Microbial Quantification Using ATP and Petrifilms for Irrigation Water Treated with Cold Plasma or Ozone
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Source
2.2. Water Treatment Systems
2.3. Sample Collection
2.4. Microbial Quantification Using Petrifilm
2.5. Microbial Quantification Using ATP Assay
2.6. Experimental Design and Statistical Analysis
3. Results
3.1. Effects of Cold Plasma and Ozone on Oxidation–Reduction Potential (ORP)
3.2. Initial Surface Water Quality
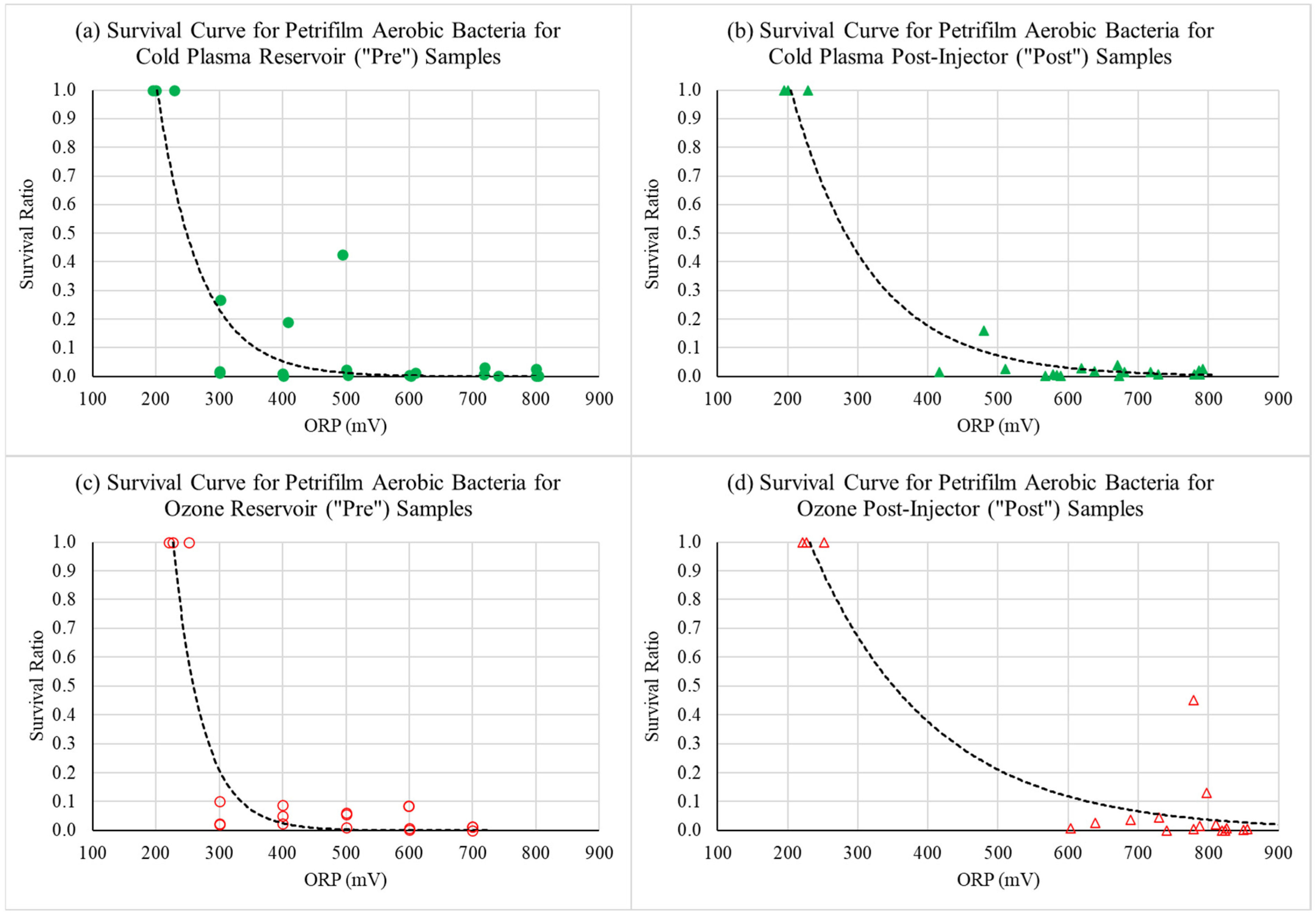
3.3. Effects of Cold Plasma and Ozone Treatment on Fungal and Bacterial Colony Counts Using Petrifilm
3.4. Effects of Cold Plasma and Ozone Treatment on Microbial ATP
3.5. Correlation Between Microbial ATP and Petrifilm Aerobic Bacterial Counts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murdoch, P.S.; Baron, J.S.; Miller, T.L. Potential effects of climate change on surface-water quality in North America. J. Am. Water Resour. Assoc. 2007, 36, 347–366. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Bai, Y.; Tian, Z.; Li, J.; Shao, X.; Mustavich, L.; Li, B. Assessment of surface water quality via multivariate statistical techniques: A case study of the Songhua River Harbin region, China. J. Hydroenviron. Res. 2013, 7, 30–40. [Google Scholar] [CrossRef]
- Jin, D.; Kong, X.; Cui, B.; Jin, S.; Xie, Y.; Wang, X.; Deng, Y. Bacterial communities and potential waterborne pathogens within the typical urban surface waters. Sci. Rep. 2018, 8, 13368. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Diversity and genetic lineages of environmental Staphylococci: A surface water overview. FEMS Microbiol. 2020, 96, fiaa191. [Google Scholar] [CrossRef]
- Bell, R.L.; Kase, J.A.; Harrison, L.M.; Balan, K.V.; Babu, U.; Chen, Y.; Macarisin, D.; Kwon, H.J.; Zheng, J.; Stevens, E.L.; et al. The Persistence of Bacterial Pathogens in Surface Water and Its Impact on Global Food Safety. Pathogens 2021, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Moorman, G. Plant pathogens in irrigation water: Challenges and opportunities. Crit. Rev. Plant Sci. 2005, 24, 189–208. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Kanarek, P.; Breza-Boruta, B.; Rolbiecki, R. Microbial composition and formation of biofilms in agricultural irrigation systems-a review. Ecohydrol. Hydrobiol. 2023, 24, 583–590. [Google Scholar] [CrossRef]
- Bucks, D.A.; Nakayama, F.S.; Gilbert, R.G. Trickle irrigation water quality and preventive maintenance. Agric. Water Manag. 1979, 2, 149–162. [Google Scholar] [CrossRef]
- Meador, D.P.; Fisher, P.R.; Guy, C.L.; Harmon, P.F.; Peres, N.A.; Teplitski, M. Using a dehydrated agar to estimate microbial water quality for horticulture irrigation. J. Environ. Qual. 2016, 45, 1445–1451. [Google Scholar] [CrossRef]
