Formation and Toxicity of Chlorine Species During Zeolite Regeneration by NaCl-NaClO After Stormwater Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Water
2.2.1. Solution
2.2.2. Stormwater
2.3. Zeolite Adsorption and Regeneration
2.3.1. Intermittent Operation
2.3.2. Continuous Operation
2.4. Acute Toxicity Analysis Methods
2.4.1. Algal Acute Toxicity Test
2.4.2. Acute Toxic Calculation Analysis
2.5. Analytical Methods
3. Results and Discussion
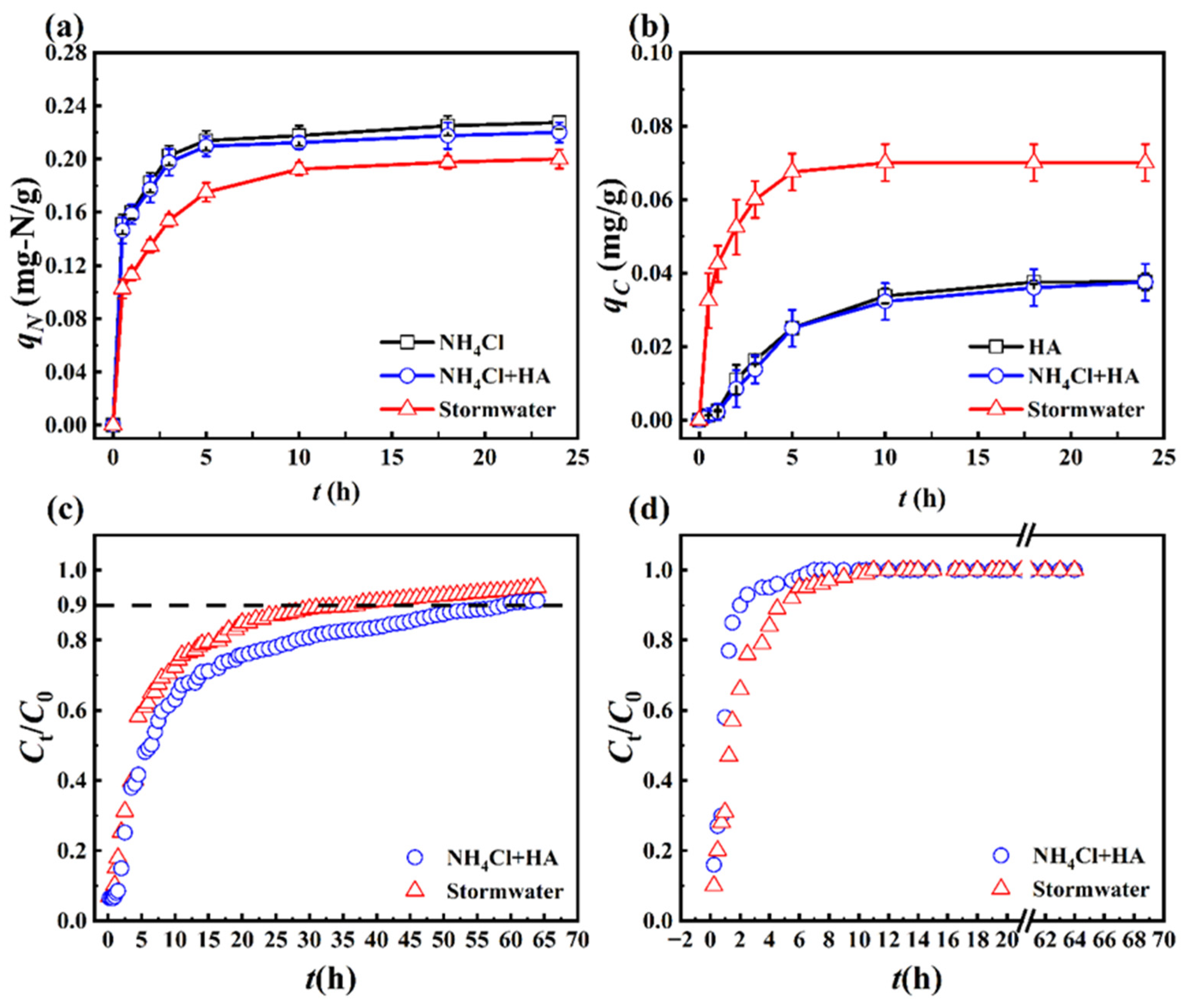
3.1. Zeolite Adsorption
3.1.1. Adsorption Isotherm
3.1.2. Intermittent Adsorption
3.1.3. Continuous Adsorption
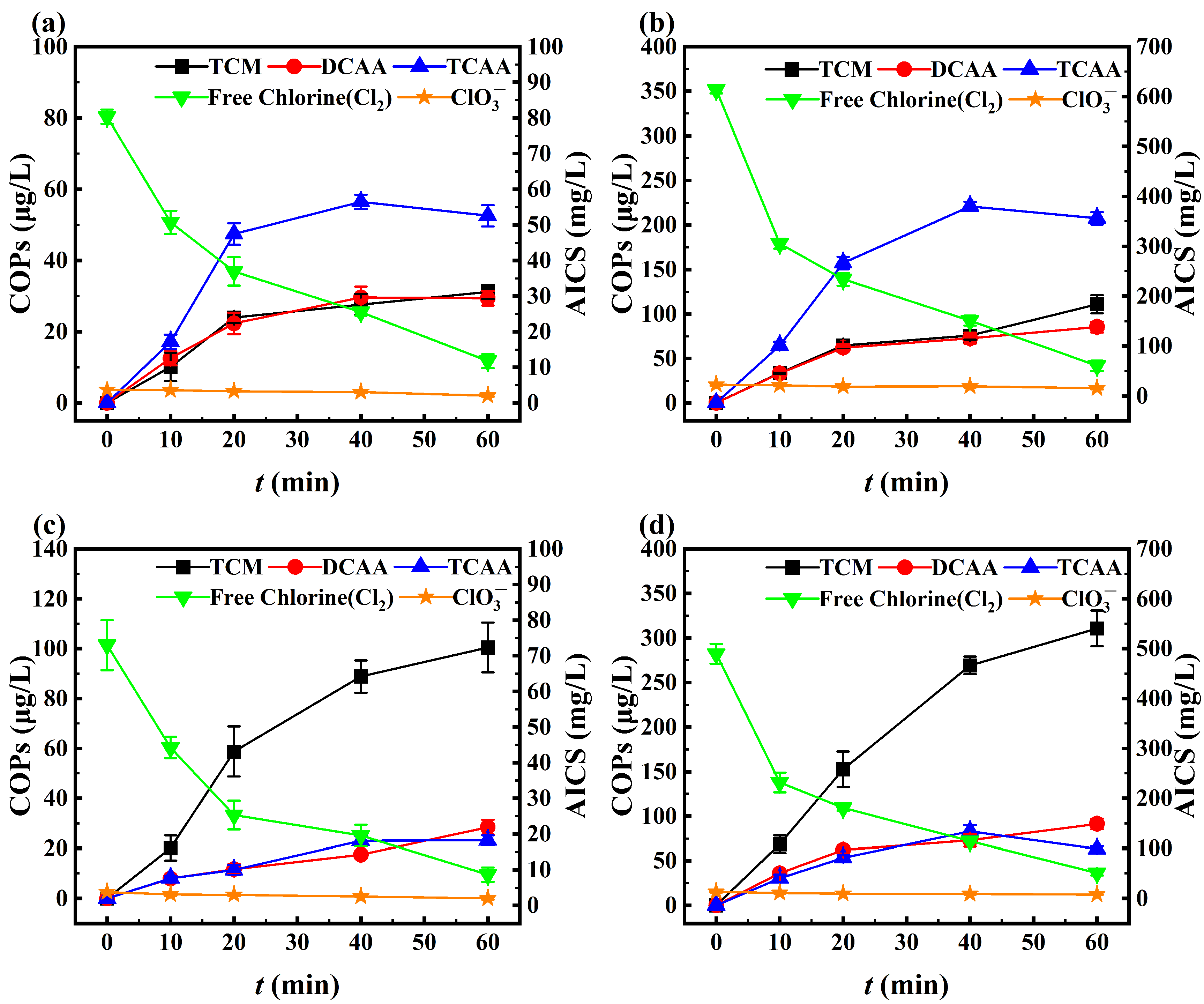
3.2. NaCl-NaClO Chlorination Reaction and Zeolite Regeneration
3.2.1. NaCl-NaClO Chlorination Reaction
