The Genesis and Hydrochemical Formation Mechanism of Karst Springs in the Central Region of Shandong Province, China
Abstract
1. Introduction
2. Study Area
3. Data Sources and Research Methods
4. Results and Discussion
4.1. Distribution and Genesis of Karst Springs
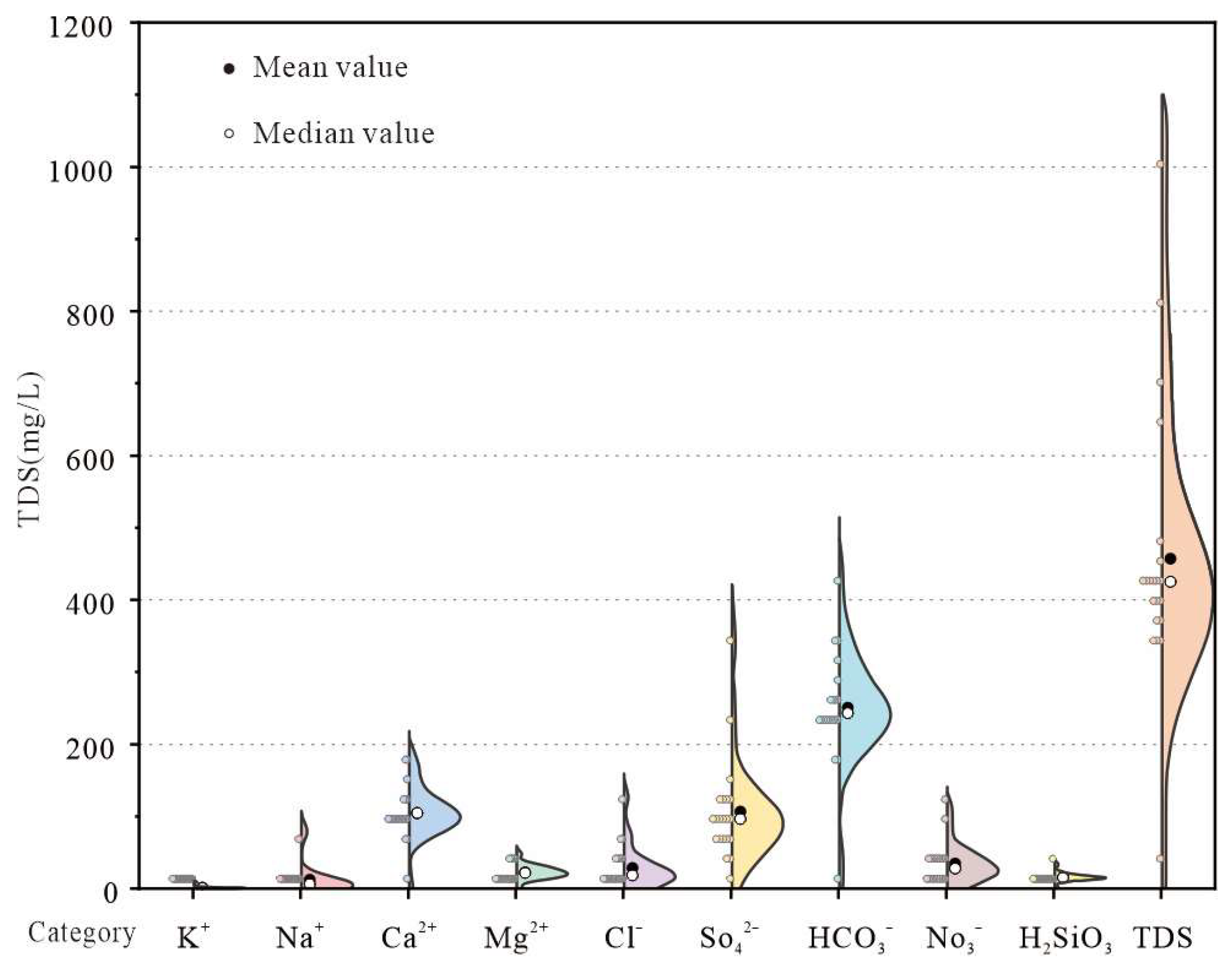
4.2. Groundwater Chemistry Characteristics
| Item | Maximum (mg·L−1) | Minimum (mg·L−1) | Mean (mg·L−1) | Coefficient of Variation/% | Standard Deviation | WHO (2011) [23] | SEL/% |
