Basic Research on the Adsorption Capacity and Enhancement of Bamboo Charcoal for the Prevention of Nitrate Groundwater Pollution
Abstract
1. Introduction
2. Materials and Methods
2.1. Bio-Char (BC), Magnesium Treated Char (Mg-BC), and Hydrogel of Magnesium Treated Bamboo Charcoal (Gel-Mg-BC)
2.2. Adsorption Experiment and Sample Preparation
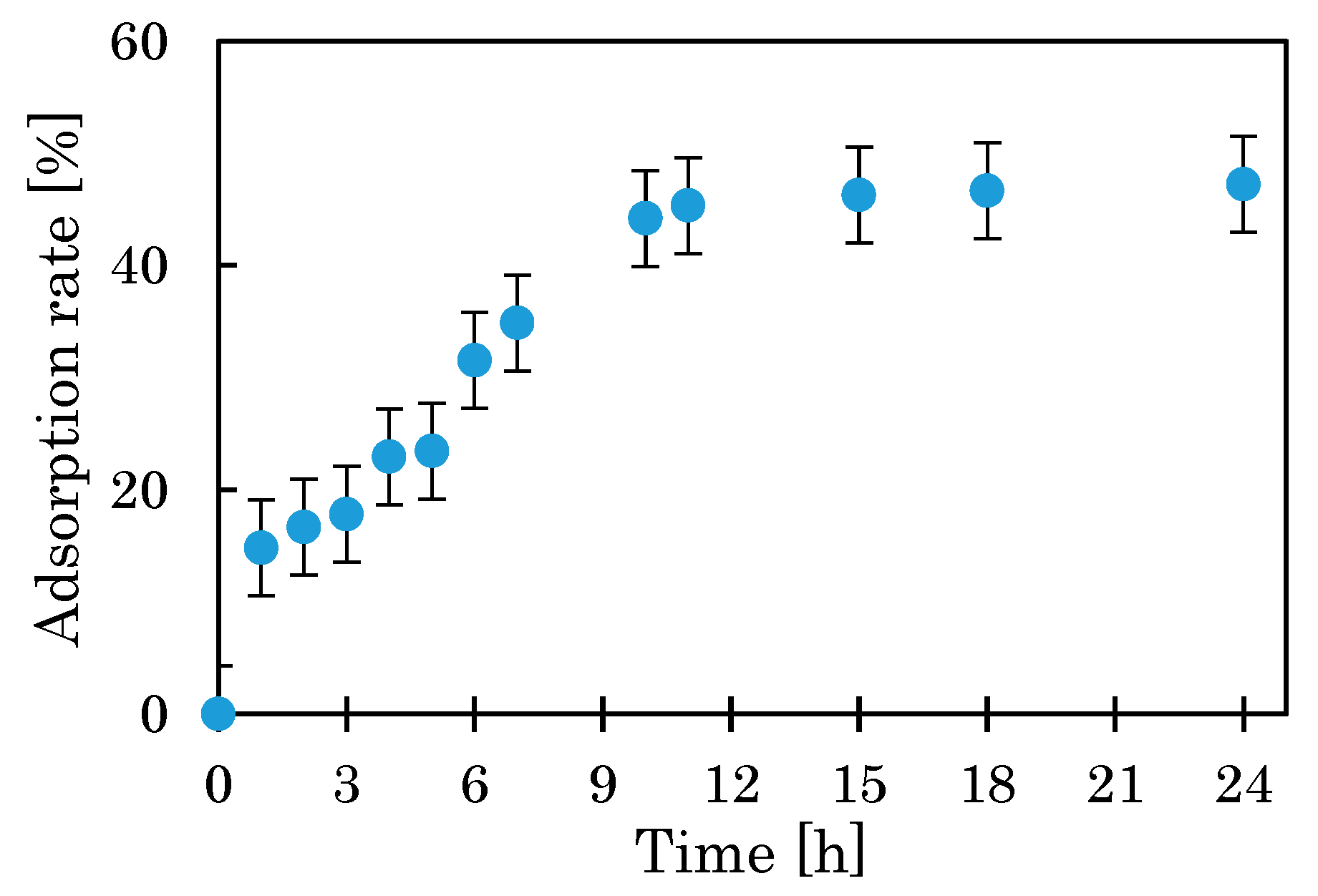
2.2.1. Evaluation of BC
- (1)
- pH dependence of nitrate adsorption
- (2)
- Effect of mixing time on nitrate adsorption
- (3)
- Effect of temperature on nitrate adsorption
- (4)
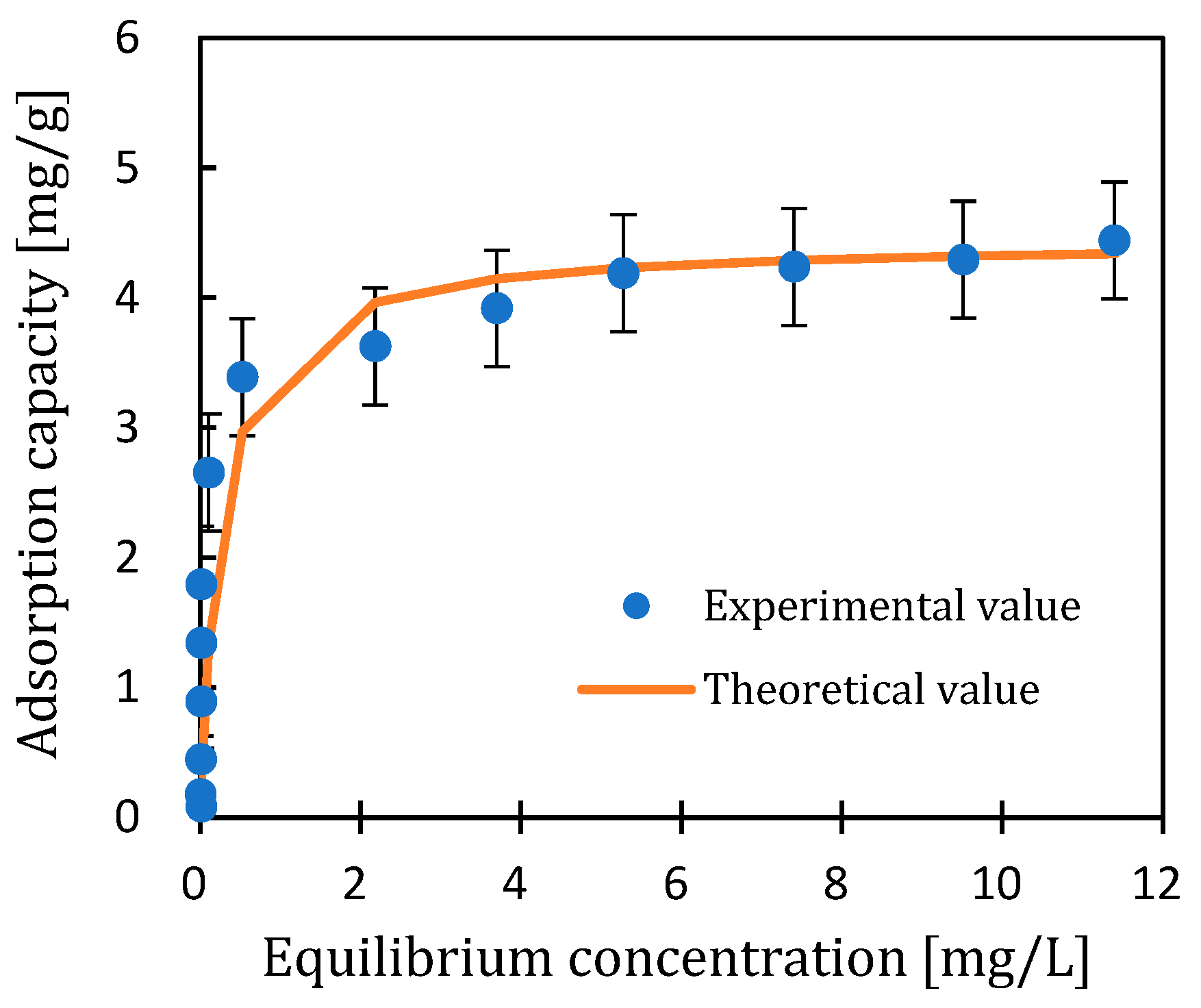
- Isothermal adsorption studies
2.2.2. Preparation of Mg-BC and Adsorption Tests
2.2.3. Preparation of Hydrogel of Mg-BC and Adsorption Test
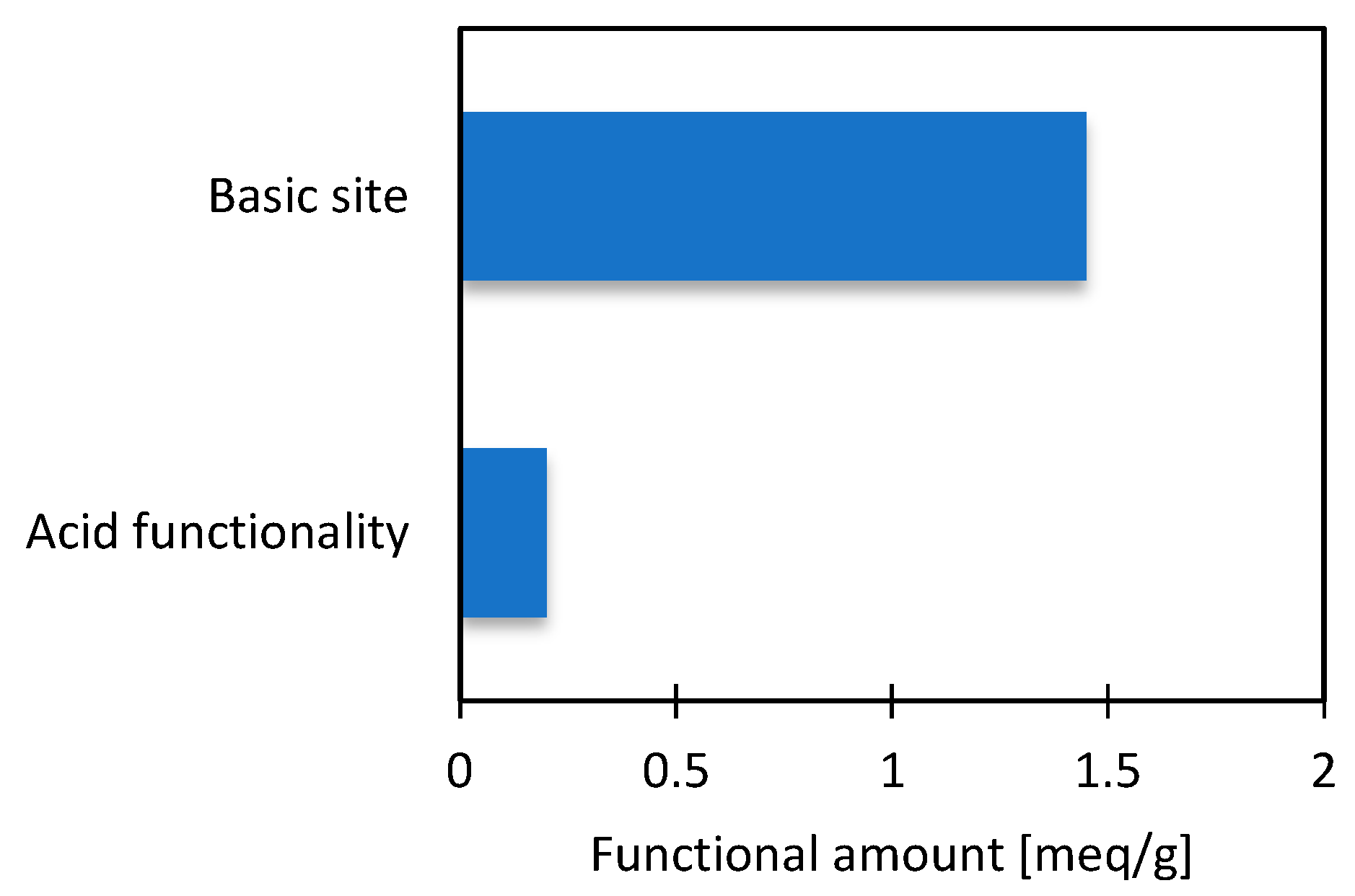
2.3. Characterization of the Material
2.4. Soil Column Experiment
2.5. Evaluation Parameter
3. Results and Discussion
3.1. BC (Biochar)
3.1.1. Effect of pH, Mixing Time, and Temperature
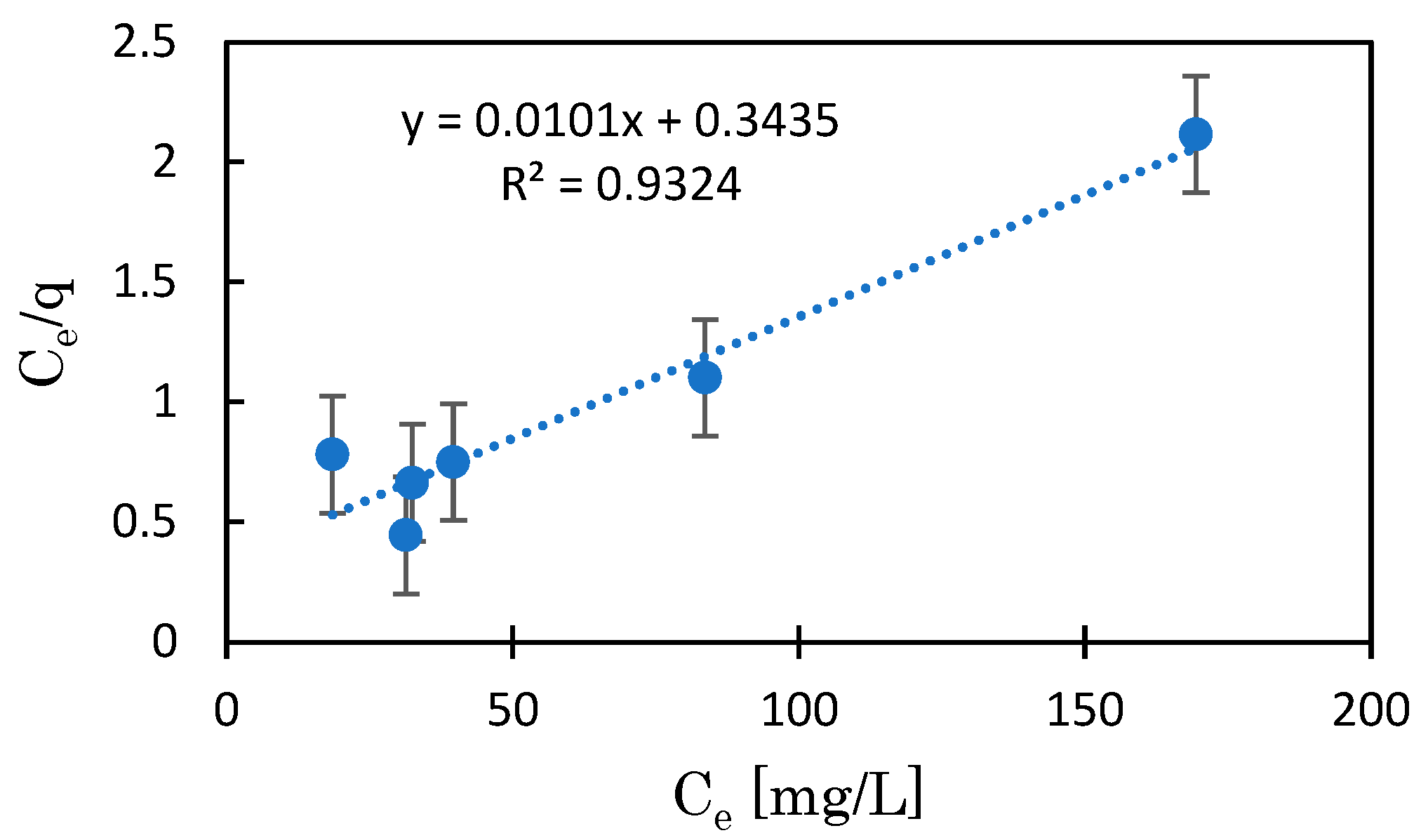
3.1.2. Isothermal Adsorption Curve
3.2. Characterization of Material
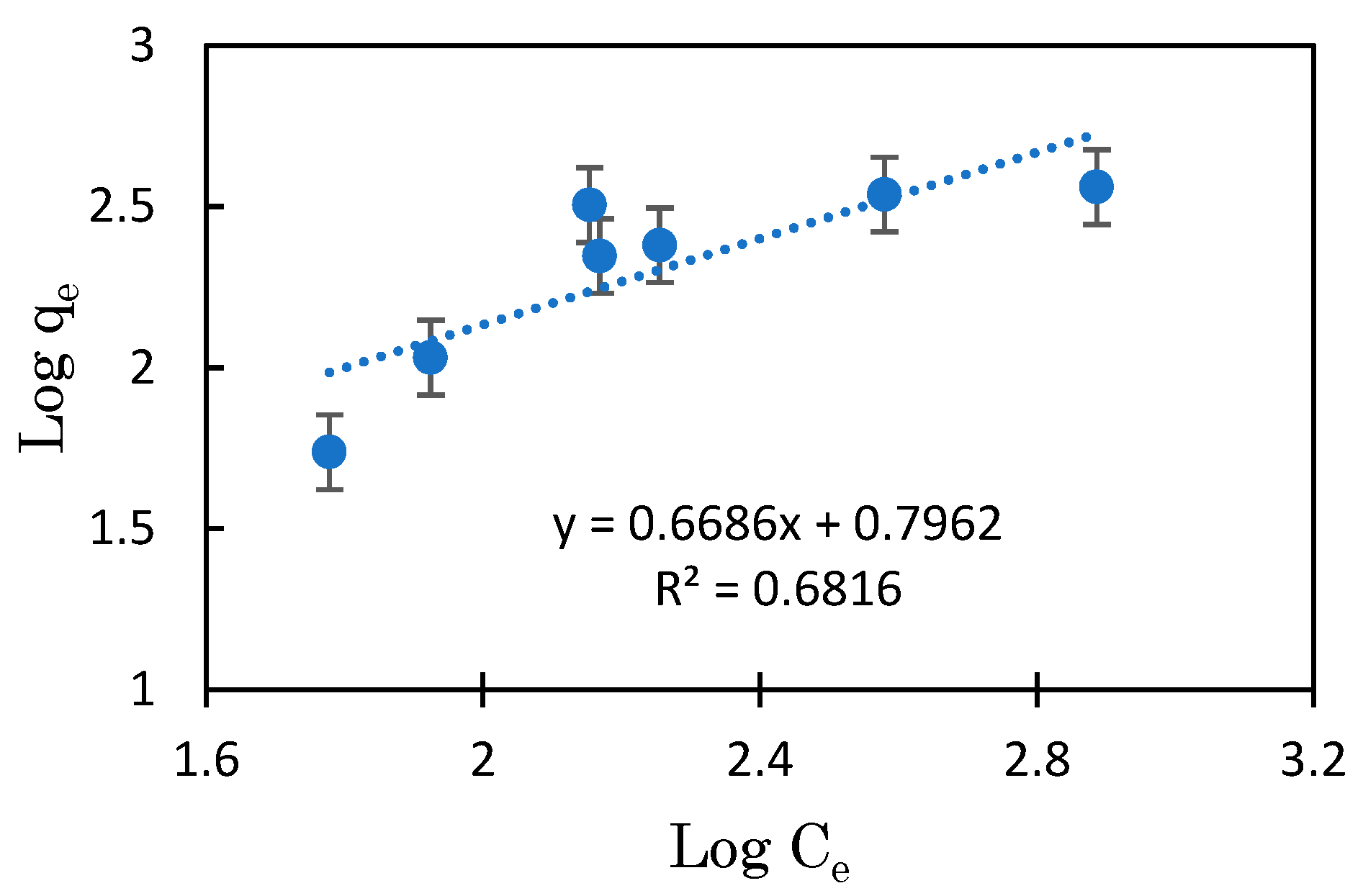
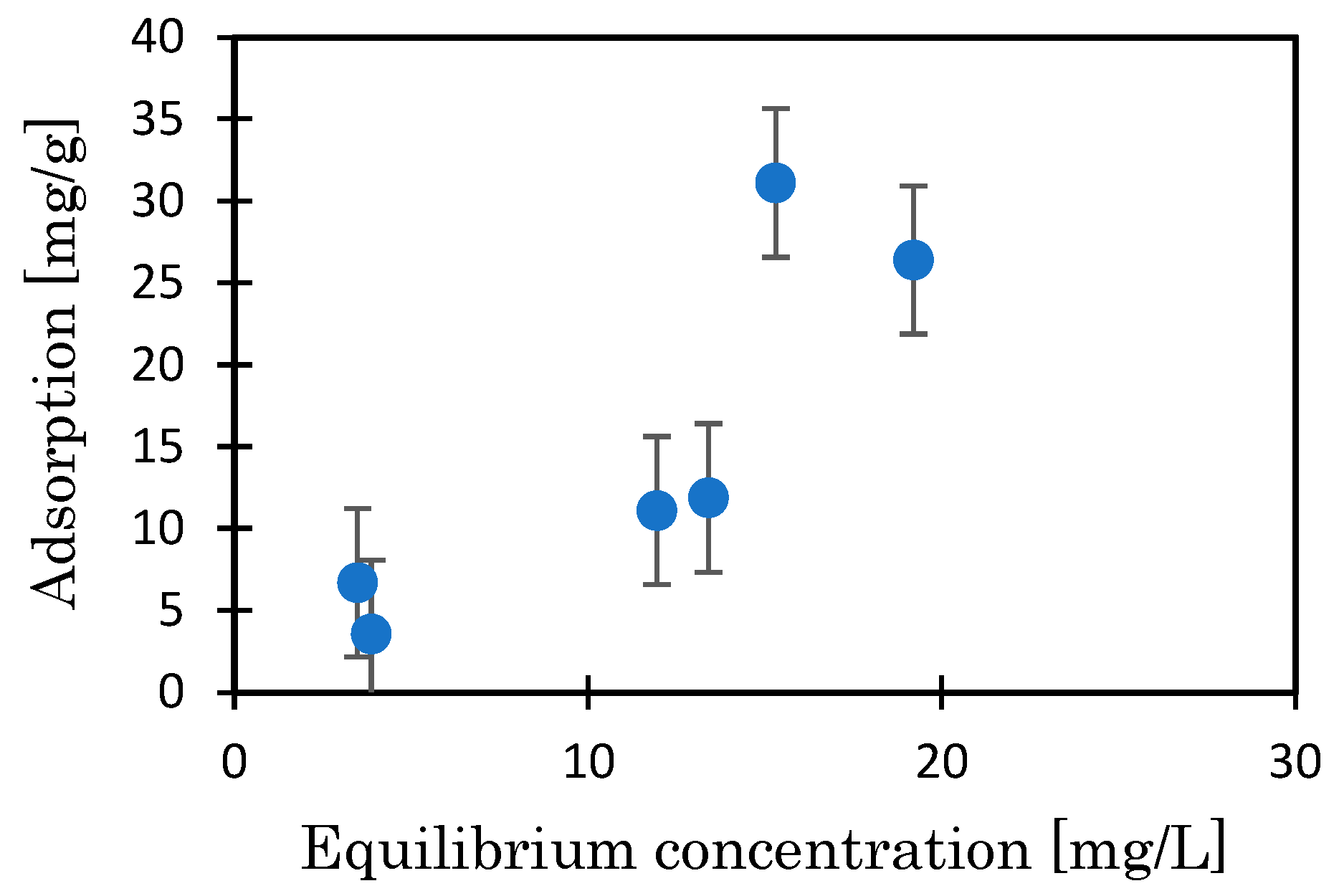
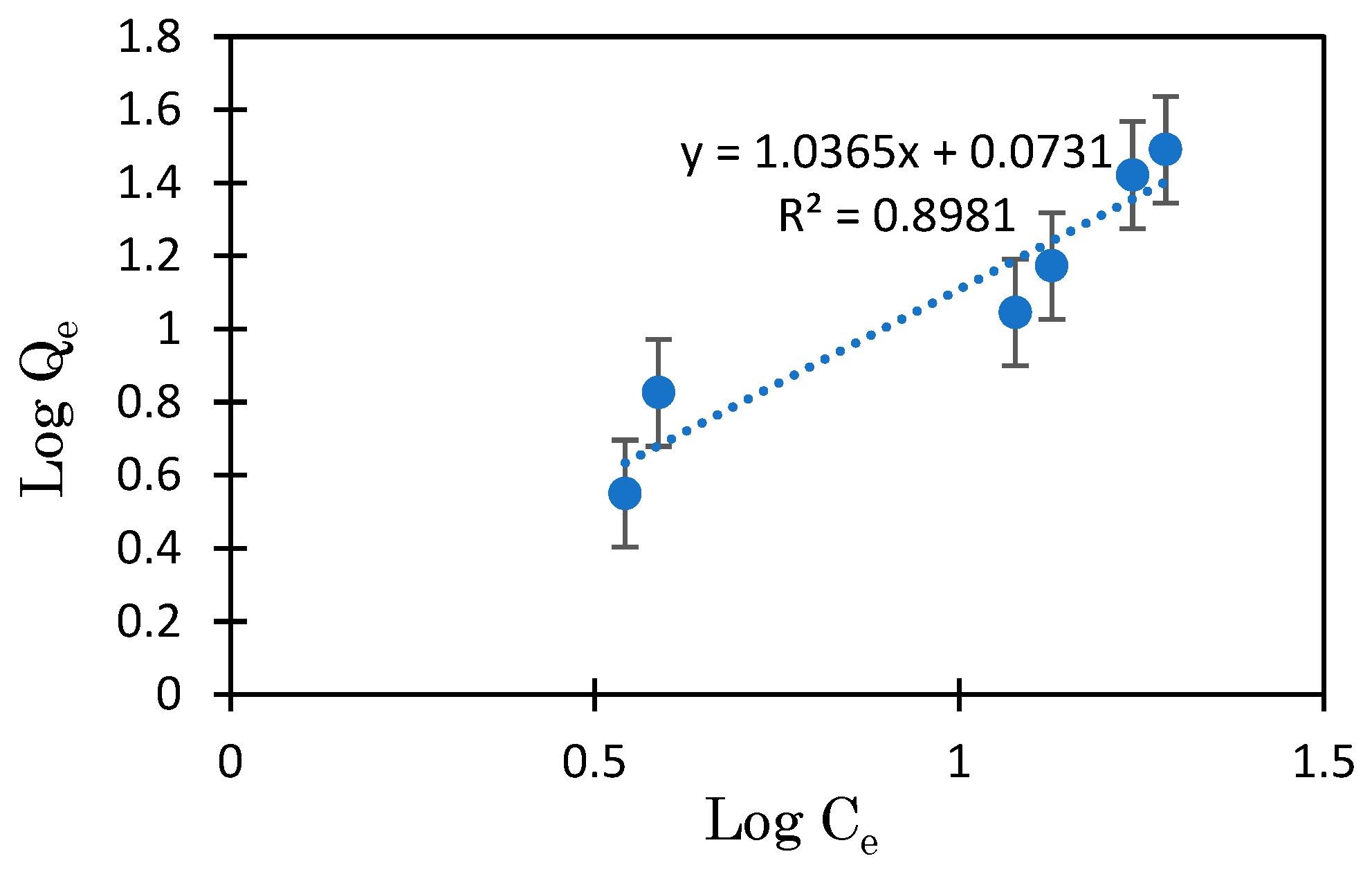