- Rogers, D.H.; Lamm, F.R.; Alam, M. Subsurface Drip Irrigation Systems Water Quality Assessment Guidelines; MF2575 SDI Water Quality Assessment Guideline; Kansas State University Agricultural Experiment Station and Cooperative Extension Service: Manhattan, KS, USA, 2003. [Google Scholar]
- Mueller, S.A.; Anderson, J.E.; Kim, B.R.; Ball, J.C. Comparison of plate counts, Petrifilm, dipslides, and adenosine triphosphate bioluminescence for monitoring bacteria in cooling-tower waters. Water Environ. Res. 2009, 81, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Vail, J.H.; Morgan, R.; Merino, C.R.; Gonzales, F.; Miller, R.; Ram, J.L. Enumeration of waterborne Escherichia coli with petrifilm plates: Comparison to standard methods. J. Environ. Qual. 2003, 32, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Meador, D.P.; Fisher, P.R.; Teplitski, M. The Use of Petrifilms to Quantify Aerobic Bacteria in Irrigation Water. Proc. Fla. State Hort. Soc. 2012, 125, 340–342. [Google Scholar]
- Fritz, B.G.; Walker, D.K.; Goveia, D.E.; Parker, A.E.; Goeres, D.M. Evaluation of Petrifilm™ aerobic count plates as an equivalent alternative to drop plating on R2A agar plates in a biofilm disinfectant efficacy test. Curr. Microbiol. 2015, 70, 450–456. [Google Scholar] [CrossRef]
- Nelson, M.T.; LaBudde, R.A.; Tomasino, S.F.; Pines, R.M. Comparison of 3M Petrifilm Aerobic Count Plates to standard plating methodology for use with AOAC antimicrobial efficacy methods 955.14, 955.15, 964.02, and 966.04 as an alternative enumeration procedure: Collaborative study. J. AOAC Int. 2013, 96, 717–722. [Google Scholar] [CrossRef]
- Berney, M.; Weilenmann, H.U.; Egli, T. Flow-cytometric study of vital cellular functions in Escherichia coli during solar disinfection (SODIS). Microbiology 2006, 152, 1719–1729. [Google Scholar] [CrossRef]
- Karl, D.M. Cellular nucleotide measurements and applications in microbial ecology. Microbiol. Rev. 1980, 44, 739–796. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, R.; Zhang, T.; Xu, J.; Zhou, X.; Yang, Y. A novel method: Using an adenosine triphosphate (ATP) luminescence-based assay to rapidly assess the biological stability of drinking water. Appl. Microbiol. Biotechnol. 2019, 103, 4269–4277. [Google Scholar] [CrossRef]
- Liu, G.; Ling, F.Q.; Magic-Knezev, A.; Liu, W.T.; Verberk, J.Q.J.C.; Van Dijk, J.C. Quantification and identification of particle-associated bacteria in unchlorinated drinking water from three treatment plants by cultivation-independent methods. Water Res. 2013, 47, 3523–3533. [Google Scholar] [CrossRef]
- Hammes, F.; Goldschmidt, F.; Vital, M.; Wang, Y.Y.; Egli, T. Measurement and interpretation of microbial adenosine triphosphate (ATP) in aquatic environments. Water Res. 2010, 44, 3915–3923. [Google Scholar] [CrossRef]
- Glaze, W.H. Drinking-water treatment with ozone. Environ. Sci. Technol. 1987, 21, 224–230. [Google Scholar] [CrossRef]