|---|---|---|---|---|---|---|---|
| pH | 8.20 | 7.10 | 7.62 | 3.28 | 0.25 | 6.5–8.5 | 0 |
| TDS | 990.00 | 334.00 | 478.61 | 36.00 | 172.31 | 1000.0 | 0 |
| K+ | 8.91 | 0.08 | 1.10 | 171.15 | 1.89 | 200.0 | 0 |
| Na+ | 82.34 | 0.68 | 12.77 | 179.14 | 22.87 | 200.0 | 0 |
| Ca2+ | 175.45 | 77.57 | 109.47 | 25.29 | 27.69 | 200.0 | 0 |
| Mg2+ | 48.16 | 12.10 | 23.54 | 36.10 | 8.50 | 150.0 | 0 |
| Cl− | 126.21 | 6.74 | 29.79 | 98.41 | 29.31 | 250.0 | 0 |
| SO42− | 342.65 | 54.40 | 111.26 | 61.21 | 68.11 | 250.0 | 5 |
| HCO3− | 427.81 | 187.53 | 261.87 | 21.73 | 56.90 | 500.0 | 0 |
| NO3− | 111.10 | 7.12 | 36.31 | 69.64 | 25.29 | 45.0 | 30 |
| H2SiO3 | 33.31 | 10.41 | 16.27 | 30.22 | 4.92 | / | / |
4.3. Hydrochemical Types of Groundwater
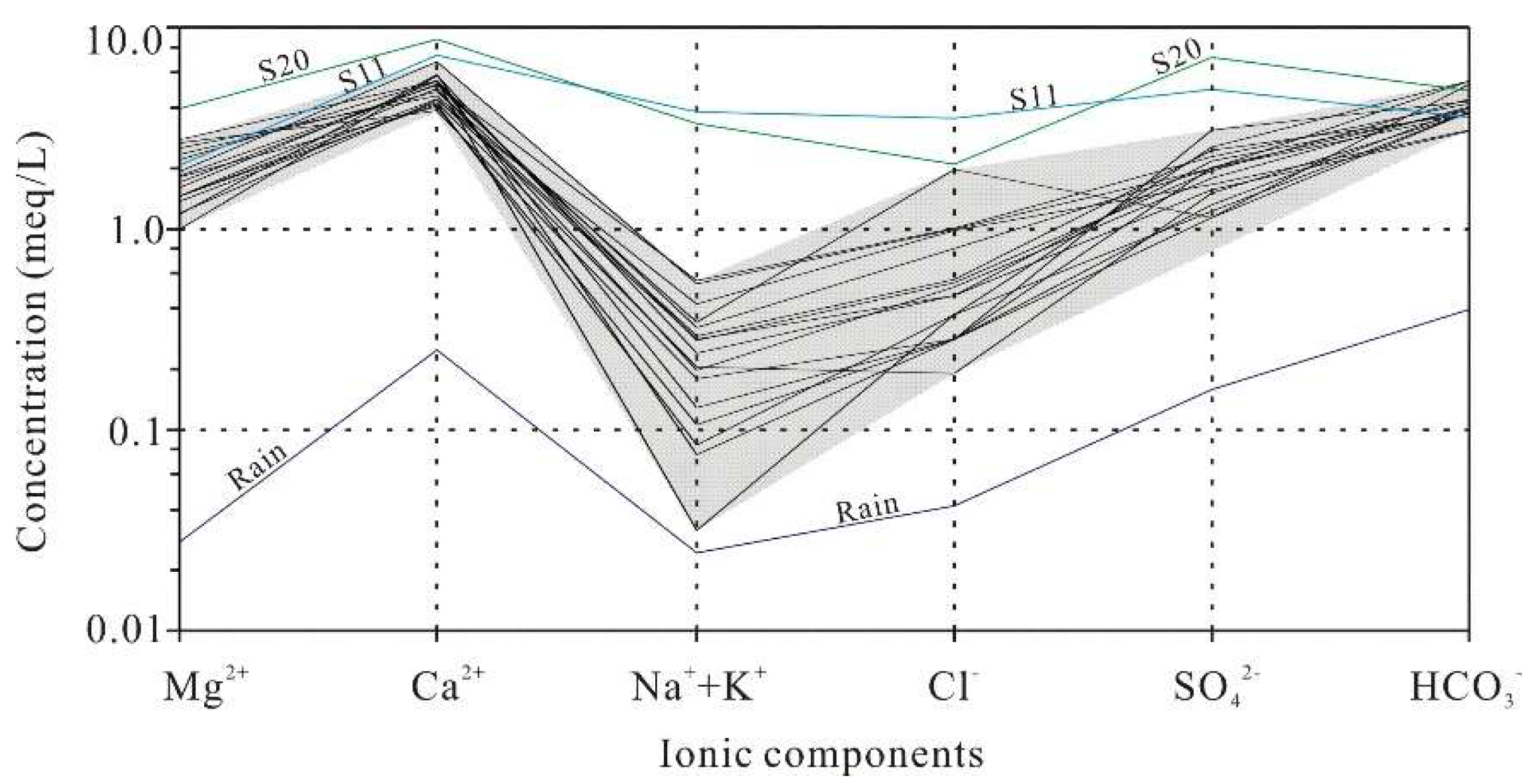
4.4. Cause Analysis of Hydrochemical Characteristics
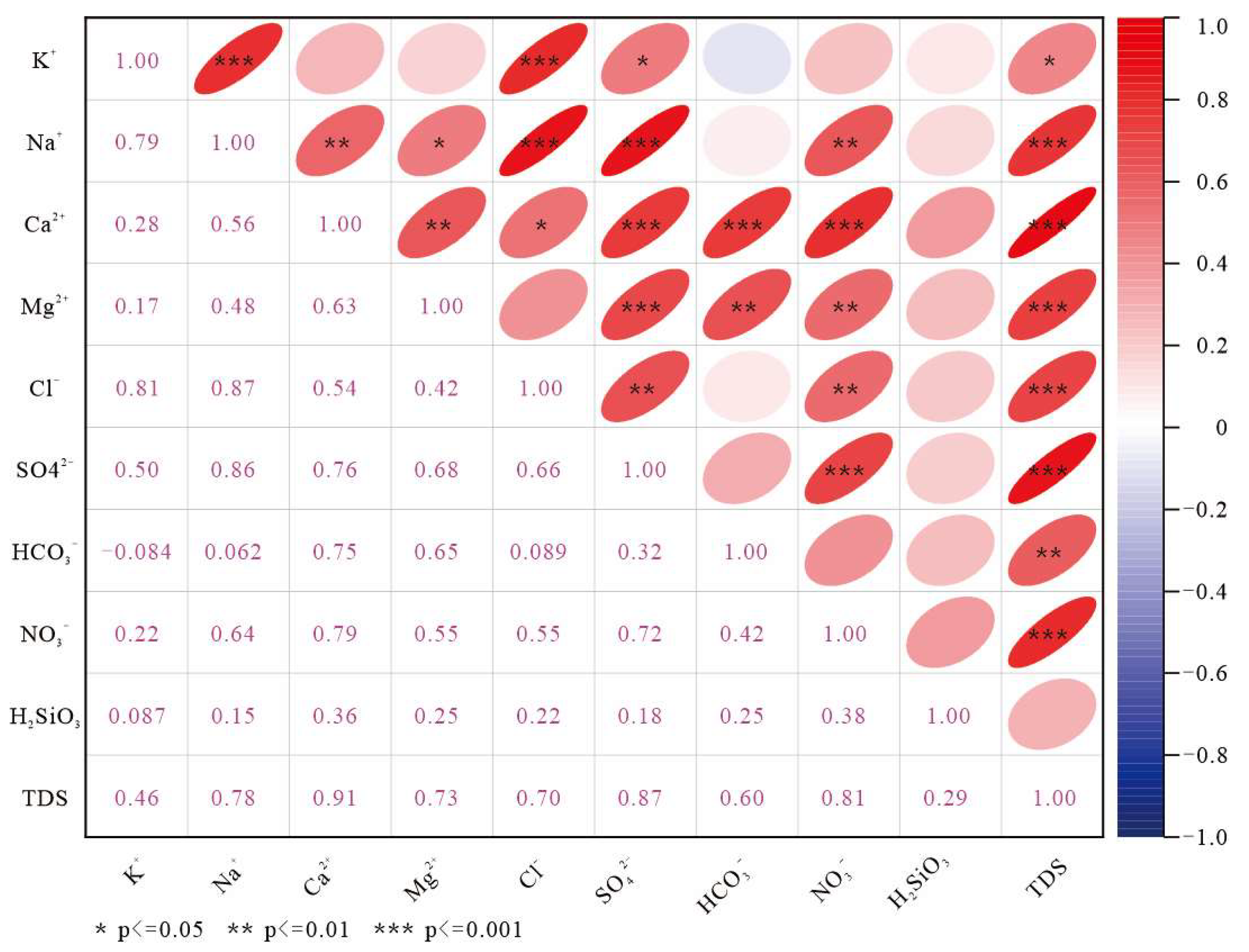
