Effects of Extreme Flooding on Soil Characteristics, Soil Enzyme Activity, and Microbial Structure in Shengjin Lake
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Communities and Sampling
2.3. Soil Chemical Analyses
2.4. Soil Enzymatic Activities Analyses
2.5. DNA Extraction, Amplification, Sequencing, and Microbial Analysis
2.6. Statistical Analyses
3. Results
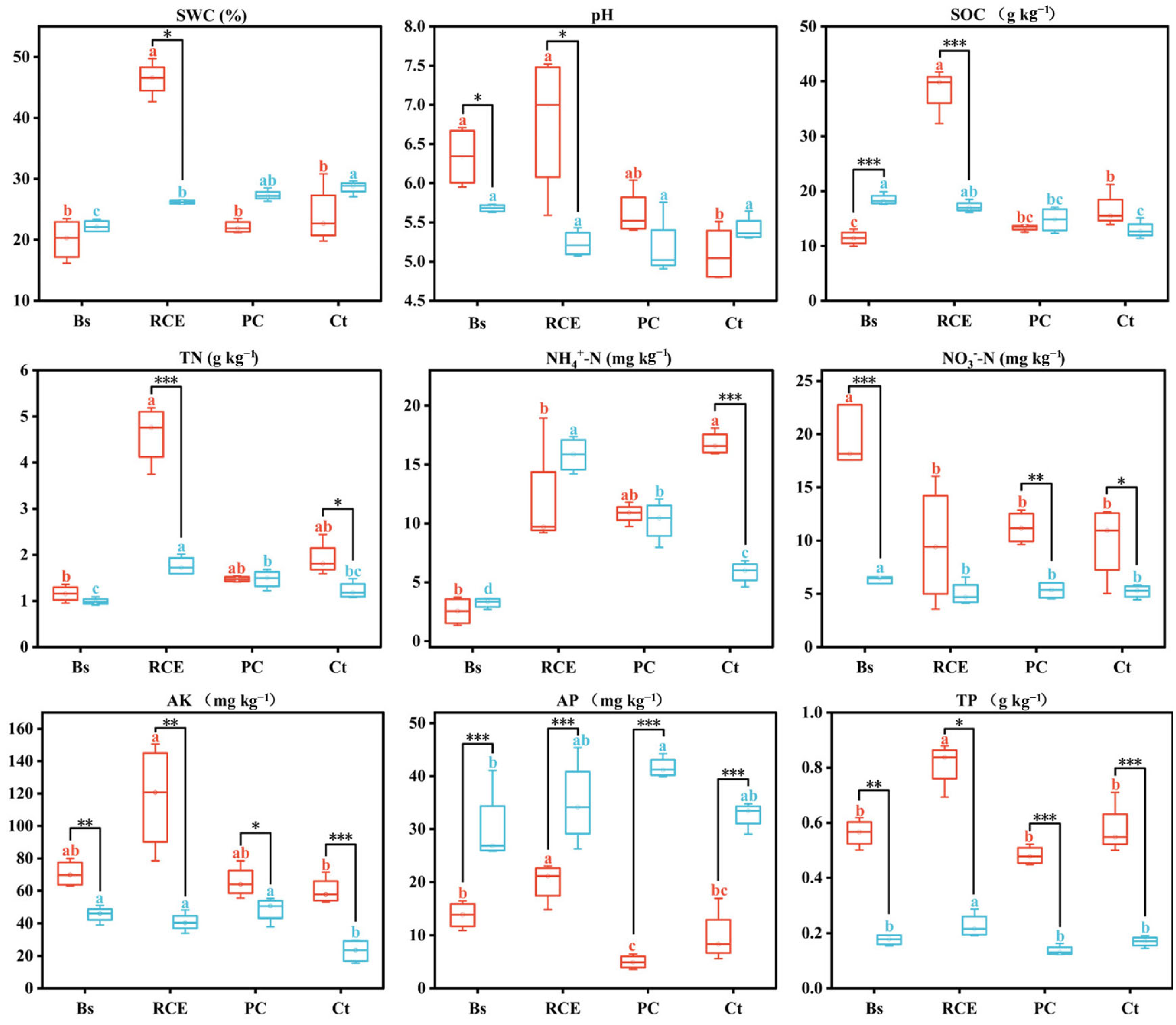
3.1. Soil Physicochemical Properties
3.2. Soil Enzyme Activities
3.3. Soil Bacterial Community Composition
3.4. Correlations Between Soil Variables and Soil Bacterial Community Structure
4. Discussion
4.1. Soil Physicochemical Properties
4.2. Soil Enzyme Activities
4.3. Soil Bacterial Community Composition
4.4. Pathways of the Effects of Vegetation Communities and Soil Factors on Composition of Soil Bacterial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunil, C.; Somashekar, R.K.; Nagaraja, B.C. Riparian vegetation assessment of Cauvery River Basin of South India. Environ. Monit. Assess. 2010, 170, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Prado, R.B.; Damasceno, G.M.S.; Aquino, F.G.A. Overview of studies on ecosystem services in riparian zones: A systematic review. Acta Limnol. Bras. 2022, 34, e19. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Q.; Liu, Z.; Jian, M.; Peng, Y.; Shen, R.; Liao, W.; Zhong, A. Hydrological alteration drives chemistry of dissolved organic matter in the largest freshwater lake of China (Poyang Lake). Water Res. 2024, 251, 121154. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Li, Y.; Wang, Y.; Zhang, B.; Yin, S.; Zhu, H.; Xing, Z. Evaluation of ecosystem service value of riparian zone using land use data from 1986 to 2012. Ecol. Indic. 2016, 69, 873–881. [Google Scholar] [CrossRef]
- Belmar, O.; Bruno, D.; Martínez-Capel, F.; Barquín, J.; Velasco, J. Effects of flow regime alteration on fluvial habitats and riparian quality in a semiarid Mediterranean basin. Ecol. Indic. 2013, 30, 52–64. [Google Scholar] [CrossRef]
- Sendek, A.; Kretz, L.; van der Plas, F.; Seele-Dilbat, C.; Schulz-Zunkel, C.; Vieweg, M.; Bondar-Kunze, E.; Weigelt, A.; Wirth, C. Topographical factors related to flooding frequency promote ecosystem multifunctionality of riparian floodplains. Ecol. Indic. 2021, 132, 108312. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine flood plains: Present state and future trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef]