- Fisher, P. Water Treatment for Pathogens and Algae. Originally Published in GMPro Magazine. 2009. Available online: http://www.plantgrower.org/uploads/6/5/5/4/65545169/water_quality_series_from_gmpro.pdf (accessed on 9 December 2024).
- Mahanta, S.; Habib, M.R.; Moore, J.M. Effect of high-voltage atmospheric cold plasma treatment on germination and heavy metal uptake by soybeans (Glycine max). Int. J. Mol. Sci. 2022, 23, 1611. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, S.; Tani, A.; Nakashima, Y.; Kitano, K. Physicochemical properties of bactericidal plasma-treated water. J. Phys. D Appl. Phys. 2016, 49, 425401. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, R.; Tian, Y.; Su, B.; Wang, K.; Yu, S.; Zhang, J.; Fang, J. Sterilization efficiency of a novel electrochemical disinfectant against Staphylococcus aureus. Environ. Sci. Technol. 2016, 50, 3184–3192. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, S.; Choe, W.; Yong, H.I.; Jo, C.; Kim, K. Plasma-functionalized solution: A potent antimicrobial agent for biomedical applications from antibacterial therapeutics to biomaterial surface engineering. ACS Appl. Mater. Interfaces 2017, 9, 43470–43477. [Google Scholar] [CrossRef]
- Raudales, R.E.; Fisher, P.R.; Hall, C.R. The cost of irrigation sources and water treatment in greenhouse production. Irrig. Sci. 2016, 35, 43–54. [Google Scholar] [CrossRef]
- Peleg, M. Microbial inactivation kinetics models, survival curves shapes, and the temporal distributions of the individual germs deactivation. Food Eng. Rev. 2024, 16, 163–178. [Google Scholar] [CrossRef]
- USEPA. Guidelines for Water Reuse. 2012. Available online: https://www3.epa.gov/region1/npdes/merrimackstation/pdfs/ar/AR-1530.pdf (accessed on 21 March 2025).
- Farrell, C.; Hassard, F.; Jefferson, B.; Leziart, T.; Nocker, A.; Jarvis, P. Turbidity composition and the relationship with microbial attachment and UV inactivation efficacy. Sci. Total Environ. 2018, 624, 638–647. [Google Scholar] [CrossRef]
- Adhikari, A.; Parraga Estrada, K.J.; Chhetri, V.S.; Janes, M.; Fontenot, K.; Beaulieu, J.C. Evaluation of ultraviolet (UV-C) light treatment for microbial inactivation in agricultural waters with different levels of turbidity. Food Sci. Nutr. 2020, 8, 1237–1243. [Google Scholar] [CrossRef]
- Foster, J.E. Plasma-based water purification: Challenges and prospects for the future. Phys. Plasmas. 2017, 24, 055501. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Cuong, L.C.; Dieu, T.V.; Ngu, T.W.; Oanh, D.T. Ozonation process and water disinfection. Vietnam J. Chem. 2018, 56, 717–720. [Google Scholar] [CrossRef]
- Gao, Y.; Francis, K.; Zhang, X. Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res. Int. 2022, 157, 111246. [Google Scholar] [CrossRef]
- Suslow, T.V. Oxidation-Reduction Potential (ORP) for Water Disinfection Monitoring, Control, and Documentation; ANR Publishing: New York, NY, USA, 2004; Available online: https://anrcatalog.ucanr.edu/pdf/8149.pdf (accessed on 21 March 2025).