3.2.2. Zeolite Regeneration
3.3. Chlorine Species Formation
3.3.1. AICS
3.3.2. COPs
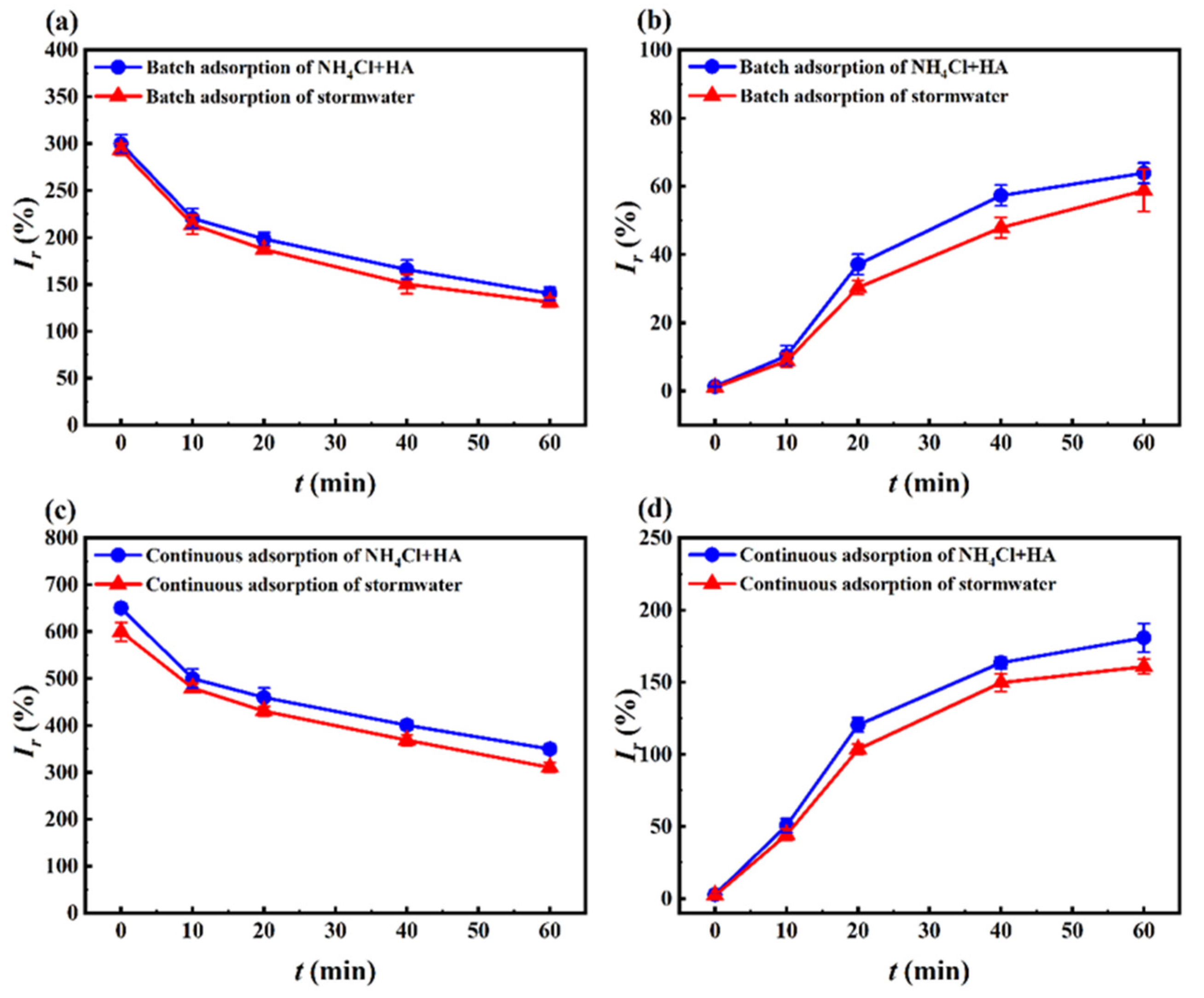
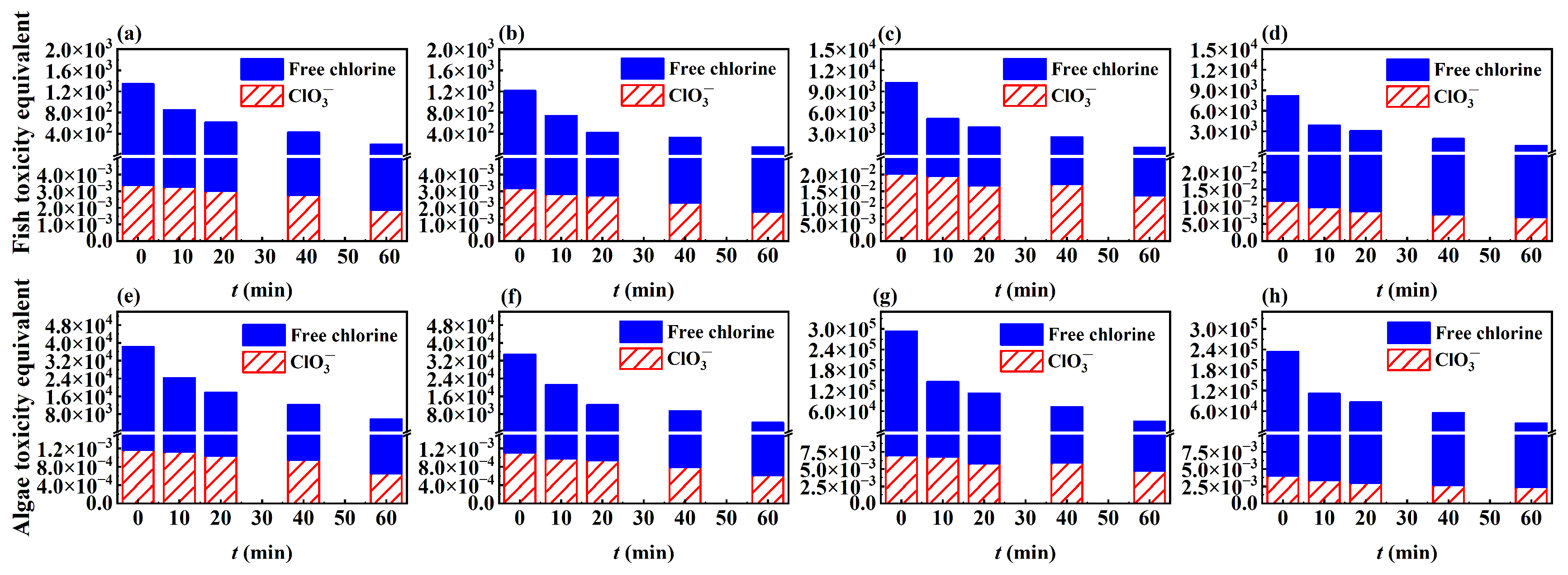
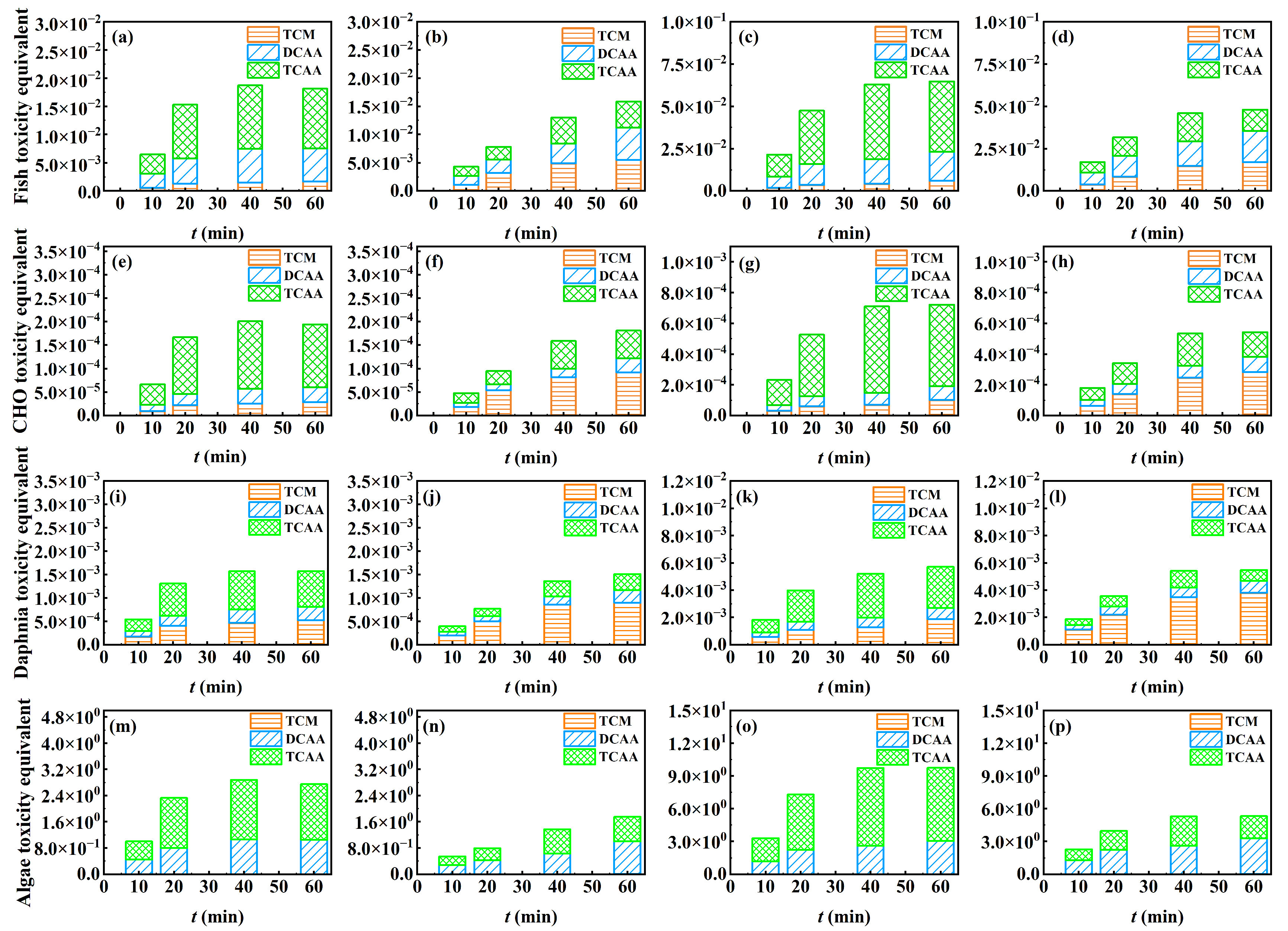
3.4. Toxicity Analysis
3.4.1. Algal Acute Toxicity Test
3.4.2. Toxicity Calculation
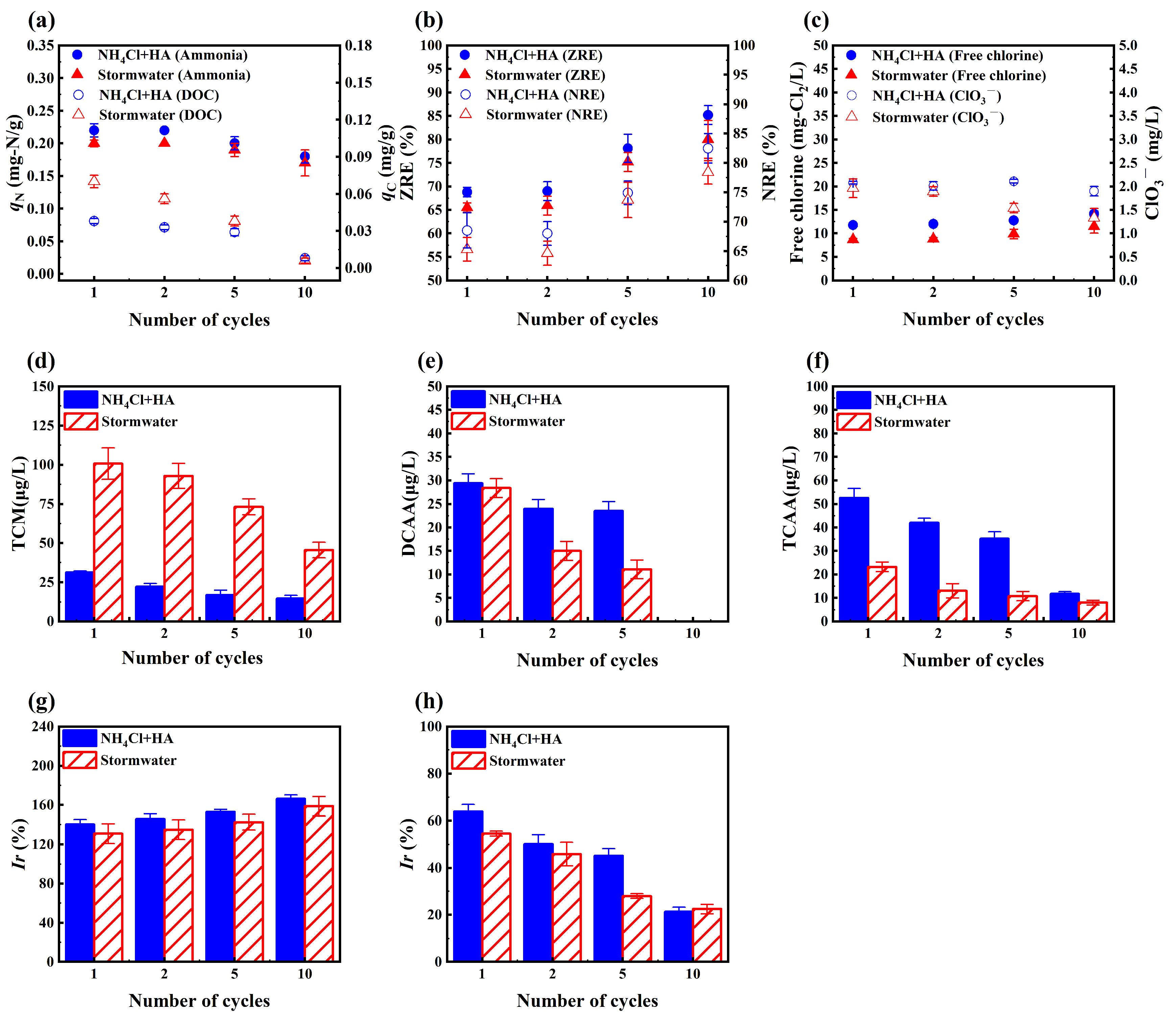
3.5. Changes in Multiple Cycles
3.5.1. Adsorption and Regeneration
3.5.2. Chlorine Species
3.5.3. Toxicity
4. Conclusions
- (1)
- The adsorption process of ammonia and HA in the NH4Cl + HA solution and stormwater by zeolite followed pseudo-second-order kinetic characteristics. Zeolite exhibited a higher adsorption capacity for HA in stormwater than in the NH4Cl + HA solution. The presence of ammonia