3.3. Mg-BC and Hydrogel of Mg-BC (Gel-Mg-BC)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, S.; Anil, A.G.; Kumar, V.; Kapoor, D.; Subramanian, S.; Singh, J.; Ramamurthy, P.C. Nitrates in the environment: A critical review of their distribution, sensing techniques. Chemosphere 2021, 287 Pt 1, 131996. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Yoshinori, H.; Nagaoka, T.; Kenji, K. Nitrogen uptake and transpiration by plant effects on nitrate leaching from granitic regosol, Nitrate Accumulation in Vegetables. Soil Sci. Plant Nutr. 2007, 53, 300–309. [Google Scholar] [CrossRef]
- Maynard, D.N.; Barker, A.V.; Minotti, P.L.; Peck, N.H. Nitrate Accumulation in Vegetables. Adv. Agron. 1976, 28, 71–118. [Google Scholar] [CrossRef]
- Anjana, S.U.; Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar] [CrossRef]
- Vieira, I.S.; Vasconselos, E.P.; Monteiro, A.A. Nitrate accumulation, yield and leaf quality of turnip greens in response to nitrogen fertilization. Nutr. Cycl. Agroecosyst. 1988, 51, 249–258. [Google Scholar] [CrossRef]
- Dangler, J.M.; Wood, C.W. Nitrogen rate, cultivar and within row spacing affect collard yield and leaf nutrient concentration. HortScience 1993, 28, 701–703. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef]
- Ahada, C.P.S.; Suthar, S. Groundwater nitrate contamination and associated human health risk assessment in southern districts of Punjab. India Environ. Sci. Pollut. Control Ser. 2018, 25, 25336–25347. [Google Scholar] [CrossRef]
