Differential Adsorption Behaviors of Light and Heavy SPM Fractions on Three Antibiotics: Implications for Lacustrine Antibiotic Migration
Abstract
1. Introduction
2. Materials and Methods
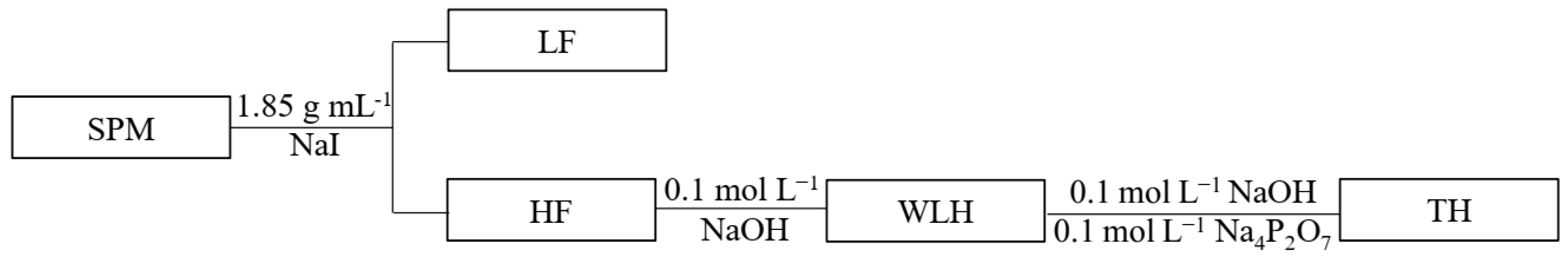
2.1. SPM Collection and Preparation
2.2. Chemicals
2.3. Experimental Design
2.3.1. Kinetic Adsorption Experiments
2.3.2. Isotherm Adsorption Experiments
2.4. Analytical Methods
2.5. Data Analysis
3. Results and Discussion
3.1. Physicochemical Properties of the Samples
3.2. Adsorption Kinetics
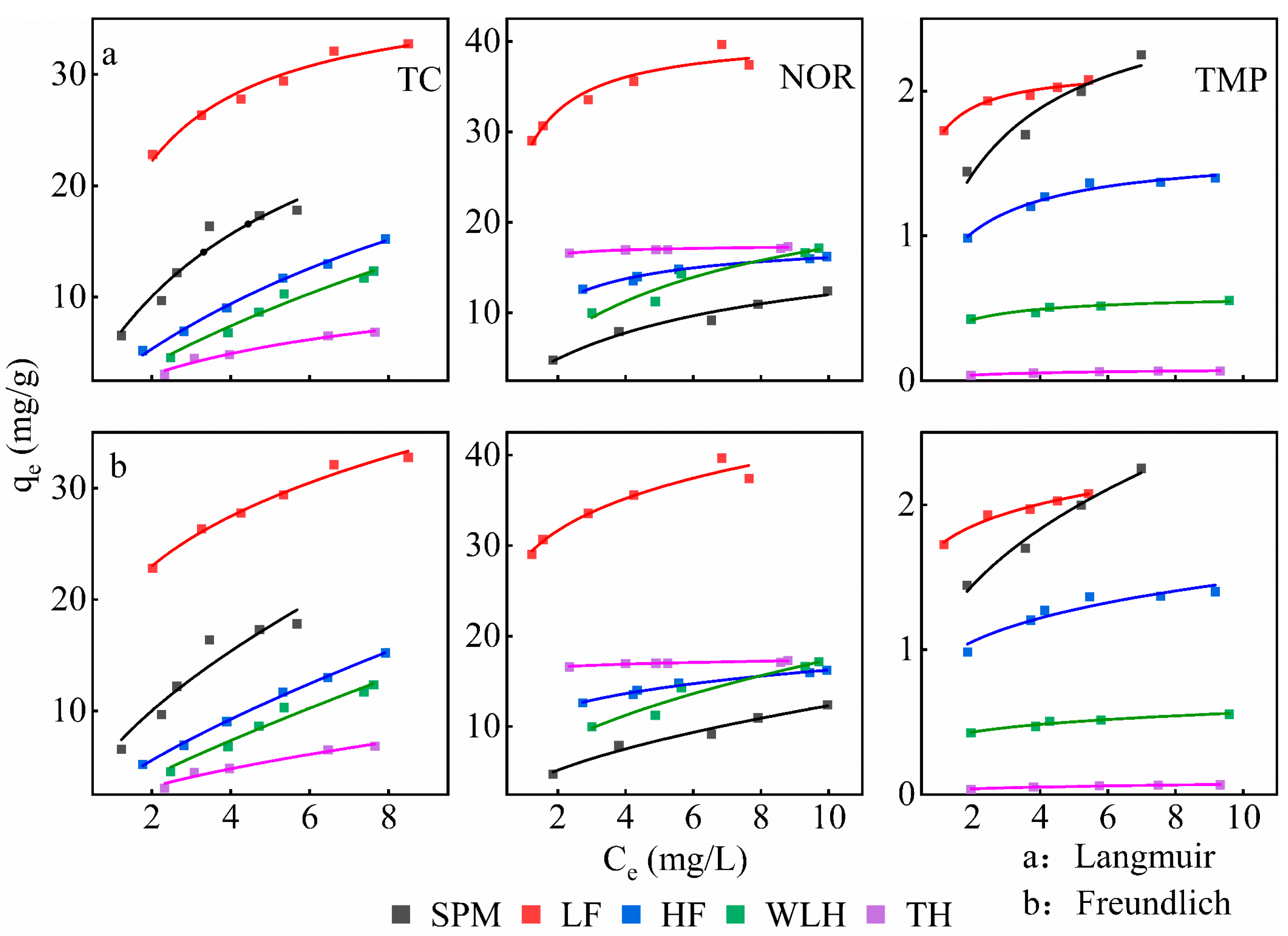
3.3. Adsorption Isotherms
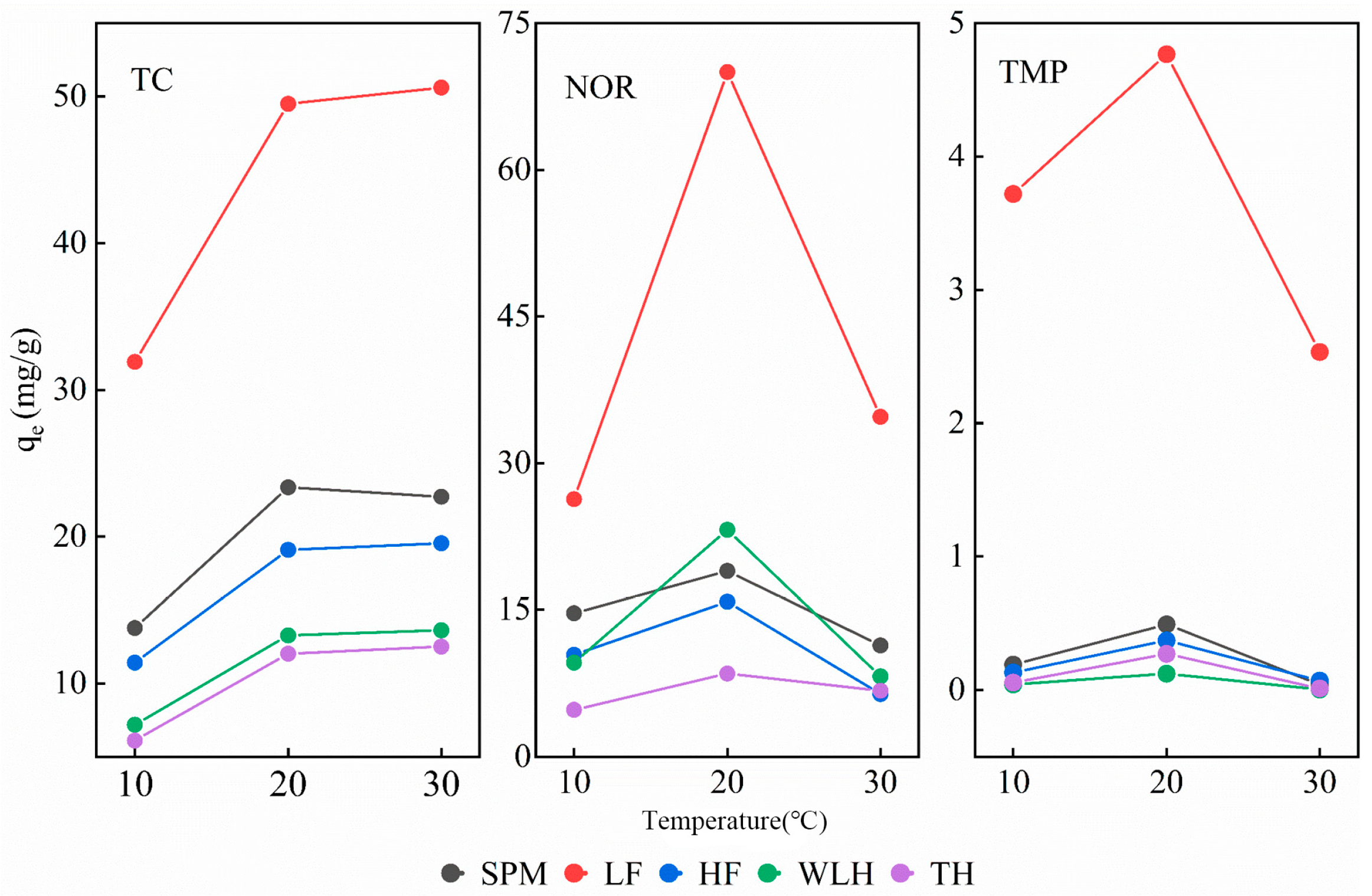
3.4. Effect of Temperature on Adsorption
3.5. Desorption Experiment
3.6. Mechanism Analysis for Adsorption System
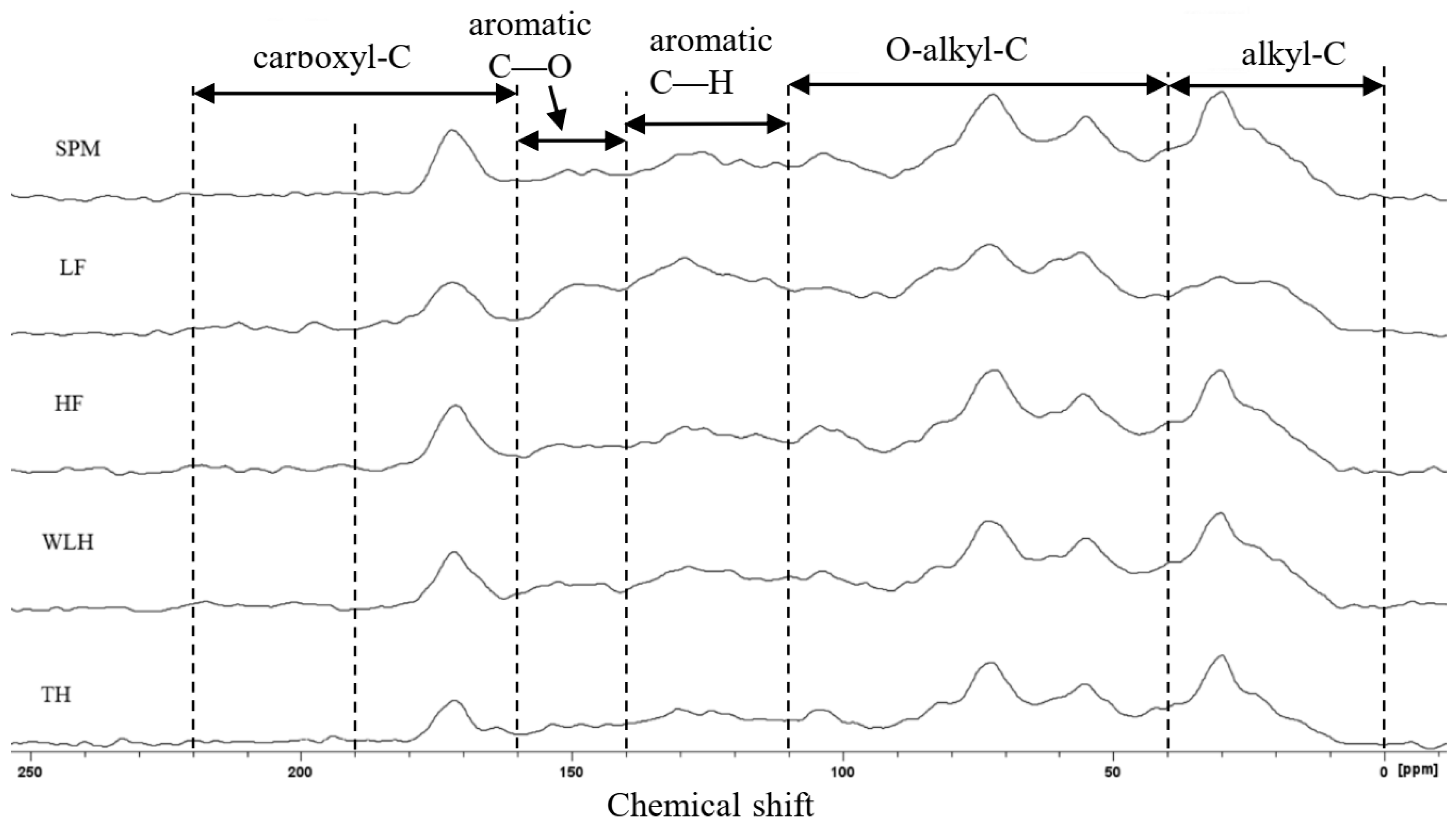
3.6.1. Analysis of Major Adsorption Functional Groups
3.6.2. The Possible Reason of Differences in Antibiotic Adsorption by LF and HF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Ying, G.; Pan, C.; Liu, Y.; Zhao, J. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Kovalakova, P.; Cizmas, L.; McDonald, T.; Marsalek, B.; Feng, M.; Sharma, V. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yang, J.; Cheng, F.; Zhang, S.; Sun, J. Adsorption and migration of sulfamethoxazole driven by suspended particulate matter in water body. Mar. Pollut. Bull. 2025, 211, 117488. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, R.; Gui, Q.; Gao, Y.; Wang, Q.; Xu, S. Fine particulate matter as a key factor promoting the spread of antibiotics in river network. Sci. Total Environ. 2024, 935, 17323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Duan, Y.; Zhang, Z.; Tu, Y.; Luo, P.; Gao, J.; Dai, C.; Zhou, L. Multimedia fate model and risk assessment of typical antibiotics in the integrated demonstration zone of the Yangtze River Delta. China Sci. Total Environ. 2022, 805, 150258. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, Z.; Su, D.; Yi, C.; Zhang, Y.; Shang, J.; Liu, Y.; Guo, R.; Chen, J.; Liao, Q. Algal blooms drive ciprofloxacin movement in lake water through adsorption: A microcosm study. Chem. Eng. J. 2025, 505, 15948. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, D.; Zheng, Y.; Yang, Y.; He, Y.; Luo, L.; Zhou, Y. Current progress in the adsorption, transport and biodegradation of antibiotics in soil. J. Environ. Manag. 2019, 251, 109598. [Google Scholar] [CrossRef]
- Xu, X.-R.; Li, X.-Y. Sorption and desorption of antibiotic tetracycline on marine sediments. Chemosphere 2010, 78, 430–436. [Google Scholar] [CrossRef]