had little impact on this process. NaCl-NaClO (0.5 g/L NaCl, ClO−: N molar ratio of 1.8, pH = 10) demonstrated a stable zeolite regeneration performance, with a ZRE of 65.0–68.8% and an NRE of 62.8–65.5%. During the regeneration process, ammonia preferentially reacted with ClO− to generate N2, while the gradual formation of COPs made it difficult for organic matter to mineralize.
- (2)
- Among the four sets of regeneration solutions, ClO3− was an inevitable AICS and degraded slowly within 1 h. TCAA and TCM were the dominant COPs in operations with the NH4Cl + HA solution and stormwater, respectively. Based on comprehensive toxicity effects and stoichiometry, the toxicity order of chlorine species in the regeneration solution was free chlorine > COPs > ClO3−. Due to the difference in adsorption capacity, the toxicity generated by continuous operations was three times that of intermittent operations. The total toxicity generated by COPs and ClO3− gradually increased over time. The zeolite regeneration using NaCl-NaClO required controlling the regeneration time to reduce the formation and toxicity of chlorine species.
- (3)
- During the 10 cycles, the zeolite adsorption capacity for ammonia and HA decreased gradually, while the ZRE and NRE increased. There was a notable accumulation of HA in the zeolites. Chlorine species continued to be formed during the regeneration process and produced a high toxicity hazard, although all of them showed a decreasing trend.