- Schaider, L.A.; Swetschinski, L.; Campbell, C.; Rudel, R.A. Environmental justice and drinking water quality: Are there socio economic disparities in nitrate levels in U.S. drinking water? Environ. Health Glob. Access Sci. Source 2019, 18, 3. [Google Scholar] [CrossRef]
- Wick, K.; Heumesser, C.; Schmid, E. Groundwater nitrate contamination: Factors and indicators. J. Environ. Manag. 2012, 111, 178–186. [Google Scholar] [CrossRef]
- Zhou, Z.; Ansems, N.; Torfs, P. A global assessment of nitrate contamination in groundwater. International Groundwater Re sources Assessment Centre. Internsh. Rep. 2015, 947, 174508. [Google Scholar] [CrossRef]
- Dahan, O.; Babad, A.; Lazarovitch, N.; Russak, E.E.; Kurtzman, D. Nitrate leaching from intensive organic farms to groundwater. Hydrol. Earth Syst. Sci. 2014, 18, 333–341. [Google Scholar] [CrossRef]
- Huijie, L.; Kartik, C.; David, S. Microbial ecology of denitrification in biological wastewater treatment. Water Res. 2014, 64, 237–254. [Google Scholar] [CrossRef]
- Scholes, R.C.; Vega, M.A.; Sharp, J.O.; Sedlak, D.L. Nitrate removal from reverse osmosis concentrate in pilot-scale. Environ. Sci. Water Res. Technol. 2021, 7, 650–661. [Google Scholar] [CrossRef]
- Yaragal, R.R.; Mutnuri, S. Nitrates removal using ion exchange resin: Batch, continuous column and pilot-scale studies. Int. J. Environ. Sci. Technol. 2023, 20, 739–754. [Google Scholar] [CrossRef]
