Restoring Lakeshore Vegetation in the Face of Hysteresis: A Water-Level and Sediment-Based Strategy for Shallow Lakes
Abstract
1. Introduction
2. Materials and Methods
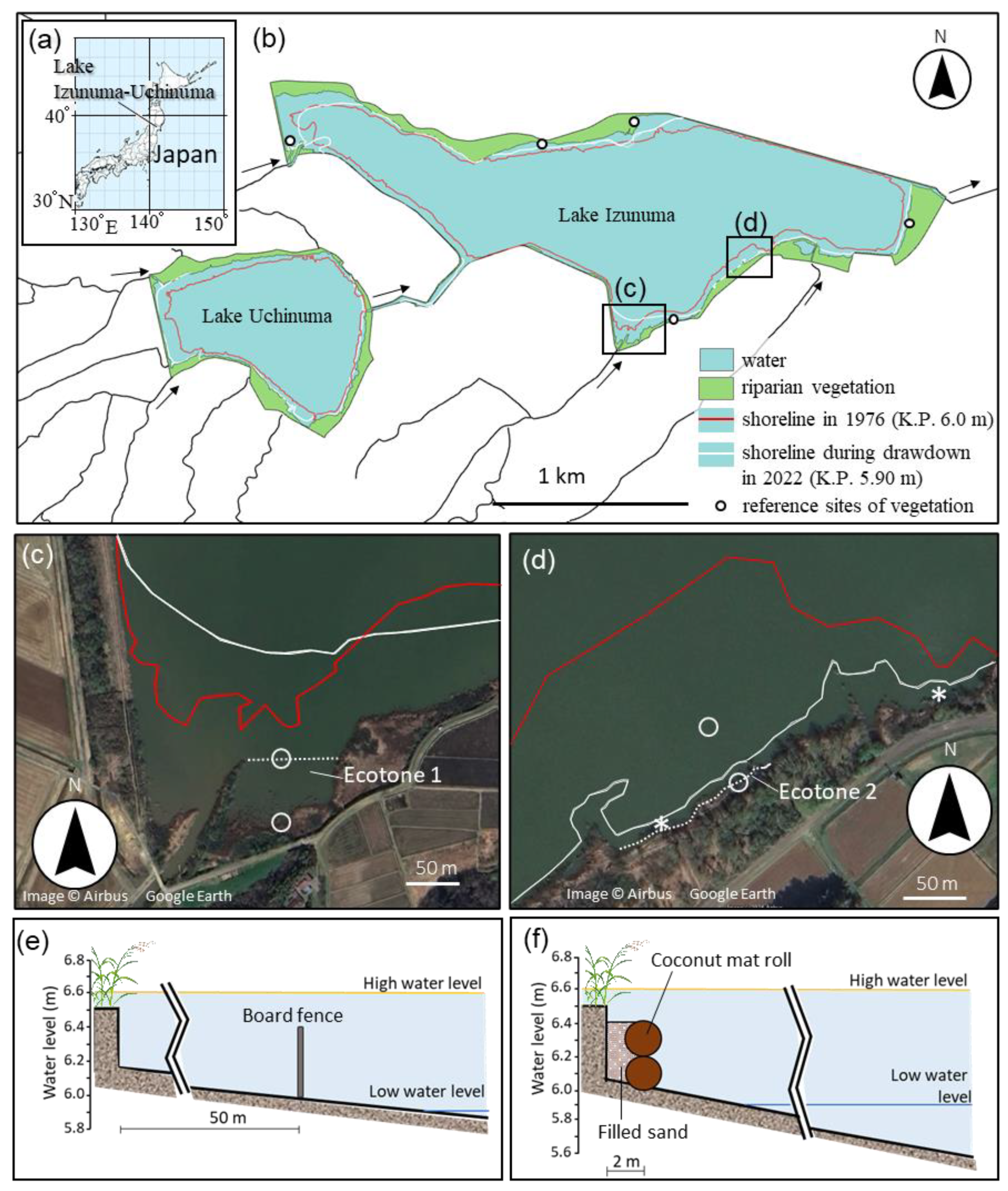
2.1. Study Site
2.2. Analysis of Water Levels and Lakebed Elevation
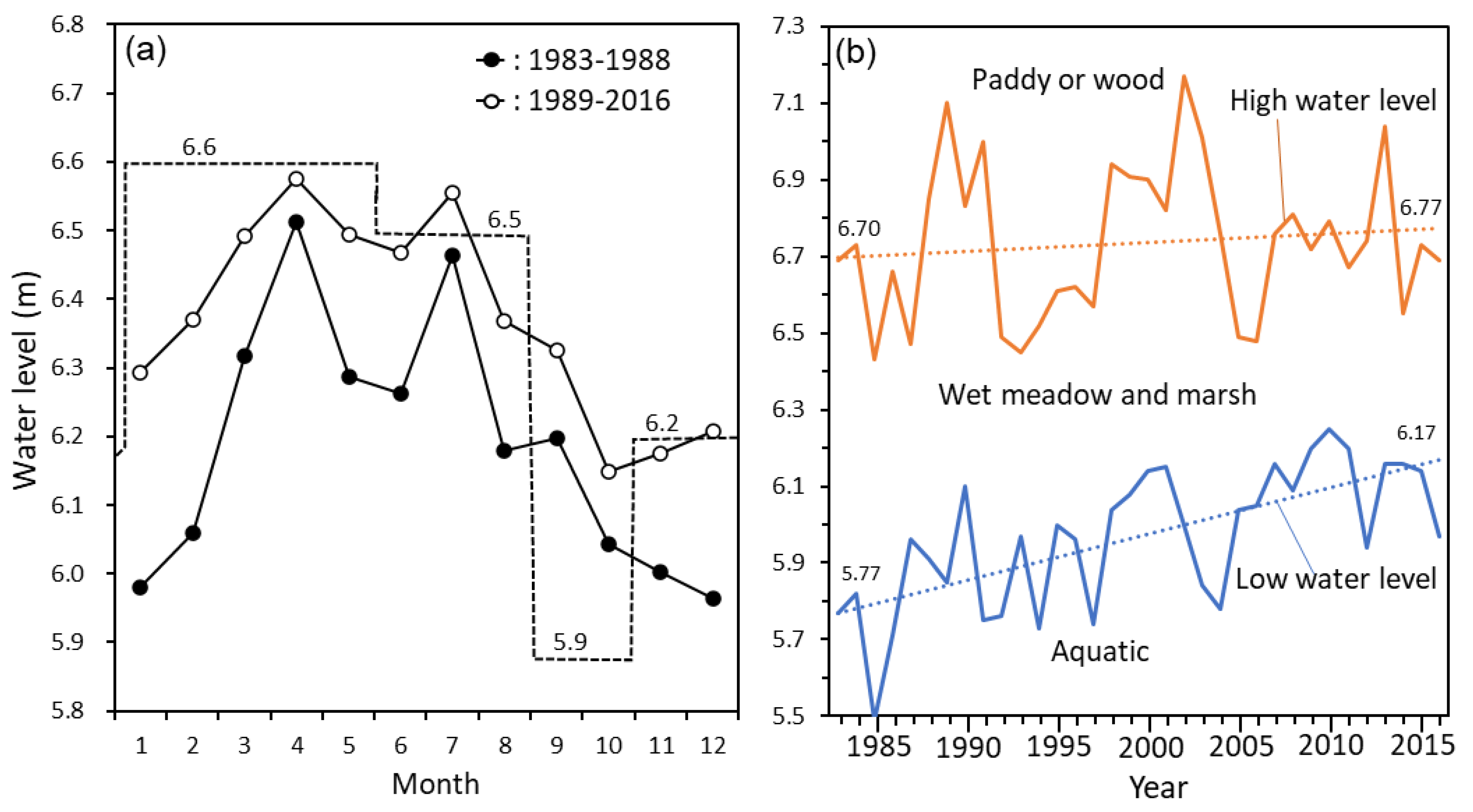
2.2.1. Water-Level Data and Long-Term Trends
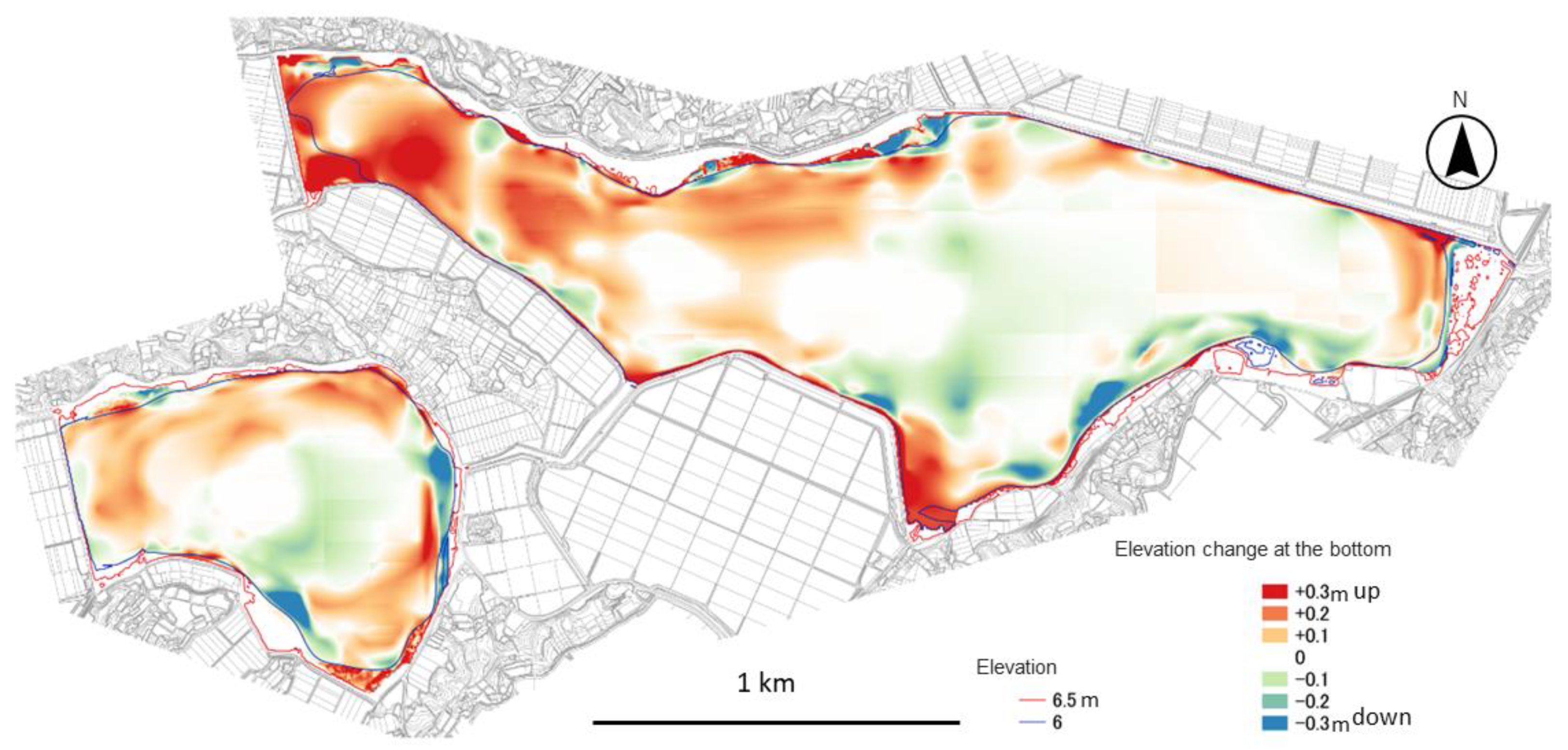
2.2.2. Creation of Bathymetric Maps and Digital Elevation Models
2.2.3. Calculation of Annual Sediment Accumulation
2.3. Future Sedimentation Simulation
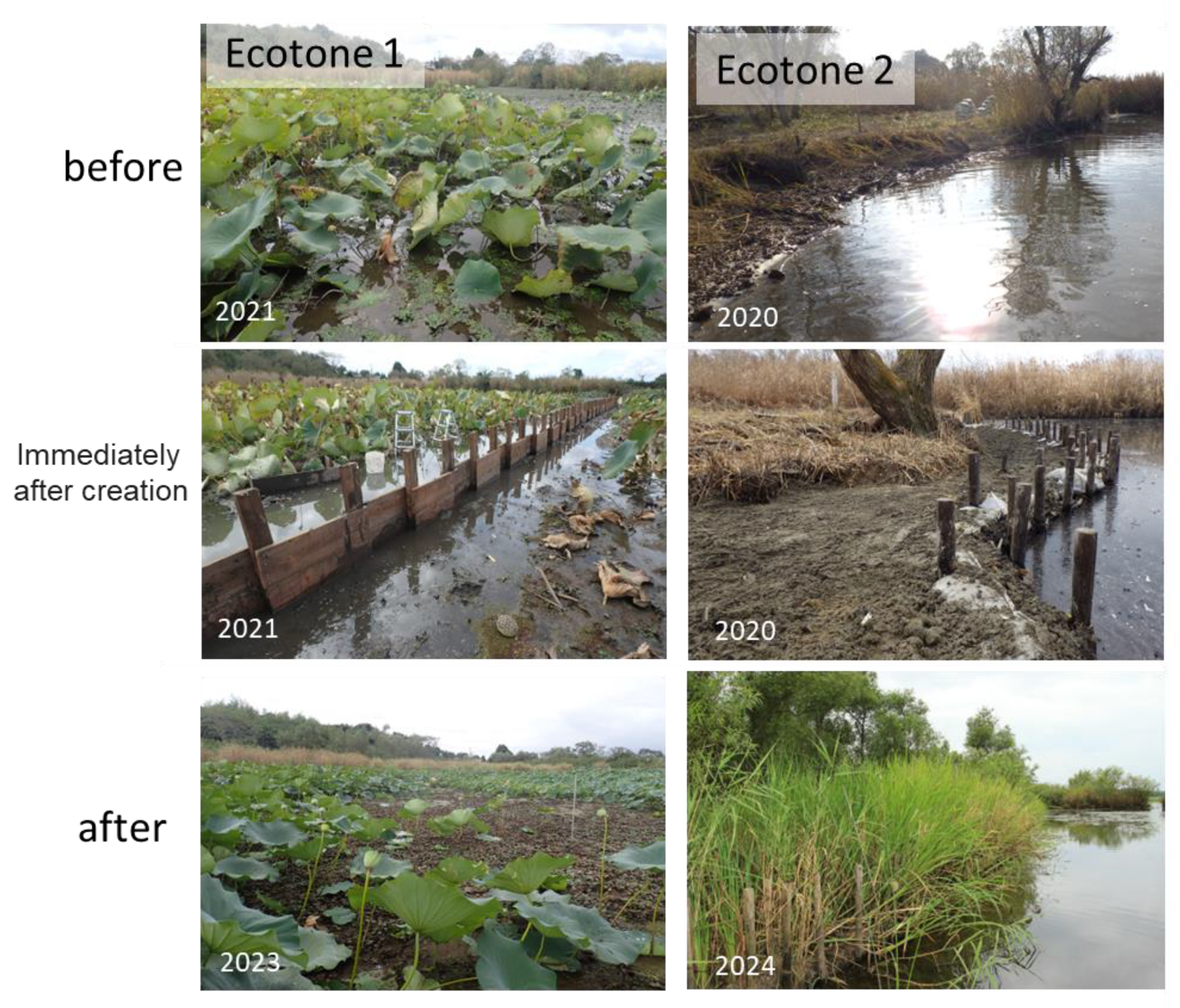
2.4. Construction of Artificial Ecotones
2.5. Evaluation of the Artificial Ecotones
2.5.1. Assessment of Z. latifolia Coverage
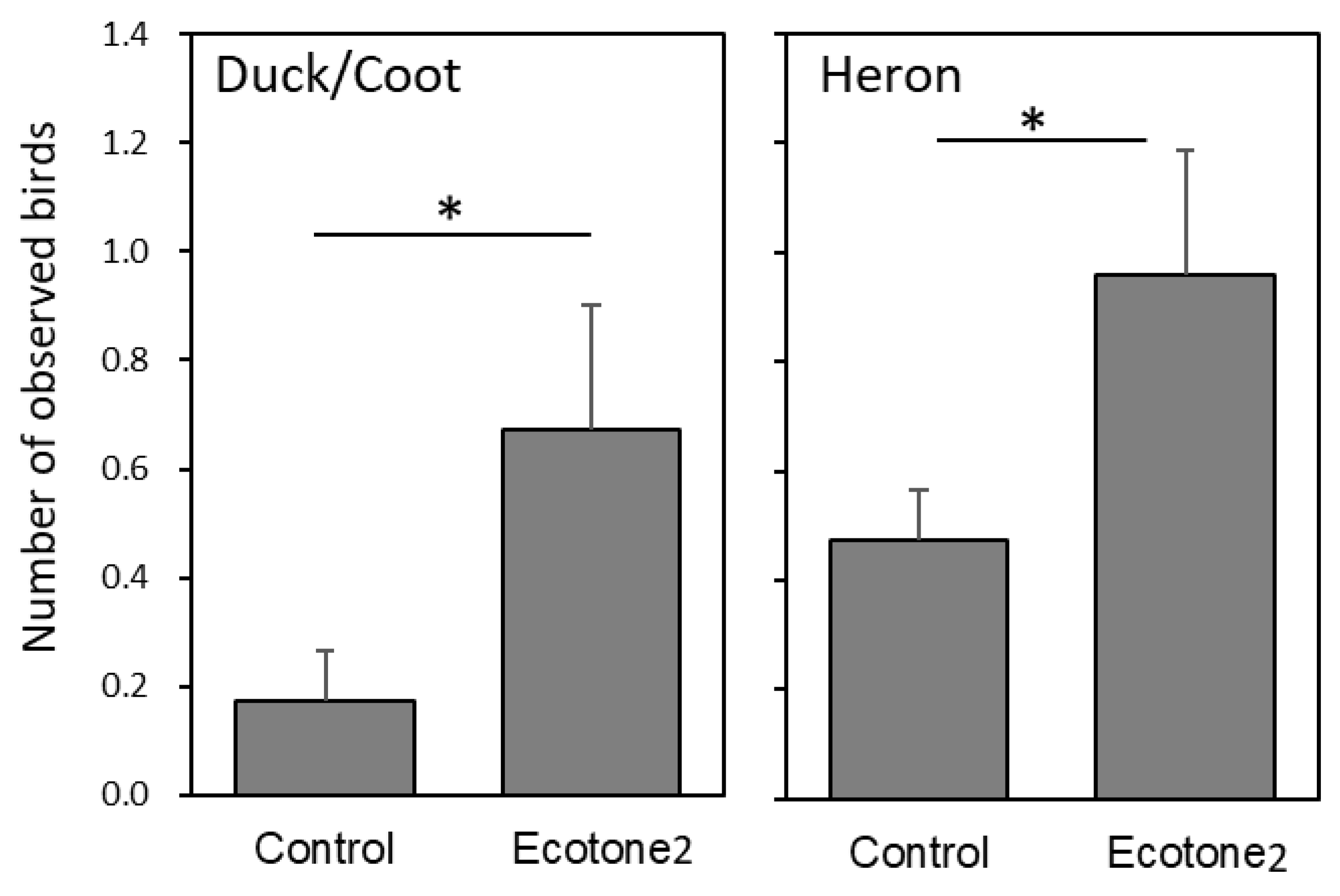
2.5.2. Waterbird Usage
2.5.3. COD Release from Sediment
3. Results
3.1. Seasonal and Long-Term Water-Level Changes
3.2. Changes in Lakebed Elevation and Future Sedimentation Simulations
3.3. Effects of the Constructed Ecotones
4. Discussion
4.1. Hysteresis from Lakeshore Vegetation Loss
4.2. A “Modern” Hydrosere Under Anthropogenic Pressure
4.3. Ecological Consequences and Biodiversity Loss
