A Review of Harmful Algal Blooms: Causes, Effects, Monitoring, and Prevention Methods
Abstract
1. Introduction
2. Methodology
3. HAB Causes
3.1. Environmental Conditions
3.1.1. Light
3.1.2. Temperature
3.1.3. Hydrodynamic Factors
3.1.4. Climate Change
3.1.5. Nutrient Enrichment Caused by Agricultural Practices
4. Impacts of HABs
4.1. Ecological
4.2. Economic
4.3. Public: Toxins Produced by HABs and Their Effects on Human Health
| Toxin | Health Effects in Humans | References |
|---|---|---|
| Anatoxin-a group | Tingling, burning, numbness, respiratory paralysis, death, drowsiness, speech disturbances, excessive salivation, and muscle pain | [17,94,95] |
| Brevetoxin | Neurotoxic Shellfish Poisoning, asthma-like symptoms, coughing, mild ciguatera or paralytic shellfish poisoning, wheezing, shortness of breath, and chest tightness | [17,93,94] |
| Ciguatoxin | Diarrhea, nausea and vomiting, sensation in the mouth, aching teeth, abdominal cramps, muscular aches and pain, itching, dizziness, difficulty concentrating or hallucinations, sweating, numbness and tingling of the mouth, toes, or fingers, fatigue, reversal of temperature, joint pain, and bradycardia | [94,96,97,98] |
| Cylindrospermopsin | Fever, headache, induced DNA strand breaks, vomiting, bloody diarrhea, inhibits eukaryotic protein synthesis, hepatocellular damage, oxidative stress, lung hemorrhage, inflammation, apoptosis, hepatic necrosis, embryo toxicity, micronucleus formation, and tubular necrosis | [94,99,100] |
| Domoic Acid | Nausea, vomiting, abdominal cramps, respiratory secretions, cardiac arrhythmias, diarrhea, dizziness, profuse respiratory difficulty, confusion and disorientation, headache, seizures, hallucinations, possibly coma, short-term memory loss, and death | [94,101] |
| Microcystin-LR | Vomiting and nausea, abdominal pain, liver cancer, hepatomegaly, intrahepatic bleeding, liver necrosis, impairs kidney function, alters hormone levels, reduces sperm quality, induces embryotoxicity, synaptic damage, disrupts immune cell function, headache, sore throat, dry cough, intrahepatic bleeding, liver necrosis, diarrhea, blistering around the mouth, liver fibrosis, cirrhosis, impairs kidney function, pneumonia, and liver cancer | [94,102,103,104] |
| Okadaic Acid | Diarrhea, nausea and vomiting, inhibits protein phosphatases and disrupts cellular regulation, abdominal cramps, induces DNA damage and cell death, chills, headache, impairs immune system function, fever, affects neuronal cells, and carcinogenesis | [96,105,106] |
| Saxitoxin | Tingling, numbness, and burning of the mouth and lips, ataxia, muscle weakness, loss of coordination, irregular heartbeat, giddiness, drowsiness, difficulty breathing, respiratory paralysis, fever, rash, diarrhea, respiratory arrest, staggering, abdominal pain, neurological symptoms, headache, nausea, vomiting, and dizziness | [96,98,107] |
5. Monitoring and Detecting HABs
6. Mitigation Strategies for HABs
6.1. Policies
6.2. Awareness/Knowledge
6.3. Practices
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- NOAA Harmful Algae Blooms (HABs). National Environmental Satellite, Data, and Information Service. Available online: https://www.nesdis.noaa.gov/harmful-algae-blooms-habs (accessed on 24 June 2025).
- Chattopadhyay, J.; Sarkar, R.R.; Pal, S. Mathematical Modelling of Harmful Algal Blooms Supported by Experimental Findings. Ecol. Complex. 2004, 1, 225–235. [Google Scholar] [CrossRef]
- Capuzzo, E.; Stephens, D.; Silva, T.; Barry, J.; Forster, R.M. Decrease in Water Clarity of the Southern and Central North Sea during the 20th Century. Glob. Change Biol. 2015, 21, 2206–2214. [Google Scholar] [CrossRef]
- Goes, J.I.; Gomes, H.D.R.; Al-Hashimi, K.; Buranapratheprat, A. Ecological Drivers of Green Noctiluca Blooms in Two Monsoonal-Driven Ecosystems. In Global Ecology and Oceanography of Harmful Algal Blooms; Glibert, P.M., Berdalet, E., Burford, M.A., Pitcher, G.C., Zhou, M., Eds.; Springer International Publishing: Cham, Germany, 2018; pp. 327–336. ISBN 978-3-319-70069-4. [Google Scholar]
- Moy, N.J.; Dodson, J.; Tassone, S.J.; Bukaveckas, P.A.; Bulluck, L.P. Biotransport of Algal Toxins to Riparian Food Webs. Environ. Sci. Technol. 2016, 50, 10007–10014. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Ruan, R.; Addy, M.; Mack, S.; Chen, P.; Zhou, Y. Life Cycle Assessment and Nutrient Analysis of Various Processing Pathways in Algal Biofuel Production. Bioresour. Technol. 2017, 230, 33–42. [Google Scholar] [CrossRef]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L.; et al. Marine Harmful Algal Blooms (HABs) in the United States: History, Current Status and Future Trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef]
- Leibach, J. Ten Fun Facts About Algae|Audubon. Available online: https://www.audubon.org/magazine/ten-fun-facts-about-algae (accessed on 12 May 2025).
- Lewin, R.; Andersen, R. Algae—Photosynthesis, Diversity, Ecology. Available online: https://www.britannica.com/science/algae/Physical-and-ecological-features-of-algae (accessed on 10 February 2025).
- Parameswarappa Jayalakshmamma, M.; Na Nagara, V.; Borgaonkar, A.; Sarkar, D.; Sadik, O.; Boufadel, M. Characterizing Microplastics in Urban Runoff: A Multi-Land Use Assessment with a Focus on 1–125 Μm Size Particles. Sci. Total Environ. 2023, 904, 166685. [Google Scholar] [CrossRef] [PubMed]
- Aure, J.; Rey, F. Oceanographic Conditions in the Sandsfjord System, Western Norway, after a Bloom of the Toxic Prymnesiophyte Prymnesium Parvum Carter in August 1990. Sarsia 1992, 76, 247–254. [Google Scholar] [CrossRef]
- Rey, F. Oppblomstringen av Chrysochromulina leadbeateri i Vestfjorden, mai-juni 1991: Rapport fra et faglig arbeidsseminar. 122 S. 1991. Available online: https://core.ac.uk/download/pdf/225955132.pdf (accessed on 24 May 2025).
- Landsberg, J.H. The Effects of Harmful Algal Blooms on Aquatic Organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Shumway, S. A Review of the Effects of Algal Blooms on Shellfish and Aquaculture. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1749-7345.1990.tb00529.x (accessed on 5 February 2025).