- Viglašová, E.; Galamboš, M.; Diviš, D.; Dankováe, Z.; Daňo, M.; Krivosudský, L.; Lengauerg, C.L.; Matik, M.; Briančinf, J.; Sojac, G. Engineered biochar as a tool for nitrogen pollutants removal: Preparation, characterization and sorption study. Desalination Water Treat. 2020, 191, 318–331. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Mekhzoum, M.E.M.; Zari, N.; Benzeid, H.; Qaiss, A.E.K.; Bouhfid, R. Biochar-supported materials for wastewater treatment. In Applied Water Science Volume 1: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 199–225. [Google Scholar] [CrossRef]
- Ravinuthala, S.; Nithya, R.; Das, S.P. Application of biochar for wastewater treatment. In BioChar: Applications for Bioremediation of Contaminated Systems; Riti, T.K., Maulin, P.S., Eds.; De Gruyter Brill: Berlin, Germany, 2022; p. 185. [Google Scholar]
- Qi, J.Y.; Sato, S. Magnesium-modified biochars for nitrate adsorption and removal in continuous flow system. Bull. Plankton Eco-Eng. Res. 2021, 1, 32–46. [Google Scholar]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solution. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Y.; Nan, H.; Pei, L.; Huang, Y.; Cao, X.; Xu, X.; Zhao, L. Metal chloride-loaded biochar for phosphorus recovery: Noteworthy roles of inherent minerals in precursor. Chemosphere 2021, 266, 128991. [Google Scholar] [CrossRef]
- Ci, F.; Tao, Z.; Ping, L.J.; Rong-Feng, W.J.; Ying-Cai, P. Health Application of magnesium modified corn biochar for phosphorus removal and recovery from swine wastewater. Int. J. Environ. Res. Public Health 2014, 11, 9217–9237. [Google Scholar] [CrossRef]
