAST (1.4.0), and a phylogenetic tree was constructed using MEGA X software (12.0.11).
2.4. Optimization of Nitrogen Removal Using RSM
To optimize nitrogen removal performance, response surface methodology (RSM) was employed. The three key parameters of temperature, pH, and inoculum concentration were selected for optimization, as they are critical factors influencing microbial activity. A Box–Behnken design was used to evaluate the interaction between these factors and their effects on ammonia nitrogen and nitrate nitrogen removal efficiency. The experimental conditions were set at three levels for each factor, as follows: temperature (15 °C, 25 °C, and 35 °C), pH (6, 8, and 10), and inoculum concentration (1%, 3%, and 5%). Statistical analysis was performed using Design Expert 13.0 software to develop a second-order polynomial regression model, which was used to predict the nitrogen removal efficiency under various conditions. The model’s significance was assessed through Analysis of Variance (ANOVA), and the optimal conditions for maximum nitrogen removal were determined. The experiments were performed in triplicate, and the results were expressed as mean ± standard deviation.
3. Results
3.1. Screening, Physiological Characteristics, and Taxonomic Research of Nitrogen Removal Bacteria
The experiment isolated two and six single colonies from heterotrophic nitrification and denitrification media, respectively, following 2–3 days of constant-temperature incubation. Morphological characterization revealed colonies with diameters of 1–2 cm, smooth surfaces, and slightly convex centers, predominantly exhibiting white or pink pigmentation (Table S1). After amplification in LB liquid medium, 2 mL of bacterial suspension was transferred to 100 mL of fresh medium for oscillatory cultivation (80 rpm), with OD600 values measured periodically to construct growth curves (Figure 1). Kinetic analysis demonstrated distinct growth patterns across the strains. The aerobic consortia (OD600 plateau ≈ 2.2) exhibited no significant lag phase, entered exponential growth at 10–20 h, and reached a stationary phase by 35 h. The strains derived from black-odor sediment displayed rapid proliferation, with exponential growth spanning 5–30 h and extended stationary phases up to 80 h. The composite bacterial powder isolates showed a 12 h lag phase, followed by exponential growth (12–25 h) and sustained stability beyond 50 h. The anaerobic sludge-derived strains exhibited minimal lag phase (0–2 h), completing exponential growth within 2–20 h and entering a stationary phase by 20 h. All strains shared a common exponential growth window (6–20 h), with OD600 values stabilizing (2.2 ± 0.1) after 35–40 h, indicating phase-dependent metabolic activity.
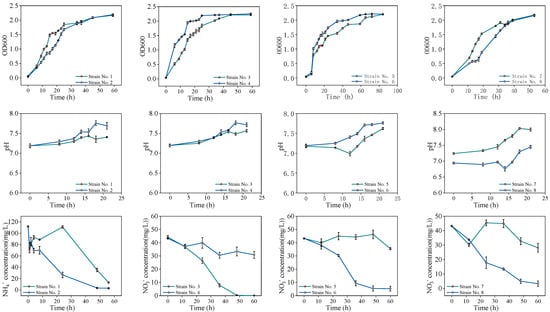
Figure 1.
Growth curves, pH changes, and denitrification performance of different microorganisms.
Ammonia and nitrate degradation assays demonstrated that Strains 3, 6, and 8 achieved nitrate removal efficiencies of 89.7%, 85.4%, and 100% at 60 h, respectively, whereas Strains 4, 5, and 7 exhibited limited degradation due to sluggish growth (low OD600). In the ammonia degradation trials, the ammonia removal rates of aerobic Strains 1 and 2 can reach 97.1% and 89.2% at 60 h, respectively. Concurrently, the pH values progressively increased over 25 h, likely driven by microbial metabolism, while urea hydrolysis, via urease, generated NH3 and CO2, with subsequent NH3 dissociation elevating OH− concentrations. Nitrate assimilation further contributed to pH rise through H+ consumption. pH stabilization under weakly alkaline conditions (≈8.5) suggested equilibrium between microbial activity and buffering capacity. Notably, OD600 peaks (0.6–0.7) correlated positively with nitrogen removal efficiency, underscoring biomass-dependent catalytic activity (Figure 1).
Phylogenetic analysis based on 16S rDNA sequencing classified Strains 2, 3, and 8 (selected for superior nitrogen removal) as Acinetobacter rudis, Ignatzschineria indica, and Staphylococcus epidermidis, respectively (Figure 2). BLAST alignment revealed 98.7%, 98.8%, and 99.9% sequence similarities to S. epidermidis strain P3 (PQ285375.1), A. rudis (NR115988.1), and I. indica (NR116120.1), respectively. A neighbor-joining phylogenetic tree (MEGA X, 1000 bootstrap replicates) was constructed using Poisson-corrected evolutionary distances (units: amino acid substitutions per site). Internal nodes were annotated with the proportion of unambiguous base positions across descendant branches. Taxonomic divergence among the three strains (distinct families/genera) implies functional diversity in nitrogen metabolism, potentially linked to niche-specific metabolic pathways.

Figure 2.
Phylogenetic tree and morphological characteristics of Strains 2, 3, and 8.