4.4.1. Correlation Analysis
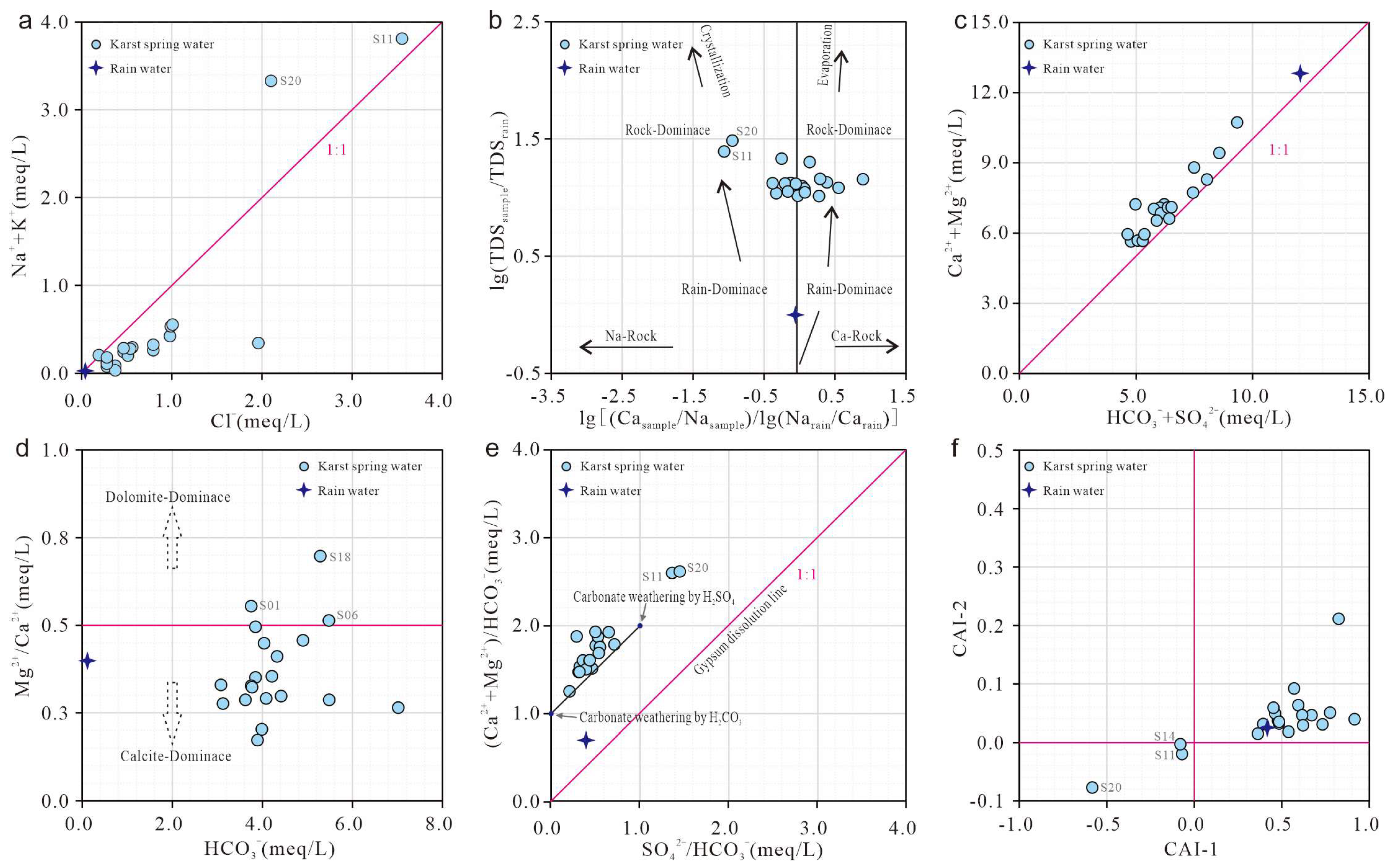
4.4.2. Natural Factors
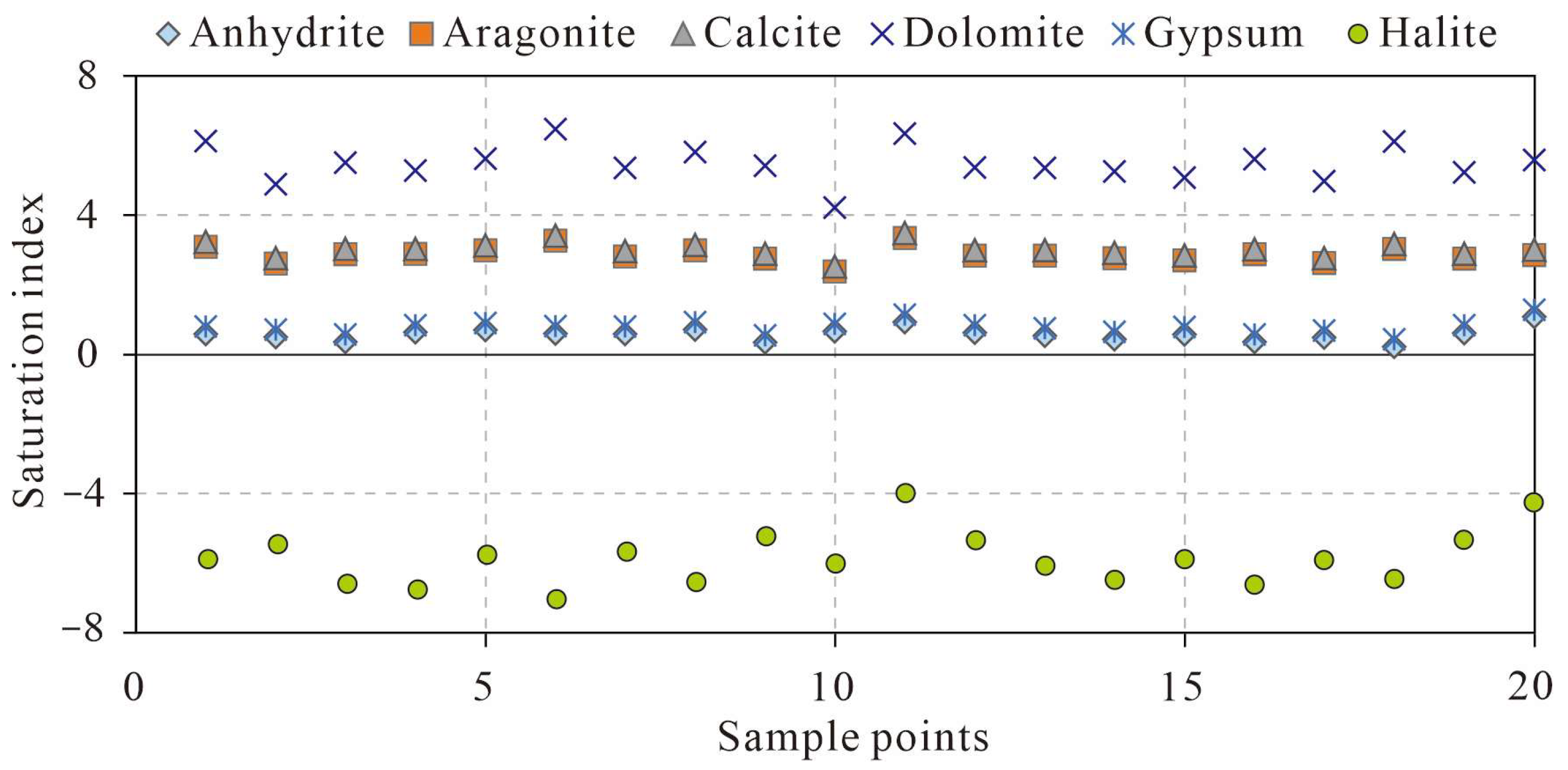
4.4.3. Saturation Index (SI)
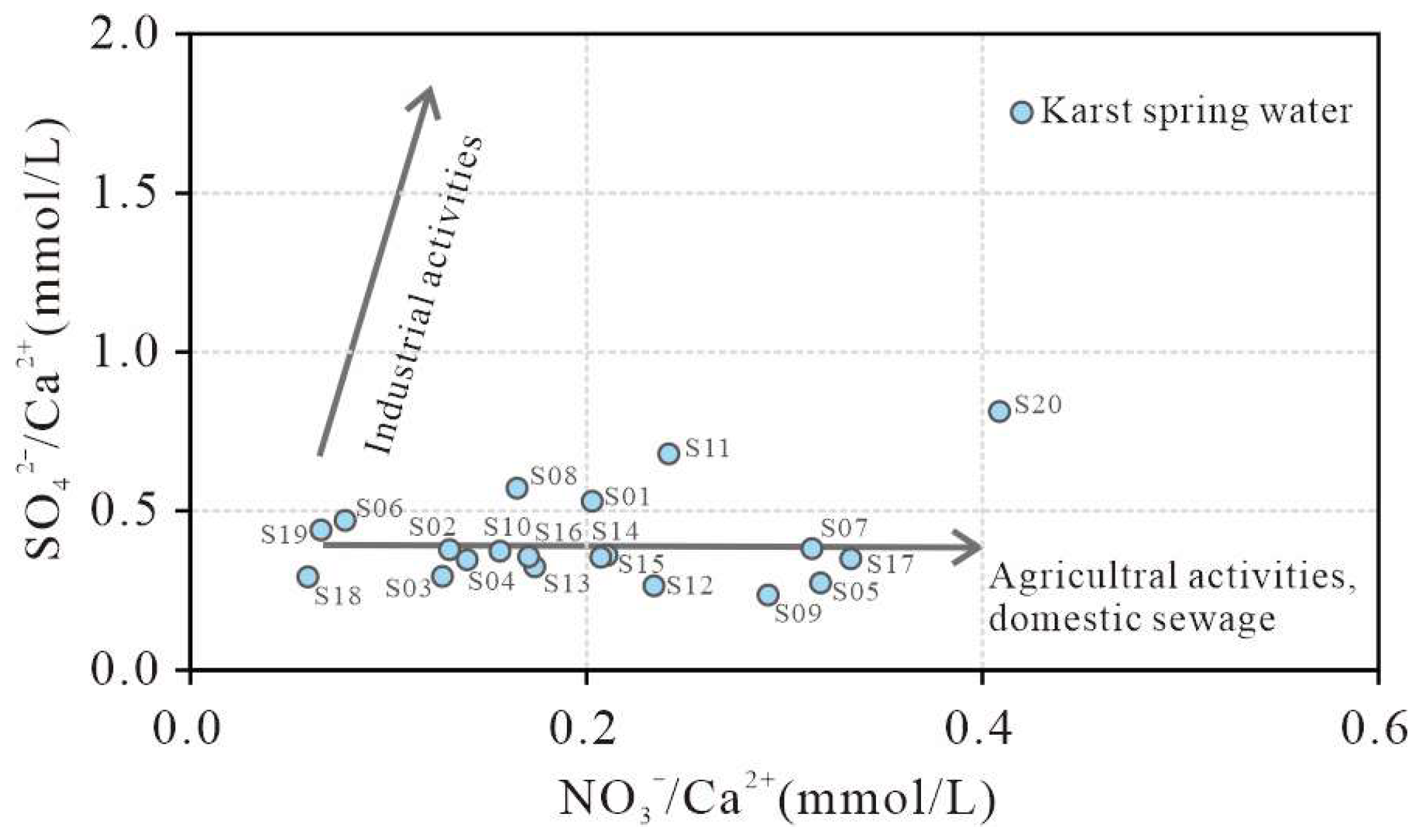
4.4.4. Human Activity
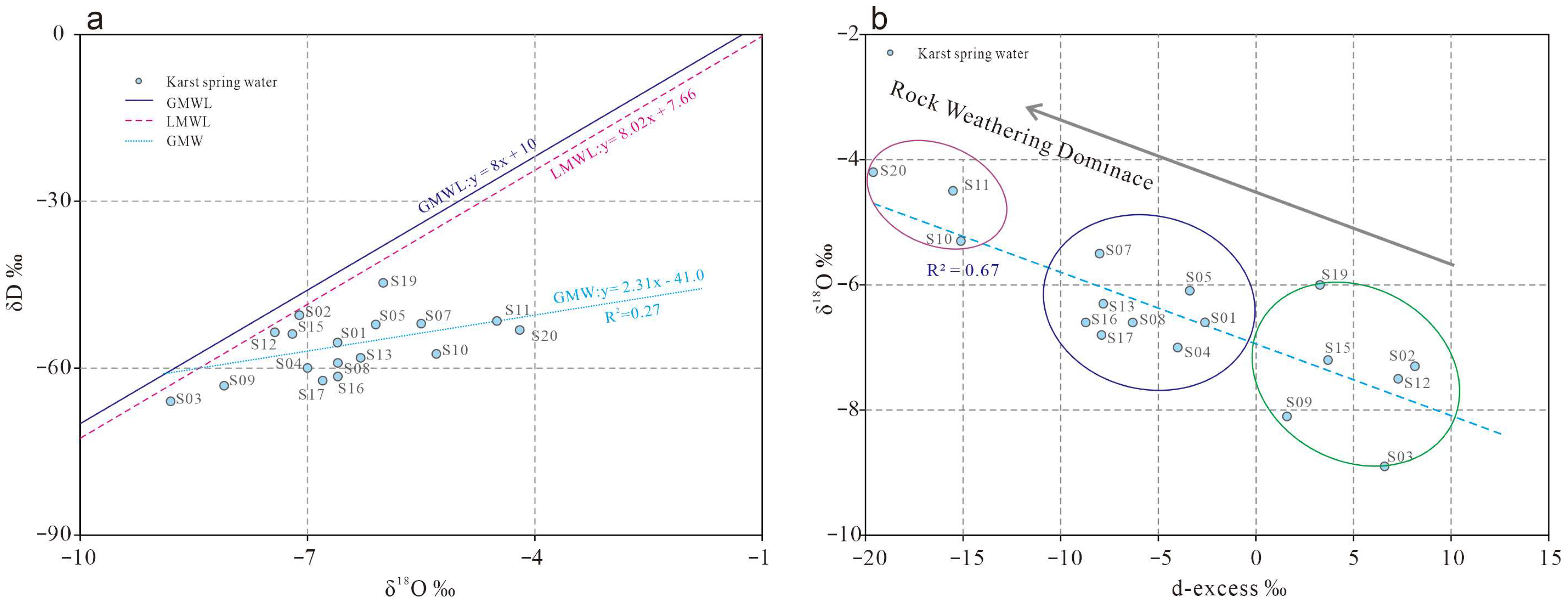
4.4.5. Isotopic Characteristics
4.5. Genetic Model of the Karst Springs
4.6. Recommendations for Sustainable Development and Management of Springs
5. Conclusions
- (1)
- Based on the geological structural characteristics of the Laiwu Basin, the controlling factors of karst spring formation were categorized into three aspects: faults, rock masses, and lithology. Consequently, the genetic types of major karst springs in the basin can be classified into four categories: lithological barriers, fault-induced water blocking, basement rock barriers, and intrusive rock barriers. The spatial distribution of these spring types varies across the basin.