- Riis, T.; Kelly-Quinn, M.; Aguiar, F.C.; Manolaki, P.; Bruno, D.; Bejarano, M.D.; Clerici, N.; Fernandes, M.R.; Franco, J.C.; Pettit, N.; et al. Global overview of ecosystem services provided by riparian vegetation. BioScience 2020, 70, 501–514. [Google Scholar] [CrossRef]
- Tahsin, S.; Medeiros, S.C.; Singh, A. Assessing the resilience of coastal wetlands to extreme hydrologic events using vegetation indices: A review. Remote Sens. 2018, 10, 1390. [Google Scholar] [CrossRef]
- Garssen, A.G.; Baattrup-Pedersen, A.; Voesenek, L.A.C.J.; Verhoeven, J.T.A.; Soons, M.B. Riparian plant community responses to increased flooding: A meta-analysis. Glob. Change Biol. 2015, 21, 2881–2890. [Google Scholar] [CrossRef]
- Ou, Y.; Rousseau, A.N.; Wang, L.; Yan, B.; Gumiere, T.; Zhu, H. Identification of the alteration of riparian wetland on soil properties, enzyme activities and microbial communities following extreme flooding. Geoderma 2019, 337, 825–833. [Google Scholar] [CrossRef]
- Garssen, A.G.; Baattrup-Pedersen, A.; Riis, T.; Raven, B.M.; Hoffman, C.C.; Verhoeven, J.T.A.; Soons, M.B. Effects of increased flooding on riparian vegetation: Field experiments simulating climate change along five European lowland streams. Glob. Change Biol. 2017, 23, 3052–3063. [Google Scholar] [CrossRef] [PubMed]
- Osunkoya, O.O.; Ali, S.; Nguyen, T.; Perrett, C.; Shabbir, A.; Navie, S.; Belgeri, A.; Dhileepan, K.; Adkins, S. Soil seed bank dynamics in response to an extreme flood event in a riparian habitat. Ecol. Res. 2014, 29, 1115–1129. [Google Scholar] [CrossRef]
- Thoms, M.C. Floodplain–river ecosystems: Lateral connections and the implications of human interference. Geomorphology 2003, 56, 335–349. [Google Scholar] [CrossRef]
- Zuijdgeest, A.L.; Zurbrügg, R.; Blank, N.; Fulcri, R.; Senn, D.B.; Wehrli, B. Seasonal dynamics of carbon and nutrients from two contrasting tropical floodplain systems in the Zambezi River basin. Biogeosciences 2015, 12, 7535–7555. [Google Scholar] [CrossRef]
- Bai, J.; Yu, L.; Du, S.; Wei, Z.; Liu, Y.; Zhang, L.; Zhang, G.; Wang, X. Effects of flooding frequencies on soil carbon and nitrogen stocks in river marginal wetlands in a ten-year period. J. Environ. Manag. 2020, 267, 110618. [Google Scholar] [CrossRef]
- Ye, F.; Ma, M.H.; Wu, S.J.; Jiang, Y.; Zhu, G.B.; Zhang, H.; Wang, Y. Soil properties and distribution in the riparian zone: The effects of fluctuations in water and anthropogenic disturbances. Eur. J. Soil Sci. 2019, 70, 664–673. [Google Scholar] [CrossRef]
- Zhang, M.; O’Connor, P.J.; Zhang, J.; Ye, X. Linking soil nutrient cycling and microbial community with vegetation cover in riparian zone. Geoderma 2021, 384, 114801. [Google Scholar] [CrossRef]