3.2. Response Surface Optimization Experiment and Verification of Single Bacteria
Based on the above denitrification and degradation experiments, five dominant strains (Strains 1, 2, 3, 6, and 8) were selected from the eight isolates for response surface optimization experiments (Figure 3, Figure 4, Figure 5 and Figures S1 and S2). Among them, there is a significant synergistic effect between temperature and inoculation amount on the nitrate nitrogen removal rate. The removal rate increases almost to the peak under the condition of weak alkali at 35 °C, and the interaction of three-dimensional surface display parameters is nonlinear. Using nitrate and ammonia nitrogen removal efficiencies as response values, a Box–Behnken design (BBD) model in Design Expert 13.0 software was employed to design experiments for three denitrifying strains and two nitrifying strains, with pH, temperature, and inoculum size as independent variables. The experimental factors and their levels are listed in Table 1. Model predictions and analyses were conducted, and the experimental design and results are summarized in Table 2.
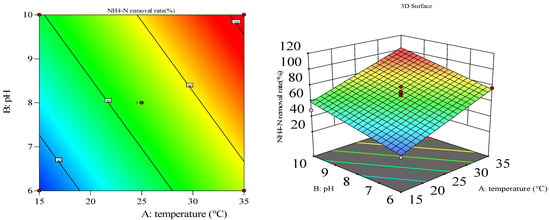
Figure 3.
Three-dimensional response surface and two-dimensional contour map of Strain No. 2.

Figure 4.
Three-dimensional response surface and two-dimensional contour map of Strain No. 3.
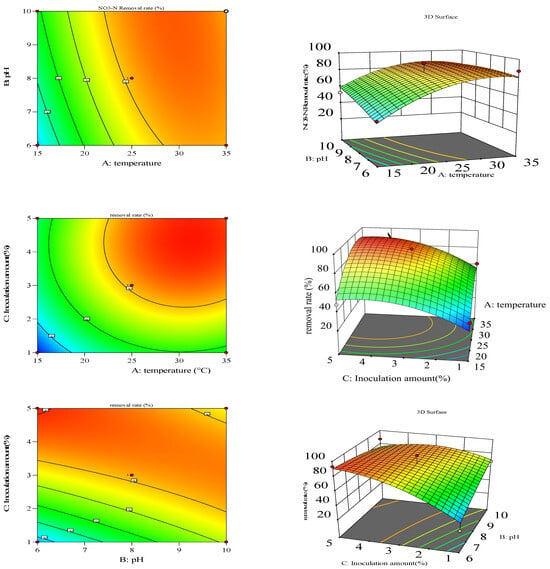
Figure 5.
Three-dimensional response surface and two-dimensional contour map of Strain No. 8.

Table 1.
Factors and levels of response surface method.

Table 2.
Response surface test results.
Using the data from Table 2, Design Expert 13.0 software was applied to process the removal results and construct multiple regression models. Appropriate functional models were used for data fitting, yielding quadratic polynomial regression equations for removal efficiency (Y) as a function of the experimental factors:
ANOVA was conducted on the models, and the results are presented in Tables S2–S6. A p-value of less than 0.01 indicates that the model is extremely significant and highly reliable. The lack-of-fit term is used to evaluate the reliability of the fitted equation, reflecting the difference between the model’s predicted values and the experimental values. A larger mean square value indicates a greater influence of the factor on Y. The correlation coefficient, R2, and the adjusted coefficient of determination, adjusted R2, represent the correlation of the model and the correlation between the model’s predicted values and the experimental values, respectively.
Based on the statistical analysis results shown in Tables S2–S6, all regression models exhibit good fitting performance and statistical significance. All models were verified for their statistical significance through the F-test. Specifically, the models in Table S2 (F = 6.94, p = 0.0091), Table S5 (F = 6.68, p = 0.0102), and Table S6 (F = 4.92, p = 0.0237) reached a significant level (p < 0.05), while those in Table S3 (F = 34.42, p < 0.0001) and Table S4 (F = 27.27, p < 0.0001) reached an extremely significant level (p < 0.001). This indicates that all models can effectively reflect the functional relationship between the process parameters and the response values.
The goodness-of-fit of the models was comprehensively verified through the coefficient of determination (R2) and the lack-of-fit test. The R2 values of each model are 0.8993 (Table S2), 0.8957 (Table S5), 0.9779 (Table S3), 0.8634 (Table S6), and 0.8629 (Table S4), respectively, showing that the equations can explain 86.29–97.79% of the response values. Meanwhile, the lack-of-fit terms of all models are not significant (p > 0.05), indicating that the regression equations can effectively fit the actual experimental data.
The regression analysis indicated that temperature was the most critical factor affecting nitrate removal efficiency (p < 0.05), with its main effect showing statistical significance across all models. Notably, the factor ranking in Table S2 revealed that inoculum size exerted a greater influence than pH (temperature > inoculum size > pH), whereas other models consistently showed temperature > pH > inoculum size. This discrepancy may be attributed to differences in experimental design or sample characteristics. Factor ranking based on mean square values closely aligned with the ANOVA results, verifying the interaction patterns between the variables. Collectively, the statistical indices demonstrate that the response surface models developed in this study possess high predictive accuracy (R2 = 0.8629–0.9779), providing reliable theoretical support for process optimization. Temperature, as the core control parameter, plays a pivotal guiding role in determining optimal intervals to enhance nitrate removal efficiency. However, the synergistic mechanisms between pH and inoculum size require further analysis under specific experimental conditions.