- Shen, Z.; Sun, Y.; Yang, Y.; Zheng, X.; Shang, J.; Liu, Y.; Guo, R.; Chen, J.; Liao, Q. Influence by varying organic matter content and forms in suspended particulate matter: Impacts on the adsorption of tetracycline and norfloxacin. Environ. Sci. Pollut. Res. 2023, 30, 112409–112421. [Google Scholar] [CrossRef]
- Onodera, M.; Kirishima, A.; Nagao, S.; Takamiya, K.; Ohtsuki, T.; Akiyama, D.; Sato, N. Desorption of radioactive cesium by seawater from the suspended particles in river water. Chemosphere 2017, 185, 806–815. [Google Scholar] [CrossRef]
- Li, H.; Duan, D.; Beckingham, B.; Yang, Y.; Ran, Y.; Grathwohl, P. Impact of trophic levels on partitioning and bioaccumulation of polycyclic aromatic hydrocarbons in particulate organic matter and plankton. Mar. Pollut. Bull. 2020, 160, 111527. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Luo, Y.; Zhu, R.; Wang, H.; Huang, B. Sorption of oxytetracycline in particulate organic matter in soils and sediments: Roles of pH, ionic strength and temperature. Sci. Total Environ. 2020, 714, 136628. [Google Scholar] [CrossRef] [PubMed]
- Conde-Cid, M.; Fernandez-Calvino, D.; Nunez-Delgado, A.; Fernandez-Sanjurjo, M.J.; Arias-Estevez, M.; Alvarez-Rodriguez, E. Estimation of adsorption/desorption Freundlich’s affinity coefficients for oxytetracycline and chlortetracycline from soil properties: Experimental data and pedotransfer functions. Ecotoxicol. Environ. Saf. 2020, 196, 110584. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, J.; Wu, C.; Zhang, J.; Tang, J.; Shang, J.; Liao, Q. Effect of particle size on adsorption of norfloxacin and tetracycline onto suspended particulate matter in lake. Environ. Pollut. 2019, 244, 549–559. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Huang, Y.; Zhang, L.; Ye, F.; Wang, J.; Shang, J.; Liao, Q. Impact of sediment characteristics on adsorption behavior of typical antibiotics in Lake Taihu, China. Sci. Total Environ. 2020, 718, 137329. [Google Scholar] [CrossRef]
- Ramnarine, R.; Voroney, R.P.; Dunfield, K.E.; Wagner-Riddle, C. Characterization of the heavy, hydrolysable and non-hydrolysable fractions of soil organic carbon in conventional and no-tillage soils. Soil Tillage Res. 2018, 181, 144–151. [Google Scholar] [CrossRef]
- Ni, J.; Luo, Y.; Wei, R.; Li, X. Distribution patterns of polycyclic aromatic hydrocarbons among different organic carbon fractions of polluted agricultural soils. Geoderma 2008, 146, 277–282. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A.; Six, J.; Kaiser, M.; Benbi, D.; Chenu, C.; Cotrufo, M.F.; Derrien, D.; Gioacchini, P.; Grand, S.; et al. Isolating organic carbon fractions with varying turnover rates in temperate agricultural soils—A comprehensive method comparison. Soil Biol. Biochem. 2018, 125, 10–26. [Google Scholar] [CrossRef]
