Mesoporous SBA-15-Supported Ceria–Cadmium Composites for Fast Degradation of Methylene Blue in Aqueous Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of SBA-15 Support
2.2. Synthesis of Cd-CeO2/SBA-15 Composites
2.3. Characterization
2.4. Photocatalytic Study
3. Results
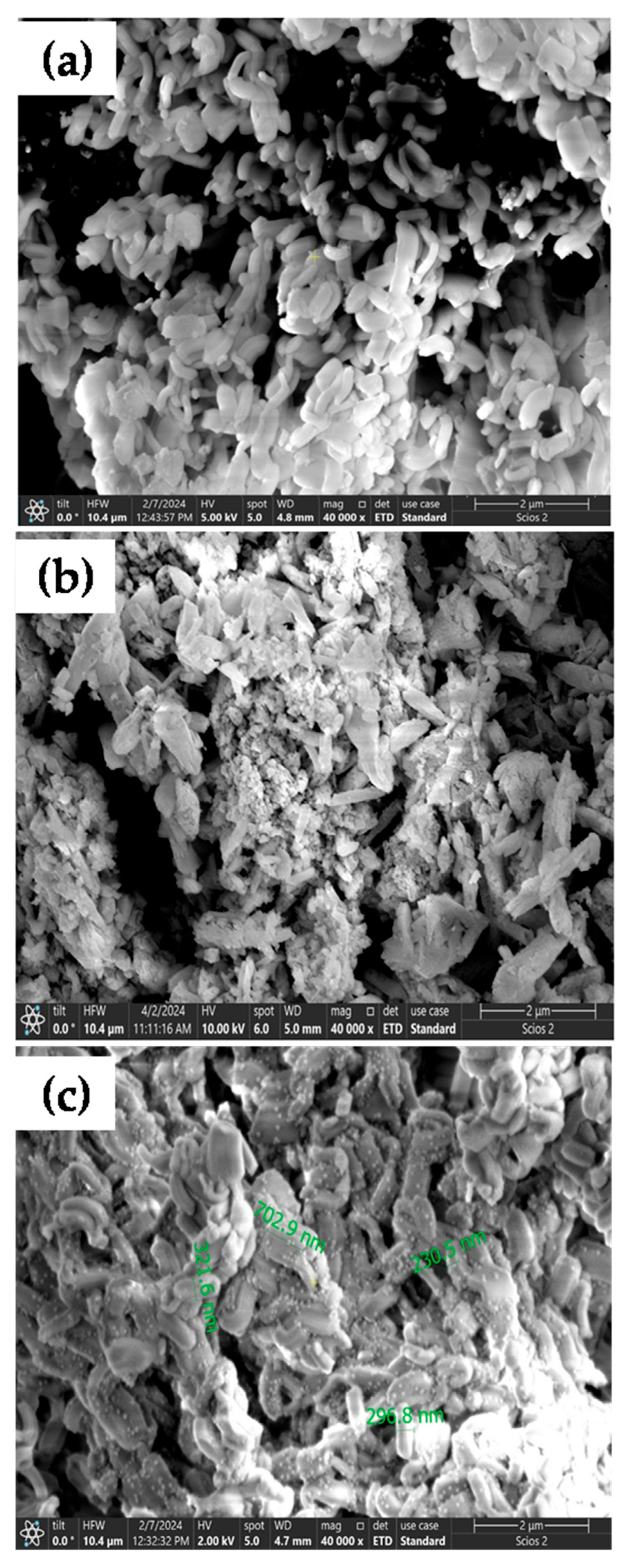
3.1. SEM/EDX Analysis
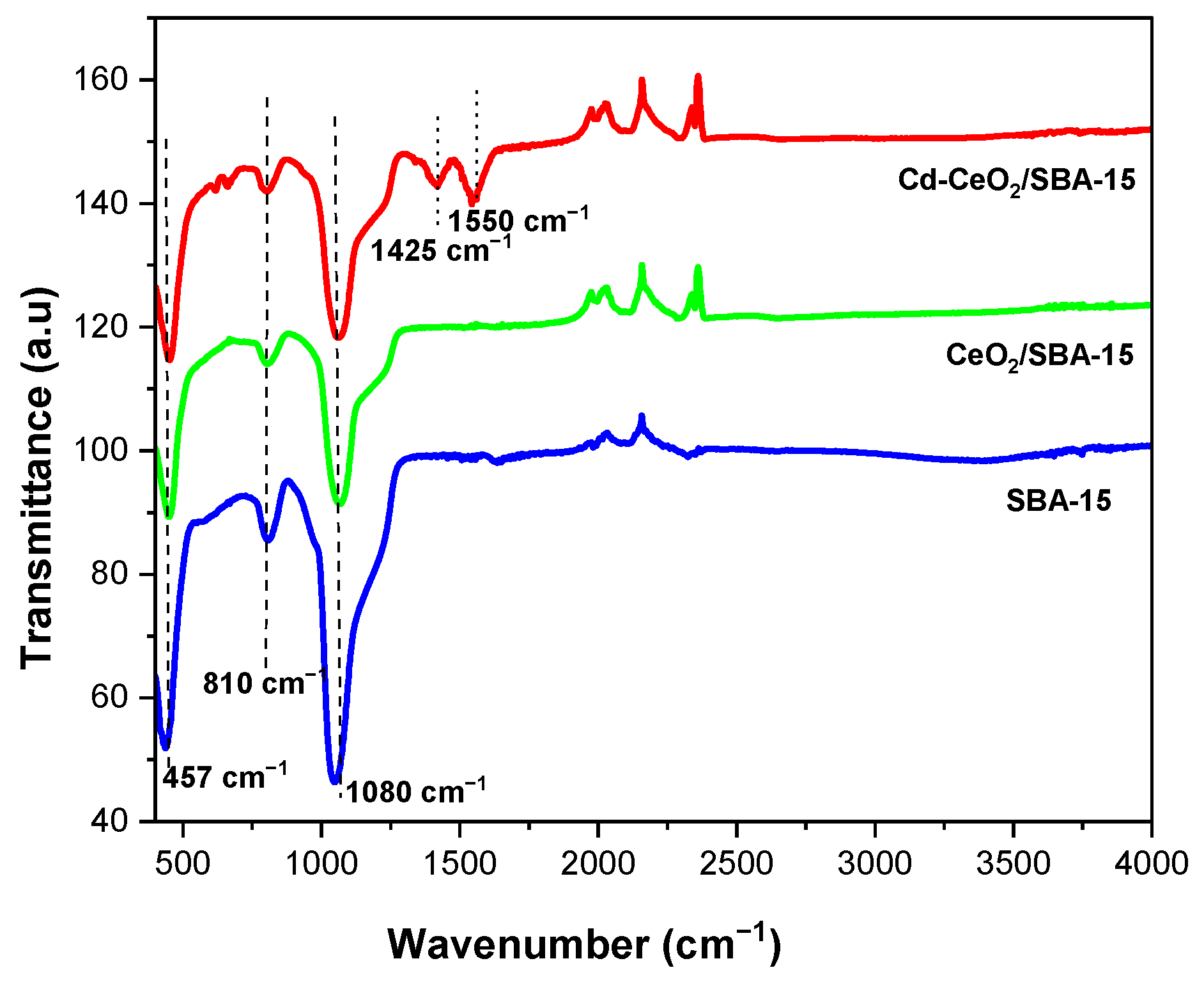
3.2. FTIR Analysis
3.3. XRD Analysis
3.4. N2 Physisorption Analysis
3.5. UV-Vis Analysis
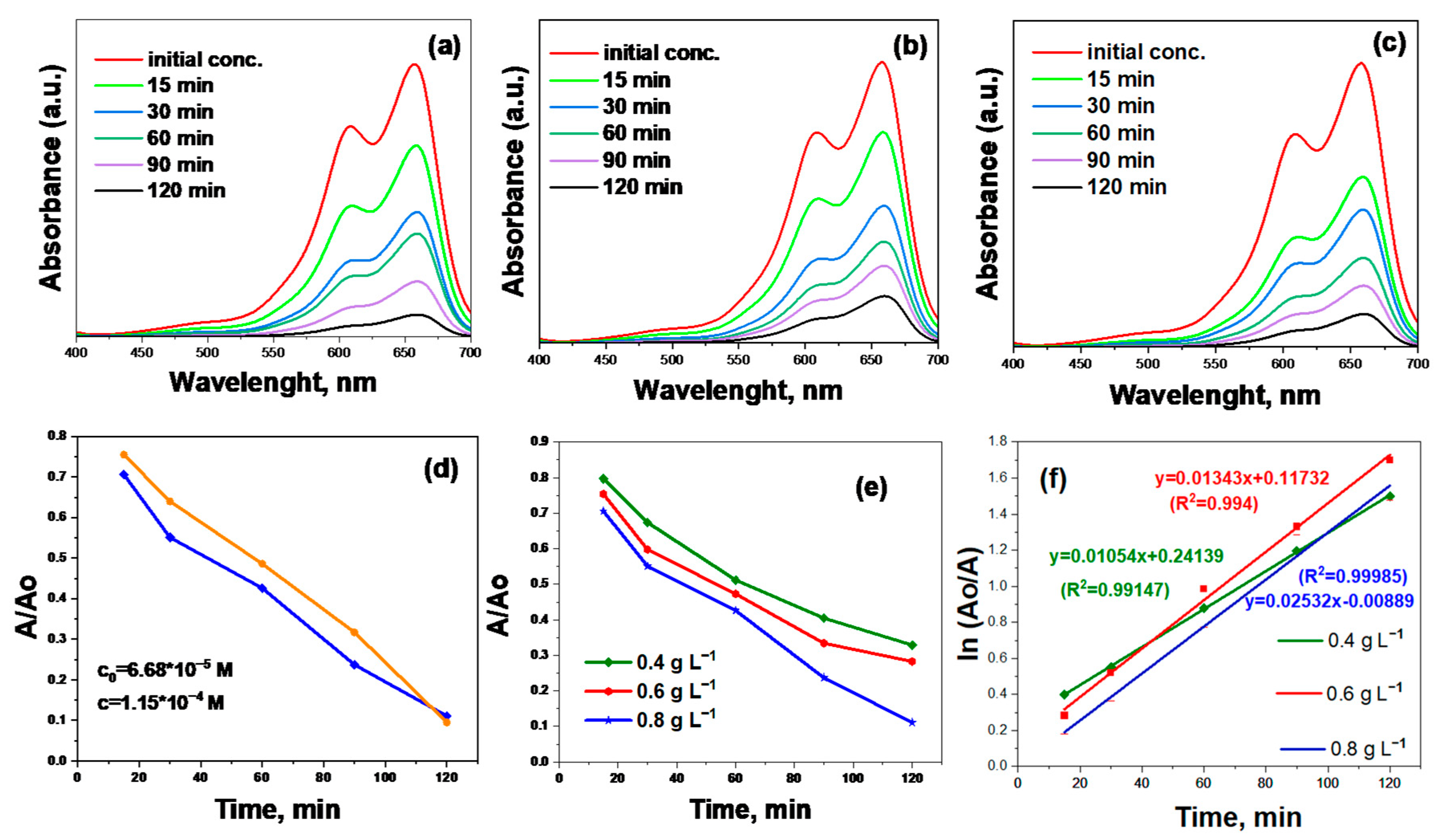
3.6. Photodegradation of MB
3.6.1. Free Radical Scavenging
3.6.2. Possible Degradation Mechanism
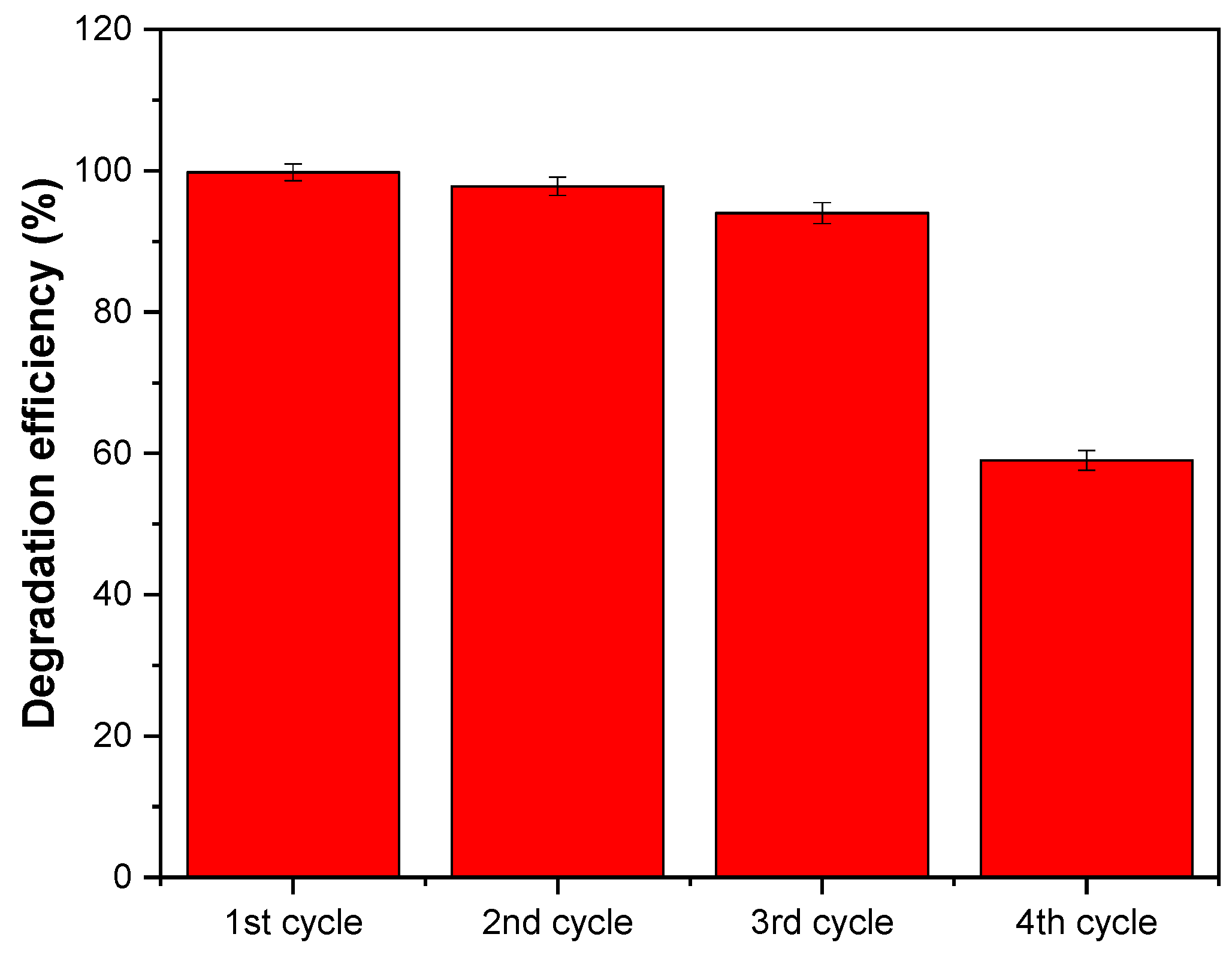
3.6.3. Recyclability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pandey, P.K.; Sarkar, J.; Srivastava, S. Catalytic Dye Degradation of Textile Dye Methylene Blue by Using Silver Nanoparticles Fabricated by Sustainable Approach. Eng. Proc. 2023, 37, 16. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Oyarce, E.; Roa, K.; Boulett, A.; Sotelo, S.; Cantero-López, P.; Sánchez, J.; Rivas, B.L. Removal of Dyes by Polymer-Enhanced Ultrafiltration: An Overview. Polymers 2021, 13, 3450. [Google Scholar] [CrossRef] [PubMed]
- Nowik-Zajac, A.; Zawierucha, I.; Lagiewka, J.; Jaksender, K.; Witt, K.; Malina, G.; Sabadash, V. Removal of Methylene Blue Dye from Aqueous Solutions Using Polymer Inclusion Membrane Containing Calix[4]pyrrole. Membranes 2024, 14, 92. [Google Scholar] [CrossRef]
- Zharylkan, S.; Sultakhan, S.; Suleimenova, M.; Azat, S.; Sailaukhanuly, Y.; Tulepov, M.; Tauanov, Z. Enhanced Adsorption of Mercury and Methylene Blue Using Silver and Surface Modified Zeolite-NaX Derived from Rice Husk. Eng. Sci. 2024, 31, 1241. [Google Scholar] [CrossRef]
- Bouchoucha, H.; Bekkouche, S.; Merouani, S.; Dehane, A.; Hamdaoui, O. Solar Chlorine Activation for Efficient Rhodamine B Removal in Strong Basic pH: Processing Conditions, Radicals Probing, and TiO2 Nanocatalyst Effect. Catalysts 2023, 13, 942. [Google Scholar] [CrossRef]