To visually examine the effects of reaction temperature, pH, inoculum size, and their interactions on nitrate and NH3-N removal, three-dimensional response surfaces and two-dimensional contour plots of selected regression models were constructed. Panels a and b illustrate the interactive effects of temperature and pH on nitrate removal by Strain 8 under optimal conditions (Figure 5). With an inoculum size of 3%, increasing temperature from 15 °C to 25 °C and pH from 6 to 8 boosted removal efficiency from 35% to 80%, with temperature showing the most pronounced effect based on the three-dimensional surface. Panels c and d display similar interactions for Strain 3, where removal efficiency rose from 25% to 70% under the same inoculum size, again highlighting temperature as the dominant factor.
Response surface methodology was applied to analyze and optimize first-order interactions significantly impacting ammonia nitrogen removal. The slope of the response surface indicated the magnitude of two-factor interactions, while contour ellipticity reflected interaction significance. Figure S1 shows the temperature–pH interaction response surface for ammonia removal. For Strain 1, the response surface exhibited a rising-then-falling trend with dense contour lines, indicating strong interactive effects between temperature and pH. For Strain 1, both factors exerted promotional effects under experimental conditions, achieving near-100% ammonia removal at pH 10 and 35 °C.
3.3. Response Surface Optimization Experiment and Verification of Composite Bacteria
Taking the removal rate of ammonia nitrogen as the response value, the composite bacteria selected, No. 2, 3, and 8, and pH, temperature, and aeration interval time as three factors, we set the initial concentration of ammonia nitrogen 75 (mg/L) using the Box–Behnken design (Figure 6) mode in the software Design Expert 13.0 to design the experiments, for which the experimental factors and levels are shown in Table 3. The prediction of the model was carried out to analyze the experimental design and results, as well as those of nitrate nitrogen and nitrite nitrogen, which are shown in Table 4. Based on the values of factors and levels in Table 3, parallel tests were conducted for each test group to reduce the operational errors. Based on the results of the removal tests, it was found that nitrate nitrogen and nitrite nitrogen residuals were lower during the ammonia nitrogen removal process, and most of the ammonia nitrogen in the device was removed as nitrogen through the processes of nitrification and denitrification, conducted using the Design Expert 13.0 software. Expert 13.0 software was used to process the data in Table 4 and construct the multiple regression model. At the same time, the appropriate function model was used to realize data fitting, and the quadratic multinomial regression equation between the removal rate (Y) and the test factors was obtained as follows:
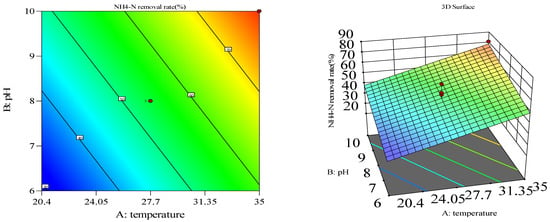
Figure 6.
Three-dimensional response surface and two-dimensional contour map of compound bacteria.

Table 3.
The factors and levels of the complex bacteria response surface method.

Table 4.
Test results of the response surface of the compound bacteria.
The model was subjected to ANOVA, and the results are shown in Table S7, where p < 0.01 indicates that the model is extremely significant and has a high degree of confidence; the misfit term is used to evaluate the reliability of the fitted equation, reflecting the magnitude of the difference between the model predicted values and the experimental values; the larger the mean squared value, the greater the effect of the factor Y; and the correlation coefficient, R2, and the corrected coefficient of determination, adjusted R2, respectively, indicate the model’s correlation and adjusted R2, which indicate the correlation of the model and the correlation between the predicted values of the model and the experimental values, respectivley.
The model’s F = 9.76, p = 0.0100, indicates that it has a high degree of fit, and R2 = 0.8300 indicates that the degree of agreement between the equation and the corresponding response value can reach 83.00%. The misfit term is not significant, and the regression model can predict the results of the experiment more accurately. The analysis of the regression model shows that the influence of the process parameters on the response value is significant; moreover, the influence of temperature on the nitrate nitrogen is the biggest. The effect of temperature on nitrate nitrogen was the most significant. According to the magnitude of the mean square value, it can be concluded that the primary and secondary factors affecting the removal rate are temperature > aeration time > pH. In order to examine more intuitively the effects of the factors of response temperature, pH, aeration time, and the interaction on the removal effect of NH3-N, the three-dimensional response surfaces and the two-dimensional contour plots of some of the regression models were plotted (Figure 3).