- (2)
- The dominant hydrochemical type of karst springs in the Laiwu Basin is Ca·Mg-HCO3·SO4, with weakly alkaline freshwater properties. Among anions, HCO3− predominates, accounting for 55.02% of total anion concentration, while Ca2+ dominates cations, constituting 71.52% of total cation concentration.
- (3)
- Dissolution of calcite-dominated carbonate rocks serves as the primary source of HCO3−, SO42−, Ca2+, and Mg2+, whereas halite dissolution contributes predominantly to Na+ and K+. Reverse cation exchange adsorption explains the weak enrichment of Ca2+ and Mg2+ and depletion of Na+ and K+ in karst spring waters. Urban domestic sewage, agricultural activities, and manure fertilization influence hydrochemical compositions. Samples S02, S09, and S19 show strong urban sewage impacts, and S11 and S20 exhibit pronounced manure-derived influences.
- (4)
- All δD and δ18O values of karst spring waters plot below the Global Meteoric Water Line (GMWL) and Local Meteoric Water Line (LMWL), confirming atmospheric precipitation as the primary recharge source. Evaporative fractionation occurred to varying degrees during infiltration.
- (5)
- Differences in topographic relief, aquifer lithology, structural attitude, and fault development result in distinct water–rock interaction intensities between northern and southern basin groundwater during flow. This is reflected in the deuterium excess (d-excess) values, which exhibit significant spatial differentiation, with higher d-excess values observed in southern basin springs compared to northern counterparts.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Darnault, C.J. Karst aquifers: Hydrogeology and exploitation. In Nato Security Through Science; Springer: Dordrecht, The Nertherlands, 2011; pp. 203–226. [Google Scholar]
- Zhang, Z.H.; Li, L.R. Groundwater Resources in China; China Cartographic Publishing House: Beijing, China, 2004. [Google Scholar]
- Liang, Y.P.; Wang, W.T.; Zhao, H.H.; Wang, W.; Tang, C.L. Variations of karst water and environmental problems in North China. Carsologica Sin. 2013, 32, 34–42. [Google Scholar]
- Hao, Y.H.; Wang, Y.J.; Zhu, Y.; Lin, Y.; Wen, J.C.; Tian, C.J.Y. Response of karst springs to climate change and anthropogenic activities: The Niangziguan Springs, China. Prog. Phys. Geogr. 2009, 33, 634–649. [Google Scholar] [CrossRef]
- Huo, X.L.; Liu, Z.F.; Duan, Q.Y.; Hao, P.M.; Zhang, Y.Y.; Hao, Y.H.; Zhan, H.B. Linkages between Large-Scale Climate Patterns and Karst Spring Discharge in Northern China. J. Hydrometeorol. 2016, 17, 713–724. [Google Scholar] [CrossRef]
- Nerantzaki, S.D.; Nikolaidis, N.P. The response of three Mediterranean karst springs to drought and the impact of climate change. J. Hydrol. 2020, 591, 125296. [Google Scholar] [CrossRef]
- Yin, X.L.; Wang, Q.B.; Feng, W. Hydro-chemical and isotopic study of the karst spring catchment in Jinan. Acta Geol. Sin. 2017, 91, 1651–1660. [Google Scholar]
- Zhao, C.H.; Liang, Y.P.; Lu, H.P.; Tang, C.L.; Shen, H.Y. Hydrogen and oxygen isotopic characteristics and influencing factors of karst water in the Niangziguan Spring Area. Geol. Sci. Technol. Inf. 2018, 37, 200–205. [Google Scholar]
- Wang, Z.H.; Liang, Y.P.; Shi, Z.M.; Zhang, S.T.; Zhao, Y.; Xie, H.; Zhao, C.H.; Tang, C.L. Currentsituation of karst groundwater environmental problems and spring source protectioninthe Gudui-Nanliang spring basin. Bull. Geol. Sci. Technol. 2023, 42, 228–230. [Google Scholar]
- Wang, Y.X. Study on ecological restoration strategy of karst spring region in north China:Taking Jinci spring as an example. Carsologica Sin. 2022, 41, 331–344. [Google Scholar]
- Liang, Y.P.; Shen, H.Y.; Gao, X.B. Review ofresearch progress of karst groundwaterin northern China. Bull. Geol. Sci. Technol. 2022, 41, 199–219. [Google Scholar]
- Liu, Y.Q.; Zhou, L.; Ma, X.M.; Lv, L.; Zheng, Y.D.; Li, W.; Meng, S.X. Evaluation on chemical characteristics and water quality of groundwaterin Feicheng Basin. J. Arid Land Resour. Environ. 2020, 34, 118–124. [Google Scholar]
- Tang, C.L.; Zhao, C.H.; Shen, H.Y.; Liang, Y.P.; Wang, Z.L. Chemical Characteristics and Causes of Groups Water in Niangziguan Spring. Environ. Sci. 2021, 42, 1416–1423. [Google Scholar]
- Qiao, X.J.; Li, B.L.; Liu, K.; Chai, X.Y.; Yu, W.J. Hydrochemical characteristic and karst development in typical karst spring area, Northern China. Front. Environ. Sci. 2024, 12, 1494730. [Google Scholar]
- Kumar, V.; Sen, S. Evaluation of spring discharge dynamics using recession curve analysis: A case study in data-scarce region, Lesser Himalayas, India. Sustain. Water Resour. Manag. 2018, 4, 539–557. [Google Scholar] [CrossRef]
- Kang, F.X.; Zheng, T.T.; Feng, Y.W.; Xu, Q.X.; Liu, B.T. Recharge coefficients and recharge mechanisms of precipitation to groundwater in karst areas of North China: A case study of Yangzhuang karst water system. Bull. Geol. Sci. Technol. 2024, 43, 268–282. [Google Scholar]
- Gao, Z.J.; Wan, Z.P.; He, K.Q.; Victor, K.Z.; Liu, J.T. Hydrochemical characteristics and controlling factors of karst groundwater in middle and upperreaches of Dawen River basin. Bull. Geol. Sci. Technol. 2022, 41, 264–272. [Google Scholar]
- Liu, Y.Q.; Wen, D.G.; Lv, L.; Li, W.; Zhang, F.C.; Wang, X.F.; Meng, S.X. Characteristics of karst groundwater flow systems of typical faulted basins in Yimeng Mountain area: A case study of Laiwu Basin. Bull. Geol. Sci. Technol. 2022, 41, 157–167. [Google Scholar]
- Wang, Z.H.; Liang, Y.P.; Shi, Z.M. Current situation of karst groundwater environmental problems and spring source protection in the Gudui-Nanliang spring basin. Bull. Geol. Sci. Technol. 2002, 23, 369–374. [Google Scholar]
- He, K.Q.; Liu, W.J.; Shao, C.F. The Comprehensive Type Classification and Proper Adjustment of Karst Water Resource in the Central-South Region of Shandong Province. Acta Geosci. Sin. 2002, 23, 369–374. [Google Scholar]
- HTSGB. Hydrogeological Summary Report of Laiwu Basin; Hydrogeological Team of Shandong Geological Bureau: Jinan, China, 1974. [Google Scholar]
- GB/T 14848-2017; Standard for Groundwater Quality. National Technical Committee for Standardization of Land and Resources: Beijing, China, 2017.