- Lang, J.M.; Rebits, B.; Newman, S.E.; Tisserat, N. Monitoring mortality of Pythium zoospores in chlorinated water using oxidation reduction potential. Plant Health Prog. 2008, 9, 9. [Google Scholar] [CrossRef]
- Igura, N.; Fujii, M.; Shimoda, M.; Hayakawa, I. Research Note: Inactivation Efficiency of Ozonated Water for Fusarium oxysporum Conidia Under Hydroponic Greenhouse Conditions. Ozone Sci. Eng. 2004, 26, 517–521. [Google Scholar] [CrossRef]
- Kobayashi, F.; Ikeura, H.; Ohsato, S.; Goto, T.; Tamaki, M. Disinfection using ozone microbubbles to inactivate Fusarium oxysporum f. sp. melonis and Pectobacterium carotovorum subsp. carotovorum. Crop Prot. 2011, 30, 1514–1518. [Google Scholar] [CrossRef]
- Runia, W.T. Disinfection of recirculation water from closed cultivation systems with ozone. Acta Hortic. 1994, 361, 388–396. [Google Scholar] [CrossRef]
- Scholtz, V.; Julák, J.; Kříha, V. The microbicidal effect of low-temperature plasma generated by corona discharge: Comparison of various microorganisms on an agar surface or in aqueous suspension. Plasma Process. Polym. 2010, 7, 237–243. [Google Scholar] [CrossRef]
- Soušková, H.; Scholtz, V.; Julák, J.; Kommová, L.; Savická, D.; Pazlarová, J. The survival of micromycetes and yeasts under the low-temperature plasma generated in electrical discharge. Folia Microbiol. 2011, 56, 77–79. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, R.; Zhang, Q.; Feng, H.; Liang, Y.; Zhang, J.; Fang, J. Assessment of the physicochemical properties and biological effects of water activated by non-thermal plasma above and beneath the water surface. Plasma Process. Polym. 2014, 12, 439–449. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y.; Xu, C.; Bai, Y. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 52, 49–56. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Wang, Y.C. Surface Germicidal Effects of Ozone for Microorganisms. AIHA J. 2010, 64, 533–537. [Google Scholar] [CrossRef]
- Ziuzina, D.; Boehm, D.; Patil, S.; Cullen, P.J.; Bourke, P. Cold plasma inactivation of bacterial biofilms and reduction of quorum sensing regulated virulence factors. PLoS ONE 2015, 10, e0138209. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of plasma-activated water with biofilms: Inactivation, dispersal effects and mechanisms of action. npj Biofilms Microbiomes 2021, 7, 11. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Jiang, A.; Guan, Q.; Sun, X.; Liu, S.; Hao, K.; Hu, W. The Effects of Cold Plasma-Activated Water Treatment on the Microbial Growth and Antioxidant Properties of Fresh-Cut Pears. Food Bioprocess Technol. 2019, 12, 1842–1851. [Google Scholar] [CrossRef]
- Thanomsub, B.; Anupunpisit, V.; Chanphetch, S.; Watcharachaipong, T.; Poonkhum, R.; Srisukonth, C. Effects of ozone treatment on cell growth and ultrastructural changes in bacteria. J. Gen. Appl. Microbiol. 2002, 48, 193–199. [Google Scholar] [CrossRef]
- Bonadonna, L.; Briancesco, R.; Giuseppina, R.L. Innovative analytical methods for monitoring microbiological and virological water quality. Microchem. J. 2019, 150, 104160. [Google Scholar] [CrossRef]
- Tian, S.; Tian, Z.; Yang, H.; Yang, M.; Zhang, Y. Detection of viable bacteria during sludge ozonation by the combination of ATP assay with PMA-Miseq sequencing. Water 2017, 9, 166. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, Y.; Du, M.; Wang, Y.; Ju, S.; Ma, R.; Jiao, Z. Subcellular mechanism of microbial inactivation during water disinfection by cold atmospheric-pressure plasma. Water Res. 2021, 188, 116513. [Google Scholar] [CrossRef]
- Siebel, E.; Wang, Y.; Thomas, E.; Frederik, H. Correlations between total cell concentration, total adenosine tri-phosphate concentration and heterotrophic plate counts during microbial monitoring of drinking water. Drink. Water Eng. Sci. Discuss. 2008, 1, 71–86. [Google Scholar] [CrossRef]