4.4. Integrated Restoration Approaches
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayer, C.A.; Fernando, E.; Jimenez, R.R.; Macfarlane, N.B.; Rapacciuolo, G.; Böhm, M.; Darwall, W.R. One-quarter of freshwater fauna threatened with extinction. Nature 2025, 638, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Schierner, F.; Zalewski, M.; Thorpe, J. Land/Inland water ecotones: Intermediate habitats critical for conservation and management. Hydrobiologia 1995, 303, 259–264. [Google Scholar] [CrossRef]
- Dai, T.; Liu, R.; Zhou, X.; Zhang, J.; Song, M.; Zou, P.; Bi, X.; Li, S. Role of Lake Aquatic—Terrestrial Ecotones in the Ecological Restoration of Eutrophic Water Bodies. Toxics 2023, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, S.; Wang, H. Periodic inundations drive community assembly of amphibious plants in floodplain lakes. Hydrobiologia 2020, 847, 4207–4217. [Google Scholar] [CrossRef]
- Chapman, L.; Chapman, C.; Chandler, M. Wetland ecotones as refugia for endangered fishes. Biol. Conserv. 1996, 78, 263–270. [Google Scholar] [CrossRef]
- Zalewski, M.; Thorpe, J.; Naiman, R. Fish and riparian ecotones: A hypothesis. Ecohydrol. Hydrobiol. 2001, 1, 11–24. [Google Scholar]
- Bateman, H.L.; Merritt, D.M. Complex riparian habitats predict reptile and amphibian diversity. Glob. Ecol. Conserv. 2020, 22, e00957. [Google Scholar] [CrossRef]
- Popova, O.; Smirnova, Y. Community of aquatic insects in forest-steppe lakes of Baraba (South of West Siberia). Contemp. Probl. Ecol. 2010, 3, 50–54. [Google Scholar] [CrossRef]
- Hansson, L.; Nicolle, A.; Brönmark, C.; Hargeby, A.; Lindström, Å.; Andersson, G. Waterfowl, macrophytes, and the clear water state of shallow lakes. Hydrobiologia 2010, 646, 101–109. [Google Scholar] [CrossRef]
- Pickens, B.; King, S. Multiscale Habitat Selection of Wetland Birds in the Northern Gulf Coast. Estuar. Coast. 2014, 37, 1301–1311. [Google Scholar] [CrossRef]
- Ostendorp, W. Die-back of reeds in Europe—A critical review of literature. Aquat. Bot. 1989, 35, 5–26. [Google Scholar] [CrossRef]
- Ostendorp, W.; Iseli, C.; Krauss, M.; Krumscheid-Plankert, P.; Moret, J.; Rollier, M.; Schanz, F. Lake shore deterioration, reed management and bank restoration in some Central European lakes. Ecol. Eng. 1995, 5, 51–75. [Google Scholar] [CrossRef]