- Lanzetta, A.; Papirio, S.; Oliva, A.; Cesaro, A.; Pucci, L.; Capasso, E.M.; Esposito, G.; Pirozzi, F. Ozonation Processes for Color Removal from Urban and Leather Tanning Wastewater. Water 2023, 15, 2362. [Google Scholar] [CrossRef]
- Shen, Y. Development of Photoreactor Design Equation for the Treatment of Dye Wastewater by UV/H2O2 Process. J. Hazard. Mater. 2002, 89, 267–277. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic Degradation of Dyes Using Semiconductor Photocatalysts to Clean Industrial Water Pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Goodarzi, N.; Ashrafi-Peyman, Z.; Khani, E.; Moshfegh, A.Z. Recent Progress on Semiconductor Heterogeneous Photocatalysts in Clean Energy Production and Environmental Remediation. Catalysts 2023, 13, 1102. [Google Scholar] [CrossRef]
- Hosseini, M.; Ghanbari, M.; Alzaidy, A.H.; Dawi, E.A.; Mahdi, M.A.; Jasim, L.S.; Sobhani, A.; Salavati-Niasari, M. Synthesis and Characterization of Fe2SiO4/Fe2O3/g-C3N4 Ternary Heterojunction Photocatalyst with Enhanced Photocatalytic Activity under Visible Light. Int. J. Hydrogen Energy 2024, 60, 1370–1382. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, F.; Zhong, Y.; Xiao, T.; Yu, Q.; Zhu, X.; Feng, W.; Qi, Z. Preparation and Photocatalytic Performance of In2O3/Bi2WO6 Type II Heterojunction Composite Materials. Molecules 2024, 29, 4911. [Google Scholar] [CrossRef] [PubMed]
- Bloh, J.Z. Intensification of Heterogeneous Photocatalytic Reactions Without Efficiency Losses: The Importance of Surface Catalysis. Catal. Lett. 2021, 151, 3105–3113. [Google Scholar] [CrossRef]
- Yang, J.; Zhan, J.; Zhu, L.; Chen, S.; Zhang, Y. Synthesis of nanotitania particles embedded in mesoporous SBA-15:Characterization and photocatalytic activity. J. Hazard. Mater. 2006, 137, 952–958. [Google Scholar] [CrossRef]
- Yarbaş, T.; Çıtak, A. A Detailed Surface Characterization Study of SBA-15 and B-SBA-15-x Mesoporous Materials Using the IGC Technique. Int. J. Adhes. Adhes. 2022, 118, 103249. [Google Scholar] [CrossRef]
- Thielemann, J.P.; Girgsdies, F.; Schlögl, R.; Hess, C. Pore Structure and Surface Area of Silica SBA-15: Influence of Washing and Scale-Up. Beilstein J. Nanotechnol. 2011, 2, 110–118. [Google Scholar] [CrossRef]