- Chai, Y.; Ma, S.; Zeng, X.; E, S.; Che, Z.; Li, L.; Duan, R.; Su, S. Long-term fertilization effects on soil organic carbon stocks in the irrigated desert soil of NW China. J. Plant Nutr. Soil Sci. 2015, 178, 622–630. [Google Scholar] [CrossRef]
- Jiang, D.; Li, J.C.; Wang, S.; Li, H.P.; Qian, L.L.; Li, B.; Cheng, X.X.; Hu, Y.M.; Hu, X. Cyclic Compound Formation Mechanisms during Pyrolysis of Typical Aliphatic Acidic Amino Acids. ACS Sustain. Chem. Eng. 2020, 8, 16968–16978. [Google Scholar] [CrossRef]
- Wei, M.; Marrakchi, F.; Yuan, C.; Cheng, X.; Jiang, D.; Zafar, F.F.; Fu, Y.; Wang, S. Adsorption modeling, thermodynamics, and DFT simulation of tetracycline onto mesoporous and high-surface-area NaOH-activated macroalgae carbon. J. Hazard. Mater. 2022, 425, 127887. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yu, H.; Chen, M.; Ge, C. Sorption of Atrazine in Tropical Soil by Biochar Prepared from Cassava Waste. Bioresources 2014, 9, 6627–6643. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, J.; Mo, Y.Z.; Shen, C.D.; Ding, P.; Wang, N.; Zhu, S.Y.; Cheng, Z.N.; He, J.Z.; Tian, Y.K.; et al. Isolation and radiocarbon analysis of elemental carbon in atmospheric aerosols using hydropyrolysis. Atmos. Environ. 2019, 198, 381–386. [Google Scholar] [CrossRef]
- Keiluweit, M.; Kleber, M. Molecular-Level Interactions in Soils and Sediments: The Role of Aromatic pi-Systems. Environ. Sci. Technol. 2009, 43, 3421–3429. [Google Scholar] [CrossRef]
- Cornelissen, G.; Gustafsson, O. Sorption of phenanthrene to environmental black carbon in sediment with and without organic matter and native sorbates. Environ. Sci. Technol. 2004, 38, 148–155. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, X.; Gao, S.; Geng, J.; Wang, X. Adsorption of tetracycline (TC) onto montmorillonite: Cations and humic acid effects. Geoderma 2012, 183, 12–18. [Google Scholar] [CrossRef]
- Angove, M.J.; Wells, J.D.; Johnson, B.B. Adsorption of cadmium(II) onto goethite and kaolinite in the presence of benzene carboxylic acids. Colloids Surf. A Physicochem. Eng. Asp. 1999, 146, 243–251. [Google Scholar] [CrossRef]
- Gu, C.; Karthikeyan, K.G. Sorption of the antibiotic tetracycline to humic-mineral complexes. J. Environ. Qual. 2008, 37, 704–711. [Google Scholar] [CrossRef]
- Ho, Y.S.; Ng, J.C.Y.; McKay, G. Kinetics of pollutant sorption by biosorbents: Review. Sep. Purif. Methods 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Wang, C.-J.; Li, Z.; Jiang, W.-T. Adsorption of ciprofloxacin on 2:1 dioctahedral clay minerals. Appl. Clay Sci. 2011, 53, 723–728. [Google Scholar] [CrossRef]