- CDC Types of Harmful Algal Blooms. Available online: https://www.cdc.gov/harmful-algal-blooms/about/types-of-harmful-algal-blooms.html (accessed on 5 December 2024).
- Yannarell, A.C.; Kent, A.D. Bacteria, Distribution and Community Structure. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Academic Press: Oxford, UK, 2009; pp. 201–210. ISBN 978-0-12-370626-3. [Google Scholar]
- Singh, V.K.; Singh, S.K.; Singh, P.K.; Verma, H.; Pandey, K.D.; Singh, P.K.; Kumar, A. Chapter 10—Impact of Pesticides Applications on the Growth and Function of Cyanobacteria. In Advances in Cyanobacterial Biology; Singh, P.K., Kumar, A., Singh, V.K., Shrivastava, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 151–161. ISBN 978-0-12-819311-2. [Google Scholar]
- CDC Harmful Algal Blooms and Your Health. Available online: https://www.cdc.gov/harmful-algal-blooms/about/index.html (accessed on 5 December 2024).
- Kibuye, F.A.; Almuhtaram, H.; Zamyadi, A.; Gaget, V.; Owen, C.; Hofmann, R.; Wert, E.C. Utility Practices and Perspectives on Monitoring and Source Control of Cyanobacterial Blooms. AWWA Water Sci. 2021, 3, e1264. [Google Scholar] [CrossRef]
- Wang, D.-Z.; Zhang, H.; Zhang, Y.; Zhang, S.-F. Marine Dinoflagellate Proteomics: Current Status and Future Perspectives. J. Proteomics 2014, 105, 121–132. [Google Scholar] [CrossRef]
- Houck, M.M.; Crispino, F.; McAdam, T. Chapter 6.4—Other Types of Evidence. In The Science of Crime Scenes; Houck, M.M., Crispino, F., McAdam, T., Eds.; Academic Press: San Diego, CA, USA, 2012; ISBN 978-0-12-386464-2. [Google Scholar]
- Assmy, P.; Smetacek, V.; Montresor, M.; Ferrante, M.I. Algal Blooms☆. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Oxford, UK, 2019; pp. 61–76. ISBN 978-0-12-811737-8. [Google Scholar]
- Research Gaps Identified to Improve Future Harmful Algal Bloom Forecasts. Available online: https://coastalscience.noaa.gov/news/research-gaps-identified-improve-future-harmful-algal-bloom-forecasts/ (accessed on 5 February 2025).
- Muller-Feuga, A.; Le Guedes, R.; Herve, A.; Durand, P. Comparison of Artificial Light Photobioreactors and Other Production Systems Using Porphyridium Cruentum. J. Appl. Phycol. 1998, 10, 83–90. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of Temperature and Light on the Growth of Algae Species: A Review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Rao, Q.; Su, H.; Ruan, L.; Deng, X.; Wang, L.; Rao, X.; Liu, J.; Xia, W.; Xu, P.; Shen, H.; et al. Stoichiometric and Physiological Mechanisms That Link Hub Traits of Submerged Macrophytes with Ecosystem Structure and Functioning. Water Res. 2021, 202, 117392. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, J.; Huo, Y.; Zhou, L.; Wu, Q.; Chen, L.; Yu, K.; He, P. Adaptability of Free-Floating Green Tide Algae in the Yellow Sea to Variable Temperature and Light Intensity. Mar. Pollut. Bull. 2015, 101, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Song, X.; Jiang, M.; Zhao, X.; Cao, X. The Inhibitory Effects of Simulated Light Sources on the Activity of Algae Cannot Be Ignored in Photocatalytic Inhibition. Chemosphere 2022, 309, 136611. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M. Exploring Temperature and Precipitation Impacts on Harmful Algal Blooms across Continental U.S. Lakes. Limnol. Oceanogr. 2020, 65, 992–1009. [Google Scholar] [CrossRef]
- Anneville, O.; Gammeter, S.; Straile, D. Phosphorus Decrease and Climate Variability: Mediators of Synchrony in Phytoplankton Changes among European Peri-Alpine Lakes. Freshw. Biol. 2005, 50, 1731–1746. [Google Scholar] [CrossRef]
- Peng, X.; Chen, M.; Chen, S.; Dasgupta, S.; Xu, H.; Ta, K.; Du, M.; Li, J.; Guo, Z.; Bai, S. Microplastics Contaminate the Deepest Part of the World’s Ocean. Geochem. Perspect. Lett. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Q.; Xi, B.; Wang, X.; Li, W.; Gao, G.; Huo, S.; Xia, X.; Jiang, T.; Ji, D.; et al. Impacts of Hydrodynamic Disturbance on Sediment Resuspension, Phosphorus and Phosphatase Release, and Cyanobacterial Growth in Lake Tai. Environ. Earth Sci. 2015, 74, 3945–3954. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H.; Zhu, Y.; Xiao, Y.; Chen, L. Effect of Flow Velocity on Phytoplankton Biomass and Composition in a Freshwater Lake. Sci. Total Environ. 2013, 447, 64–71. [Google Scholar] [CrossRef]
- Matson, P.G.; Boyer, G.L.; Bridgeman, T.B.; Bullerjahn, G.S.; Kane, D.D.; McKay, R.M.L.; McKindles, K.M.; Raymond, H.A.; Snyder, B.K.; Stumpf, R.P.; et al. Physical Drivers Facilitating a Toxigenic Cyanobacterial Bloom in a Major Great Lakes Tributary. Limnol. Oceanogr. 2020, 65, 2866–2882. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhang, H.; Lei, P.; Tang, W.; Yin, W.; Li, J.; Zhong, H.; Li, K. Algal Blooms in the Middle and Lower Han River: Characteristics, Early Warning and Prevention. Sci. Total Environ. 2020, 706, 135293. [CrossRef]
- Zhu, C.; Chi, Z.; Bi, C.; Zhao, Y.; Cai, H. Hydrodynamic Performance of Floating Photobioreactors Driven by Wave Energy. Biotechnol. Biofuels 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- San, L.; Long, T.; Liu, C.C.K. Algal Bioproductivity in Turbulentwater: An Experimental Study. Water 2017, 9, 304. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, T.; Liang, D.; Chen, J.M. Experimental Study of Water and Dissolved Pollutant Runoffs on Impervious Surfaces. J. Hydrodyn. 2016, 28, 162–165. [Google Scholar] [CrossRef]