- Brönmark, C.; Hansson, L. Environmental issues in lakes and ponds: Current state and perspectives. Environ. Conserv. 2002, 29, 290–307. [Google Scholar] [CrossRef]
- Nishihiro, J.; Washitani, I. Quantitative evaluation of water-level effects on “regeneration safe-sites” for lakeshore plants in Lake Kasumigaura, Japan. Lake Reserv. Manag. 2009, 25, 217–223. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeppesen, E.; Liu, X.; Qin, B.; Shi, K.; Zhou, Y.; Thomaz, S.; Deng, J. Global loss of aquatic vegetation in lakes. Earth-Sci. Rev. 2017, 173, 259–265. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Keddy, P.A. Wetland Ecology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Valk, A.; Squires, L.; Welling, C. Assessing the impacts of an increase in water level on wetland vegetation. Ecol. Appl. 1994, 4, 525–534. [Google Scholar] [CrossRef]
- Theuerkauf, E.; Braun, K. Rapid water level rise drives unprecedented coastal habitat loss along the Great Lakes of North America. J. Great Lakes Res. 2021, 47, 945–954. [Google Scholar] [CrossRef]
- Harris, S.; Marshall, W. Ecology of water₋level manipulations on a northern marsh. Ecology 1963, 44, 331–343. [Google Scholar] [CrossRef]
- Nakamura, K.; Kadokura, N.; Munakta, Y.; Shimatani, Y.; Uda, T. Restoration of lakeshore vegetation by artificial floating island. Environ. Syst. Res. 1999, 27, 305–314. [Google Scholar] [CrossRef][Green Version]
- Coops, H.; Hosper, S.H. Water-level management as a tool for the restoration of shallow lakes in the Netherlands. Lake Reserv. Manag. 2002, 18, 293–298. [Google Scholar] [CrossRef]
- Nishihiro, J.; Nishihiro, M.; Washitani, I. Restoration of wetland vegetation using soil seed banks: Lessons from a project in Lake Kasumigaura, Japan. Landsc. Ecol. Eng. 2006, 2, 171–176. [Google Scholar] [CrossRef]
- Abrahams, C. Sustainable shorelines: The management and re-vegetation of drawdown zones. Lakes Reserv. Res. Manag. 2008, 13, 235–246. [Google Scholar]
- Haskell, D.; Bales, A.; Webster, C.; Meyer, M.; Flaspohler, D. Restoring hardwood trees to lake riparian areas using three planting treatments. Restor. Ecol. 2017, 25, 933–941. [Google Scholar] [CrossRef]