- (4)
- In engineering applications, it is advisable to collect the regeneration solution separately for the treatment of stable COPs and ClO3−. Future research could focus on exploring the way the regeneration solution treatment as well as the quantitative detection and toxicity evaluation of other unregulated yet harmful substances during the regeneration.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AICS | Active inorganic chlorine species |
| BDL | Below detection limit |
| CHO | Chinese hamster ovary |
| COD | Chemical oxygen demand |
| COPs | Chlorinated organic products |
| DCAA | Dichloroacetic acid |
| DCAN | Dichloroacetonitrile |
| DOC | Dissolved organic carbon |
| DOM | Dissolved organic matter |
| ECD | Electron capture detector |
| ECOSAR | Ecological structure activity relationship |
| HA | Humic acid |
| MCAA | Monochloroacetic acid |
| NOM | Natural organic matter |
| NRE | Nitrogen removal efficiency |
| SCOD | Soluble chemical oxygen demand |
| SS | Suspended solids |
| TCAA | Trichloroacetic acid |
| TCAN | Trichloroacetonitrile |
| TCM | Trichloromethane |
| TN | Total nitrogen |
| TP | Total phosphorus |
| U.S. EPA | United State Environmental Protection Agency |
| WHO | World Health Organization |
| ZRE | Zeolite regeneration efficiency |
References
- Kirshen, P.; Aytur, S.; Hecht, J.; Walker, A.; Burdick, D.; Jones, S.; Fennessey, N.; Bourdeau, R.; Mather, L. Integrated urban water management applied to adaptation to climate change. Urban Clim. 2018, 24, 247–263. [Google Scholar] [CrossRef]
- Shi, M.; Geng, B.; Zhao, T.; Wang, F. Influence of atmospheric deposition on surface water quality and DBP formation potential as well as control technology of rainwater DBPs: A review. Environ. Sci. Water Res. Technol. 2021, 7, 2156–2165. [Google Scholar] [CrossRef]
- Xu, J.; Wu, X.; Ge, X.; Tian, Y.; Ma, X.; Li, Y.; Xu, X.; Li, Z. Variations of Concentration Characteristics of Rainfall Runoff Pollutants in Typical Urban Living Areas. Bull. Environ. Contam. Toxicol. 2021, 106, 608–613. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Yu, Y.; Chen, Y.; Du, L.; Qu, X.; Peng, W.; Zhang, M.; Gui, C. Effect of Urban Stormwater Road Runoff of Different Land Use Types on an Urban River in Shenzhen, China. Water 2019, 11, 2545. [Google Scholar] [CrossRef]
- Song, Y.; Du, X.; Ye, X. Analysis of Potential Risks Associated with Urban Stormwater Quality for Managed Aquifer Recharge. Int. J. Environ. Res. Public Health 2019, 16, 3121. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lee, L.Y.; Lim, F.Y.; Lyu, Z.; Zhu, H.; Ong, S.L.; Hu, J. Water treatment residual: A critical review of its applications on pollutant removal from stormwater runoff and future perspectives. J. Environ. Manag. 2020, 259, 109649. [Google Scholar] [CrossRef]
- State Environmental Protection Administration, Environmental Quality Standard for Surface Water: GB3838-2002. 2002. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/shjzlbz/200206/t20020601_66497.shtml (accessed on 20 June 2025).
- Wang, K.; Zhou, Z.; Qiang, J.; Yu, S.; Wang, X.; Yuan, Y.; Zhao, X.; Qin, Y.; Xiao, K. Emerging wastewater treatment strategy for efficient nitrogen removal and compact footprint by coupling mainstream nitrogen separation with chemical coagulation and biological aerated filter. Bioresour. Technol. 2021, 320, 124389. [Google Scholar] [CrossRef]
- Nyström, F.; Nordqvist, K.; Herrmann, I.; Hedström, A.; Viklander, M. Laboratory scale evaluation of coagulants for treatment of stormwater. J. Water Process Eng. 2020, 36, 101271. [Google Scholar] [CrossRef]
- Zong, Y.; Jin, X.; Li, Y.; Shang, Y.; Wang, Y.; Jin, P.; Wang, X.C.; Guo, F.; Li, D. The coagulation behavior and mechanism of low-coagulability organic matter (LCOM). Sep. Purif. Technol. 2024, 328, 125055. [Google Scholar] [CrossRef]
- Qin, Y.; Zhou, C.; Yu, S.; Pang, H.; Guo, J.; Wei, J.; Wang, L.; Xing, Y.; An, Y.; Zhou, Z. Optimization of a compact on-site stormwater runoff treatment system: Process performance and reactor design. Chemosphere 2023, 315, 137767. [Google Scholar] [CrossRef]
- Lucke, T.; Drapper, D.; Hornbuckle, A. Urban stormwater characterisation and nitrogen composition from lot-scale catchments—New management implications. Sci. Total. Environ. 2018, 619, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; Zhou, Z.; Wang, K.; Qiu, Z.; Zhi, H.; Yuan, Y.; Zhang, Y.; Jiang, Y.; Zhao, X.; Wang, Z.; et al. Coupling ammonia nitrogen adsorption and regeneration unit with a high-load anoxic/aerobic process to achieve rapid and efficient pollutants removal for wastewater treatment. Water Res. 2020, 170, 115280. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, D.; Wang, Z.-W. The potential of using biological nitrogen removal technique for stormwater treatment. Ecol. Eng. 2017, 106, 482–495. [Google Scholar] [CrossRef]
- Morales-Figueroa, C.; Castillo-Suárez, L.A.; Linares-Hernández, I.; Martínez-Miranda, V.; Teutli-Sequeira, E.A. Treatment processes and analysis of rainwater quality for human use and consumption regulations, treatment systems and quality of rainwater. Int. J. Environ. Sci. Technol. 2023, 20, 9369–9392. [Google Scholar] [CrossRef]
- Xi, J.; Zhou, Z.; Yuan, Y.; Xiao, K.; Qin, Y.; Wang, K.; An, Y.; Ye, J.; Wu, Z. Enhanced nutrient removal from stormwater runoff by a compact on-site treatment system. Chemosphere 2022, 290, 133314. [Google Scholar] [CrossRef]
- Lahav, O.; Schwartz, Y.; Nativ, P.; Gendel, Y. Sustainable removal of ammonia from anaerobic-lagoon swine waste effluents using an electrochemically-regenerated ion exchange process. Chem. Eng. J. 2013, 218, 214–222. [Google Scholar] [CrossRef]