- Yang, G.; Tang, X.; Wilhelm, S.W.; Pan, W.; Rui, Z.; Xu, L.; Zhong, C.; Hu, X. Intermittent Disturbance Benefits Colony Size, Biomass and Dominance of Microcystis in Lake Taihu under Field Simulation Condition. Harmful Algae 2020, 99, 101909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiao, Y.; Li, Z.; Wang, S.; Guo, J.; Lu, L. Turbulence Exerts Nutrients Uptake and Assimilation of Bloom-Forming Dolichospermum through Modulating Morphological Traits: Field and Chemostat Culture Studies. Sci. Total Environ. 2019, 671, 329–338. [Google Scholar] [CrossRef]
- Zhao, G.; Gao, X.; Zhang, C.; Sang, G. The Effects of Turbulence on Phytoplankton and Implications for Energy Transfer with an Integrated Water Quality-Ecosystem Model in a Shallow Lake. J. Environ. Manag. 2020, 256, 109954. [Google Scholar] [CrossRef]
- Kang, L.; He, Y.; Dai, L.; He, Q.; Ai, H.; Yang, G.; Liu, M.; Jiang, W.; Li, H. Interactions between Suspended Particulate Matter and Algal Cells Contributed to the Reconstruction of Phytoplankton Communities in Turbulent Waters. Water Res. 2019, 149, 251–262. [Google Scholar] [CrossRef]
- Liu, P.; Qian, L.; Wang, H.; Zhan, X.; Lu, K.; Gu, C.; Gao, S. New Insights into the Aging Behavior of Microplastics Accelerated by Advanced Oxidation Processes. Environ. Sci. Technol. 2019, 53, 3579–3588. [Google Scholar] [CrossRef]
- Wu, X.; Noss, C.; Liu, L.; Lorke, A. Effects of Small-Scale Turbulence at the Air-Water Interface on Microcystis Surface Scum Formation. Water Res. 2019, 167, 115091. [Google Scholar] [CrossRef]
- Marrasé, C.; Costello, J.H.; Granata, T.; Strickler, J.R. Grazing in a Turbulent Environment: Energy Dissipation, Encounter Rates, and Efficacy of Feeding Currents in Centropages Hamatus. Proc. Natl. Acad. Sci. USA 1990, 87, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shen, L.; Zhang, L.; Li, J.; Chen, M. Study of a Hydrodynamic Threshold System for Controlling Dinoflagellate Blooms in Reservoirs. Environ. Pollut. 2021, 278, 116822. [Google Scholar] [CrossRef] [PubMed]
- Burford, M.A.; Johnson, S.A.; Cook, A.J.; Packer, T.V.; Taylor, B.M.; Townsley, E.R. Correlations between Watershed and Reservoir Characteristics, and Algal Blooms in Subtropical Reservoirs. Water Res. 2007, 41, 4105–4114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Mu, Z.; Wang, H.; Zhao, F.; Li, Y.; Lin, L. Water Residence Time in a Typical Tributary Bay of the Three Gorges Reservoir. Water 2019, 11, 1585. [Google Scholar] [CrossRef]
- Chuo, M.; Ma, J.; Liu, D.; Yang, Z. Effects of the Impounding Process during the Flood Season on Algal Blooms in Xiangxi Bay in the Three Gorges Reservoir, China. Ecol. Model. 2019, 392, 236–249. [Google Scholar] [CrossRef]
- Gao, X.; Kong, B.; Vigil, R.D. Multiphysics Simulation of Algal Growth in an Airlift Photobioreactor: Effects of Fluid Mixing and Shear Stress. Bioresour. Technol. 2018, 251, 75–83. [Google Scholar] [CrossRef]
- Soares, M.C.S.; Marinho, M.M.; Huszar, V.L.M.; Branco, C.W.C.; Azevedo, S.M.F.O. The Effects of Water Retention Time and Watershed Features on the Limnology of Two Tropical Reservoirs in Brazil. Lakes Reserv. Sci. Policy Manag. Sustain. Use 2008, 13, 257–269. [Google Scholar] [CrossRef]
- Song, Y. Hydrodynamic Impacts on Algal Blooms in Reservoirs and Bloom Mitigation Using Reservoir Operation Strategies: A Review. J. Hydrol. 2023, 620, 129375. [Google Scholar] [CrossRef]
- Amorim, C.A.; Moura, A.D.N. Ecological Impacts of Freshwater Algal Blooms on Water Quality, Plankton Biodiversity, Structure, and Ecosystem Functioning. Sci. Total Environ. 2021, 758, 1585. [Google Scholar] [CrossRef]
- Raine, R.; Berdalet, E.; Yamazaki, H.; Jenkinson, I.; Reguera, B. Key Questions and Recent Research Advances on Harmful Algal Blooms in Stratified Systems. In Global Ecology and Oceanography of Harmful Algal Blooms; Glibert, P.M., Berdalet, E., Burford, M.A., Pitcher, G.C., Zhou, M., Eds.; Springer International Publishing: Cham, UK, 2018; pp. 165–186. ISBN 978-3-319-70069-4. [Google Scholar]
- Azadi, F.; Ashofteh, P.-S.; Chu, X. Evaluation of the Effects of Climate Change on Thermal Stratification of Reservoirs. Sustain. Cities Soc. 2021, 66, 102531. [Google Scholar] [CrossRef]
- Ishikawa, M.; Gurski, L.; Bleninger, T.; Rohr, H.; Wolf, N.; Lorke, A. Hydrodynamic Drivers of Nutrient and Phytoplankton Dynamics in a Subtropical Reservoir. Water 2022, 14, 1544. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, P.; Liu, D.; Ma, J.; Ji, D.; Cui, Y. Hydrodynamic Mechanisms Underlying Periodic Algal Blooms in the Tributary Bay of a Subtropical Reservoir. Ecol. Eng. 2018, 120, 6–13. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Elmore, S.; Van Gerven, T.; Stankiewicz, A. Hydrodynamic Evaluations in High Rate Algae Pond (HRAP) Design. Chem. Eng. J. 2013, 217, 231–239. [Google Scholar] [CrossRef]
- Sarat Chandra, T.; Aditi, S.; Maneesh Kumar, M.; Mukherji, S.; Modak, J.; Chauhan, V.S.; Sarada, R.; Mudliar, S.N. Growth and Biochemical Characteristics of an Indigenous Freshwater Microalga, Scenedesmus Obtusus, Cultivated in an Airlift Photobioreactor: Effect of Reactor Hydrodynamics, Light Intensity, and Photoperiod. Bioprocess Biosyst. Eng. 2017, 40, 1057–1068. [Google Scholar] [CrossRef]
- US EPA. Climate Change and Freshwater Harmful Algal Blooms. Available online: https://www.epa.gov/habs/climate-change-and-freshwater-harmful-algal-blooms (accessed on 12 May 2025).