- Janauer, G. Ecohydrological control of macrophytes in floodplain lakes. Ecohydrol. Hydrobiol. 2006, 6, 19–24. [Google Scholar] [CrossRef]
- Chen, F.; Lu, S.; Hu, X.; He, Q.; Feng, C.; Xu, Q.; Chen, N.; Ngo, H.; Guo, H. Multi-dimensional habitat vegetation restoration mode for lake riparian zone, Taihu, China. Ecol. Eng. 2019, 134, 56–64. [Google Scholar] [CrossRef]
- Zohary, T.; Ostrovsky, I. Ecological Impacts of Excessive Water Level Fluctuations in Stratified Freshwater Lakes. Inland Waters 2011, 1, 47–59. [Google Scholar] [CrossRef]
- Osland, M.J. Managing Invasive Plants During Wetland Restoration: The Role of Disturbance, Plant Strategies, and Environmental Filters. Dissertation, Duke University. Available online: https://hdl.handle.net/10161/1200 (accessed on 9 February 2025).
- Oertli, B.; Parris, K.M. Review: Toward management of urban ponds for freshwater biodiversity. Ecosphere 2019, 10, e02810. [Google Scholar] [CrossRef]
- Lake Izunuma-Uchinuma Nature Restoration Council. Lake Izunuma-Uchinuma Nature Regeneration Project Phase 2. Available online: https://www.pref.miyagi.jp/documents/23928/783455.pdf (accessed on 16 January 2025).