- Ruchomski, L.; Ozimek, J.; Siedliska, K.; Raftopoulos, K.N.; Pielichowski, K. Characterization of Ti/SBA-15 Composites Synthesized by Chemical Vapour Deposition of Organic Titanium Compounds. Crystals 2023, 13, 288. [Google Scholar] [CrossRef]
- Jang, M.; Choi, S.; Kim, Y.; Cha, J.; Kim, A.-R.; Jeong, H.; Kim, Y.; Choi, S.H.; Nam, S.W.; Lim, J.; et al. Effect of CeO2 Redox Properties on the Catalytic Activity of Pt-CeOx over Irreducible SiO2 Support for Methylcyclohexane (MCH) Dehydrogenation. Appl. Surf. Sci. 2023, 627, 157134. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A review on oxygen storage capacity of CeO2-based materials: Influence factors, measurement techniques, and applications in reactions related to catalytic utomotive emissions control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; De Los Milagros Ballari, M.; Kubacka, A.; Cassano, A.E.; Alfano, O.M.; Fernández-García, M. Acetaldehyde Degradation under UV and Visible Irradiation Using CeO2–TiO2 Composite Systems: Evaluation of the Photocatalytic Efficiencies. Chem. Eng. J. 2014, 255, 297–306. [Google Scholar] [CrossRef]
- Seeharaj, P.; Saenman, T.; Phiwhom, T.; Muangsuwan, C.; Srinives, S.; Kim-Lohsoontorn, P. Improvement of Surface Properties of Metal Doped-CeO2Nanospindle Catalysts for Direct Synthesis of Dimethyl Carbonate from CO2 and Methanol. J. Environ. Chem. Eng. 2023, 11, 109813. [Google Scholar] [CrossRef]
- Qamar, M.T.; Iqbal, S.; Aslam, M.; Alhujaily, A.; Bilal, A.; Rizwan, K.; Farooq, H.M.U.; Sheikh, T.A.; Bahadur, A.; Awwad, N.S.; et al. Transition Metal Doped CeO2 for Photocatalytic Removal of 2-Chlorophenol in the Exposure of Indoor White Light and Antifungal Activity. Front. Chem. 2023, 11, 1126171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, T.; Zhang, L.; Wu, H.; Guo, J.; Hu, X. One-Pot Synthesized NiFe2O4/CeO2 Composite Catalyst for Efficient Degradation of Methylene Blue via Photocatalysis under Visible Light. Catal. Commun. 2023, 185, 106814. [Google Scholar] [CrossRef]