- Gobler, C.J. Climate Change and Harmful Algal Blooms: Insights and Perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Reimer, J.E.; Maas, C.M.; Galella, J.G.; Utz, R.M.; Duan, S.; Kryger, J.R.; Yaculak, A.M.; et al. Freshwater Salinization Syndrome: From Emerging Global Problem to Managing Risks. Biogeochemistry 2021, 154, 255–292. [Google Scholar] [CrossRef]
- Qiu, Y.; Ma, Z.; Liu, X.; Zheng, R.; Xiao, Y.; Wang, M. The Detrimental Effect of High Salinity on the Growth and Microcystins Contamination of Microcystis Aeruginosa. Water 2022, 14, 2871. [Google Scholar] [CrossRef]
- Pan, Y.; Amenorfenyo, D.K.; Dong, M.; Zhang, N.; Huang, X.; Li, C.; Li, F. Effects of Salinity on the Growth, Physiological and Biochemical Components of Microalga Euchlorocystis Marina. Front. Mar. Sci. 2024, 11, 1402071. [Google Scholar] [CrossRef]
- Yao, C.-H.; Ai, J.-N.; Cao, X.-P.; Xue, S. Salinity Manipulation as an Effective Method for Enhanced Starch Production in the Marine Microalga Tetraselmis subcordiformis. Bioresour. Technol. 2013, 146, 663–671. [Google Scholar] [CrossRef]
- Lan, J.; Liu, P.; Hu, X.; Zhu, S. Harmful Algal Blooms in Eutrophic Marine Environments: Causes, Monitoring, and Treatment. Water 2024, 16, 2525. [Google Scholar] [CrossRef]
- Le, K.T.N.; Maldonado, J.F.G.; Nguyen, T.-L.; Goitom, E.; Trigui, H.; Ndiaye, N.A.; Terrat, Y.; Shapiro, B.J.; Husk, B.; Zamyadi, A.; et al. The Short-Term Effect of Nitrogen on Freshwater Cyanobacteria and Cyanotoxins. Front. Water 2024, 6, 1432183. [Google Scholar] [CrossRef]
- Kudela, R.M.; Seeyave, S.; Cochlan, W.P. The Role of Nutrients in Regulation and Promotion of Harmful Algal Blooms in Upwelling Systems. Prog. Oceanogr. 2010, 85, 122–135. [Google Scholar] [CrossRef]
- Oduor, N.A.; Munga, C.N.; Ong’anda, H.O.; Botwe, P.K.; Moosdorf, N. Nutrients and Harmful Algal Blooms in Kenya’s Coastal and Marine Waters: A Review. Ocean Coast. Manag. 2023, 233, 106454. [Google Scholar] [CrossRef]
- Wang, J.; Dong, J.; Tang, M.; Yao, J.; Li, X.; Kong, D.; Zhao, K. Identification and Detection of Microplastic Particles in Marine Environment by Using Improved Faster R–CNN Model. J. Environ. Manag. 2023, 345, 118802. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Onodera, S.-I.; Saito, M.; Shimizu, Y. Long-Term Variations in Water Balance by Increase in Percent Imperviousness of Urban Regions. J. Hydrol. 2021, 602, 126767. [Google Scholar] [CrossRef]
- Wells, M.L.; Karlson, B. Harmful Algal Blooms in a Changing Ocean. In Global Ecology and Oceanography of Harmful Algal Blooms; Glibert, P.M., Berdalet, E., Burford, M.A., Pitcher, G.C., Zhou, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 77–90. ISBN 978-3-319-70069-4. [Google Scholar]
- Ragueneau, O.; Conley, D.J.; Leynaert, A.; Longphuirt, S.N.; Slomp, C.P. Role of Diatoms in Silica Cycling and Coastal Marine Food Webs. SCOPE 2006, 66, 163–195. [Google Scholar]
- Shetye, S.; Pratihary, A.; Shenoy, D.; Kurian, S.; Gauns, M.; Uskaikar, H.; Naik, B.; Nandakumar, K.; Borker, S. Rice Husk as a Potential Source of Silicate to Oceanic Phytoplankton. Sci. Total Environ. 2023, 879, 162941. [Google Scholar] [CrossRef]
- Davidson, K.; Gowen, R.J.; Harrison, P.J.; Fleming, L.E.; Hoagland, P.; Moschonas, G. Anthropogenic Nutrients and Harmful Algae in Coastal Waters. J. Environ. Manag. 2014, 146, 206–216. [Google Scholar] [CrossRef]
- Heffer, P.; Prud’homme, M. Global Nitrogen Fertiliser Demand and Supply: Trend, Current Level and Outlook. In Proceedings of the International Nitrogen Initiative Conference, Melbourne, Australia, 4 December 2016. [Google Scholar]
- Paerl, H.W. Coastal Eutrophication and Harmful Algal Blooms: Importance of Atmospheric Deposition and Groundwater as “New” Nitrogen and Other Nutrient Sources. Limnol. Oceanogr. 2003, 42, 1154–1165. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G.; Kudela, R. Mitigating the Expansion of Harmful Algal Blooms Across the Freshwater-to-Marine Continuum. Environ. Sci. Technol. 2018, 52, 5519–5529. [Google Scholar] [CrossRef] [PubMed]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, Eutrophication and Harmful Algal Blooms along the Freshwater to Marine Continuum. WIREs Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- FAO Inorganic Fertilizers 1961–2019. Available online: https://www.fao.org/statistics/highlights-archive/highlights-detail/New-FAOSTAT-data-release-Inorganic-fertilizers-(1961-2019)/en (accessed on 5 March 2025).
- Parameswarappa Jayalakshmamma, M.; Na Nagara, V.; Borgaonkar, A.; Sarkar, D.; Obropta, C.; Boufadel, M. Temporal and Spatial Distribution of Microplastics in Green Infrastructures: Rain Gardens. Chemosphere 2024, 362, 142543. [Google Scholar] [CrossRef] [PubMed]
- Sprouse, L.; Kryston, A.; Lebu, S.; Muoghalu, C.; Woods, C.; Manga, M. Septic Systems in North Carolina: A Neglected Half of the State? PLoS Water 2024, 3, e0000304. Available online: https://journals.plos.org/water/article?id=10.1371/journal.pwat.0000304 (accessed on 12 May 2025). [CrossRef]
- Brewton, R.A.; Kreiger, L.B.; Tyre, K.N.; Baladi, D.; Wilking, L.E.; Herren, L.W.; Lapointe, B.E. Septic System–Groundwater–Surface Water Couplings in Waterfront Communities Contribute to Harmful Algal Blooms in Southwest Florida. Sci. Total Environ. 2022, 837, 155319. [Google Scholar] [CrossRef]
- Hitting Us Where It Hurts: The Untold Story of Harmful Algal Blooms. Available online: https://www.fisheries.noaa.gov/west-coast/science-data/hitting-us-where-it-hurts-untold-story-harmful-algal-blooms (accessed on 5 March 2025).
- Alvarez, S.; Brown, C.E.; Garcia Diaz, M.; O’Leary, H.; Solís, D. Non-Linear Impacts of Harmful Algae Blooms on the Coastal Tourism Economy. J. Environ. Manag. 2024, 351, 119811. [Google Scholar] [CrossRef]
- NCCOS Harmful Algal Blooms: NOAA State of the Science Fact Sheet. Available online: https://coastalscience.noaa.gov/news/hab-noaa-fact-sheet/ (accessed on 3 June 2025).
- Neese, A.W. Climate Change Could Cost Ohio Billions. Available online: https://www.axios.com/local/columbus/2022/07/26/climate-change-could-cost-ohio-billions (accessed on 3 June 2025).