- Hidayat, E.; Sarbani, N.M.M.; Samitsu, S.; Nugroho, F.A.A.; Lahiri, S.K.; Aoyagi, M.; Harada, H. Evaluation of slow-release fertilizers derived from hydrogel beads: Sodium alginate-poly (acrylic acid) and humic acid-encapsulated struvite for soil salinity amelioration. Arab. J. Chem. 2024, 17, 105877. [Google Scholar] [CrossRef]
- Ramalingam, S.; Subramanian, A. Effective Removal of Nitrates from the Drinking Water by Chemical and Electrochemical Methods. Eng. Sci. 2021, 15, 80–88. [Google Scholar] [CrossRef]
- Harada, H.; Maisarah, N.M.S.; Aoyagi, M.; Nishimoto, J. Adsorption of Nitrate Ions Using Magnesium-Loaded Bamboo Powder and Nano-Sized Crushed Oyster Shells. Separations 2025, 12, 76. [Google Scholar] [CrossRef]
- Ito, M.; Yamazaki, N. Effect of a carbide of defatted rice bran treated with iron (III) chloride on retention of nitrate-nitrogen, growth of melon and radish and leaching of nitrate nitrogen in sand dune area. Jpn. J. Soil Sci. Plant Nutr. 2008, 79, 155–161. [Google Scholar] [CrossRef]
- Mizuta, K.; Matsumoto, T.; Hatate, Y.; Nishihara, K.; Nakanishi, T. Removal of nitrate-nitrogen from drinking water using bamboo powder charcoal. Bioresour. Technol. 2004, 95, 255–257. [Google Scholar] [CrossRef]