- Patel, K.F.; Fansler, S.J.; Campbell, T.P.; Bond-Lamberty, B.; Smith, A.P.; RoyChowdhury, T.; McCue, L.A.; Varga, T.; Bailey, V.L. Soil texture and environmental conditions influence the biogeochemical responses of soils to drought and flooding. Commun. Earth Environ. 2021, 2, 127. [Google Scholar] [CrossRef]
- Unger, I.M.; Motavalli, P.P.; Muzika, R.-M. Changes in soil chemical properties with flooding: A field laboratory approach. Agric. Ecosyst. Environ. 2009, 131, 105–110. [Google Scholar] [CrossRef]
- Gutknecht, J.L.M.; Goodman, R.M.; Balser, T.C. Linking soil process and microbial ecology in freshwater wetland ecosystems. Plant Soil 2006, 289, 17–34. [Google Scholar] [CrossRef]
- Ren, C.J.; Zhang, W.; Zhong, Z.K.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Differential responses of soil microbial biomass, diversity, and compositions to elevation gradient depend on plant and soil characteristics. Sci. Total Environ. 2018, 610, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Bai, J.; Wang, W.; Zhang, G.; Jia, J.; Cui, B.; Liu, X. Effects of soil moisture on carbon mineralization in floodplain wetlands with different flooding frequencies. J. Hydrol. 2019, 574, 1074–1084. [Google Scholar] [CrossRef]
- Lan, Z.; Chen, Y.; Shen, R.; Cai, Y.; Luo, H.; Jin, B.; Chen, J. Effects of flooding duration on wetland plant biomass: The importance of soil nutrients and season. Freshw. Biol. 2020, 66, 211–222. [Google Scholar] [CrossRef]
- Taylor, J.P.; Wester, D.B.; Smith, L.M. Soil disturbance, flood management, and riparian woody plant establishment in the Rio Grande floodplain. Wetlands 1999, 19, 372–382. [Google Scholar] [CrossRef]
- Unger, I.M.; Kennedy, A.C.; Muzika, R.-M. Flooding effects on soil microbial communities. Appl. Soil Ecol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Fox, A.D.; Cao, L.; Zhang, Y.; Barter, M.; Zhao, M.; Meng, F.; Wang, S. Declines in the tuber-feeding waterbird guild at Shengjin Lake National Nature Reserve, China—A barometer of submerged macrophyte collapse. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 82–91. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Xu, W.; Wang, X.; Ren, Y.; Ye, X. Change in the Ecological Stoichiometry of Carex thunbergii in Response to Seasonal Dynamics and Environmental Factors in Shengjin Lake, China. Diversity 2024, 16, 198. [Google Scholar] [CrossRef]
- Anhui Provincial Department of Water Resources. 22 May 2022. Available online: http://yc.wswj.net/ahyc/ (accessed on 22 May 2022).