- Sharma, P.; Kumari, D.; Subrahmanyam, M. TiO2 supported over SBA-15: An efficient photocatalyst for the pesticide degradation using solar light. Chemosphere 2008, 73, 1562–1569. [Google Scholar] [CrossRef]
- Calzada, L.A.; Castellanos, R.; García, L.A.; Klimova, T.E. TiO2, SnO2 and ZnO catalysts supported on mesoporous SBA-15 versus unsupported nanopowders in photocatalytic degradation of methylene blue. Microporous Mesoporous Mater. 2019, 285, 247–258. [Google Scholar] [CrossRef]
- Ngomade, S.B.; Fotsop, C.G.; Ngueteu, M.L.T.; Tamo, A.K.; Nche, G.N.-A.; Anagho, S.G. Catalytic Performances of CeO2@SBA-15 as Nanostructured Material for Biodiesel Production from Podocarpus Falcatus Oil. Chem. Eng. Res. Des. 2023, 194, 789–800. [Google Scholar] [CrossRef]
- Kaminski, P. The Application of FTIR in Situ Spectroscopy Combined with Methanol Adsorption to the Study of Mesoporous Sieve SBA-15 with Cerium-Zirconium Oxides Modified with Gold and Copper Species. Arab. J. Chem. 2017, 13, 851–862. [Google Scholar] [CrossRef]
- Göl, S.C.; Akbay, E. The effect of metal-titaniainteraction on photodegradation in sba-15-supported metal-titaniaphotocatalysts. Chem. Ind. Chem. Eng. Q. 2023, 29, 281–289. [Google Scholar] [CrossRef]
- Mihai, G.D.; Meynen, V.; Mertens, M.; Bilba, N.; Cool, P.; Vansant, E.F. ZnO nanoparticles supported on mesoporous MCM-41 and SBA-15: A comparative physicochemical and photocatalytic study. J. Mater. Sci. 2010, 45, 5786–5794. [Google Scholar] [CrossRef]
- Liang, B.; Zhu, P.; Gu, J.; Yuan, W.; Xiao, B.; Hu, H.; Rao, M. Advancing Adsorption and Separation with Modified SBA-15: A Comprehensive Review and Future Perspectives. Molecules 2024, 29, 3543. [Google Scholar] [CrossRef]
- Fan, J.; Jiang, X.; Min, H.; Li, D.; Ran, X.; Zou, L.; Sun, Y.; Li, W.; Yang, J.; Teng, W. Facile Preparation of Cu–Mn/CeO2/SBA-15 Catalysts Using Ceria as an Auxiliary for Advanced Oxidation Processes. J. Mater. Chem. A 2014, 2, 10654. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Basati, S. Synthesis, characterization and photocatalytic activity of CeO2-SBA-15. Iran. J. Catal. 2012, 2, 51–55. [Google Scholar]