- Carias, J.; Vásquez-Lavín, F.; Barrientos, M.; Ponce Oliva, R.D.; Gelcich, S. Economic Valuation of Harmful Algal Blooms (HAB): Methodological Challenges, Policy Implications, and an Empirical Application. J. Environ. Manag. 2024, 365, 121566. [Google Scholar] [CrossRef]
- Milutinović, A.; Sedmak, B.; Horvat-Znidarsic, I.; Suput, D. Renal Injuries Induced by Chronic Intoxication with Microcystins. Cell. Mol. Biol. Lett. 2002, 7, 139–141. [Google Scholar]
- Botha, N.; van de Venter, M.; Downing, T.G.; Shephard, E.G.; Gehringer, M.M. The Effect of Intraperitoneally Administered Microcystin-LR on the Gastrointestinal Tract of Balb/c Mice. Toxicon 2004, 43, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: Oxfordshire, UK, 2021; ISBN 978-0-367-53332-8. [Google Scholar]
- Lun, Z.; Hai, Y.; Kun, C. Relationship Between Microcystin in Drinking Water and Colorectal Cancer. Biomed. Environ. Sci. 2002, 15, 166–171. [Google Scholar]
- Lad, A.; Breidenbach, J.D.; Su, R.C.; Murray, J.; Kuang, R.; Mascarenhas, A.; Najjar, J.; Patel, S.; Hegde, P.; Youssef, M.; et al. As We Drink and Breathe: Adverse Health Effects of Microcystins and Other Harmful Algal Bloom Toxins in the Liver, Gut, Lungs and Beyond. Life 2022, 12, 418. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.D.; Colvara, W.A.; Rantin, F.T.; Kalinin, A.L. Microcystin-LR: How It Affects the Cardio-Respiratory Responses to Hypoxia in Nile Tilapia, Oreochromis niloticus. Chemosphere 2011, 84, 154–159. [Google Scholar] [CrossRef]
- Qiu, T.; Xie, P.; Liu, Y.; Li, G.; Xiong, Q.; Hao, L.; Li, H. The Profound Effects of Microcystin on Cardiac Antioxidant Enzymes, Mitochondrial Function and Cardiac Toxicity in Rat. Toxicology 2009, 257, 86–94. [Google Scholar] [CrossRef]
- US EPA. What Are the Effects of HABs. Available online: https://www.epa.gov/habs/what-are-effects-habs (accessed on 5 March 2025).
- Washington State Department of Health Provisional Health Advisory Level for Anatoxin-a and Saxitoxin in Drinking Water. 2024. Available online: https://doh.wa.gov/sites/default/files/2025-01/334-552.pdf (accessed on 5 March 2025).
- Florida Health Charts Saxitoxin Poisoning (Paralytic Shellfish Poisoning). Available online: https://www.flhealthcharts.gov/ChartsDashboards/rdPage.aspx?rdReport=NonVitalIndNoGrpCounts.DataViewer&cid=8615 (accessed on 3 June 2025).
- Kimura, A. Ciguatera Fish Poisoning Fact Sheet. Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/CiguateraFishPoisoningFactSheet.pdf (accessed on 5 March 2025).
- NYC Health Cigualtera Fish Poisoning—NYC Health. Available online: https://www.nyc.gov/site/doh/health/health-topics/cigualtera-fish-poisoning.page (accessed on 3 June 2025).
- Traylor, J.; Murray, B.P.; Singhal, M. Ciguatera Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Heudorf, U.; Neitzert, V.; Spark, J. Particulate Matter and Carbon Dioxide in Classrooms—The Impact of Cleaning and Ventilation. Int. J. Hyg. Environ. Health 2009, 212, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.M.; Bedard, K.; Breining, T.; Cribb, A.E. Disruption of the Endoplasmic Reticulum by Cytotoxins in LLC-PK1 Cells. Toxicol. Lett. 2005, 159, 154–163. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Ouyang, Z.; Su, Z.; Wang, D.; Yu, X. Neuroprotective Effects of Adenosine Isolated from Cordyceps Cicadae against Oxidative and ER Stress Damages Induced by Glutamate in PC12 Cells. Environ. Toxicol. Pharmacol. 2016, 44, 53–61. [Google Scholar] [CrossRef]
- Jiménez-Cárcamo, D.; Garcia, C.; Contreras, H. Toxins of Okadaic Acid-Group Increase Malignant Properties in Cells of Colon Cancer. Available online: https://www.mdpi.com/2072-6651/12/3/179 (accessed on 3 June 2025).
- Valdiglesias, V.; Prego-Faraldo, M.V.; Pásaro, E.; Méndez, J.; Laffon, B. Okadaic Acid: More than a Diarrheic Toxin. Mar. Drugs 2013, 11, 4328–4349. [Google Scholar] [CrossRef] [PubMed]
- Landwehr, J. FDA Issues Warning Over Paralytic Shellfish Poisoning After Oyster, Clam Recall—What to Know. Available online: https://www.health.com/fda-shellfish-paralytic-poisoning-recall-8663030 (accessed on 3 June 2025).
- Griffiths, D.; Saker, M. The Palm Island Mystery Disease 20 Years on: A Review of Research on the Cyanotoxin Cylindrospermopsin. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/tox.10103?casa_token=pgVhOOhMR7cAAAAA:L2U6nDjB91iYknda9QA4VUP66J92sKHSDey80O70p7w_Cf43Azzbah8qmZMLmXRiXJw_EaNS0DOJ3sn5DA (accessed on 5 March 2025).