- Guo, W.; Zhou, Z.; Chen, J.; Zheng, X.; Ye, X. Effects of extreme flooding on aquatic vegetation cover in Shengjin Lake, China. Hydrol. Process. 2022, 36, e14459. [Google Scholar] [CrossRef]
- Bao, S. Soil and Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Dick, R.P. Methods of Soil Enzymology; Soil Science Society of America: Madison, WI, USA, 2011; p. 95. [Google Scholar] [CrossRef]
- Guan, S.Y. Soil Enzyme and Its Analysis Method; Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Lefcheck, J.S.; Freckleton, R. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2015, 7, 573–579. [Google Scholar] [CrossRef]
- Shipley, B. Confirmatory path analysis in a generalized multilevel context. Ecology 2009, 90, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, A.R.; Hill, P.W.; Chadwick, D.R.; Jones, D.L. Typology of extreme flood event leads to differential impacts on soil functioning. Soil Biol. Biochem. 2019, 129, 153–168. [Google Scholar] [CrossRef]
- Eltohamy, K.M.; Menezes-Blackburn, D.; Klumpp, E.; Liu, C.; Jin, J.; Xing, C.; Lu, Y.; Liang, X. Microbially induced soil colloidal phosphorus mobilization under anoxic conditions. Environ. Sci. Technol. 2024, 58, 7554–7566. [Google Scholar] [CrossRef]
- Wu, G.; Liang, F.; Wu, Q.; Feng, X.-G.; Shang, W.-D.; Li, H.-W.; Li, X.-X.; Che, Z.; Dong, Z.-R.; Song, H. Soil pH differently affects N2O emissions from soils amended with chemical fertilizer and manure by modifying nitrification and denitrification in wheat-maize rotation system. Biol. Fertil. Soils 2024, 60, 101–113. [Google Scholar] [CrossRef]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; van Agtmaal, M.; et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef]
- Jiru, E.B.; Wegari, H.T. Soil and water conservation practice effects on soil physicochemical properties and crop yield in Ethiopia: Review and synthesis. Ecol. Process. 2022, 11, 21. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, Y.; Zhang, K.; Xu, X.; Zhao, Y.; Bai, T.; Zhao, Y.; Wang, H.; Sheng, X.; Bloszies, S.; et al. Intermediate soil acidification induces highest nitrous oxide emissions. Nat. Commun. 2024, 15, 2695. [Google Scholar] [CrossRef]
- Bao, B.; Chang, M.; Jin, S.; Li, X.; Xie, H. Periodic flooding decoupled the relations of soil C, N, P, and K ecological stoichiometry in a coastal shelterbelt forest of eastern China. Forests 2023, 14, 2270. [Google Scholar] [CrossRef]
- Zuccarini, P.; Sardans, J.; Asensio, L.; Peñuelas, J. Altered activities of extracellular soil enzymes by the interacting global environmental changes. Glob. Change Biol. 2023, 29, 2067–2091. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lu, S.; Wang, C.; Mu, J.; Zhang, Y.; Wang, X. Changes in soil organic carbon fractions and enzyme activities in response to tillage practices in the Loess Plateau of China. Soil Tillage Res. 2021, 209, 104940. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, L.; Liu, J.; Wan, Y.; Yang, R.; Mou, J.; He, Q.; Tang, S.; Dan, X.; Wu, Y.; et al. Land use change from natural tropical forests to managed ecosystems reduces gross nitrogen production rates and increases the soil microbial nitrogen limitation. Environ. Sci. Technol. 2024, 58, 2786–2797. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Liao, Y.; Pan, C.; Li, X. Positive effects of intercropping on soil phosphatase activity depend on the application scenario: A meta-analysis. Soil Tillage Res. 2024, 235, 105914. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Gong, P.; He, X.; Liu, H.; Li, L.; Wang, C.; Li, P.; Wei, J.; Yu, X. Enhanced irrigation volume reduces salinity and improves deep root zone soil nutrients, phosphatase activity and changes root traits of fruit trees. Agric. Water Manag. 