- Ajmal, Z.; Muhmood, A.; Usman, M.; Kizito, S.; Lu, J.; Dong, R.; Wu, S. Phosphate removal from aqueous solution using iron oxides: Adsorption, desorption and regeneration characteristics. J. Colloid Interface Sci. 2018, 528, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Zhang, L.; Song, C.; Zhang, M.; Zhu, H.; Zhang, L. Characterization of modified wheat straw, kinetic and equilibrium study about copper ion and methylene blue adsorption in batch mode. Carbohydr. Polym. 2010, 79, 1140–1149. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Bao, N.; Cheng, C.; Ren, L.; Zhang, C. Textural properties and surface chemistry of lotus stalk-derived activated carbons prepared using different phosphorus oxyacids: Adsorption of trimethoprim. J. Hazard. Mater. 2012, 235, 367–375. [Google Scholar] [CrossRef]
- Liu, L.; Wan, Y.; Xie, Y.; Zhai, R.; Zhang, B.; Liu, J. The removal of dye from aqueous solution using alginate-halloysite nanotube beads. Chem. Eng. J. 2012, 187, 210–216. [Google Scholar] [CrossRef]
- Salihi, E.C.; Wang, J.; Kabacaoglu, G.; Kirkulak, S.; Siller, L. Graphene oxide as a new generation adsorbent for the removal of antibiotics from waters. Sep. Sci. Technol. 2021, 56, 453–461. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Huang, D.; Liu, C.; Zhang, C.; Deng, R.; Wang, R.; Xue, W.; Luo, H.; Zeng, G.; Zhang, Q.; Guo, X. Cr(VI) removal from aqueous solution using biochar modified with Mg/Al-layered double hydroxide intercalated with ethylenediaminetetraacetic acid. Bioresour. Technol. 2019, 276, 127–132. [Google Scholar] [CrossRef]
- Deng, R.; Huang, D.; Zeng, G.; Wan, J.; Xue, W.; Wen, X.; Liu, X.; Chen, S.; Li, J.; Liu, C.; et al. Decontamination of lead and tetracycline from aqueous solution by a promising carbonaceous nanocomposite: Interaction and mechanisms insight. Bioresour. Technol. 2019, 283, 277–285. [Google Scholar] [CrossRef]
- Chao, Y.; Zhu, W.; Wu, X.; Hou, F.; Xun, S.; Wu, P.; Ji, H.; Xu, H.; Li, H. Application of graphene-like layered molybdenum disulfide and its excellent adsorption behavior for doxycycline antibiotic. Chem. Eng. J. 2014, 243, 60–67. [Google Scholar] [CrossRef]