- Poniedziałek, B.; Rzymski, P.; Kokociński, M. Cylindrospermopsin: Water-Linked Potential Threat to Human Health in Europe. Environ. Toxicol. Pharmacol. 2012, 34, 651–660. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Marañón, E. Cell Size as a Key Determinant of Phytoplankton Metabolism and Community Structure. Annu. Rev. Mar. Sci. 2015, 7, 241–264. [Google Scholar] [CrossRef]
- Trainer, V.L.; Moore, S.K.; Hallegraeff, G.; Kudela, R.M.; Clement, A.; Mardones, J.I.; Cochlan, W.P. Pelagic Harmful Algal Blooms and Climate Change: Lessons from Nature’s Experiments with Extremes. Harmful Algae 2020, 91, 101591. [Google Scholar] [CrossRef]
- Segura, A.M.; Piccini, C.; Nogueira, L.; Alcántara, I.; Calliari, D.; Kruk, C. Increased Sampled Volume Improves Microcystis Aeruginosa Complex (MAC) Colonies Detection and Prediction Using Random Forests. Ecol. Indic. 2017, 79, 347–354. [Google Scholar] [CrossRef]
- Gholizadeh, M.H.; Melesse, A.M.; Reddi, L. A Comprehensive Review on Water Quality Parameters Estimation Using Remote Sensing Techniques. Sensors 2016, 16, 1298. [Google Scholar] [CrossRef] [PubMed]
- Sòria-Perpinyà, X.; Vicente, E.; Urrego, P.; Pereira-Sandoval, M.; Ruíz-Verdú, A.; Delegido, J.; Soria, J.M.; Moreno, J. Remote Sensing of Cyanobacterial Blooms in a Hypertrophic Lagoon (Albufera of València, Eastern Iberian Peninsula) Using Multitemporal Sentinel-2 Images. Sci. Total Environ. 2020, 698, 134305. [Google Scholar] [CrossRef]
- Astuti, W.; Govindaraju, R. Characterization and Modeling of Harmful Algal Blooms: A Review. J. Hydraul. Eng. 2025, 151, 03125001. [Google Scholar] [CrossRef]
- Xu, S.; Lyu, P.; Zheng, X.; Yang, H.; Xia, B.; Li, H.; Zhang, H.; Ma, S. Monitoring and Control Methods of Harmful Algal Blooms in Chinese Freshwater System: A Review. Environ. Sci. Pollut. Res. 2022, 29, 56908–56927. [Google Scholar] [CrossRef]
- Busch, J.A.; Andree, K.B.; Diogène, J.; Fernández-Tejedor, M.; Toebe, K.; John, U.; Krock, B.; Tillmann, U.; Cembella, A.D. Toxigenic Algae and Associated Phycotoxins in Two Coastal Embayments in the Ebro Delta (NW Mediterranean). Harmful Algae 2016, 55, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Luerssen, R.M.; Thomas, A.C.; Hurst, J. Relationships between Satellite-Measured Thermal Features and Alexandrium-Imposed Toxicity in the Gulf of Maine. Deep Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 2656–2673. [Google Scholar] [CrossRef]
- Stumpf, R.P.; Culver, M.E.; Tester, P.A.; Tomlinson, M.; Kirkpatrick, G.J.; Pederson, B.A.; Truby, E.; Ransibrahmanakul, V.; Soracco, M. Monitoring Karenia Brevis Blooms in the Gulf of Mexico Using Satellite Ocean Color Imagery and Other Data. Harmful Algae 2003, 2, 147–160. [Google Scholar] [CrossRef]
- Anderson, D.M. Approaches to Monitoring, Control and Management of Harmful Algal Blooms (HABs). Ocean Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef]
- Brooks, C. A Breakthrough in Harmful Algal Bloom Monitoring with FlowCam. Available online: https://www.fluidimaging.com/blog/flow-imaging-microscopy-breakthrough-harmful-algal-bloom-monitoring-with-flowcam (accessed on 12 March 2025).
- Monitoring Harmful Algal Blooms Using Drones. Available online: https://www.unh.edu/unhtoday/monitoring-harmful-algal-blooms-using-drones (accessed on 12 March 2025).
- Arias, F.; Zambrano, M.; Galagarza, E.; Broce, K. Mapping Harmful Algae Blooms: The Potential of Hyperspectral Imaging Technologies. Remote Sens. 2025, 17, 608. [Google Scholar] [CrossRef]
- Gupta, A.; Hantush, M.M.; Govindaraju, R.S. Sub-Monthly Time Scale Forecasting of Harmful Algal Blooms Intensity in Lake Erie Using Remote Sensing and Machine Learning. Sci. Total Environ. 2023, 900, 165781. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yang, J.; Li, Y.; Gao, L. Harmful Algal Bloom Warning Based on Machine Learning in Maritime Site Monitoring. Knowl.-Based Syst. 2022, 245, 108569. [Google Scholar] [CrossRef]
- Cruz, R.C.; Costa, P.R.; Vinga, S.; Krippahl, L.; Lopes, M.B. A Review of Recent Machine Learning Advances for Forecasting Harmful Algal Blooms and Shellfish Contamination. J. Mar. Sci. Eng. 2021, 9, 283. [Google Scholar] [CrossRef]
- Nagkoulis, N.; Vasiloudis, G.; Moumtzidou, A.; Gialampoukidis, I.; Vrochidis, S.; Kompatsiaris, I. Forecasting Lakes’ Chlorophyll Concentrations Using Satellite Images and Generative Adversarial Networks. Water Resour. Res. 2024, 60, e2024WR037138. [Google Scholar] [CrossRef]
- Park, J.; Patel, K.; Lee, W.H. Recent Advances in Algal Bloom Detection and Prediction Technology Using Machine Learning. Sci. Total Environ. 2024, 938, 173546. [Google Scholar] [CrossRef]