The response surface method was used to analyze and optimize the first-level interactions that had a significant effect on the ammonia nitrogen removal effect, in which the tilt of the response surface indicated the degree of influence of the two-factor interactions on the response values and the ellipticity of the contour lines indicated whether the interactions were significant or not. Figure 3 shows the response surface plots of the interactions of temperature and pH. As can be seen from Figure 3, both temperature and pH promoted the degradation of ammonia nitrogen in the range, and the highest nitrate nitrogen removal rate was calculated using Design Expert software for temperature 34.85 °C, pH 9.94, aeration interval of 5h, and composite bacterial good bacteria. Under this condition, the maximum predicted removal rate was 81.82%.
4. Discussion
4.1. Functional Diversity and Phylogenetic Divergence of Denitrifying Consortia
The distinct growth kinetics observed among denitrifying strains (e.g., rapid proliferation in black-odor sediment isolates vs. lag phases in composite-powder-derived strains) highlight niche-specific adaptations linked to their environmental origins [40]. Aerobic consortia exhibited immediate exponential growth, likely reflecting their adaptation to oxygen-rich habitats, while anaerobic sludge-derived strains displayed minimal lag phases, aligning with their metabolic readiness for low-oxygen conditions [41,42]. The phylogenetic divergence of high-efficiency strains (Staphylococcus epidermidis, Acinetobacter rudis, and Ignatzschineria indica) into distinct taxonomic families underscores functional diversity in nitrogen metabolism [43,44,45,46]. For instance, S. epidermidis (Strain 8) and I. indica (Strain 3) achieved 89.72% and 100% nitrate removal, respectively, suggesting genus-specific enzymatic pathways (e.g., urease activity driving pH elevation) [43,44]. The correlation between OD600 peaks (0.65–0.7) and nitrogen removal efficiency further supports biomass-dependent catalytic capacity, with pH stabilization (~8.5) reflecting dynamic equilibrium between microbial metabolism and buffering systems [47]. In the study of screening aerobic denitrifying bacteria and their growth kinetics characteristics, some studies have screened strains from environmental samples and analyzed their growth rates and metabolites [41,42]. In contrast, the strains involved in this study have a more diverse range of sources, including black-odorous sediments, composite powders, etc. The study of strains is not limited to growth rate, but also related to nitrogen removal efficiency and related physiological and biochemical characteristics, such as the relationship between OD600 peak and nitrogen removal efficiency and the dynamic balance of microbial metabolism and buffering system reflected by pH stability.
4.2. Single-Strain Optimization: Temperature as a Dominant Control Parameter
Response surface modeling revealed temperature as the most critical factor influencing nitrogen removal across all strains, with F-test significance (p < 0.0001) and R2 values (0.8629–0.9779) confirming model reliability. Strain-specific optimal conditions diverged markedly, as follows: Strain 8 achieved maximal nitrate removal (77.13%) at 20.4 °C and pH 6, whereas Strain 3 required 35 °C and pH 10 for peak performance (75.8%). Aerobic ammonia-oxidizing Strains 1 and 2 showed extreme pH and temperature optima (pH 9–10, 34–35 °C), achieving >97% ammonia removal, likely due to enhanced urease and nitrification enzyme activity under alkaline, thermophilic conditions [48]. The interaction between pH and temperature (evidenced by elliptical contour plots) further emphasized synergistic effects, as follows: Strain 1’s ammonia removal efficiency surged from 35% to 80% when pH and temperature shifted from acidic/mesophilic (pH 6, 15 °C) to alkaline/thermophilic (pH 8, 25 °C) conditions. These findings align with metabolic theory, where elevated temperatures accelerate enzymatic rates until reaching denaturation thresholds, while alkaline pH facilitates ammonia volatilization and nitrification [49,50]. Another study simulated the coupled dynamics of denitrification, bacteria, transcripts, and enzymes at the river–groundwater interface through computational models [48]. This study is mainly based on experimental observations of the growth of different denitrifying strains and physiological and biochemical indicators during nitrogen removal processes, rather than simulating reaction processes in complex environments through models.
4.3. Synergistic Effects in Composite Consortia: Balancing Aeration and Environmental Parameters
Composite consortia (Strains 2, 3, and 8) exhibited enhanced ammonia removal (81.82% predicted efficiency) under optimized conditions (34.85 °C, pH 9.94, 5 h aeration intervals), outperforming individual strains in stability and adaptability. The reduced nitrite/nitrate accumulation (<10% residual) suggests efficient coupling of nitrification and denitrification pathways, likely mediated by cross-feeding (e.g., Strain 10’s aerobic nitrification products fueling Strain 6’s anaerobic denitrification) [51]. The aeration interval emerged as a novel critical factor (p < 0.01), with prolonged intervals favoring anoxic denitrification phases [52]. The simplified linear model (Y = 53.94 + 15.05A + 9.64B − 9.41C) contrasted with single-strain quadratic models, implying reduced parameter sensitivity in consortia due to functional redundancy. However, temperature retained dominance (MS = 15.05), highlighting its universal role in microbial kinetics. Practical applications should prioritize dynamic aeration control and thermophilic-alkaline regimes to harness these synergies for wastewater treatment [53,54].