- Zhao, H.; Xue, Y.; Long, L.; Hu, X. Adsorption of nitrate onto biochar derived from agricultural residuals. Water Sci. Technol. 2018, 77, 548–554. [Google Scholar] [CrossRef]
- Wang, Z.H.; Guo, H.Y.; Shen, F.; Yang, G.; Zhang, Y.Z.; Zeng, Y.M.; Wang, L.L.; Xiao, H.; Deng, S.H. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Li, A.Y.; Deng, H.; Ye, C.H.; Wu, Y.Q.; Linmu, Y.D.; Hang, H.L. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks. Bioresour. Technol. 2019, 276, 183–189. [Google Scholar] [CrossRef]
- Han, W.; Chen, A.; Li, Z.; Zhou, R.; Zhang, K.; Zhao, W.; Zhang, X. Development of Agro-Based Engineered Biochars for Low Concentration Nitrate Adsorption: Modification Selection, Performance, Factors, Mechanism, and Reliability. Water Air Soil Pollut. 2025, 236, 356. [Google Scholar] [CrossRef]
- Rajeswari, A.; Amalraj, A.; Pius, A. Adsorption studies for the removal of nitrate using chitosan/PEG and chitosan/PVA polymer composites. J. Water Process Eng. 2016, 9, 123–134. [Google Scholar] [CrossRef]
- Gizaw, A.; Zewge, F.; Kumar, A.; Mekonnen, A.; Tesfaye, M. A comprehensive review on nitrate and phosphate removal and recovery from aqueous solutions by adsorption. AQUA—Water Infrastruct. Ecosyst. Soc. 2021, 70, 921–947. [Google Scholar] [CrossRef]
- He, H.; Huang, Y.; Yan, M.; Xie, Y.; Li, Y. Synergistic effect of electrostatic adsorption and ion exchange for efficient removal of nitrate. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 123973. [Google Scholar] [CrossRef]