- Sun, J.; Cui, L.; Gao, Y.; He, Y.; Liu, H.; Huang, Z. Environmental application of magnetic cellulose derived from Pennisetum sinese Roxb for efficient tetracycline removal. Carbohydr. Polym. 2021, 251, 117004. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.; Ge, C.; Muller, K.; Yu, H.; Huang, P.; Li, J.; Tsang, D.C.W.; Bolan, N.S.; Rinklebe, J.; et al. Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems. Bioresour. Technol. 2018, 263, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, H.; Zheng, X.; Yu, T.; Chen, Y. Tetracycline-induced effects on the nitrogen transformations in sediments: Roles of adsorption behavior and bacterial activity. Sci. Total Environ. 2019, 695, 133811. [Google Scholar] [CrossRef]
- Fernandez-Calvino, D.; Bermudez-Couso, A.; Arias-Estevez, M.; Carlos Novoa-Munoz, J.; Fernandez-Sanjurjo, M.J.; Alvarez-Rodriguez, E.; Nunez-Delgado, A. Competitive adsorption/desorption of tetracycline, oxytetracycline and chlortetracycline on two acid soils: Stirred flow chamber experiments. Chemosphere 2015, 134, 361–366. [Google Scholar] [CrossRef]
- Leal, R.M.P.; Figueira, R.F.; Tornisielo, V.L.; Regitano, J.B. Occurrence and sorption of fluoroquinolones in poultry litters and soils from sao Paulo State, Brazil. Sci. Total Environ. 2012, 432, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Bialk-Bielinska, A.; Maszkowska, J.; Mrozik, W.; Bielawska, A.; Kolodziejska, M.; Palavinskas, R.; Stepnowski, P.; Kumirska, J. Sulfadimethoxine and sulfaguanidine: Their sorption potential on natural soils. Chemosphere 2012, 86, 1059–1065. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Seibicke, T.; Schulten, H.R.; Leinweber, P. Sorption of sulfonamide pharmaceutical antibiotics on whole soils and particle-size fractions. J. Environ. Qual. 2004, 33, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, Y.; Shen, G.; Zhang, H.; Yuan, Z.; Zhang, W. Adsorption/desorption behavior and mechanisms of sulfadiazine and sulfamethoxazole in agricultural soil systems. Soil Tillage Res. 2019, 186, 233–241. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sarmah, A.K. Characterisation of agricultural waste-derived biochars and their sorption potential for sulfamethoxazole in pasture soil: A spectroscopic investigation. Sci. Total Environment. 2015, 502, 471–480. [Google Scholar] [CrossRef]
- Wang, F.; Ren, F.; Mu, P.; Zhu, Z.; Sun, H.; Ma, C.; Xiao, C.; Liang, W.; Chen, L.; Li, A. Hierarchical porous spherical-shaped conjugated microporous polymers for the efficient removal of antibiotics from water. J. Mater. Chem. A 2017, 5, 11348–11356. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, B.; Shen, J.; Yan, P.; Kang, J.; Wang, W.; Bi, L.; Zhu, X.; Li, Y.; Wang, S.; et al. Preparation of novel N-doped biochar and its high adsorption capacity for atrazine based on pi-pi electron donor-acceptor interaction. J. Hazard. Mater. 2022, 432, 128757. [Google Scholar] [CrossRef]