- The Miyagi Prefectural Izunuma-Uchinuma Environmental Foundation. A floral list around Lake Izunuma-Uchinuma. Izunuma-Uchinuma Wetl. Res. 2010, 4, 41–61. [Google Scholar] [CrossRef]
- Ueda, K.; Fujimoto, Y. Seasonal occurrences, reproductive seasons, and habitat environments of an endangered damselfly Paracercion plagiosum (Odonata: Coenagrionidae) in ponds neighboring Lake Izunuma–Uchinuma, Japan. Jpn. J. Entomol. 2022, 25, 153–164. [Google Scholar]
- Fujimoto, Y.; Takahashi, K.; Shindo, K.; Saitoh, K.; Mitsuzuka, M.; Shimada, T. Recovery of the endangered bitterling Acheilognathus typus in Lake Izunuma-Uchinuma after the removal of largemouth bass (Micropterus salmoides). Jpn. J. Ichthyol. 2021, 68, 61–66. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Takahashi, K.; Shindo, K.; Fujiwara, T.; Arita, K.; Saitoh, K.; Shimada, T. Success in population control of the invasive largemouth bass Micropterus salmoides through removal at spawning sites in a Japanese shallow lake. Manag. Biol. Invas. 2021, 12, 997–1011. [Google Scholar] [CrossRef]
- Shitara, H. Geographical and topographical position of Lake Izunuma-Uchinuma. In Report on Environmental Conservation Measures for Izunuma-Uchinuma; Izunuma-Uchinuma Environmental Conservation Measures Study Committee: Sendai, Japan, 1992; pp. 1–3. [Google Scholar]
- Naito, T. Interactions between swans and aquatic plants. In Report on Environmental Conservation Measures for Izunuma-Uchinuma; Izunuma-Uchinuma Environmental Conservation Measures Study Committee: Sendai, Japan, 1992; pp. 265–281. [Google Scholar]
- Takahashi, Y.; Fujimoto, Y. Survey of shape and area of Lake Izunuma-Uchinuma, Miyagi Prefecture, Japan using aerial photographs taken in 2007. Izunuma-Uchinuma Wetl. Res. 2018, 12, 17–25. [Google Scholar]
- Biodiversity Center of Japan. Monitoring Site 1000 Inland Waters Survey Report. Available online: https://www.biodic.go.jp/moni1000/findings/reports/pdf/h27_inland_waters.pdf (accessed on 16 January 2025).
- Kamata, K.; Hraide, A.; Nishita, M.; Fujimoto, Y.; Shindo, K. Searching for spawning areas of Micropterus salmoides by Side scan sonar and inspecting the validity of this method in Lake Izunuma. Izunuma-Uchinuma Wetl. Res. 2009, 3, 31–40. [Google Scholar]
- Nakagawa, H.; Hibino, K. Survey of Bottom Sediments in Lake Izunuma-Uchinuma. In Lake Izunuma-Uchinuma Environmental Conservation Science Research Report; Izunuma-Uchinuma Environmental Conservation Academic Investigation Committee: Sendai, Japan, 1988; pp. 157–199. [Google Scholar]
- Naito, T.; Shibasaki, T.; Sugawara, K.; Iizumi, S. Plants of Lake Izunuma-Uchinuma. In Lake Izunuma-Uchinuma Environmental Conservation Science Research Report; Izunuma-Uchinuma Environmental Conservation Academic Investigation Committee: Sendai, Japan, 1988; pp. 201–262. [Google Scholar]
- Tsuji, M.; Hiratsuka, A. Special Issue “Ecological restoration of riverine ecotone”. Jpn. Soc. Reveget. Technol. 2008, 33, 548–553. [Google Scholar] [CrossRef]
- Japan Sediments Management Association. Sediment Investigation and Testing Manual: Technical Materials, 4th ed.; Japan Sediments Management Association: Tokyo, Japan, 2016. [Google Scholar]
- Tan, W.; Xing, J.; Yang, S.; Yu, G.; Sun, P.; Jiang, Y. Long term aquatic vegetation dynamics in Longgan Lake using Landsat time series and their responses to water level fluctuation. Water 2020, 12, 2178. [Google Scholar] [CrossRef]
- Wilcox, D.A.; Nichols, S.J. The effects of water-level fluctuations on vegetation in a Lake Huron wetland. Wetlands 2008, 28, 487–501. [Google Scholar] [CrossRef]
- Evtimova, V.; Donohue, I. Water-level fluctuations regulate the structure and functioning of natural lakes. Freshw. Biol. 2016, 61, 251–264. [Google Scholar] [CrossRef]
- Wen, J.; Li, B.; Xiao, H.; Gong, C.; Gao, A.; Wang, Y.; Li, D.; Zeng, H.; Li, Y.; Yuan, G.; et al. Floating Mat Formation Makes Zizania latifolia More Competitive under the Conditions of Continuous Significant Water Level Rise. Plants 2023, 12, 1193. [Google Scholar] [CrossRef]
- Johnston, C.A. Sediment and nutrient retention by freshwater wetlands: Effects on surface water quality. Crit. Rev. Environ. Control 1991, 21, 491–565. [Google Scholar] [CrossRef]
