A Pilot-Scale Study on Cross-Tube Ozone Catalytic Oxidation of Biochemical Tailwater in an Industrial Park in Suzhou (China)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Analytical Methods
2.3. Experimental Procedure
2.4. Catalyst Characterization
3. Results
3.1. Influence of Critical Parameters
3.1.1. Influence of HRT
3.1.2. Influence of Ozone Dosage and O3/CODin
3.1.3. Influence of pH
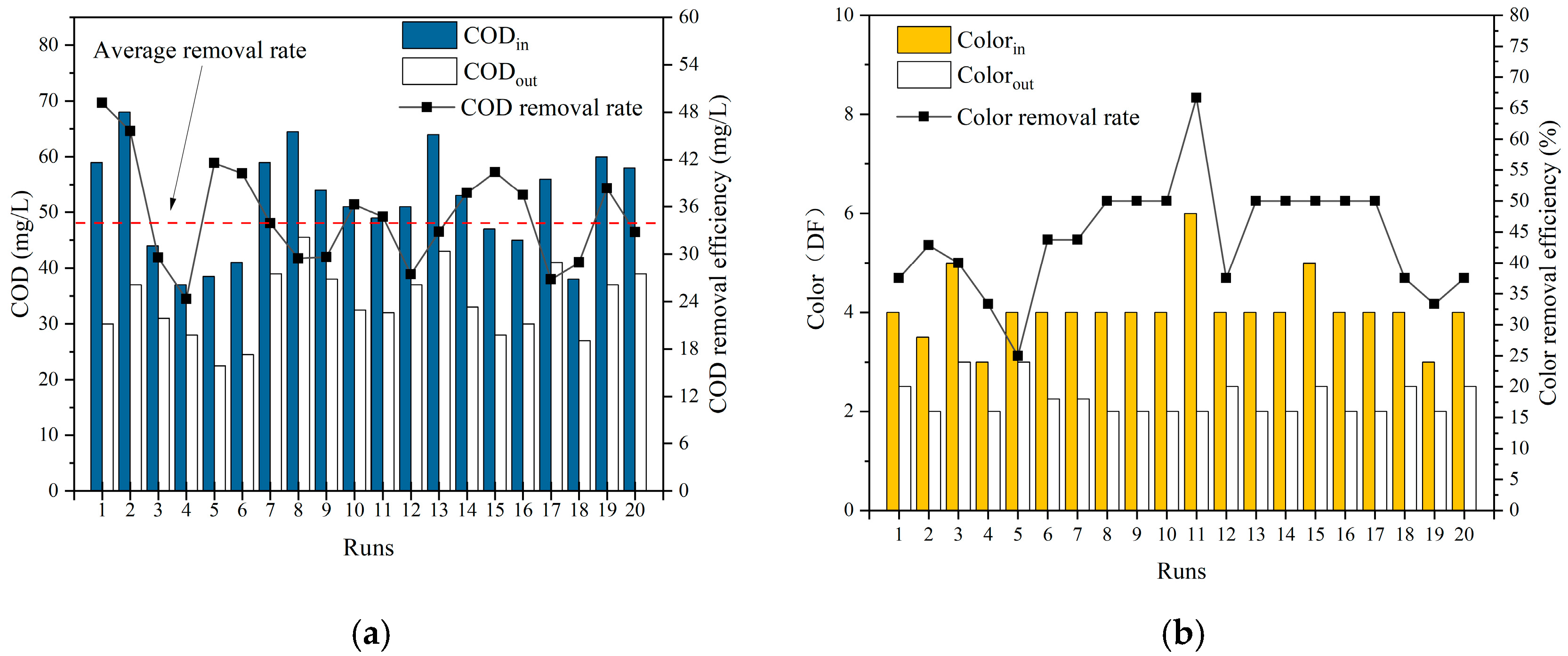
3.2. Continuous Operation Effect Verification
3.3. Pilot-Scale Reactor Kinetic Analysis
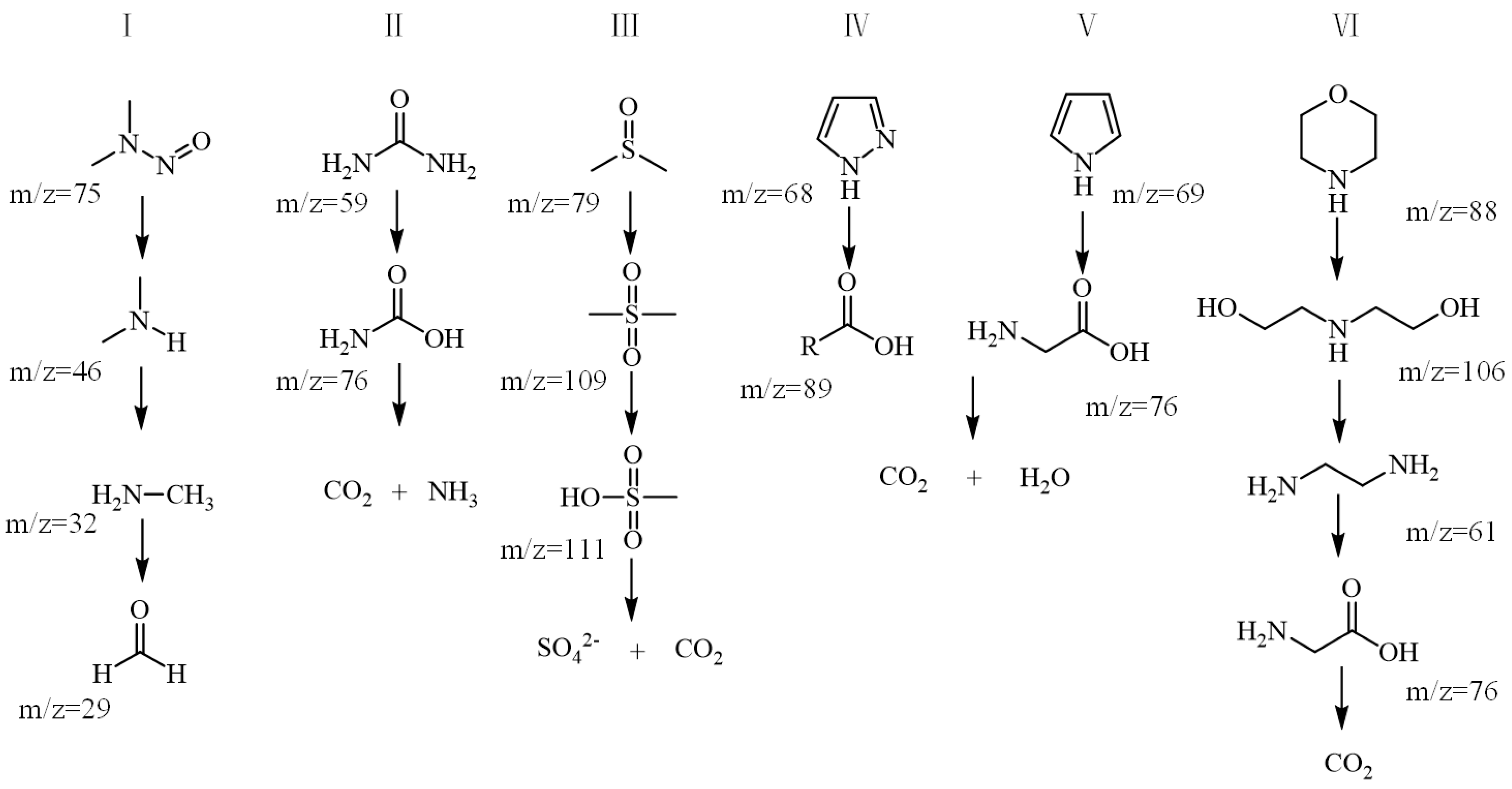
3.4. Proposed Degradation Pathways for Different Types of Pollutants
- Nitrogen-containing organics
- Sulphur-containing compounds
- Heterocyclic compounds
- Amines
3.5. Cost Analysis Estimates and Comparisons
4. Conclusions
- The cross-tube ozone catalytic oxidation device developed in this study, under the optimized parameters (HRT = 25 min, ozone dosage 43 mg/L), has a stable COD removal efficiency of 35.47% for the biochemical tail water of industrial parks, with the average value of COD in the effluent being below 50 mg/L. It also has a strong ability to resist shock loads. When the influent COD ≤ 40 mg/L, the effluent can be reduced to 30 mg/L or below. The colority removal efficiency exceeded 34%, with an average effluent colority of 2.38 DF. These results are markedly superior to those achieved by conventional tower-type ozonation processes.
- By synergistically optimizing the HRT and ozone dosage, ozone utilization efficiency was markedly enhanced. The O3/CODin ratio remained stable between 1.24 and 2.27, while the ozone dosage was cut by 46% and the retention time was shortened by 60% compared to conventional processes. At the same time, the unit treatment cost dropped to only 0.486 USD/m3—34.5% lower than the original process (0.874 USD/m3)—thereby achieving both high efficiency and cost-effectiveness.
- The device adopts a self-made biotite-based iron–manganese modified catalyst, which has both high catalytic activity and a low cost; the innovative design of the lateral aeration tangential cross-flow reactor improves the gas–liquid mass transfer efficiency and reduces the floor space, which provides technological support for the intensive design and stable operation of the catalytic ozone oxidation system in the actual project.