- Zameni, L.; Sadegh-Zadeh, F.; Jalili, B.; Bahmanyar, M.A. Adsorption of nitrate from aqueous solution by biochar and Fe–coated biochar. Water Soil Manag. Model. 2024, 4, 70–84. [Google Scholar]







| Physical Properties | Value |
|---|---|
| Particle size (D50) | Approximately 10 μm |
| Apparent density | 350–550 g/L |
| Moisture | 12% |
| Ash content | 4% |
| Components | P2O5 | MgO | K2O | CaO | TN |
|---|---|---|---|---|---|
| Compositions (mg/100 g) | 1.2 | 700.4 | 773.2 | 227.5 | 0.8 |
| Adsorbent | Model | Q∞ (cal) | K | R2 | n | Q∞ (Graph) |
|---|---|---|---|---|---|---|
| BC | Langmuir | 4.44 | 3.82 | 0.995 | n.d. | n.d. |
| Mg-BC | Langmuir | 99.09 | 0.023 | 0.932 | n.d. | n.d. |
| Freundlich | n.d. | 1.18 | 0.681 | 0.668 | 86–90 | |
| Gel-Mg-BC | Freundlich | n.d. | 6.25 | 0.8981 | 1.0365 | 25–35 |
| Reference | Adsorbent Materials | Adsorption Capacity [mg/g] |
|---|---|---|
| [16] | Mg-Bio char | 9.13 |
| [19] | Water hyacinth/Mg-modified biochar | 19.1 |
| [20] | MgO-biochar nanocomposites | 95 |
| [26] | Rice bran treated with iron (III) chloride | 2.77 |
| [27] | Bamboo powder charcoal | 1.25 |
| [28] | Biochar derived from agricultural residuals | 14.46 |
| [29] | Lanthanum-oak sawdust biochar | 8.94 (Char) → 100.0 (La-Char) |
| [30] | Mg-loaded cassava straw biochar | 24.04 |
| [31] | HCl impregnation of corn straw-biochar | 1.958 |