- Niri, M.V.; Mahvi, A.H.; Alimohammadi, M.; Shirmardi, M.; Golastanifar, H.; Mohammadi, M.J.; Naeimabadi, A.; Khishdost, M. Removal of natural organic matter (NOM) from an aqueous solution by NaCl and surfactant-modified clinoptilolite. J. Water Health 2015, 13, 394–405. [Google Scholar] [CrossRef]
- Liu, G.-H.; Wang, Y.; Zhang, Y.; Xu, X.; Qi, L.; Wang, H. Modification of natural zeolite and its application to advanced recovery of organic matter from an ultra-short-SRT activated sludge process effluent. Sci. Total. Environ. 2019, 652, 1366–1374. [Google Scholar] [CrossRef]
- Hong, N.; Li, Y.; Liu, J.; Yang, M.; Liu, A. A snapshot on trihalomethanes formation in urban stormwater: Implications for its adequacy as an alternative water resource. J. Environ. Chem. Eng. 2022, 10, 107180. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, Y.; Li, X.; Shuang, C.; Pan, Y.; Li, Y.; Li, A. Effect of ammonia on acute toxicity and disinfection byproducts formation during chlorination of secondary wastewater effluents. Sci. Total. Environ. 2022, 826, 153916. [Google Scholar] [CrossRef]
- Li, X.-F.; Mitch, W.A. Drinking Water Disinfection Byproducts (DBPs) and Human Health Effects: Multidisciplinary Challenges and Opportunities. Environ. Sci. Technol. 2018, 52, 1681–1689. [Google Scholar] [CrossRef]
- Feretti, D.; Zerbini, I.; Ceretti, E.; Villarini, M.; Zani, C.; Moretti, M.; Fatigoni, C.; Orizio, G.; Donato, F.; Monarca, S. Evaluation of chlorite and chlorate genotoxicity using plant bioassays and in vitro DNA damage tests. Water Res. 2008, 42, 4075–4082. [Google Scholar] [CrossRef]
- Kali, S.; Khan, M.; Ghaffar, M.S.; Rasheed, S.; Waseem, A.; Iqbal, M.M.; Niazi, M.B.K.; Zafar, M.I. Occurrence, influencing factors, toxicity, regulations, and abatement approaches for disinfection by-products in chlorinated drinking water: A comprehensive review. Environ. Pollut. 2021, 281, 116950. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Chu, W.; Krasner, S.W.; Yu, Y.; Fang, C.; Xu, B.; Gao, N. The stability of chlorinated, brominated, and iodinated haloacetamides in drinking water. Water Res. 2018, 142, 490–500. [Google Scholar] [CrossRef]
- Wang, X.-X.; Liu, B.-M.; Lu, M.-F.; Li, Y.-P.; Jiang, Y.-Y.; Zhao, M.-X.; Huang, Z.-X.; Pan, Y.; Miao, H.-F.; Ruan, W.-Q. Characterization of algal organic matter as precursors for carbonaceous and nitrogenous disinfection byproducts formation: Comparison with natural organic matter. J. Environ. Manag. 2021, 282, 111951. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, L.; Xue, Q.; Liu, J.; Hou, L.; Ding, L. Removal of ammonium from swine wastewater by zeolite combined with chlorination for regeneration. J. Environ. Manag. 2015, 160, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, Z.; An, Y.; Du, S.; Ruan, D.; Zhao, C.; Ren, N.; Tian, X. Optimization for zeolite regeneration and nitrogen removal performance of a hypochlorite-chloride regenerant. Chemosphere 2017, 178, 565–572. [Google Scholar] [CrossRef]
- Narbaitz, R.M.; Akerele, G.O.; Sartaj, M.; Downey, J. Chlorine regeneration of a zeolite ion-exchange column for ammonia removal from an explosives-impacted mining wastewater. Environ. Technol. 2025, 46, 1–14. [Google Scholar] [CrossRef]
- Kukučka, M.; Stojanović, N.K.; Foglar, L. Enhancement of synthetic zeolite (Crystal-Right) adsorption characteristics toward NH4+ by regeneration. Desalin. Water Treat. 2017, 80, 326–336. [Google Scholar] [CrossRef]
- Pandian, A.M.K.; Rajamehala, M.; Singh, M.V.P.; Sarojini, G.; Rajamohan, N. Potential risks and approaches to reduce the toxicity of disinfection by-product—A review. Sci. Total Environ. 2022, 822, 153323. [Google Scholar] [CrossRef]
- Rodak, C.M.; Moore, T.L.; David, R.; Jayakaran, A.D.; Vogel, J.R. Urban stormwater characterization, control, and treatment. Water Environ. Res. 2019, 91, 1034–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.T.; Zhu, S.L.; Yang, D.H.; An, Y.; Zhou, Z.; Yu, S.Q. Pilot Study on On-site Rapid Treatment Technology of Initial Rainwater. China Water Wastewater 2022, 38, 108–113. [Google Scholar] [CrossRef]
- Du, Q.; Liu, S.; Cao, Z.; Wang, Y. Ammonia removal from aqueous solution using natural Chinese clinoptilolite. Sep. Purif. Technol. 2005, 44, 229–234. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 2011. [Google Scholar]
- Li, C.; Luo, G.; Liu, Y. Comparison on the formation and toxicity for chlorinated products during the oxidation of acetic acid (CH3COOH) by three widely used advanced oxidation processes (AOPs) at the presence of Cl−. J. Environ. Chem. Eng. 2023, 11, 111501. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, X.; Zhu, Y.; Huang, Y.; Chen, B. Ecotoxicological effects of DBPs on freshwater phytoplankton communities in co-culture systems. J. Hazard. Mater. 2022, 421, 126679. [Google Scholar] [CrossRef]
- Lau, S.S.; Wei, X.; Bokenkamp, K.; Wagner, E.D.; Plewa, M.J.; Mitch, W.A. Assessing Additivity of Cytotoxicity Associated with Disinfection Byproducts in Potable Reuse and Conventional Drinking Waters. Environ. Sci. Technol. 2020, 54, 5729–5736. [Google Scholar] [CrossRef]
- Wagner, E.D.; Plewa, M.J. CHO cell cytotoxicity and genotoxicity analyses of disinfection by-products: An updated review. J. Environ. Sci. 2017, 58, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Tomazic–Jezic, V.; Lucas, A.; Lamanna, A.; Stratmeyer, M. Quantitation of Natural Rubber Latex Proteins: Evaluation of Various Protein Measurement Methods. Toxicol. Mech. Methods 1999, 9, 153–164. [Google Scholar] [CrossRef]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Baig, U.; Faizan, M.; Sajid, M. Effective removal of hazardous pollutants from water and deactivation of water-borne pathogens using multifunctional synthetic adsorbent materials: A review. J. Clean. Prod. 2021, 302, 126735. [Google Scholar] [CrossRef]