- Several different types of degradation products in the experimental wastewater were analyzed by LC-MS, and five possible degradation pathways were listed and discussed. Based on the detected intermediates, it was deduced that ozone and •OH generated a series of intermediates by triggering the conversion pathways of hydroxylation, cyclization, and ring-opening of organic pollutants. The catalyst’s neutral pHpzc (7.2 ± 0.2) optimally matched the wastewater’s inherent near-neutrality (mean pH 7.35), maintaining surface charge balance for maximized •OH generation. This synergy resulted in the stable operation and deep oxidation of recalcitrant pollutants to CO2/H2O.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Fu, L.; Tan, Y.; Zhou, Y.; Wu, C. Application progress of catalytic ozonation in industrial effluents treatment. Ind. Water Treat. 2022, 42, 56–65. [Google Scholar]
- Zhang, J.; Liu, P.; Ren, Y.; Du, Y.; Geng, C.; Ma, J.; Zhao, F. Treatment of shale gas produced water by magnetic CuFe2O4/TNTs hybrid heterogeneous catalyzed ozone: Efficiency and mechanisms. J. Hazard. Mater. 2022, 423, 127124. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhuo, Q.; Yu, J.; Qi, B.; Yu, B.; Fu, H. Constructing electron-enriched Cu-Mn binuclear sites for enhanced catalytic ozonation toward wastewater purification. Chem. Eng. J. 2024, 501, 157680. [Google Scholar] [CrossRef]
- An, W.; Li, X.; Ma, J.; Ma, L. Advanced treatment of industrial wastewater by ozonation with iron-based monolithic catalyst packing: From mechanism to application. Water Res. 2023, 235, 119860. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.; Wang, Y.; Liang, W.; He, S.; Yue, L. Performance study of CeO2-Co3O4/Z in ozone catalytic oxidation of antibiotic wastewater. Mater. Lett. 2025, 390, 138386. [Google Scholar] [CrossRef]
- Dai, M.; Yang, C.; Yang, L.; Lin, Y.; He, S.; Li, X.; Niu, Q.; Wu, S. Performances and mechanisms of catalytic ozonation for paint wastewater via catalysts of Fe-Mn deposited within graphitized beads of γ-Al2O3. J. Clean. Prod. 2025, 495, 145099. [Google Scholar] [CrossRef]
- Gao, E.; Xu, J.; Liu, F.; Wu, Z.; Zhu, J.; Wang, W.; Li, J.; Yao, S.; Wu, Z. Highly efficient mineralization of phenol through catalytic ozonation using urchin-like CuxCe1Oy-BTC catalysts derived from metal-organic frameworks. J. Environ. Sci. 2025, 154, 575–589. [Google Scholar] [CrossRef]
- Zhong, J.; feng Mao, X.; Wang, G.; Li, H.; Li, J.; Qu, S.; Zhao, J. Synthesis of Cu/Mn/Ce polymetallic oxide catalysts and catalytic ozone treatment of wastewater. RSC Adv. 2024, 14, 35993–36004. [Google Scholar] [CrossRef]
- Mao, H.; Ye, S.; Wang, C.; Liu, J.; Guo, Y. Co-Mn bimetallic oxide catalyzed continuously ozonation of lignin to high-value added aromatic aldehydes under mild condition. Ind. Crops Prod. 2025, 226, 120644. [Google Scholar] [CrossRef]
- Chen, W.; Bao, Y.; Li, X.; Huang, J.; Tang, Y.; Li, L. Mineralization of salicylic acid via catalytic ozonation with Fe-Cu@ SiO2 core-shell catalyst: A two-stage first order reaction. Chemosphere 2019, 235, 470–480. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, T.; Liang, M.; Wang, Y.; Xu, Z.; Zhang, D.; Li, L. Ni-Fe layered double hydroxides catalized ozonation of synthetic wastewater containing Bisphenol A and municipal secondary effluent. Chemosphere 2019, 235, 143–152. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, J.; Tang, M.; Wang, D.; Nie, Y.; Yang, L.; Dai, C.; Yang, C.; Lu, L. Surface acidity and basicity of Mg/Al hydrotalcite for 2, 4-dichlorophenoxyacetic acid degradation with ozone: Mineralization, mechanism, and implications to practical water treatment. J. Hazard. Mater. 2021, 402, 123475. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J. Catalytic ozonation of sulfamethoxazole over Fe3O4/Co3O4 composites. Chemosphere 2019, 234, 14–24. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Q.; Tang, Y.; Hu, B.; Yang, X.; Hu, Q. ZnO-MgO/Al2O3adsorption-ozone catalytic oxidation treatment of refractory organic wastewater. Environ. Chem. 2021, 40, 818–827. [Google Scholar]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Chen, B.; Liu, B.; Hounjet, L.J.; Cao, Y.; Stoyanov, S.R.; Yang, M.; Zhang, B. Advanced oxidation processes in microreactors for water and wastewater treatment: Development, challenges, and opportunities. Water Res. 2022, 211, 118047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Li, Z.X.; Yin, S.J.; Liu, W.J.; Ju, P.P.; Kong, X.H.; Liu, Y.F. Progress in the application of ozonation catalyzed by nano-catalysts. Appl. Chem. Ind. 2024, 53, 218–221+227. [Google Scholar]
- Zheng, Y.; Yu, Y.; Li, Y.; Wu, C.; Liu, W.; Liu, C. Adsorption of phosphorus or ammonia nitrogen by ceramsite made from waterworks sludge. Chin. J. Environ. Eng. 2015, 9, 756–762. [Google Scholar]
- Xian, G.; Fei, M.; Dong, T. Adsorption treatment of Cd2+ wastewater with clay based ceramsite. Ind. Water Treat. 2011, 31, 49–52. [Google Scholar]
- HJ 828-2017; Water Quality—Determination of Chemical Oxygen Demand—Potassium Dichromate Method. China Environmental Science Press: Beijing, China, 2017.