- Murugadoss, G.; Mab, J.; Ning, X.; Kumar, M.R. Selective metal ions doped CeO2 nanoparticles for excellent photocatalytic activity under sun light and supercapacitor application. Inorg. Chem. Commun. 2019, 109, 107577. [Google Scholar] [CrossRef]
- Chen, L.F.; Arellano, U.; Wang, J.A.; Balcázar, L.M.; Sotelo, R.; Solis, S.; Azomosa, M.; González, J.; Vargas, O.A.G.; Song, Y. Oxygen Defect, Electron Transfer and Photocatalytic Activity of Ag/CeO2/SBA-15 Hybrid Catalysts. Catal. Today 2021, 394–396, 62–80. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, S.C.; Xue, D.; Chen, Q.; Li, Z.C. Preparation of nanocrystalline CeO2 by nanocasting with mesoporous silica. J. Phys. Conf. Ser. 2009, 152, 012070. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W. Visible Light-Responsive CeO2/MoS2 Composite for Photocatalytic Hydrogen Production. Catalysts 2022, 12, 1185. [Google Scholar] [CrossRef]
- Srinivasan, N.R.; Majumdar, P.; Eswar, N.K.R.; Bandyopadhyaya, R. Photocatalysis by morphologically tailored mesoporous silica (SBA-15) embedded with SnO2nanoparticles: Experiments and model. Appl. Catal. A Gen. 2015, 498, 107–116. [Google Scholar] [CrossRef]
- Abdullah, N.; Ainirazali, N.; Chong, C.C.; Razak, H.A.; Setiabudi, H.D.; Chin, S.Y.; Jalil, A.A. Effect of Ni Loading on SBA-15 Synthesized from Palm Oil Fuel Ash Waste for Hydrogen Production via CH4 Dry Reforming. Int. J. Hydrogen Energy 2019, 45, 18411–18425. [Google Scholar] [CrossRef]
- Gonzalez, A.E.; Rangel, R.; Solís-Garcia, A.; Venezia, A.M.; Zepeda, T.A. FTIR Investigation under Reaction Conditions during CO Oxidation over Ru(x)-CeO2 Catalysts. Mol. Catal. 2020, 493, 111086. [Google Scholar] [CrossRef]
- Sareen, S.; Mutreja, V.; Singh, S.; Pal, B. Fine CuO Anisotropic Nanoparticles Supported on Mesoporous SBA-15 for Selective Hydrogenation of Nitroaromatics. J. Colloid Interface Sci. 2015, 461, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Li, J.J.; Tian, H.; Hao, Z.P.; Qiao, S.Z. Synthesis of Mesoporous Co/Ce-SBA-15 Materials and Their Catalytic Performance in the Catalytic Oxidation of Benzene. Mater. Res. Bull. 2007, 43, 2599–2606. [Google Scholar] [CrossRef]