5. Conclusions
In this study, six denitrifying and two nitrifying strains were isolated from black-odor sediment, wastewater treatment plants, and active bacterial powder samples. The growth kinetics and nitrogen removal performance of the eight strains were systematically evaluated. Strains 8, 6, and 3 exhibited significant nitrate removal efficiencies of 89.7%, 85.4%, and 100% at 60 h, respectively. For ammonia degradation, Strains 1 and 2 achieved 97.1% and 89.2% at 60 h, respectively. Response surface optimization further enhanced the performance of the selected strains as follows: Strain 8 achieved 77.1% nitrate removal at 24 h under optimal conditions (20.4 °C, pH 6, 5% inoculum), while Strain 3 reached 75.8% nitrate removal (35 °C, pH 10, 5% inoculum). Notably, Strain 2 demonstrated near-complete ammonia removal (99.9%) at 24 h after aeration optimization. Phylogenetic analysis via 16S rDNA sequencing identified Strain 8 as Staphylococcus epidermidis (99.9% similarity to PQ285375.1), Strain 2 as Acinetobacter rudis (98.7% similarity to NR115988.1), and Strain 3 as Ignatzschineria indica (98.830% similarity to NR116120.1). Composite consortia (Strains 2, 3, and 8), optimized at 34.8 °C, pH 9.9, and 5 h aeration intervals, achieved 81.8% ammonia removal with negligible residual nitrate/nitrite, indicating efficient coupling of nitrification and denitrification pathways. These findings highlight the potential of tailored microbial consortia and parameter optimization for sustainable bioremediation of nitrogen-contaminated ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17142101/s1, Figure S1: Three-dimensional response surface and two-dimensional contour map of Strain No. 1; Figure S2: Three-dimensional response surface and two-dimensional contour map of Strain No. 6; Table S1: Basic morphology of bacteria; Table S2: Analysis of Variance (ANOVA) of the quadratic model of strain No. 8; Table S3: Analysis of Variance (ANOVA) of the quadratic model of strain No. 3; Table S4: Analysis of Variance (ANOVA) of the quadratic model of strain No. 2; Table S5: Analysis of Variance (ANOVA) of the quadratic model of strain No. 6; Table S6: Analysis of Variance (ANOVA) of the quadratic model of strain No. 1. Table S7: Analysis of Variance (ANOVA) of the quadratic model of Compound bacteria.
Author Contributions
D.W. and L.C. designed the research and wrote the manuscript. D.W. conceived the experiments. S.S. refined the experimental design and methods. D.W. analyzed the data. H.F., S.S., A.L., D.W., X.W. and L.Z. reviewed and edited the manuscript. H.F. supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Chinese Research Academy of Environmental Sciences Youth Exploration Fund (2025YSKY-47), the National Natural Science Foundation of China (No. 42407511), the key R&D sub-project (2024YFD1600203), and the study on the integrated equipment sewage treatment station for black-odor water bodies (local consulting service project-2023-local scientific research-0730).
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, upon reasonable request.
Conflicts of Interest
Author Shishun Sun was employed by the company Chinese Research Academy of Environmental Sciences Environmental Technology & Engineering Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhang, W.; Zhu, J.X.; Liu, Z.W.; Liu, L. The Eutrophication-related Index of Drinking Water Sources Based on the Oxidation-Reduction Potential. Bioresources 2024, 19, 4941–4959. [Google Scholar] [CrossRef]
- Mycielski, R.; Blaszczyk, M.; Jackowska, A.; Olkowska, H. Denitrification of high concentrations of nitrites and nitrates in synthetic medium with different sources of organic carbon. II. Ethanol. Acta Microbiol. Pol. 1983, 32, 381–388. [Google Scholar] [PubMed]
- Wang, S.H.; Ma, Y.K.; Zhang, X.Y.; Yu, Y.; Zhou, X.H.; Shen, Z.Y. Nitrogen transport and sources in urban stormwater with different rainfall characteristics. Sci. Total Environ. 2022, 837, 155902. [Google Scholar] [CrossRef]
- Guo, J.X.; Wang, L.C.; Yang, L.; Deng, J.C.; Zhao, G.M.; Guo, X.Y. Spatial-temporal characteristics of nitrogen degradation in typical Rivers of Taihu Lake Basin, China. Sci. Total Environ. 2020, 713, 136456. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Das, P.; Mehra, A.; Chattopadhyay, S. A Comprehensive Review on the Biofilm-Mediated Removal of Nitrogen and Chemical Oxygen Demand from Different Wastewater Sources. Clean-Soil Air Water 2024, 52, e202300282. [Google Scholar] [CrossRef]