- GB 11893-89; Water Quality—Determination of Total Phosphorus—Ammonium Molybdate Spectrophotometric Method. China Standards Press: Beijing, China, 1989.
- Rakness, K.; Gordon, G.; Langlais, B.; Masschelein, W.; Matsumoto, N.; Richard, Y.; Robson, C.M.; Somiya, I. Guideline for measurement of ozone concentration in the process gas from an ozone generator. J. Int. Ozone Assoc. 1996, 18, 209–229. [Google Scholar] [CrossRef]
- Mahmood, Z.; Garg, S.; Yuan, Y.; Xie, L.; Wang, Y.; Waite, T.D. Performance evaluation and optimization of a suspension-type reactor for use in heterogeneous catalytic ozonation. Water Res. 2024, 254, 121410. [Google Scholar] [CrossRef] [PubMed]
- Fallah, N.; Bloise, E.; Santoro, D.; Mele, G. State of art and perspectives in catalytic ozonation for removal of organic pollutants in water: Influence of process and operational parameters. Catalysts 2023, 13, 324. [Google Scholar] [CrossRef]
- Long, J.; Guo, Y.; Yu, G.; Komarneni, S.; Wang, Y. Evaluation of the effect of catalysts on ozone mass transfer and pollutant abatement during laboratory catalytic ozonation experiments: Implications for practical water and wastewater treatment. ACS EST Eng. 2022, 3, 387–397. [Google Scholar] [CrossRef]
- Psaltou, S.; Mitrakas, M.; Zouboulis, A. Heterogeneous catalytic ozonation: Solution pH and initial concentration of pollutants as two important factors for the removal of micropollutants from water. Separations 2022, 9, 413. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Alnaief, M.; Al-Qodah, Z.; Al-Shannag, M.; Elnakar, H.; AlJbour, N.; Alu’datt, M.; Alrosan, M.; Ezelden, E. On the performance of electrocoagulation treatment of high-loaded gray water: Kinetic modeling and parameters optimization via response surface methodology. Appl. Water Sci. 2025, 15, 114. [Google Scholar] [CrossRef]
- GB 8978-1996; Integrated Wastewater Discharge Standard. China Standards Press: Beijing, China, 1996.