- Janus, R.; Wądrzyk, M.; Lewandowski, M.; Natkański, P.; Łątka, P.; Kuśtrowski, P. Understanding Porous Structure of SBA-15 upon Pseudomorphic Transformation into MCM-41: Non-Direct Investigation by Carbon Replication. J. Ind. Eng. Chem. 2020, 92, 131–144. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Lee, J.-H.; Ansari, M.O.; Lee, J.; Cho, M.H. Electrochemically Active Biofilm Assisted Synthesis of Ag@CeO2 Nanocomposites for Antimicrobial Activity, Photocatalysis and Photoelectrodes. J. Colloid Interface Sci. 2014, 431, 255–263. [Google Scholar] [CrossRef]
- Taddesse, A.M.; Bekele, T.; Diaz, I.; Adgo, A. Polyaniline Supported CdS/CeO2/Ag3PO4 Nanocomposite: An “A-B” Type Tandem n-n Heterojunctions with Enhanced Photocatalytic Activity. J. Photochem. Photobiol. A Chemistry 2020, 406, 113005. [Google Scholar] [CrossRef]
- Abrahim, Z.; Taddesse, A.M.; Bogale, Y.; Bezu, Z.; Teju, E. Polyaniline-Supported g-C3N4/ZnO/Ag2CrO4 Composite for Photodegradation of Methylene Blue under Visible Light Irradiation. J. Photochem. Photobiol. 2024, 21, 100239. [Google Scholar] [CrossRef]
- Ashiegbu, D.C.; Potgieter, H.J. ZnO-Based Heterojunction Catalysts for the Photocatalytic Degradation of Methyl Orange Dye. Heliyon 2023, 9, e20674. [Google Scholar] [CrossRef]
- Arzaee, N.A.; Betti, N.; Al-Amiery, A.; Isahak, W.N.R.W. The Role of Tin Species in Doped Iron (III) Oxide for Photocatalytic Degradation of Methyl Orange Dye under UV Light. Heliyon 2023, 9, e18076. [Google Scholar] [CrossRef]
- Lv, F.; Miao, Y.; Yang, D.; Mao, B.; Bian, Z.; Zhu, F. In Situ Etching Synthesis of TiO2-SBA-15 Nanocomposite Enhancing Adsorption and Photocatalytic Degradation. Catalysts 2022, 12, 1334. [Google Scholar] [CrossRef]
- Ali, N.S.; Alismaeel, Z.T.; Majdi, H.S.; Salih, H.G.; Abdulrahman, M.A.; Saady, N.M.C.; Albayati, T.M. Modification of SBA-15 Mesoporous Silica as an Active Heterogeneous Catalyst for the Hydroisomerization and Hydrocracking of n-Heptane. Heliyon 2022, 8, e09737. [Google Scholar] [CrossRef] [PubMed]