- Yan, B.; Niu, C.H. Modeling and site energy distribution analysis of levofloxacin sorption by biosorbents. Chem. Eng. J. 2017, 307, 631–642. [Google Scholar] [CrossRef]
- Xue, D.-M.; Qi, S.-C.; Zeng, Q.-Z.; Lu, R.-J.; Long, J.-H.; Luo, C.; Liu, X.-Q.; Sun, L.-B. Fabrication of nitrogen-doped porous carbons derived from ammoniated copolymer precursor: Record-high adsorption capacity for indole. Chem. Eng. J. 2019, 374, 1005–1012. [Google Scholar] [CrossRef]
- Tang, J.; Lv, H.; Gong, Y.; Huang, Y. Preparation and characterization of a novel graphene/biochar composite for aqueous phenanthrene and mercury removal. Bioresour. Technol. 2015, 196, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Niu, C.H. Adsorption behavior of norfloxacin and site energy distribution based on the Dubinin-Astakhov isotherm. Sci. Total Environ. 2018, 631–632, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, Z.; Wang, Y.; Liu, Y.Q.; Luo, R.; Shang, J.G.; Liao, Q.J.H. Migration of two antibiotics during resuspension under simulated wind-wave disturbances in a water-sediment system. Chemosphere 2018, 192, 234–243. [Google Scholar] [CrossRef]
- Lv, S.; Rong, F.; Hu, S.; Wang, G.; Liu, J.; Hou, G.; Xu, Y.; Li, M.; Liu, A. Competitive adsorption and desorption of three antibiotics in distinct soil aggregate size fractions. Ecotoxicol. Environ. Saf. 2023, 259, 115002. [Google Scholar] [CrossRef]
- Chen, W.; Duan, L.; Wang, L.; Zhu, D. Adsorption of hydroxyl- and amino-substituted aromatics to carbon manotubes. Environ. Sci. Technol. 2008, 42, 6862–6868. [Google Scholar] [CrossRef]
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry: Part A: Structure and Mechanisms; Springer: New York, NY, USA, 2007. [Google Scholar]
- Wang, Z.; Yu, X.; Pan, B.; Xing, B. Norfloxacin Sorption and Its Thermodynamics on Surface-Modified Carbon Nanotubes. Environ. Sci. Technol. 2010, 44, 978–984. [Google Scholar] [CrossRef]
- Ecke, F.; Golovko, O.; Hörnfeldt, B.; Ahrens, L. Trophic fate and biomagnification of organic micropollutants from staple food to a specialized predator. Environ. Res. 2024, 261, 119686. [Google Scholar] [CrossRef]




| Adsorbate | Electrostatic Potential Surfaces | pKa | Structure |
|---|---|---|---|
| TC C22H25ClN2O8 480.90 g/mol | 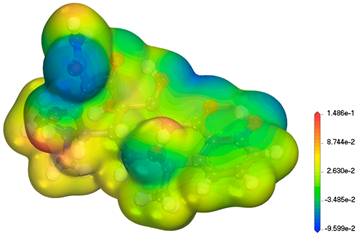 | 3.30 7.70 9.70 | 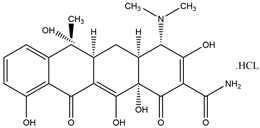 |
| Norfloxacin NOR C16H18FN3O3 319.33 g/mol |  | 6.23 8.55 | 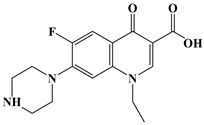 |
| TMP C41H76N2O15 290.32 g/mol | 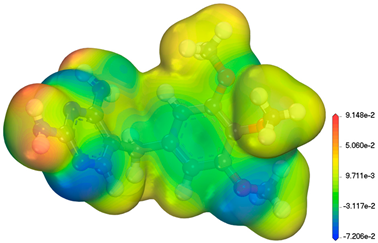 | 7.16 | 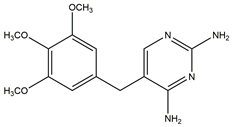 |