- Gilbert, P.M. Eutrophication, harmful algae and biodiversity—Challenging paradigms in a world of complex nutrient changes. Mar. Pollut. Bull. 2017, 124, 591–606. [Google Scholar] [CrossRef]
- Sanoja-López, K.A.; Loor-Molina, N.S.; Luque, R. Nitrifying bacteria for the remediation of organic nitrogen-contaminated waters: A review. Biofuels Bioprod. Biorefining-Biofpr 2025, 19, 250–259. [Google Scholar] [CrossRef]
- Chen, X.; Duan, F.; Yu, X.; Xie, Y.Y.; Wang, Z.B.; Ni, S.Q. Uncovering pathway and mechanism of simultaneous thiocyanate detoxicity and nitrate removal through anammox and denitrification. NPJ Clean Water 2024, 7, 109. [Google Scholar] [CrossRef]
- Ning, M.Y.; Li, X.; Lu, Z.D.; Yang, Y.L.; Liu, W.L. Heterotrophic nitrification-aerobic denitrification (HNAD) in marine aquaculture wastewater treatment: Nitrogen removal performance, mechanism and microbial community characteristics. J. Water Process Eng. 2025, 70, 107006. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gao, D.W.; Peng, Y.Z.; Wang, P.; Yang, Q. Nitrification-denitrification via nitrite for nitrogen removal from high nitrogen soybean wastewater with on-line fuzzy control. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2004, 49, 121–127. [Google Scholar] [CrossRef]
- Ju, C.J.; Niyazi, S.; Cao, W.Y.; Wang, Q.; Chen, R.P.; Yu, L. Characteristics and comparisons of the aerobic and anaerobic denitrification of a Klebsiella oxytoca strain: Performance, electron transfer pathway, and mechanism. J. Environ. Manag. 2023, 338, 117787. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lu, D.W.; Qin, B.D.; Liu, Q.L.; Zhao, Y.M.; Liu, H.L.; Ma, J. Highly efficient nitrogen removal of a coldness-resistant and low nutrient needed bacterium, Janthinobacterium sp. M-11. Bioresour. Technol. 2018, 256, 366–373. [Google Scholar] [CrossRef]
- Sander, E.M.; Virdis, B.; Freguia, S. Bioelectrochemical nitrogen removal as a polishing mechanism for domestic wastewater treated effluents. Water Sci. Technol. 2017, 76, 3150–3159. [Google Scholar] [CrossRef] [PubMed]
- Cecconet, D.; Zou, S.Q.; Capodaglio, A.G.; He, Z. Evaluation of energy consumption of treating nitrate-contaminated groundwater by bioelectrochemical systems. Sci. Total Environ. 2018, 636, 881–890. [Google Scholar] [CrossRef]
- Cecconet, D.; Sabba, F.; Anastasi, V.; Bolognesi, S.; Callegari, A.; He, Z.; Capodaglio, A.G. Integrated experimental and modeling evaluation of removal efficiency and energy consumption for an autotrophic denitrifying biocathode. Environ. Sci.-Water Res. Technol. 2022, 8, 1466–1477. [Google Scholar] [CrossRef]
- Munasinghe-Arachchige, S.P.; Abeysiriwardana-Arachchige, I.S.A.; Delanka-Pedige, H.M.K.; Cooke, P.; Nirmalakhandan, N. Nitrogen-fertilizer recovery from urban sewage via gas permeable membrane: Process analysis, modeling, and intensification. Chem. Eng. J. 2021, 411, 128443. [Google Scholar] [CrossRef]
- Liu, Z.J.; Kieffer, J.M.; Kingery, W.L.; Huddleston, D.H.; Hossain, F. Watershed modeling of dissolved oxygen and biochemical oxygen demand using a hydrological simulation Fortran program. J. Environ. Sci. Health Part Toxic Hazard. Subst. Environ. Eng. 2007, 42, 2023–2032. [Google Scholar] [CrossRef]
- Li, J.N.; Feng, Y.J.; Qiu, Y.; Chen, D.H.; Liang, D.D.; Zhou, J.J.; Liu, G.H. Recovery of electron and carbon source from agricultural waste corncob by microbial electrochemical system to enhance wastewater denitrification. Sci. Total Environ. 2023, 878, 162926. [Google Scholar] [CrossRef]
- Pan, Y.; Hua, T.W.; Sun, R.Z.; Fu, Y.Y.; Xiao, Z.C.; Wang, J.; Yu, H.Q. Machine Learning-Assisted Optimization of Mixed Carbon Source Compositions for High-Performance Denitrification. Environ. Sci. Technol. 2024, 58, 12498–12508. [Google Scholar] [CrossRef]
- Ren, G.B.; Zhou, M.H.; Zhang, Q.Z.; Xu, X.; Li, Y.C.; Su, P.; Paidar, M.; Bouzek, K. Cost-efficient improvement of coking wastewater biodegradability by multi-stages flow through peroxi-coagulation under low current load. Water Res. 2019, 154, 336–348. [Google Scholar] [CrossRef]
- Maxwell, B.M.; Birgand, F.; Schipper, L.A.; Barkle, G.; Rivas, A.A.; Helmers, M.J.; Christianson, L.E. High-frequency, in situ sampling of field woodchip bioreactors reveals sources of sampling error and hydraulic inefficiencies. J. Environ. Manag. 2020, 272, 110996. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.L.; Wan, N.; Shi, J.X.; Tang, Y.J.; Hu, H. Exploration of simulation-based optimization and impact factors in short-cut nitrification-denitrification start-up in coal pyrolysis wastewater. J. Water Process Eng. 2024, 63, 105482. [Google Scholar] [CrossRef]