- Kragović, M.; Stojmenović, M.; Petrović, J.; Loredo, J.; Pašalić, S.; Nedeljković, A.; Ristović, I. Influence of alginate encapsulation on point of zero charge (pHpzc) and thermodynamic properties of the natural and Fe(III)-modified zeolite. Procedia Manuf. 2019, 32, 286–293. [Google Scholar] [CrossRef]
- Yan, H.; Lu, P.; Pan, Z.; Wang, X.; Zhang, Q.; Li, L. Ce/SBA-15 as a heterogeneous ozonation catalyst for efficient mineralization of dimethyl phthalate. J. Mol. Catal. A Chem. 2013, 377, 57–64. [Google Scholar] [CrossRef]
- Li, Z.; Chen, S.; Liu, L.; Qian, D.; Yuan, M.; Yu, J.; Chen, Z.; Yang, J.; Su, X.; Hu, J. Formation mechanism of persistent free radicals during pyrolysis of Fenton-conditioned sewage sludge: Influence of NOM and iron. Water Res. 2024, 254, 121376. [Google Scholar] [CrossRef]
- Hendrasarie, N.; Ramadhani, A.N.; Rosariawari, F.; Amalia, A. Hybrid Biofilter-Adsorption Performance to Removal Organic Matter and Color on Black Soybean Sauce Wastewater. IOP Conf. Ser. Earth Environ. Sci. 2025, 1454, 012030. [Google Scholar] [CrossRef]
- Flyunt, R.; Leitzke, A.; Mark, G.; Mvula, E.; Reisz, E.; Schick, R.; von Sonntag, C. Determination of •OH, O2•−, and hydroperoxide yields in ozone reactions in aqueous solution. J. Phys. Chem. B 2003, 107, 7242–7253. [Google Scholar] [CrossRef]
- Zhang, S.; Quan, X.; Zheng, J.-F.; Wang, D. Probing the interphase “HO zone” originated by carbon nanotube during catalytic ozonation. Water Res. 2017, 122, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, L.; Shen, Z.; Liao, S.; An, W. Operation of iron-based monolithic catalyst packing ozonation for industry wastewater advanced treatment: The importance of gas–liquid flow patterns and wastewater flow regimes. Chem. Eng. J. 2024, 494, 153117. [Google Scholar] [CrossRef]