- Duda, S.; Baron, J.L.; Wagener, M.M.; Vidic, R.D.; Stout, J.E. Lack of correlation between Legionella colonization and microbial population quantification using heterotrophic plate count and adenosine triphosphate bioluminescence measurement. Environ. Monit. Assess. 2015, 187, 393. [Google Scholar] [CrossRef]
- Helmi, K.; David, F.; Di Martino, P.; Jaffrezic, M.P.; Ingrand, V. Assessment of flow cytometry for microbial water quality monitoring in cooling tower water and oxidizing biocide treatment efficiency. J. Microbiol. Methods 2018, 152, 201–209. [Google Scholar] [CrossRef]
- Arroyo, M.G.; Ferreira, A.M.; Frota, O.P.; Rigotti, M.A.; Andrade, D.; Brizzotti, N.S.; Peresi, J.T.M.; Castilho, E.M.; Almeida, M.T.G. Effectiveness of ATP bioluminescence assay for presumptive identification of microorganisms in hospital water sources. BMC Infect Dis. 2017, 17, 458. [Google Scholar] [CrossRef]
- Turner, D.E.; Daugherity, E.K.; Altier, C.; Maurer, K.J. Efficacy and limitations of an ATP-based monitoring system. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 90–195. [Google Scholar]
- Lanpher, K.B.; Popendorf, K.J. Variability of microbial particulate ATP concentrations in subeuphotic microbes due to underlying metabolic strategies in the South Pacific Ocean. Front. Mar. Sci. 2021, 8, 655898. [Google Scholar] [CrossRef]
- Maal-Bared, R.; McCracken, M.; Busawon, B.; Simpson, D. Monitoring adenosine triphosphate concentrations in a chloraminated drinking water distribution system for risk and asset management. Water 2023, 15, 1636. [Google Scholar] [CrossRef]
- Chen, W.S.; Abkar, L.; Mohseni, M. Evaluating ATP testing for distribution system monitoring: Comparison to HPC, impact of chlorine quenching, and hold time dependency. J. Biol. Eng. 2024, 18, 63. [Google Scholar] [CrossRef]
- Park, E.; Lee, C.; Bisesi, M.; Lee, J. Efficiency of peracetic acid in inactivating bacteria, viruses, and spores in water determined with ATP bioluminescence, quantitative PCR, and culture-based methods. J. Water Health 2014, 12, 13–23. [Google Scholar] [CrossRef]




| Parameter | Mean ± Standard Error | |
|---|---|---|
| Petrifilm (CFU/mL) | Aerobic bacteria | 42,550 ± 13,316 |
| Yeasts and molds | 510 ± 219 | |
| ATP (RLU) | Total | 1374 ± 363 |
| Free | 507 ± 193 | |
| Microbial | 867 ± 198 | |
| ORP (mV) | 221 ± 9 | |
| DO (mg/L) | 5.87 ± 0.56 | |
| DO saturation (%) | 66.8 ± 6.3 | |
| Temp °C | 21.8 ± 0.2 | |
| pH | 7.33 ± 0.06 | |
| EC (mS/cm) | 0.27 ± 0.03 | |
| Turbidity (NTU) | 3.93 ± 0.13 |
| Method | Correlation Coefficient | p-Value | 95% Confidence Interval |
|---|---|---|---|
| Pearson’s correlation (Power = 0.13) | 0.39 | 0.0003 | 0.19–0.57 |
| Spearman’s rank correlation (ρ) | 0.39 | 0.0004 | 0.16–0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakulla, D.; Fisher, P.R. Microbial Quantification Using ATP and Petrifilms for Irrigation Water Treated with Cold Plasma or Ozone. Water 2025, 17, 1856. https://doi.org/10.3390/w17131856
Thakulla D, Fisher PR. Microbial Quantification Using ATP and Petrifilms for Irrigation Water Treated with Cold Plasma or Ozone. Water. 2025; 17(13):1856. https://doi.org/10.3390/w17131856
Chicago/Turabian StyleThakulla, Dharti, and Paul R. Fisher. 2025. "Microbial Quantification Using ATP and Petrifilms for Irrigation Water Treated with Cold Plasma or Ozone" Water 17, no. 13: 1856. https://doi.org/10.3390/w17131856
APA StyleThakulla, D., & Fisher, P. R. (2025). Microbial Quantification Using ATP and Petrifilms for Irrigation Water Treated with Cold Plasma or Ozone. Water, 17(13), 1856. https://doi.org/10.3390/w17131856