2024, 302, 109001. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, C.; Kang, E.; Li, X.; Deng, Y.; Feng, X. Plant–microbe interactions underpin contrasting enzymatic responses to wetland drainage. Nat. Clim. Change 2024, 14, 1078–1086. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Dobies, T.; Cesarz, S.; Hobbie, S.E.; Meyer, R.J.; Worm, K.; Reich, P.B. Plant diversity effects on soil food webs are stronger than those of elevated CO₂ and N deposition in a long-term grassland experiment. Proc. Natl. Acad. Sci. USA 2013, 110, 6889–6894. [Google Scholar] [CrossRef]
- Doering, M.; Freimann, R.; Antenen, N.; Roschi, A.; Robinson, C.T.; Rezzonico, F.; Smits, T.H.; Tonolla, D. Microbial communities in floodplain ecosystems in relation to altered flow regimes and experimental flooding. Sci. Total Environ. 2021, 788, 147497. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- He, H.; Miao, Y.; Gan, Y.; Wei, S.; Tan, S.; Rask, K.A.; Wang, L.; Dai, J.; Chen, W.; Ekelund, F. Soil bacterial community response to long-term land use conversion in Yellow River Delta. Appl. Soil Ecol. 2020, 156, 103709. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Yin, M.; Ma, X.; Yu, X.; Guo, X.; Du, N.; Eller, F.; Guo, W. Soil salinity, not plant genotype or geographical distance, shapes soil microbial community of a reed wetland at a fine scale in the Yellow River Delta. Sci. Total Environ. 2023, 856, 159136. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Abadie, M.; Blaud, A.; Carswell, A.; Misselbrook, T.H.; Clark, I.M.; Hirsch, P.R. Effects of urease and nitrification inhibitors on soil N, nitrifier abundance and activity in a sandy loam soil. Biol. Fertil. Soils 2020, 56, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, Z.; Ou, J.; Liu, F.; Cai, G.; Tan, K.; Wang, X. Nitrogen substitution practice improves soil quality of red soil (Ultisols) in South China by affecting soil properties and microbial community composition. Soil Tillage Res. 2024, 240, 106089. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant–soil feedback: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef]
- Martínez-Arias, C.; Witzell, J.; Solla, A.; Martin, J.A.; Rodríguez-Calcerrada, J. Beneficial and pathogenic plant–microbe interactions during flooding stress. Plant Cell Environ. 2022, 45, 2875–2897. [Google Scholar] [CrossRef]
- Crawford, K.M.; Bauer, J.T.; Comita, L.S.; Eppinga, M.B.; Johnson, D.J.; Mangan, S.A.; Queenborough, S.A.; Strand, A.E.; Suding, K.N.; Umbanhowar, J.; et al. When and where plant–soil feedback may promote plant coexistence: A meta-analysis. Ecol. Lett. 2019, 22, 1274–1284. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Xu, W.; Ren, Y.; Zhang, N.; Zhou, X.; Ye, X. Effects of Extreme Flooding on Soil Characteristics, Soil Enzyme Activity, and Microbial Structure in Shengjin Lake. Water 2025, 17, 1789. https://doi.org/10.3390/w17121789
Duan X, Xu W, Ren Y, Zhang N, Zhou X, Ye X. Effects of Extreme Flooding on Soil Characteristics, Soil Enzyme Activity, and Microbial Structure in Shengjin Lake. Water. 2025; 17(12):1789. https://doi.org/10.3390/w17121789
Chicago/Turabian StyleDuan, Xinyi, Wenjing Xu, Yujing Ren, Nan Zhang, Xiaotao Zhou, and Xiaoxin Ye. 2025. "Effects of Extreme Flooding on Soil Characteristics, Soil Enzyme Activity, and Microbial Structure in Shengjin Lake" Water 17, no. 12: 1789. https://doi.org/10.3390/w17121789
APA StyleDuan, X., Xu, W., Ren, Y., Zhang, N., Zhou, X., & Ye, X. (2025). Effects of Extreme Flooding on Soil Characteristics, Soil Enzyme Activity, and Microbial Structure in Shengjin Lake. Water, 17(12), 1789. https://doi.org/10.3390/w17121789