- Hai, J.; Zhang, G.; Cheng, J. Estimation of mass transfer coefficient in ozone absorption by linear least square fitting and Simplex search methods. J. Cent. South Univ. 2012, 19, 3396–3399. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, J.; Allinson, M.; Gray, S.; Scales, P.J. A chemical credit framework to predict the removal performance of organic chemicals of concern from water through an ozonation process. Water Res. 2023, 232, 119671. [Google Scholar] [CrossRef]
- Song, Y.; Peng, J.; Zhao, Z.; Qin, W.; Jiang, J.; Ma, J. Novel insights into the control of N-nitrosodimethylamine (NDMA) and bromate formation during heterogeneous catalytic ozonation. Chem. Eng. J. 2023, 474, 145913. [Google Scholar] [CrossRef]
- Kim, J.Y.U.; Bessegato, G.G.; de Souza, B.C.; da Silva, J.J.; Zanoni, M.V.B. Efficient treatment of swimming pool water by photoelectrocatalytic ozonation: Inactivation of Candida parapsilosis and mineralization of Benzophenone-3 and urea. Chem. Eng. J. 2019, 378, 122094. [Google Scholar] [CrossRef]
- Jabesa, A.; Ghosh, P. A comparative study on the removal of dimethyl sulfoxide from water using microbubbles and millibubbles of ozone. J. Water Process Eng. 2021, 40, 101937. [Google Scholar] [CrossRef]
- Tekle-Röttering, A.; Lim, S.; Reisz, E.; Lutze, H.V.; Abdighahroudi, M.S.; Willach, S.; Schmidt, W.; Tentscher, P.R.; Rentsch, D.; McArdell, C.S. Reactions of pyrrole, imidazole, and pyrazole with ozone: Kinetics and mechanisms. Environ. Sci. Water Res. Technol. 2020, 6, 976–992. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, S.; Mao, Y.; Zhou, Y.; Cheng, X. Ce-modified NH2-MIL-88B enhances catalytic ozonation for effective antibiotic degradation. Environ. Res. 2025, 275, 121388. [Google Scholar] [CrossRef]
- Strotmann, U.; Weberruß, U.; Bias, W. Degradation of morpholine in several biodegradation tests and in wastewater treatment plants. Chemosphere 1993, 26, 1729–1742. [Google Scholar] [CrossRef]