- Yang, S.; Yang, F.L. Nitrogen removal via short-cut simultaneous nitrification and denitrification in an intermittently aerated moving bed membrane bioreactor. J. Hazard. Mater. 2011, 195, 318–323. [Google Scholar] [CrossRef]
- Zhang, C.; Sha, H.; Lv, Z.; Hu, X.M. Enhancement of Pulsed Electric Field on Anammox Process to Reduce the Higher Nitrogen Loading Shock. Waste Biomass Valorization 2023, 14, 2167–2177. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Weng, T.-Y.; Yeh, T.-Y.; Chou, P.-J.; Whang, L.-M. Nitrogen removal strategy for real swine wastewater by combining partial nitrification-denitrification process with anammox. Chemosphere 2024, 364, 143116. [Google Scholar] [CrossRef]
- Fu, H.M.; Jiang, X.W.; Sun, C.P.; Li, S.J.; Weng, X.; Peng, M.W.; Yan, P.; Xu, X.W.; Chen, Y.P.; Shen, Y. Exploring the physical disruptions of anammox granular sludge under propylene glycol stress: Implications for nitrogen removal and long-term stability. J. Water Process Eng. 2025, 71, 107405. [Google Scholar] [CrossRef]
- Wang, X.L.; Han, Q.H.; Yu, H.Y.; Lin, S.S. Enhancement of the reactivation process of long-term starved anammox granular sludge with gravel balls: Microbial succession and metabolic impact. Environ. Res. 2024, 263, 120227. [Google Scholar] [CrossRef]
- Li, B.Y.; Song, Y.X.; Liu, C.H.; He, Y.Q.; Ma, B. Rapid cultivation of anammox bacteria by forming free cells in a membrane bioreactor. Water Environ. Res. 2021, 93, 1640–1650. [Google Scholar] [CrossRef]
- Wang, W.Y.; Wang, R.; Abbas, G.; Wang, G.; Zhao, Z.G.; Deng, L.W.; Wang, L. Aggregation enhances the activity and growth rate of anammox bacteria and its mechanisms. Chemosphere 2022, 291, 132907. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, H.; Lyu, Y.K.; Wang, Y. Nitrogen removal by a metal-resistant bacterium, Pseudomonas putida ZN1, capable of heterotrophic nitrification-aerobic denitrification. J. Chem. Technol. Biotechnol. 2019, 94, 1165–1175. [Google Scholar] [CrossRef]
- Wang, J.L.; Gong, B.Z.; Wang, Y.M.; Wen, Y.H.; Zhou, J.; He, Q. The potential multiple mechanisms and microbial communities in simultaneous nitrification and denitrification process treating high carbon and nitrogen concentration saline wastewater. Bioresour. Technol. 2017, 243, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Martikainen, P.J. Heterotrophic nitrification-An eternal mystery in the nitrogen cycle. Soil Biol. Biochem. 2022, 168, 108611. [Google Scholar] [CrossRef]
- Han, K.; Yeum, Y.; Yun, G.; Kim, Y.W.; Park, C.W.; Kim, Y. Evaluating the efficacy of slow-releasing carbon source tablets for in situ biological heterotrophic denitrification of groundwater. Chemosphere 2022, 304, 135268. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.F.; Feng, Y.L.; Li, H.R.; Jiang, S.W.; Yao, Y.S.; Liu, M.Y.; Wang, J.W. A study of the mechanism of the degradation of dodecylamine in mineral processing wastewater by deep-sea microflora. J. Water Process Eng. 2025, 70, 107081. [Google Scholar] [CrossRef]
- Gupta, R.K.; Poddar, B.J.; Nakhate, S.P.; Chavan, A.R.; Singh, A.K.; Purohit, H.J.; Khardenavis, A.A. Role of heterotrophic nitrifiers and aerobic denitrifiers in simultaneous nitrification and denitrification process: A nonconventional nitrogen removal pathway in wastewater treatment. Lett. Appl. Microbiol. 2022, 74, 159–184. [Google Scholar] [CrossRef]
- Qin, Y.L.; Liang, Z.L.; Ai, G.M.; Liu, W.F.; Tao, Y.; Jiang, C.Y.; Liu, S.J.; Li, D.F. Heterotrophic nitrification by Alcaligenes faecalis links organic and inorganic nitrogen metabolism. Isme J. 2024, 18, wrae174. [Google Scholar] [CrossRef]
- Maharjan, A.K.; Mori, K.; Nishida, K.; Toyama, T. Nitrogen removal from ammonium-contaminated groundwater using dropping nitrification-cotton-based denitrification reactor. Water Supply 2022, 22, 462–473. [Google Scholar] [CrossRef]
- Li, Y.C.; Dong, W.Y.; Hou, Z.L.; Zhao, Z.L.; Xie, J.; Wang, H.J.; Huang, X.; Peng, Y.Z. Intermittent hydroxylamine dosing to strengthen stability of partial nitrification and nitrogen removal efficiency through continuous-flow anaerobic–aerobic-anoxic reactor treating municipal wastewater. Bioresour. Technol. 2024, 406, 130947. [Google Scholar] [CrossRef]