| Sample | SPM | LF | HF | WLH | TH |
|---|---|---|---|---|---|
| Total organic carbon (g/kg) | 71.4 | 426.9 | 61.2 | 56.7 | 36.7 |
| C (%) | 2.19 | 12.91 | 1.85 | 1.74 | 1.11 |
| N (%) | 0.227 | 0.818 | 0.176 | 0.144 | 0.093 |
| CEC (mg/100 g) | 104.894 | 112.413 | 101.132 | 120.319 | 88.934 |
| Mass ratio (%) | 100.00 | 1.66 | 96.67 | 90.12 | 78.56 |
| Specific surface area (mg2/g) | 16.587 | 15.229 | 10.848 | 4.211 | 14.205 |
| Antibiotics | Sample | qe,exp (mg) | Pseudo-First-Order Dynamics | Pseudo-Second-Order Dynamics | ||||
|---|---|---|---|---|---|---|---|---|
| qe,cal | k1 | R2 | qe,cal | k2 | R2 | |||
| TC | SPM | 21.798 | 15.747 | 21.867 | 0.941 | 21.867 | 108.534 | 0.987 |
| LF | 46.316 | 31.246 | 44.924 | 0.831 | 44.924 | 762.495 | 0.964 | |
| HF | 19.037 | 12.406 | 18.258 | 0.774 | 18.258 | 52.723 | 0.959 | |
| WLH | 19.653 | 13.104 | 19.482 | 0.910 | 19.482 | 67.071 | 0.980 | |
| TH | 26.160 | 13.867 | 25.720 | 0.713 | 25.720 | 282.517 | 0.989 | |
| NOR | SPM | 19.942 | 12.712 | 19.849 | 0.886 | 19.849 | 100.986 | 0.992 |
| LF | 29.981 | 15.071 | 30.202 | 0.940 | 30.202 | 822.482 | 0.999 | |
| HF | 17.057 | 16.729 | 16.821 | 0.857 | 16.821 | 51.306 | 0.984 | |
| WLH | 21.892 | 17.048 | 22.533 | 0.926 | 22.533 | 159.206 | 0.998 | |
| TH | 24.957 | 14.103 | 23.918 | 0.717 | 23.918 | 195.818 | 0.978 | |
| TMP | SPM | 3.204 | 0.097 | 3.200 | 0.694 | 3.200 | 15.330 | 0.999 |
| LF | 6.157 | 0.548 | 6.195 | 0.716 | 6.195 | −49.181 | 0.999 | |
| HF | 2.806 | 0.176 | 2.818 | 0.430 | 2.818 | 12.923 | 0.999 | |
| WLH | 1.102 | 2.357 | 1.105 | 0.654 | 1.105 | 0.158 | 0.999 | |
| TH | 1.334 | 0.372 | 1.345 | 0.770 | 1.345 | 0.187 | 0.999 | |
| Antibiotics | Sample | Linear | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kd | Koc | R2 | qm | KL | R2 | KF | n−1 | R2 | ||
| TC | SPM | 2.617 | 36.653 | 0.848 | 35.289 | 0.199 | 0.925 | 6.521 | 0.618 | 0.894 |
| LF | 1.545 | 3.619 | 0.922 | 38.089 | 0.698 | 0.967 | 19.253 | 0.257 | 0.978 | |
| HF | 1.643 | 26.846 | 0.993 | 39.055 | 0.079 | 0.995 | 3.359 | 0.731 | 0.997 | |
| WLH | 1.469 | 25.908 | 0.947 | 47.641 | 0.046 | 0.964 | 2.356 | 0.820 | 0.957 | |
| TH | 0.661 | 18.011 | 0.918 | 12.767 | 0.157 | 0.963 | 2.134 | 0.586 | 0.945 | |
| NOR | SPM | 0.894 | 12.521 | 0.953 | 18.749 | 0.177 | 0.955 | 3.584 | 0.535 | 0.968 |
| LF | 1.413 | 3.310 | 0.855 | 40.731 | 1.918 | 0.916 | 28.470 | 0.153 | 0.931 | |
| HF | 0.461 | 7.533 | 0.935 | 18.147 | 0.781 | 0.971 | 10.457 | 0.191 | 0.978 | |
| WLH | 1.060 | 18.695 | 0.913 | 26.414 | 0.186 | 0.915 | 5.822 | 0.475 | 0.923 | |