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Xiao, Y.; Liu, K.; Hao, Q.C.; Xiao, D.; Zhu, Y.C.; Yin, S.Q.; Zhang, Y.H. Hydrogeochemical insights into the signatures, genesis and sustainable perspective of nitrate enriched groundwater in the piedmont of Hutuo watershed, China. Catena 2022, 212, 106020. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Zhou, L.; Lv, L.; Li, W.; Wang, X.F.; Zheng, Y.D.; Li, C.S. Hydrochemical characteristics and control factors of pore-water in the middle and upper reaches of Muwen River. Environ. Sci. 2023, 44, 1429–1439. [Google Scholar]
- Chander, K.S.; Anand, K.; Satyanarayan, S.; Alok, K.; Pankaj, K.; Javed, M. Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. J. Geochem. Explor. 2017, 175, 59–71. [Google Scholar]
- Zhang, T.; Cai, W.T.; Li, Y.Z.; Zhang, Z.Y.; Geng, T.T.; Bian, C.; Zhao, M.; Cai, Y.M. Major ionic features and their possible controls in the water of the Niyang River Basin. Environ. Sci. 2017, 38, 4537–4545. [Google Scholar]
- Liu, P.; Nils, H.; Carsten, D.; Sun, Y.; Xu, Z. Hydro-geochemical paths of multi-layer groundwater system in coal mining regions—Using multivariate statistics and geochemical modeling approaches. Sci. Total Environ. 2017, 601–602, 1–14. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, F.X.; Zhang, W.Q.; Xu, Q.Y.; Qin, P.; Zhao, Q. Identification and genetic mechanism of recharge sources in groundwater-rich area of Changxiao karst water system in Jinan City. Bull. Geol. Sci. Technol. 2024, 43, 292–305. [Google Scholar]
- He, J.; Zhang, Y.K.; Zhao, Y.Q.; Han, S.B.; Liu, Y.Q.; Zhang, T. Hydrochemical characteristics and possible controls of groundwater in the Xialatuo Basin section of the Xianshui River. Environ. Sci. 2019, 40, 1236–1244. [Google Scholar]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 17, 1088–1090. [Google Scholar] [CrossRef]
- Li, Z.J.; Yang, Q.C.; Yang, Y.S.; Ma, H.; Wang, H.; Luo, J.N.; Bian, J.M.; Jordi, D.M. Isotopic and geochemical interpretation of groundwater under the influences of anthropogenic activities. J. Hydrogeol. 2019, 576, 685–697. [Google Scholar] [CrossRef]
- Andres, M.; Paul, S. Groundwater chemistry and the Gibbs Diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar]
- Gaillardet, J.; Dupre, B.; Louvat, P.; Allegre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Gan, Y.Q.; Zhao, K.; Deng, Y.M.; Liang, X.; Ma, T.; Wang, Y.X. Groundwater flow and hydrogeochemical evolution in the Jianghan Plain, central China. Hydrogeol. J. 2018, 26, 1609–1623. [Google Scholar] [CrossRef]
- Wu, Y.; Gibson, C.E. Mechanisms controlling the water chemistry of small lakes in northern Ireland. Water Res. 1996, 30, 178–182. [Google Scholar] [CrossRef]
- Zhu, B.Q.; Yu, J.J.; Qin, X.G.; Patrick, R.; Xiong, H.G. Climatic and geological factors contributing to the natural water chemistry in an arid environment from watersheds in northern Xinjiang, China. Geomorphology 2012, 153–154, 102–114. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.X.; Sun, Z.Y.; Zheng, C.M.; Ma, T.; Prommer, H. Geochemical evolution of groundwater in carbonate aquifers in Taiyuan, northern China. Appl. Geochem. 2011, 26, 884–897. [Google Scholar] [CrossRef]
- Pu, J.B.; Yuan, D.X.; Xiao, Q.; Zhao, H.P. Hydrogeochemical characteristics in karst subterranean streams: A case history from Chongqing, China. Carbonates Evaporites 2015, 30, 307–319. [Google Scholar] [CrossRef]
- Xie, Y.T.; Hao, Y.P.; Li, J.; Guo, Y.L.; Xiao, Q.; Huang, F. Influence of anthropogenic sulfuric acid on different lithological carbonate weathering and the related carbon sink budget: Examples from Southwest China. Water 2023, 15, 2933. [Google Scholar] [CrossRef]
- Thakur, T.; Rishi, M.S.; Naik, P.K.; Sharma, P. Elucidating hydrochemical properties of groundwater for drinking and agriculture in parts of Punjab, India. Environ. Earth Sci. 2016, 75, 467. [Google Scholar] [CrossRef]
- Gao, Z.J.; Liu, J.T.; Feng, J.G.; Wang, M.; Wu, G.W. Hydrogeochemical characteristics and the suitability of Groundwater in the alluvial-diluvial Plain of Southwest Shandong Province, China. Water 2019, 11, 1577. [Google Scholar] [CrossRef]