- Burkholder, J.M.; Noga, E.J.; Hobbs, C.H.; Glasgow, H.B. New “phantom” Dinoflagellate Is the Causative Agent of Major Estuarine Fish Kills. Nature 1992, 358, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, H.B., Jr.; Burkholder, J.M.; Schmechel, D.E.; Tester, P.A.; Rublee, P.A. Insidious Effects of a Toxic Estuarine Dino Flagellate on Fish Survival and Human Health. J. Toxicol. Environ. Health 1995, 46, 501–522. [Google Scholar] [CrossRef]
- Grattan, L.M.; Oldach, D.; Perl, T.M.; Lowitt, M.H.; Matuszak, D.L.; Dickson, C.; Parrott, C.; Shoemaker, R.C.; Kauffman, C.L.; Wasserman, M.P.; et al. Learning and Memory Difficulties after Environmental Exposure to Waterways Containing Toxin-Producing Pfiesteria or Pfiesteria-like Dinoflagellates. Lancet 1998, 352, 532–539. [Google Scholar] [CrossRef]
- Haselow, D.T.; Brown, E.; Tracy, J.K.; Magnien, R.; Grattan, L.M.; Morris, J.G., Jr.; Oldach, D.W. Gastrointestinal and Respiratory Tract Symptoms Following Brief Environmental Exposure to Aerosols during a Pfiesteria-Related Fish Kill. J. Toxicol. Environ. Health—Part A 2001, 63, 553–564. [Google Scholar] [CrossRef]
- Hudnell, H.K. The State of U.S. Freshwater Harmful Algal Blooms Assessments, Policy and Legislation. Toxicon 2010, 55, 1024–1034. [Google Scholar] [CrossRef]
- Shoemaker, R.C. Residential and Recreational Acquisition of Possible Estuary-Associated Syndrome: A New Approach to Successful Diagnosis and Treatment. Environ. Health Perspect. 2001, 109, 791–796. [Google Scholar] [CrossRef] [PubMed][Green Version]
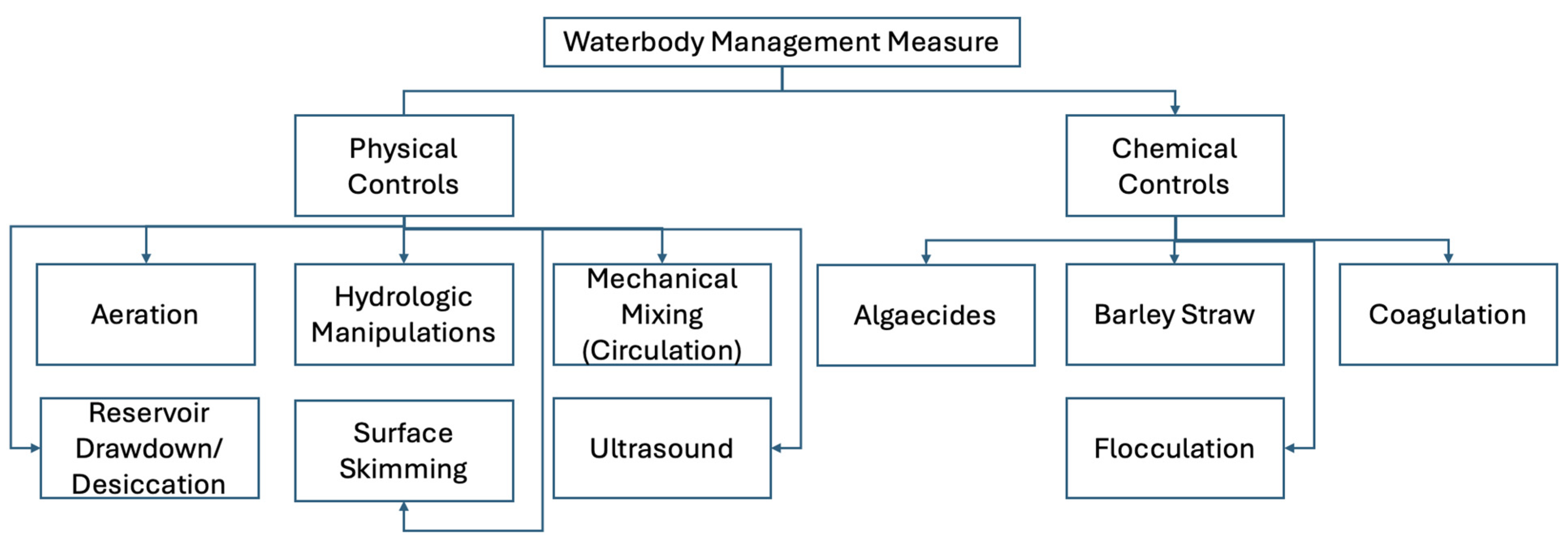
- United States Environmental Protection Agency. Control Measures for Cyanobacterial HABs in Surface Water. Available online: https://www.epa.gov/habs/control-measures-cyanobacterial-habs-surface-water (accessed on 13 March 2025).
- Jacobi, A.L.; Thuneibat, M.; Vigar, M.K.; Rutt, C.; Andújar, A.; Roberts, V.A. Public Awareness and Concern about Harmful Algal Blooms—United States, 2020. J. Water Health 2024, 22, 1337–1346. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, S.; Xiong, C.; Yan, B.; Wang, Z.; Li, K.; Olivier, I.; Wang, H. Flocculation of Chlorella Vulgaris–Induced Algal Blooms: Critical Conditions and Mechanisms. Environ. Sci. Pollut. Res. 2022, 29, 78809–78820. [Google Scholar] [CrossRef] [PubMed]

| Nutrient | HAB Role | Processes/Conditions | Dominant Algal Species | References |
|---|---|---|---|---|
| Nitrogen (N) | Can limit nutrients and is a primary influence for algal growth | Urban runoff and high inputs from agriculture for coastal waters, atmospheric deposition, and upwelling for ocean water | Dinoflagellates, Cyanobacteria, Diatoms | [66,67,68] |
| Phosphorus (P) | The main limiting nutrient | Runoff from agriculture and sewage discharge for estuaries and coastal lagoons, and High P inputs for freshwater | Dinoflagellates, Cyanobacteria | [69,70,71] |
| Silicon (Si) | Encourages diatom growth | Abundant silicon from riverine inputs for rivers and nutrient upwelled waters for upwelling zones | Diatoms | [72,73,74] |
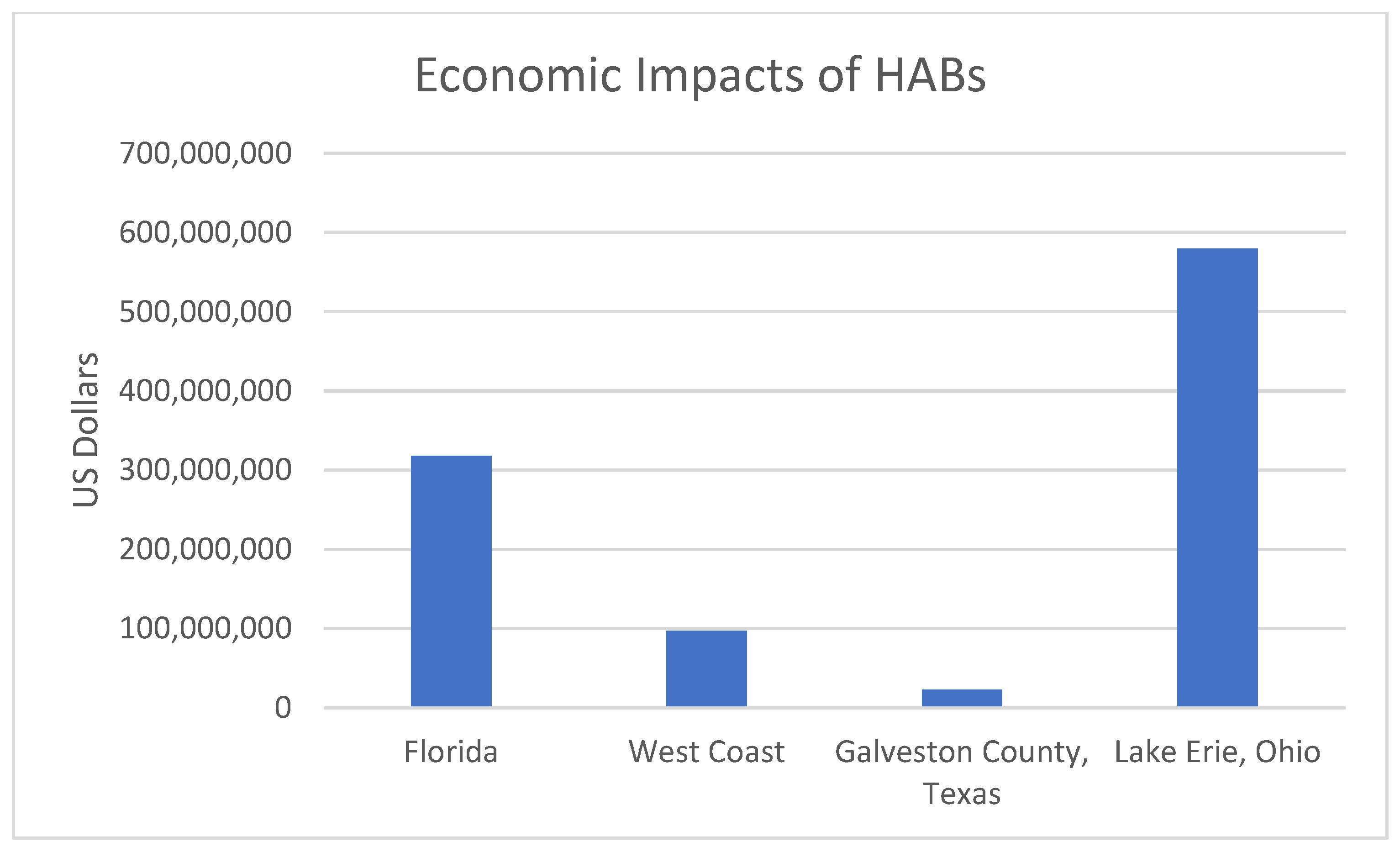
| Cost (USD M) | Occurrence | Impact | Year (s) | Location |
|---|---|---|---|---|
| 97.5 | Fisheries loss due to a toxic bloom of Pseudo-nitzschia | Loss of Dungess crab landings | 2015 | U.S. West Coast (California, Oregon, and Washington) |
| 40 | Coastal communities’ loss due to a toxic bloom of Pseudo-nitzschia | Loss of tourism spending | 2015 | U.S. West Coast (California, Oregon, and Washington) |
| 10 | Levels of mycrocystin impacted drinking water | Loss of shoreline property value services | 2014 | Toledo Ohio |
| 5.58 | Summer-long blooms of cyanobacteria decreased fishing licenses purchases | On estimated lost fishing expenditures | 2011–2014 | Lake Erie |
| 10.3 | Red tide banned oyster harvesting | Drop in Oyster Landings | 2011 | Texas |
| 235–470 | Brown tides caused prevention of seagrasses from recovering | Potential loss to local Economy | 2011 | Indian River Lagoon, Florida |
| 5 | Fisheries failure due to red tides of Alexandrium | On fisheries failure disaster relief | 2009 | Massachusetts, Maine and New Hampshire |
| 4 | Fisheries failure due to red tides of Alexandrium | On fisheries failure disaster relief | 2006 | Massachusetts, and Maine |
| 50 | Caused by red tides of Alexandrium and floods | Lost Income | 2005 | Maine |
| 0.785 | Cleanup efforts due to red tides | On red-tide clean-up and response costs | 2004–2007 | Four Florida Counties (Pinellas, Sarasota, Lee and Collier) |