- Sareen, S.; Mutreja, V.; Pal, B.; Singh, S. Homogeneous Dispersion of Au Nanoparticles into Mesoporous SBA-15 Exhibiting Improved Catalytic Activity for Nitroaromatic Reduction. Microporous Mesoporous Mater. 2014, 202, 219–225. [Google Scholar] [CrossRef]
- Katsina, A.U.; Mihai, S.; Matei, D.; Cursaru, D.-L.; Şomoghi, R.; Nistor, C.L. Construction of Pt@BiFeO3 Xerogel-Supported O-g-C3N4 Heterojunction System for Enhanced Visible-Light Activity towards Photocatalytic Degradation of Rhodamine B. Gels 2023, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Sultanov, F.; Daulbayev, C.; Azat, S.; Kuterbekov, K.; Bekmyrza, K.; Bakbolat, B.; Bigaj, M.; Mansurov, Z. Influence of Metal Oxide Particles on Bandgap of 1D Photocatalysts Based on SrTiO3/PAN Fibers. Nanomaterials 2020, 10, 1734. [Google Scholar] [CrossRef]
- Verma, M.; Kumar, A.; Thakur, V.K.; Maurya, A.; Kumar, S.; Singh, S.; Srivastav, S.K. Efficient and Rapid Sunlight-Driven Photocatalytic Degradation of Methylene Blue Dye Using Multiferroic BiFeO3 Nanoparticles. J. Sol-Gel Sci. Technol. 2025, 113, 356–373. [Google Scholar] [CrossRef]
- Venkatesh, D.; Pavalamalar, S.; Silambarasan, R.; Anbalagan, K. Synergistic Excited State Involved Catalytic Reduction of (NH3-Trz)[Fe(Dipic)2] Complex by SnO2/TiO2 Nanocomposite. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2712–2728. [Google Scholar] [CrossRef]
- Cheng, M.; Zhao, C.; Wu, Z.; Liu, L.; Wang, H. Degradation of Dye Wastewater by a Novel mBT-MPR Visible Light Photocatalytic System. Int. J. Environ. Res. Public Health 2023, 20, 571. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, H. Noncompensated Codoping TiO2 Nanowires: The Enhanced Visible Light Photocatalytic Properties. Int. J. Photoenergy 2014, 2014, 568185. [Google Scholar] [CrossRef]
- Bouziani, M.; Bouziani, A.; Hsini, A.; Bianchi, C.L.; Falletta, E.; Di Michele, A.; Çelik, G.; Hausler, R. Synergistic Photocatalytic Degradation of Methylene Blue and Ibuprofen Using Co3O4-Decorated Hexagonal Boron Nitride (hBN) Composites under Sun-Like Irradiation. Chemosphere 2025, 371, 144061. [Google Scholar] [CrossRef]