- Xu, W.L.; Chen, J.Y.; Jian, Y.; Pan, Z.C.; Mou, Z.S. Treatment of Sewage Using a Constructed Soil Rapid Infiltration System Combined with Pre-Denitrification. Int. J. Environ. Res. Public Health 2018, 15, 2005. [Google Scholar] [CrossRef]
- Bydalek, F.; Webster, G.; Barden, R.; Weightman, A.J.; Kasprzyk-Hordern, B.; Wenk, J. Microbial community and antimicrobial resistance niche differentiation in a multistage, surface flow constructed wetland. Water Res. 2024, 254, 121408. [Google Scholar] [CrossRef]
- LaPara, T.M.; Konopka, A.; Alleman, J.E. Energy spilling by thermophilic aerobes in potassium-limited continuous culture. Water Res. 2000, 34, 2723–2726. [Google Scholar] [CrossRef]
- Höhne, A.; Müller, B.M.; Schulz, H.; Dara, R.; Posselt, M.; Lewandowski, J.; McCallum, J.L. Fate of trace organic compounds in the hyporheic zone: Influence of microbial metabolism. Water Res. 2022, 224, 119056. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, J.; Tang, Z.; Wan, S.; Hu, J.; Li, B.; Wang, J.; Li, F. Unveiling the nitrogen metabolism mechanism for nitrogen retention in compost via in-situ ammonia recycling strategy. J. Environ. Manag. 2025, 379, 124863. [Google Scholar] [CrossRef] [PubMed]
- Holguin, G.; Bashan, Y. Nitrogen-fixation by Azospirillum brasilense Cd is promoted when co-cultured with a mangrove rhizosphere bacterium (Staphylococcus sp.). Soil Biol. Biochem. 1996, 28, 1651–1660. [Google Scholar] [CrossRef]
- Alitaleshi, F.; Daghbandan, A.; Pendashteh, A. Performance of rice husk biocarrier on ammonia nitrogen removal in the MBBR treating aquaculture wastewater using biological attached growth process: Performance and kinetic study. J. Environ. Chem. Eng. 2024, 12, 111446. [Google Scholar] [CrossRef]
- He, J.; Hong, L.; Song, M.; Zhang, Y.; Zhang, W.; Zhang, L.; Zhou, D.; Chen, Z.; Yu, Y.; Chen, H.; et al. Diverse Acinetobacter species and Plasmid-Driven spread of carbapenem resistance in pharmaceutical settings in China. Environ. Int. 2025, 198, 109373. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Zhong, X.; Jiang, J.; Zhu, F.; Huang, S.; Zhang, Z.; Wu, Y.; Xue, S. Metabolism of Penicillium oxalicum-mediated microbial community reconstructed by nitrogen improves stable aggregates formation in bauxite residue: A field-scale demonstration. J. Clean. Prod. 2025, 493, 144963. [Google Scholar] [CrossRef]
- Chen, M.; Xia, Y.; Qiu, Z.; Zhu, S.; Yin, P.; Zhao, Y.; Luo, X. Enzyme-responsive aptasensor based on the functionalized fluorescent protein chromophore derivative for the detection of alkaline phosphatase activity. Sens. Actuators B Chem. 2024, 417, 136178. [Google Scholar] [CrossRef]
- Ma, Q.; Yuan, Y.; Wu, E.; Wang, H.; Dang, K.; Feng, Y.; Ivanistau, A.; Feng, B. Endogenous bioactive gibberellin/abscisic acids and enzyme activity synergistically promote the phytoremediation of alkaline soil by broomcorn millet (Panicum miliaceum L.). J. Environ. Manag. 2022, 305, 114362. [Google Scholar] [CrossRef]
- Wan, W.; Wang, Y.; Tan, J.; Qin, Y.; Zuo, W.; Wu, H.; He, H.; He, D. Alkaline phosphatase-harboring bacterial community and multiple enzyme activity contribute to phosphorus transformation during vegetable waste and chicken manure composting. Bioresour. Technol. 2020, 297, 122406. [Google Scholar] [CrossRef]
- Yang, L.; Abudu, A.; Zhu, K.; Han, T.; Duan, C.; Chen, Y.; Li, X.; Shi, G.; Zhu, C.; Li, G.; et al. Acute alkalinity stress induces functional damage and alters immune metabolic pathways in the gill tissue of spotted scat (Scatophagus argus). Aquaculture 2025, 599, 742186. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Zhou, C.; Huang, X.; Chen, Y.; Wang, S.; Liu, G. Enhanced nitrogen removal via partial nitrification/denitrification coupled Anammox using three stage anoxic/oxic biofilm process with intermittent aeration. Water Res. 2024, 255, 121491. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.-N.; Yin, Y.; Guo, Z.; Wang, Q.; Xu, Y.; Zhang, F.; Xiao, Y.; Liu, C. Partial nitrification-denitrification and enrichment of paracoccus induced by iron-chitosan beads addition in an intermittently-aerated activated sludge system. J. Environ. Manag. 2024, 353, 120189. [Google Scholar] [CrossRef]
- Leite, W.; Magnus, B.S.; Guimarães, L.B.; Gottardo, M.; Belli Filho, P. Feasibility of thermophilic anaerobic processes for treating waste activated sludge under low HRT and intermittent mixing. J. Environ. Manag. 2017, 201, 335–344. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).