| 4.4 | Emergency room visits due to respiratory illness due to red tides | On emergency room visits | 2001–2002 | Sarasota County, Florida |
| 3.7 | Impacts from golden algal blooms on tourism and recreation | Total economic impacts on tourism and recreation | 2001 | Possum Kingdom Lake, Texas |
| 1.4 | Impacts from golden algal blooms on tourism and recreation | Total economic impacts on tourism and recreation | 2003 | Possum Kingdom Lake, Texas |
| 96 | Willingness to purchase seafood | Loss of consumer surplus in | 2001 | Delaware, Maryland, North Carolina, and Virginia |
| 22–25.4 | Impacts of red tide | On total economic impact | 2000 | Galveston County, Texas |
| Policy Mechanism | Description | Example | References |
|---|---|---|---|
| Prevention | Aims to proactively minimize the likelihood of HAB occurrences by reducing nutrient and pollutant inputs—particularly nitrogen and phosphorus—from agriculture, urban runoff, and wastewater. Prevention policies also include ecosystem-based management, land-use regulations, and watershed protection plans. | The Law of the Sea Convention (UNCLOS) obligates countries to prevent aquatic pollution. In the U.S., the Clean Water Act Section 319 supports nutrient reduction through state-level Nonpoint Source Management Programs. Agricultural buffer zones and green infrastructure (e.g., wetlands) are applied to intercept nutrients before they reach waterways. | [86,96] |
| Monitoring and Forecasting | Real-time and remote HAB monitoring and Early warning systems | NOAA HAB Forecasts, satellite imagery, sensor buoys, citizen science apps, local water alerts, and risk dashboards | [1,7,23] |
| Restoration (Control) | Initiated after a HAB is detected. Includes interventions to contain or mitigate active blooms using physical (e.g., clay flocculation), biological (e.g., algicidal bacteria), or chemical (e.g., hydrogen peroxide) methods. Also includes policy-enforced emergency response protocols and nutrient input controls to prevent bloom worsening. | Shipping regulations enforce the Polluter Pays Principle, compelling polluters to pay for ecosystem damages. National contingency plans—like the U.S. HABHRCA framework—coordinate bloom response. Some countries use real-time monitoring and mobile alert systems to issue health advisories and shellfish bans. | [1,80] |
| Regulatory | Nutrient load limits, agricultural runoff controls, and land use and development regulations | Clean Water Act (CWA), Total Maximum Daily Loads (TMDLs), NPDES permits; Fertilizer use restrictions, manure management plans, buffer zone requirements and zoning laws, riparian setback mandates, wetland conservation | [96,136] |
| Amelioration | Refers to localized and often temporary mitigation actions designed to alleviate HAB impacts on human health, aquatic life, and recreation. Includes cleanup operations, public education, beach closures, shellfish harvesting bans, and water aeration systems to reduce anoxia caused by bloom decay. | Beach algae removal programs, such as those in Florida, use raking, vacuum systems, and controlled disposal. Shellfish monitoring programs, like Canada’s Molluscan Shellfish Sanitation Program, close contaminated areas. Public signage and mobile alerts reduce exposure to aerosolized toxins near affected shores. | [98] |
| Education and Outreach | Public awareness campaigns, transparency, and access to data | HAB warning signs, septic maintenance education, lawn care best practices, and Open-access portals, community science hubs | [1,136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brenckman, C.M.; Parameswarappa Jayalakshmamma, M.; Pennock, W.H.; Ashraf, F.; Borgaonkar, A.D. A Review of Harmful Algal Blooms: Causes, Effects, Monitoring, and Prevention Methods. Water 2025, 17, 1980. https://doi.org/10.3390/w17131980
Brenckman CM, Parameswarappa Jayalakshmamma M, Pennock WH, Ashraf F, Borgaonkar AD. A Review of Harmful Algal Blooms: Causes, Effects, Monitoring, and Prevention Methods. Water. 2025; 17(13):1980. https://doi.org/10.3390/w17131980
Chicago/Turabian StyleBrenckman, Christina M., Meghana Parameswarappa Jayalakshmamma, William H. Pennock, Fahmidah Ashraf, and Ashish D. Borgaonkar. 2025. "A Review of Harmful Algal Blooms: Causes, Effects, Monitoring, and Prevention Methods" Water 17, no. 13: 1980. https://doi.org/10.3390/w17131980
APA StyleBrenckman, C. M., Parameswarappa Jayalakshmamma, M., Pennock, W. H., Ashraf, F., & Borgaonkar, A. D. (2025). A Review of Harmful Algal Blooms: Causes, Effects, Monitoring, and Prevention Methods. Water, 17(13), 1980. https://doi.org/10.3390/w17131980