- Dhanda, E.; Nain, A.; Dahiya, S. A Highly Effective PANI@(Ce-Er) Dual Doped ZnO (PCEZ) Nanocomposite as UV-Light Driven Photocatalyst for Degradation of MB Dye: Synthesis and Characterization. Phys. Scr. 2024, 99, 085043. [Google Scholar] [CrossRef]
- Mohammadi, R.; Alizadehlarijan, M. Proficient Adsorption, Photodegradation and Sonodegradation of Methylene Blue by Fe3O4/Graphene Nanocomposite. Iran. J. Anal. Chem. 2023, 10, 61–71. [Google Scholar] [CrossRef]





| Catalyst | SBET (m2/g) | Pore Size Distribution | |
|---|---|---|---|
| DP (nm) | VP (cm3/g) | ||
| SBA-15 | 581.27 | 4.74 | 0.638 |
| CeO2/SBA-15 | 403.13 | 3.71 | 0.447 |
| Cd-CeO2/SBA-15 | 330.17 | 3.04 | 0.367 |
| Photocatalyst | MB (mg/L) | Dosage (g L−1) | Lamp Power (W) | Volume (L) | k (min−1) | EE/O (kWh m−3) | Reference |
|---|---|---|---|---|---|---|---|
| Cd–CeO2/SBA-15 | 28.8 | 0.8 | 150 | 0.05 | 0.0253 | 281.8 | This work |
| N-TiO2 | 10 | 1 | 450 | 0.1 | - | 1.6 × 104 | [58] |
| Co3O4-hBN | 10 | 1 | 300 | 0.1 | 0.015 | 5670 | [59] |
| ZnO | 10 | 0.2 | 5 | 0.15 | 0.03 | 47 | [60] |
| Fe3O4/Graphene | 20 | 0.4 | 36 | 0.1 | 0.41 | 9.29 | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, D.; Katsina, A.U.; Cursaru, D.-L.; Mihai, S. Mesoporous SBA-15-Supported Ceria–Cadmium Composites for Fast Degradation of Methylene Blue in Aqueous Systems. Water 2025, 17, 1834. https://doi.org/10.3390/w17121834
Matei D, Katsina AU, Cursaru D-L, Mihai S. Mesoporous SBA-15-Supported Ceria–Cadmium Composites for Fast Degradation of Methylene Blue in Aqueous Systems. Water. 2025; 17(12):1834. https://doi.org/10.3390/w17121834
Chicago/Turabian StyleMatei, Dănuţa, Abubakar Usman Katsina, Diana-Luciana Cursaru, and Sonia Mihai. 2025. "Mesoporous SBA-15-Supported Ceria–Cadmium Composites for Fast Degradation of Methylene Blue in Aqueous Systems" Water 17, no. 12: 1834. https://doi.org/10.3390/w17121834
APA StyleMatei, D., Katsina, A. U., Cursaru, D.-L., & Mihai, S. (2025). Mesoporous SBA-15-Supported Ceria–Cadmium Composites for Fast Degradation of Methylene Blue in Aqueous Systems. Water, 17(12), 1834. https://doi.org/10.3390/w17121834