| TH | 0.085 | 2.316 | 0.765 | 17.453 | 8.163 | 0.902 | 16.266 | 0.027 | 0.874 | |
| TMP | SPM | 0.159 | 2.227 | 0.997 | 2.760 | 0.534 | 0.900 | 1.136 | 0.345 | 0.973 |
| LF | 0.076 | 0.178 | 0.883 | 2.162 | 3.384 | 0.968 | 2.162 | 3.384 | 0.968 | |
| HF | 0.051 | 0.833 | 0.713 | 1.590 | 0.901 | 0.966 | 0.914 | 0.207 | 0.879 | |
| WLH | 0.016 | 0.282 | 0.833 | 0.594 | 1.217 | 0.924 | 0.384 | 0.166 | 0.942 | |
| TH | 0.004 | 0.109 | 0.842 | 0.088 | 0.372 | 0.992 | 0.031 | 0.372 | 0.940 | |
| Sample | Desorption Ratios (%) | ||
|---|---|---|---|
| TC | NOR | TMP | |
| SPM | 4.45 | 5.12 | 10.21 |
| LF | 3.21 | 2.21 | 7.21 |
| HF | 4.83 | 6.35 | 28.12 |
| WLH | 7.89 | 7.35 | 30.32 |
| TH | 8.91 | 8.28 | 44.01 |
| Sample | alkyl-C 0–45 ppm | O-alkyl-C 45–110 ppm | Aromatic C-H 110–140 ppm | Aromatic C-O 140–160 ppm | Carboxyl-C 160–220 ppm | Aliphatic- C (A) | Aromatic-C (B) | A/B | Polar-C Fractions | |
|---|---|---|---|---|---|---|---|---|---|---|
| -COOH 160–190 | -CHO/-C=O 190–220 | |||||||||
| SPM | 26.27 | 45.71 | 13.69 | 5.48 | 8.85 | 0 | 71.98 | 19.17 | 3.75 | 60.04 |
| LF | 17.55 | 43.8 | 20.79 | 8.62 | 7.86 | 1.38 | 61.35 | 29.41 | 2.09 | 61.66 |
| HF | 25.43 | 46.07 | 14 | 5.82 | 8.69 | 0 | 71.5 | 19.82 | 3.61 | 60.58 |
| WLH | 27.41 | 44.43 | 14.48 | 6.07 | 7.61 | 0 | 71.84 | 20.55 | 3.50 | 58.11 |
| TH | 26.58 | 45.88 | 14.33 | 5.46 | 7.39 | 0.36 | 72.46 | 19.79 | 3.66 | 59.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, H.; Gao, J.; Su, D.; Wang, Y.; Gao, J.; Wang, Y.; Li, H.; Liao, Q.; Zheng, Y. Differential Adsorption Behaviors of Light and Heavy SPM Fractions on Three Antibiotics: Implications for Lacustrine Antibiotic Migration. Water 2025, 17, 1859. https://doi.org/10.3390/w17131859
Tu H, Gao J, Su D, Wang Y, Gao J, Wang Y, Li H, Liao Q, Zheng Y. Differential Adsorption Behaviors of Light and Heavy SPM Fractions on Three Antibiotics: Implications for Lacustrine Antibiotic Migration. Water. 2025; 17(13):1859. https://doi.org/10.3390/w17131859
Chicago/Turabian StyleTu, Haoran, Jinlong Gao, Di Su, Yifeng Wang, Jinyu Gao, Yuran Wang, Hao Li, Qianjiahua Liao, and Yufen Zheng. 2025. "Differential Adsorption Behaviors of Light and Heavy SPM Fractions on Three Antibiotics: Implications for Lacustrine Antibiotic Migration" Water 17, no. 13: 1859. https://doi.org/10.3390/w17131859
APA StyleTu, H., Gao, J., Su, D., Wang, Y., Gao, J., Wang, Y., Li, H., Liao, Q., & Zheng, Y. (2025). Differential Adsorption Behaviors of Light and Heavy SPM Fractions on Three Antibiotics: Implications for Lacustrine Antibiotic Migration. Water, 17(13), 1859. https://doi.org/10.3390/w17131859