- Araújo, C.V.; Nascimento, R.B.; Oliveira, C.A.; Strotmann, U.J.; da Silva, E.M. The use of Microtox® to assess toxicity removal of industrial effluents from the industrial district of Camaçari (BA, Brazil). Chemosphere 2005, 58, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Quaff, A.; Venkatesh, S.; Venkatesh, K. Degradation of azo dye by ozone oxidation: Cost analysis and buffering effects on dye decomposition. Natl. Acad. Sci. Lett. 2021, 44, 339–341. [Google Scholar] [CrossRef]
- Chiavola, A.; Salvati, C.; Bongirolami, S.; Di Marcantonio, C.; Boni, M.R. Techno-economic evaluation of ozone-oxidation for sludge reduction at the full-scale. Comparison between the application to the return activated sludge (RAS) and the sludge digestion unit. J. Water Process Eng. 2021, 42, 102114. [Google Scholar] [CrossRef]
- Wu, C.-H.; Ng, H.-Y. Degradation of CI Reactive Red 2 (RR2) using ozone-based systems: Comparisons of decolorization efficiency and power consumption. J. Hazard. Mater. 2008, 152, 120–127. [Google Scholar] [CrossRef] [PubMed]
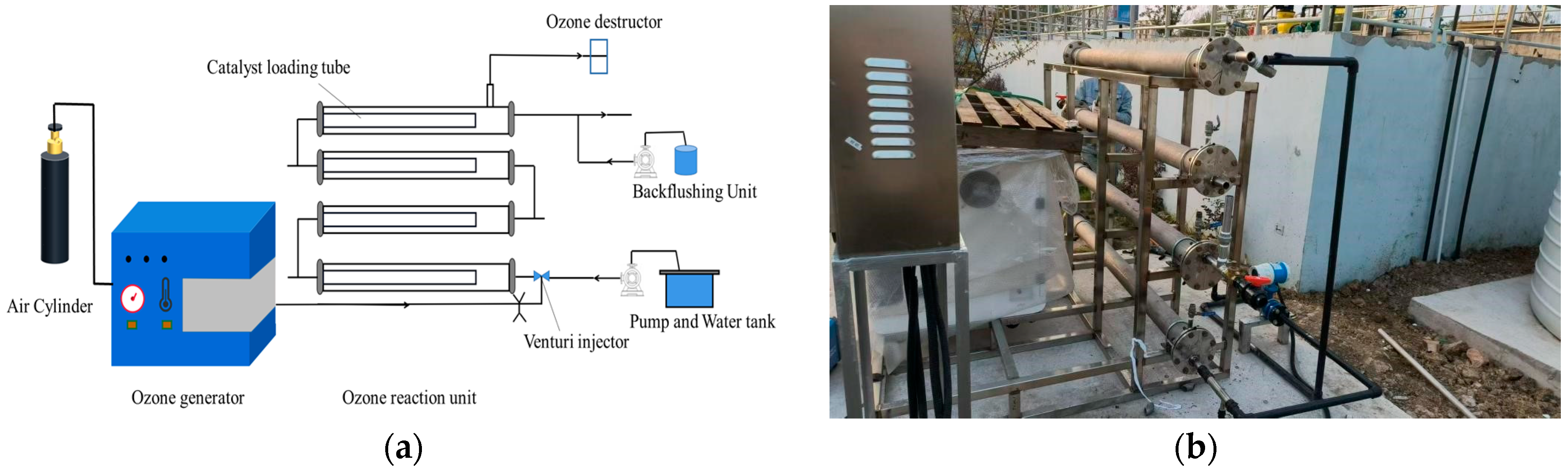
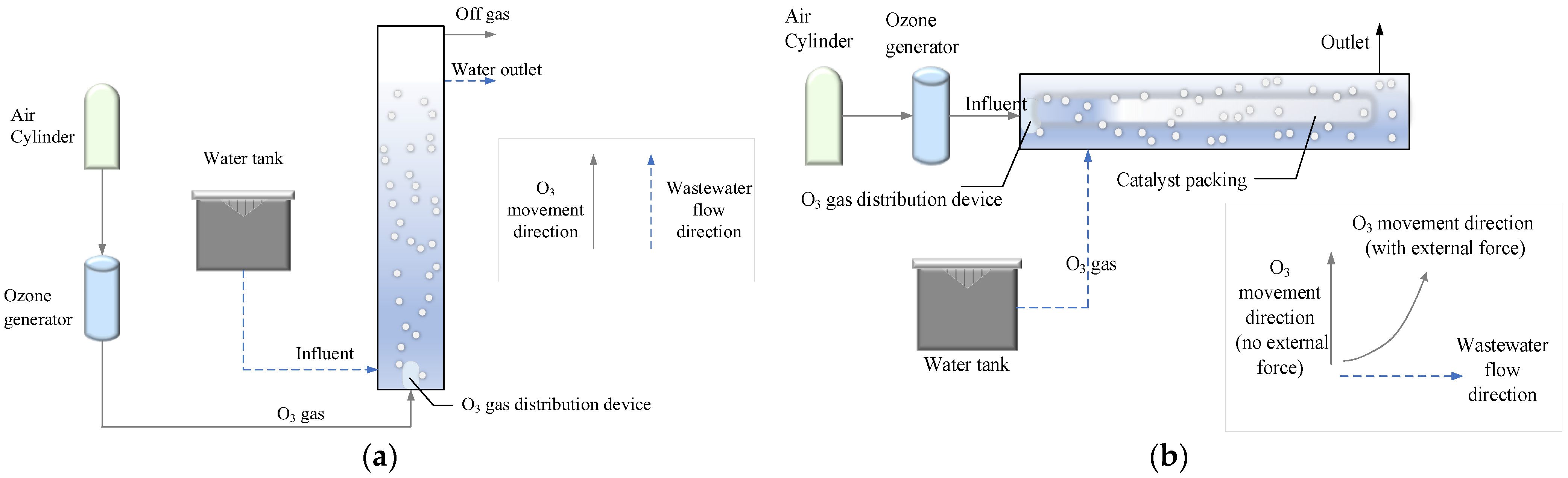
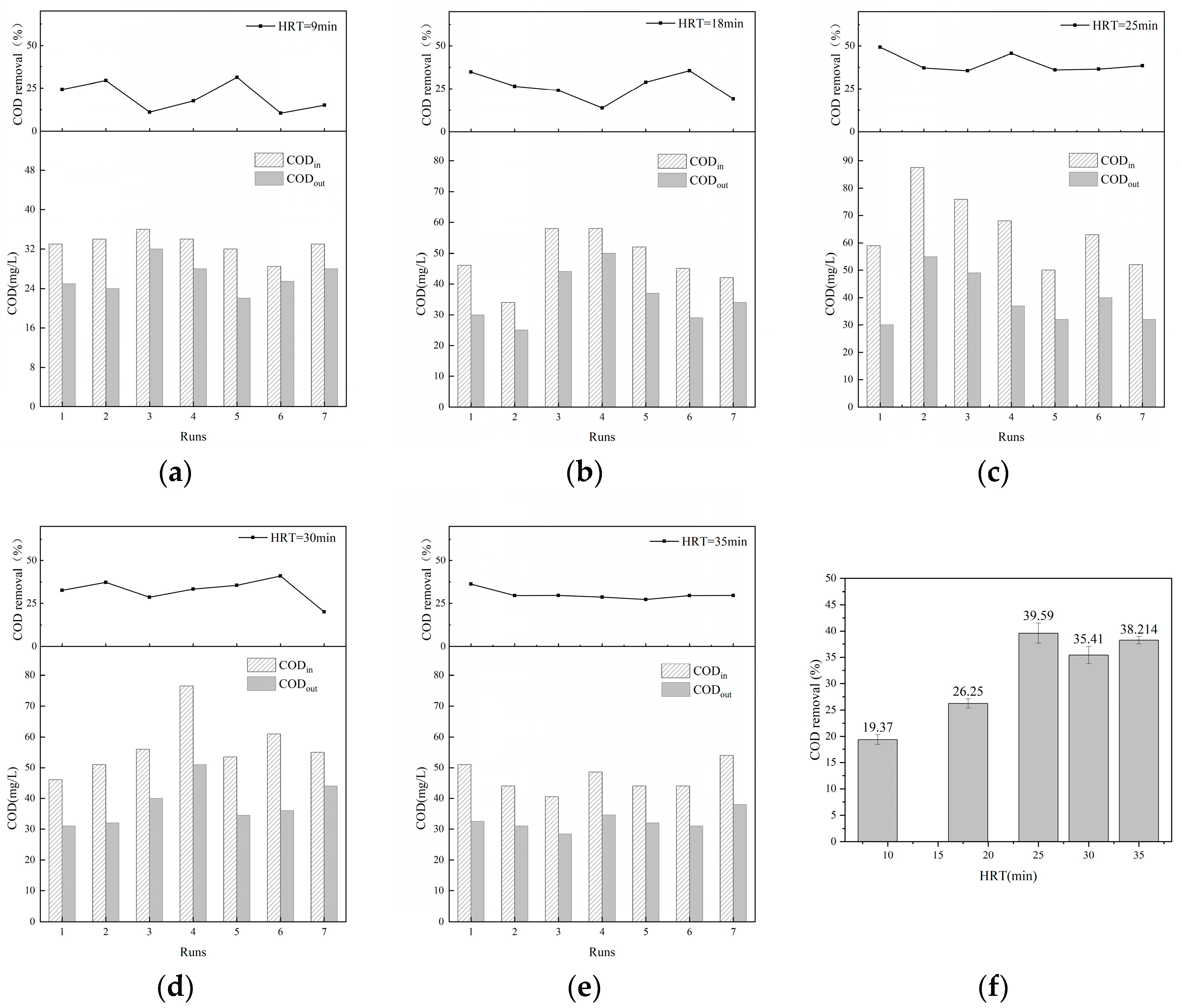
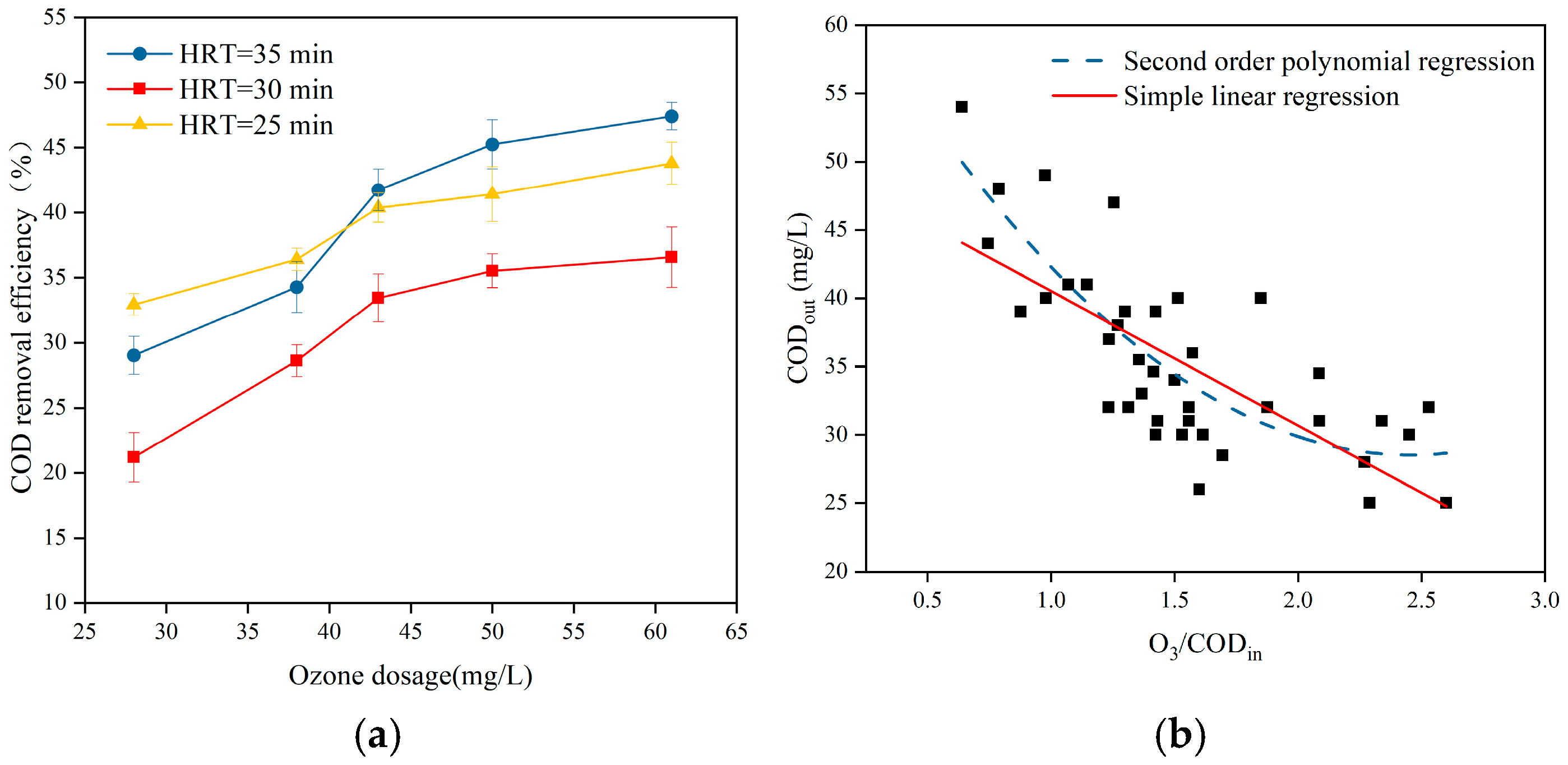


| Parameter | pH | COD (mg/L) | NH3-N (mg/L) | TP (mg/L) | SS (mg/L) | Colority (DF) |
|---|---|---|---|---|---|---|
| Average value ± standard deviation | 7.35 ± 0.16 | 56.21 ± 14.20 | 0.89 ± 0.52 | 0.16 ± 0.17 | 6.06 ± 1.48 | 4.08 ± 0.77 |
| Parameter | Influent | Effluent | Average Removal Efficiency | ||
|---|---|---|---|---|---|
| Range | Average Value | Range | Average Value | ||
| COD (mg/L) | 82.5~29.7 | 56.22 | 57.5~22.5 | 36.28 | 35.47% |
| TP (mg/L) | 0.77~0.02 | 0.16 | 0.65~0.015 | 0.13 | 18.75% |
| SS (mg/L) | 11~3.5 | 6.06 | 7.5~2.5 | 4.44 | 26.68% |
| pH | 6.94~7.9 | 7.35 | 6.9~7.66 | 7.24 | - |
| Colority (DF) | 6~2 | 4.27 | 4~2 | 2.38 | 44.26% |
| Compound | Chemical Structure | Type |
|---|---|---|
| N-nitrosodimethylamine (NDMA) | 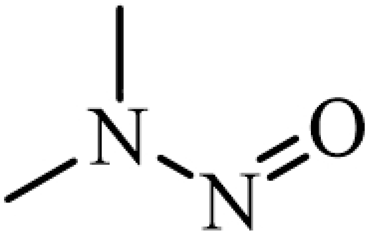 | Nitrogen-containing organics |