- Alomar, T.; Qiblawey, H.; Almomani, F.; Al-Raoush, R.I.; Han, D.S.; Ahmad, N.M. Recent advances on humic acid removal from wastewater using adsorption process. J. Water Process Eng. 2023, 53, 103679. [Google Scholar] [CrossRef]
- Yao, A.K.Z.; Jiun, L.W.; Yong, L.C.; Shi, C.Y.; Seng, O.B. Ammonium sorption and regeneration using Mg-modified zeolites: A study on the interferences of competing ions from aquaculture effluent. J. Water Process. Eng. 2022, 48, 102909. [Google Scholar] [CrossRef]
- Jha, V.K.; Hayashi, S. Modification on natural clinoptilolite zeolite for its NH4+ retention capacity. J. Hazard. Mater. 2009, 169, 29–35. [Google Scholar] [CrossRef]
- Moussavi, G.; Talebi, S.; Farrokhi, M.; Sabouti, R.M. The investigation of mechanism, kinetic and isotherm of ammonia and humic acid co-adsorption onto natural zeolite. Chem. Eng. J. 2011, 171, 1159–1169. [Google Scholar] [CrossRef]
- Croué, J.P.; Lefebvre, E.; Martin, B.; Legube, B. Removal of Dissolved Hydrophobic and Hydrophilic Organic Substances during Coagulation/Flocculation of Surface Waters. Water Sci. Technol. 1993, 27, 143–152. [Google Scholar] [CrossRef]
- Shi, X.; Xu, C.; Hu, H.; Tang, F.; Sun, L. Characterization of dissolved organic matter in the secondary effluent of pulp and paper mill wastewater before and after coagulation treatment. Water Sci. Technol. 2016, 74, 1346–1353. [Google Scholar] [CrossRef]
- Driss, K.; Bouhelassa, M. Modeling drinking water chlorination at the breakpoint: I. Derivation of breakpoint reactions. Desalin. Water Treat. 2014, 52, 5757–5768. [Google Scholar] [CrossRef]
- Chuang, Y.-H.; Chou, C.-S.; Chu, Y.-L. Unveiling the critical pathways of hydroxyl radical formation in breakpoint chlorination: The role of trichloramine and di-chloramine interactions. Environ. Sci. Technol. 2024, 58, 21086–21096. [Google Scholar] [CrossRef]
- Gao, J.-N.; Ma, Q.-Q.; Wang, Z.-W.; Rittmann, B.E.; Zhang, W. Direct electrosynthesis and separation of ammonia and chlorine from waste streams via a stacked membrane-free electrolyzer. Nat. Commun. 2024, 15, 8455. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Li, A.-P.; Lin, Y.-L.; Ling, X.; Cheng, M. Degradation kinetics and DBP formation during chlorination of metribuzin. J. Taiwan Inst. Chem. Eng. 2017, 80, 255–261. [Google Scholar] [CrossRef]
- Stefán, D.; Erdélyi, N.; Izsák, B.; Záray, G.; Vargha, M. Formation of chlorination by-products in drinking water treatment plants using breakpoint chlorination. Microchem. J. 2019, 149, 104008. [Google Scholar] [CrossRef]
- Liu, Z.; Tao, Y.; Zhang, Z.; He, J.; Yang, K.; Ma, J. Active chlorine mediated ammonia oxidation in an electrified SnO2–Sb filter: Reactivity, mechanisms and response to matrix effects. Sep. Purif. Technol. 2023, 312, 123369. [Google Scholar] [CrossRef]
- Nikolaou, A.D.; Lekkas, T.D. The role of natural organic matter during formation of chlorination by-products: A review. Acta Hydroch. Hydrob. 2001, 29, 63–77. [Google Scholar] [CrossRef]
- Du, Y.; Wu, Q.-Y.; Lu, Y.; Hu, H.-Y.; Yang, Y.; Liu, R.; Liu, F. Increase of cytotoxicity during wastewater chlorination: Impact factors and surrogates. J. Hazard. Mater. 2017, 324, 681–690. [Google Scholar] [CrossRef]
- Asami, M.; Kosaka, K.; Kunikane, S. Bromate, chlorate, chlorite and perchlorate in sodium hypochlorite solution used in water supply. J. Water Supply: Res. Technol. AQUA 2009, 58, 107–115. [Google Scholar] [CrossRef]
- Meng, X.; Khoso, S.A.; Lyu, F.; Wu, J.; Kang, J.; Liu, H.; Zhang, Q.; Han, H.; Sun, W.; Hu, Y. Study on the influence and mechanism of sodium chlorate on COD reduction of minerals processing wastewater. Miner. Eng. 2019, 134, 1–6. [Google Scholar] [CrossRef]
- Rougé, V.; Lee, Y.; von Gunten, U.; Allard, S. Kinetic and mechanistic understanding of chlorite oxidation during chlorination: Optimization of ClO2 pre-oxidation for disinfection byproduct control. Water Res. 2022, 220, 118515. [Google Scholar] [CrossRef]
- van Wijk, D.J.; Kroon, S.G.; Garttener-Arends, I.C. Toxicity of Chlorate and Chlorite to Selected Species of Algae, Bacteria, and Fungi. Ecotoxicol. Environ. Saf. 1998, 40, 206–211. [Google Scholar] [CrossRef]
- Jakab-Nácsa, A.; Stomp, D.; Farkas, L.; Kaptay, G. Large NaCl-effect on the Decomposition Rate of Chlorate Ions in HCl-containing Brine Solutions and Its Consequences for the Chlor-alkali Industry. Period. Polytech. Chem. Eng. 2021, 65, 238–242. [Google Scholar] [CrossRef]
- Busch, M.; Simic, N.; Ahlberg, E. Exploring the mechanism of hypochlorous acid decomposition in aqueous solutions. Phys. Chem. Chem. Phys. 2019, 21, 19342–19348. [Google Scholar] [CrossRef]
- Lu, W.; Chen, N.; Feng, C.; Deng, Y.; Zhang, J.; Chen, F. Treatment of polluted river sediment by electrochemical oxidation: Changes of hydrophilicity and acute cytotoxicity of dissolved organic matter. Chemosphere 2020, 243, 125283. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Zhao, X.; Qu, J.; Fan, M. Formation of disinfection by-products in the chlorination of ammonia-containing effluents: Significance of Cl2/N ratios and the DOM fractions. J. Hazard. Mater. 2011, 190, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Song, Z.-M.; Wu, D.-X.; Liang, J.-K.; Wang, W.-L.; Hu, W.; Yu, Y. Comparative molecular transformations of dissolved organic matter induced by chlorination and ammonia/chlorine oxidation process. Environ. Pollut. 2023, 339, 122771. [Google Scholar] [CrossRef]