| [32] | Chitosan/PVA | 35.03 |
| [33] | Chitosan hydrogel | 92.1 |
| [34] | Chitosan/zeolite/Fe3+/Mg2+ bead | 62.23 |
| [35] | Bio Char Fe Coated | 43.66 |
| This study | Mg-Bamboo charcoal | 99.0 |
| This study | Gel-Mg-Bamboo charcoal | 32.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarbani, N.M.M.; Harada, H.; Aoyagi, M.; Nishimoto, J.; Yonemura, S. Basic Research on the Adsorption Capacity and Enhancement of Bamboo Charcoal for the Prevention of Nitrate Groundwater Pollution. Water 2025, 17, 1979. https://doi.org/10.3390/w17131979
Sarbani NMM, Harada H, Aoyagi M, Nishimoto J, Yonemura S. Basic Research on the Adsorption Capacity and Enhancement of Bamboo Charcoal for the Prevention of Nitrate Groundwater Pollution. Water. 2025; 17(13):1979. https://doi.org/10.3390/w17131979
Chicago/Turabian StyleSarbani, Nur Maisarah Mohamad, Hiroyuki Harada, Mitsuru Aoyagi, Jun Nishimoto, and Seiichiro Yonemura. 2025. "Basic Research on the Adsorption Capacity and Enhancement of Bamboo Charcoal for the Prevention of Nitrate Groundwater Pollution" Water 17, no. 13: 1979. https://doi.org/10.3390/w17131979
APA StyleSarbani, N. M. M., Harada, H., Aoyagi, M., Nishimoto, J., & Yonemura, S. (2025). Basic Research on the Adsorption Capacity and Enhancement of Bamboo Charcoal for the Prevention of Nitrate Groundwater Pollution. Water, 17(13), 1979. https://doi.org/10.3390/w17131979