| Urea | 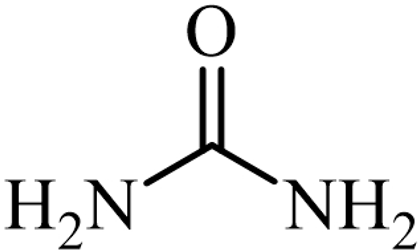 | |
| Dimethyl sulfoxide (DMSO) | 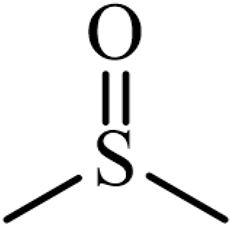 | Sulphur-containing compounds |
| Pyrrole | 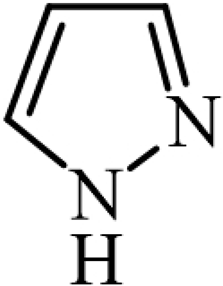 | Heterocyclic compounds |
| Pyrazole |  | |
| Morpholine | 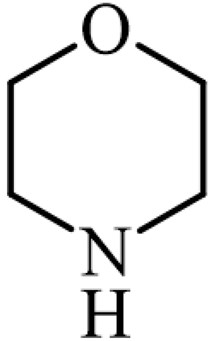 | Amines |
| Scheme | Ozone Dosage (mg/L) | HRT (min) | ΔCOD (mg/L) |
|---|---|---|---|
| Former ozone catalytic oxidation | 81 ± 5 | 70 | 20~10 |
| Cross-tube ozone catalytic oxidation | 42 ± 2 | 25 | 30~15 |
| Scheme | Items | Cost (USD/m3) | Total Operational Costs (USD/m3) |
|---|---|---|---|
| Former ozone catalytic oxidation | Maintenance of equipment | 0.026 | 0.121 |
| Electricity bill for operational costs | 0.028 | ||
| Liquid oxygen | 0.050 | ||
| Catalyst costs | 0.017 | ||
| Cross-tube ozone catalytic oxidation | Maintenance of equipment | 0.024 | 0.067 |
| Catalyst costs | 0.009 | ||
| Electricity bill for operational costs | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, P.; Cui, K.; Sun, S.; Wang, J. A Pilot-Scale Study on Cross-Tube Ozone Catalytic Oxidation of Biochemical Tailwater in an Industrial Park in Suzhou (China). Water 2025, 17, 1953. https://doi.org/10.3390/w17131953
Wei P, Cui K, Sun S, Wang J. A Pilot-Scale Study on Cross-Tube Ozone Catalytic Oxidation of Biochemical Tailwater in an Industrial Park in Suzhou (China). Water. 2025; 17(13):1953. https://doi.org/10.3390/w17131953
Chicago/Turabian StyleWei, Pengyu, Kangping Cui, Shijie Sun, and Jiao Wang. 2025. "A Pilot-Scale Study on Cross-Tube Ozone Catalytic Oxidation of Biochemical Tailwater in an Industrial Park in Suzhou (China)" Water 17, no. 13: 1953. https://doi.org/10.3390/w17131953
APA StyleWei, P., Cui, K., Sun, S., & Wang, J. (2025). A Pilot-Scale Study on Cross-Tube Ozone Catalytic Oxidation of Biochemical Tailwater in an Industrial Park in Suzhou (China). Water, 17(13), 1953. https://doi.org/10.3390/w17131953