- Fan, B.L.; Zhao, Z.Q.; Tao, F.X.; Liu, B.J.; Tao, Z.H.; Gao, S.; Zhang, Z.H. Characteristics of carbonate, evaporite and silicate weathering in Huanghe River basin: A comparison among the upstream, midstream and downstream. J. Asian Earth Sci. 2014, 96, 17–26. [Google Scholar] [CrossRef]
- Pu, J.B.; Yuan, D.X.; Jiang, Y.J.; Gou, P.F.; Yin, J.J. Hydrogeochemistry and environmental meaning of Chongqing subterranean karst streams in China. Adv. Water Sci. 2010, 21, 628–636. [Google Scholar]
- Mohamed, O.A.; Tiziano, B.; Abdillahi, E.A.; Mahamoud, A.C.; Moussa, M.A.; Omar, A.D.; Youssouf, D.S.; Nima, M.E.; Ali, D.K.; Ibrahim, H.K.; et al. Origin of nitrate and sulfate sources in volcano-sedimentary aquifers of the East Africa Rift System: An example of the Ali-Sabieh groundwater (Republic of Djibouti). Sci. Total Environ. 2022, 804, 150072. [Google Scholar]
- Wen, D.G. Groundwater resources attribute based on environmental isotopes. Earth Sci.-J. China Univ. Geosci. 2002, 27, 141–147. [Google Scholar]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; Lewis Publishers: NewYork, NY, USA, 1997; pp. 1–312. [Google Scholar]
- Liu, Y.Q.; Zhou, L.; Li, W.; Zhu, Q.J.; Xu, M.; Lv, L.; Deng, Q.J.; He, J.; Wang, X.F. The Characteristics and Genetic Analysis of the Paleogene Semi- Consolidated Water-Bearing Formation on the Northwestern Margin of Laiwu Basin, Shandong Province. Acta Geosci. Sin. 2018, 39, 737–748. [Google Scholar]
- Yin, G.; Ni, S.J.; Gao, Z.Y.; Shi, Z.M.; Yan, Q.S. Variation of isotope compositions and deuterium excess of brines in Sichuan Basin. J Miner. Pet. 2008, 28, 56–62. [Google Scholar]






| Spring | Discharge (L·s−1) | Main Controlling Factors | Origin | TDS(mg/L)/pH | Water Types | Typical Section |
|---|---|---|---|---|---|---|
| S01 | 14.85 | Water resistance controlled by lithology | Water-resisting by detrital rocks of ∈3-4g | 420/8.00 | HCO3·SO4-Ca·Mg |  |
| S02 | 5.0 | Water-resisting by detrital rocks of E2-3d | 353/7.50 | HCO3·SO4-Ca | ||
| S08 | 6.0 | Water resistance controlled by faults | Water-resisting by Shengshuian fault | 468/7.80 | HCO3-Ca | 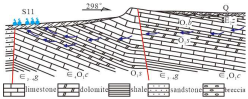 |
| S09 | 5.56 | Water-resisting by Tai’an fault | 426/7.50 | HCO3·Cl-Ca·Mg | ||
| S10 | 6.0 | Water-resisting by Changjia fault | 407/7.10 | HCO3·SO4-Ca | ||
| S11 | 85.5 | Water-resisting by Nanliu fault | 800/8.20 | SO4·HCO3·Cl-Ca·Na | ||
| S13 | 13.86 | Water resistance controlled by metamorphic rock basement | Water-resisting by Archean metamorphic basement | 386/7.60 | HCO3·SO4-Ca·Mg |  |
| S14 | 5.0 | 335/7.60 | Ca·Mg-HCO3·SO4 | |||
| S15 | 17.0 | 425/7.40 | HCO3·SO4-Ca·Mg | |||
| S16 | 2.0 | 334/7.70 | HCO3·SO4-Ca·Mg | |||
| S19 | 37.7 | Water resistance controlled by magmatic rock mass | Water-resisting by Yanshanian rock mass | 430/7.50 | HCO3·SO4-Ca·Mg |  |
| S20 | 15.0 | Water-resisting by Yanshanian rock mass | 990/7.50 | HCO3·SO4-Ca·Mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhou, L.; Ma, X.; Wen, D.; Li, W.; Shi, Z. The Genesis and Hydrochemical Formation Mechanism of Karst Springs in the Central Region of Shandong Province, China. Water 2025, 17, 1805. https://doi.org/10.3390/w17121805
Liu Y, Zhou L, Ma X, Wen D, Li W, Shi Z. The Genesis and Hydrochemical Formation Mechanism of Karst Springs in the Central Region of Shandong Province, China. Water. 2025; 17(12):1805. https://doi.org/10.3390/w17121805
Chicago/Turabian StyleLiu, Yuanqing, Le Zhou, Xuejun Ma, Dongguang Wen, Wei Li, and Zheming Shi. 2025. "The Genesis and Hydrochemical Formation Mechanism of Karst Springs in the Central Region of Shandong Province, China" Water 17, no. 12: 1805. https://doi.org/10.3390/w17121805
APA StyleLiu, Y., Zhou, L., Ma, X., Wen, D., Li, W., & Shi, Z. (2025). The Genesis and Hydrochemical Formation Mechanism of Karst Springs in the Central Region of Shandong Province, China. Water, 17(12), 1805. https://doi.org/10.3390/w17121805