- Nguyen, H.V.-M.; Lee, H.-S.; Lee, S.-Y.; Hur, J.; Shin, H.-S. Changes in structural characteristics of humic and fulvic acids under chlorination and their association with trihalomethanes and haloacetic acids formation. Sci. Total. Environ. 2021, 790, 148142. [Google Scholar] [CrossRef]
- Feng, W.; Ma, W.; Zhao, Q.; Li, F.; Zhong, D.; Deng, L.; Zhu, Y.; Li, Z.; Zhou, Z.; Wu, R.; et al. The mixed-order chlorine decay model with an analytical solution and corresponding trihalomethane generation model in drinking water. Environ. Pollut. 2023, 335, 122227. [Google Scholar] [CrossRef]
- Cai, L.; Huang, H.; Li, Q.; Deng, J.; Ma, X.; Zou, J.; Li, G.; Chen, G. Formation characteristics and acute toxicity assessment of THMs and HAcAms from DOM and its different fractions in source water during chlorination and chloramination. Chemosphere 2023, 329, 138696. [Google Scholar] [CrossRef] [PubMed]
- Malamis, S.; Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 252, 428–461. [Google Scholar] [CrossRef]
- Feng, H.; Liao, X.; Yang, R.; Chen, S.; Zhang, Z.; Tong, J.; Liu, J.; Wang, X. Generation, toxicity, and reduction of chlorinated byproducts: Overcome bottlenecks of electrochemical advanced oxidation technology to treat high chloride wastewater. Water Res. 2023, 230, 119531. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Chen, B.; Jiang, Y.; Tao, Y.; Zhu, X.; Cai, Z. Toxicity of 17 Disinfection By-products to Different Trophic Levels of Aquatic Organisms: Ecological Risks and Mechanisms. Environ. Sci. Technol. 2021, 55, 10534–10541. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Wang, Q.; Shi, Z.; Fang, Y.; Shi, S.; Wang, J.; Wu, C. Advanced treatment of wet-spun acrylic fiber manufacturing wastewater using three-dimensional electrochemical oxidation. J. Environ. Sci. 2016, 50, 21–31. [Google Scholar] [CrossRef]
- Han, J.; Zhang, X. Evaluating the Comparative Toxicity of DBP Mixtures from Different Disinfection Scenarios: A New Approach by Combining Freeze-Drying or Rotoevaporation with a Marine Polychaete Bioassay. Environ. Sci. Technol. 2018, 52, 10552–10561. [Google Scholar] [CrossRef]
- Chen, H.; Lin, T.; Chen, W.; Tao, H.; Xu, H. Removal of disinfection byproduct precursors and reduction in additive toxicity of chlorinated and chloraminated waters by ozonation and up-flow biological activated carbon process. Chemosphere 2019, 216, 624–632. [Google Scholar] [CrossRef]
- Xu, X.; Chen, G.; Wei, J.; Qin, Y.; Tang, R.; Wang, L.; Wei, H.; Zhou, C.; Wu, J.; Zhao, X.; et al. Effects of competitive cations and dissolved organic matter on ammonium exchange and up-concentration properties of ion exchangers from domestic wastewater under multicycle exchange—Regeneration operation. Sep. Purif. Technol. 2024, 345, 127274. [Google Scholar] [CrossRef]
- Koryabkina, N.; Bergendahl, J.; Thompson, R.; Giaya, A. Adsorption of disinfection byproducts on hydrophobic zeolites with regeneration by advanced oxidation. Microporous Mesoporous Mater. 2007, 104, 77–82. [Google Scholar] [CrossRef]
- Osio-Norgaard, J.; Srubar, W.V. Zeolite Adsorption of Chloride from a Synthetic Alkali-Activated Cement Pore Solution. Materials 2019, 12, 2019. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Liu, Z.; Yan, B.; Zhang, J.; Liu, D.; Xu, Y.; Wang, P.; Cui, F.; Liu, Z. Formation kinetics of disinfection byproducts in algal-laden water during chlorination: A new insight into evaluating disinfection formation risk. Environ. Pollut. 2019, 245, 63–70. [Google Scholar] [CrossRef] [PubMed]

| Water Quality Indicator | Unit | Raw Initial Stormwater | Stormwater After Coagulation–Precipitation |
|---|---|---|---|
| DOC | mg/L | 20.6 ± 0.1 | 10.6 ± 0.1 |
| COD | mg/L | 104 ± 1 | 46 ± 1 |
| SCOD | mg/L | 80 ± 1 | 35 ± 1 |
| TN | mg/L | 10.05 ± 0.05 | 9.5 ± 0.05 |
| Ammonia | mg-N/L | 10.05 ± 0.02 | 9.50 ± 0.02 |
| NO3− | mg-N/L | BDL * | BDL |
| NO2− | mg-N/L | BDL | BDL |
| Polysaccharide | mg/L | 12.24 ± 0.2 | BDL |
| Protein | mg/L | 5.16 ± 0.3 | BDL |
| Humic acid | mg/L | 18.93 ± 0.3 | 5.36 ± 0.3 |
| Alkalinity (CaCO3) | mg/L | 244.97 ± 0.2 | 219.84 ± 0.2 |
| Turbidity | NTU | 70.1 ± 0.05 | 5.13 ± 0.05 |
| SS | mg/L | 138 ± 0.5 | 10 ± 0.5 |
| Cl− | mg/L | 46.5 ± 1 | 45.0 ± 1 |
| pH | / | 7.50 ± 0.1 | 7.50 ± 0.1 |
| Model | Equation | Parameter | Ammonia (N) | HA (DOC) |
|---|---|---|---|---|
| Langmuir | qm (mg/g) | 1.69 ± 0.01 | 0.110 ± 0.002 | |
| kL (L/mg) | 0.116 ± 0.002 | 0.071 ± 0.005 | ||
| R2 | 0.996 | 0.966 | ||
| Freundlich | 1/n | 0.730 ± 0.003 | 0.519 ± 0.005 | |
| kF (mg/g) | 0.168 ± 0.002 | 0.012 ± 0.002 | ||
| R2 | 0.994 | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, W.; Li, C.; Cao, X.; Zhu, Y.; Liu, Y. Formation and Toxicity of Chlorine Species During Zeolite Regeneration by NaCl-NaClO After Stormwater Adsorption. Water 2025, 17, 1955. https://doi.org/10.3390/w17131955
Lei W, Li C, Cao X, Zhu Y, Liu Y. Formation and Toxicity of Chlorine Species During Zeolite Regeneration by NaCl-NaClO After Stormwater Adsorption. Water. 2025; 17(13):1955. https://doi.org/10.3390/w17131955
Chicago/Turabian StyleLei, Wanlin, Chenxi Li, Xinyue Cao, Yuhao Zhu, and Yan Liu. 2025. "Formation and Toxicity of Chlorine Species During Zeolite Regeneration by NaCl-NaClO After Stormwater Adsorption" Water 17, no. 13: 1955. https://doi.org/10.3390/w17131955
APA StyleLei, W., Li, C., Cao, X., Zhu, Y., & Liu, Y. (2025). Formation and Toxicity of Chlorine Species During Zeolite Regeneration by NaCl-NaClO After Stormwater Adsorption. Water, 17(13), 1955. https://doi.org/10.3390/w17131955