- Wu, D.; Hua, Z. The effect of vegetation on sediment resuspension and phosphorus release under hydrodynamic disturbance in shallow lakes. Ecol. Eng. 2014, 69, 55–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, X.; Zhang, L.; Song, K.; Yao, X.; Gu, L.; Pang, C. The influence of aquatic vegetation on flow structure and sediment deposition: A field study in Dongting Lake, China. J. Hydrol. 2020, 584, 124644. [Google Scholar] [CrossRef]
- Yuan, Y.; Bingner, R.L.; Locke, M.A. A review of effectiveness of vegetative buffers on sediment trapping in agricultural areas. Ecohydrology 2009, 2, 321–336. [Google Scholar] [CrossRef]
- Yoshimura, S. Limnology; Sanseido: Tokyo, Japan, 1937. [Google Scholar]
- Wilcox, D.A. Implications of hydrologic variability on the succession of plants in Great Lakes wetlands. Aquat. Ecosyst. Health Manag. 2004, 7, 223–231. [Google Scholar] [CrossRef]
- Topping, D.; Rubin, D.; Nelson, J.; Kinzel, P.; Corson, I. Colorado River sediment transport: 2. Systematic Bed-elevation and grain-size effects of sand supply limitation. Water Resour. Res. 2000, 36, 543–570. [Google Scholar] [CrossRef]
- Marburg, A.; Turner, M.; Kratz, T. Natural and anthropogenic variation in coarse wood among and within lakes. J. Ecol. 2006, 94, 558–568. [Google Scholar] [CrossRef]
- Ono, Y.; Insects of Lake Izunuma-Uchinuma. Lake Izunuma-Uchinuma Environmental Conservation Science Research Report; Izunuma-Uchinuma Environmental Conservation Academic Investigation Committee: Sendai, Japan, 1988; pp. 329–334. [Google Scholar]
- Kurechi, M.; Takara, S.; Kosugi, M.; Koyama, H.; Sato, T.; Takahashi, O.; Hayashi, K.; Iwai, S.; Yamaki, H.; Hiraizumi, H. On the environmental utilization during the breeding season of little grebe and Eurasian coot. In Report on the Conservation and Management Plan for the Birds of Lake Izunuma-Uchinuma; Japanese Wild Bird Society and the Miyagi Prefectural Board of Education: Sendai, Japan, 1981; pp. 181–202. [Google Scholar]
- Nishimura, O. Analysis of Formation Mechanism of Bottom Environment and Development of Sedimentation Control Technology in Shallow Closed Water Area. Final Research Results Report of Environment Research and Technology Development Fund (B-1004); the Ministry of the Environment: Tokyo, 2013. Available online: https://www.env.go.jp/policy/kenkyu/suishin/kadai/syuryo_report/pdf/B-1004.pdf (accessed on 16 January 2025).
- Fujimoto, Y.; Shimada, T.; Inoue, K.; Takahashi, Y.; Hayami, H. Below-average water level in Lake Izunuma-Uchinuma in Miyagi Pref, Japan, in the winter of 2016/17 induced whooper swan feeding activity, leading to reduced coverage of lotus vegetation and increased dissolved oxygen in the water. Jpn. J. Conserv. Ecol. 2020, 25, 99–108. [Google Scholar] [CrossRef]
- Zhao, F.; Mizuno, K.; Tabeta, S.; Hayami, H.; Fujimoto, Y.; Shimada, T. Survey of freshwater mussels using high-resolution acoustic imaging sonar and deep learning–based object detection in the Lake Izunuma, Japan. Aquat. Conserv. 2024, 34, e4040. [Google Scholar] [CrossRef]
- Fujimoto, Y. Extinction of a Vallisneria denseserrulata population by the expansion of Nelumbo nucifera in a pond around Lake Izunuma-Uchinuma. Izunuma-Uchinuma Wetl. Res. 2011, 5, 13–19. [Google Scholar] [CrossRef]
- Nishihiro, J.; Munemitsu, A.; Yamanouchi, T.; Takamura, N. Time-declining potential of aquatic plant recovery from the propagule banks of lake sediments. Jpn. J. Conserv. Ecol. 2016, 21, 147–154. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Hayami, H.; Fujimoto, Y. A record of Eichhornia crassipes (Mart.) Solms from Lake Izunuma, Miyagi Prefecture, Japan and the possibility of its establishment due to global warming. Izunuma-Uchinuma Wetl. Res. 2022, 16, 33–38. [Google Scholar] [CrossRef]
- Nishihiro, J. Effects of lake water-level control on lakeshore plant regeneration (Ohshima Award). Jpn. J. Conserv. Ecol. 2011, 16, 139–148. [Google Scholar] [CrossRef]
- Hirano, T.; Higuchi, H. The relationship between river width and the occurrence of riparian bird species in winter. Strix 1988, 7, 203–212. [Google Scholar]
- Zhou, J.; Zhou, L.; Xu, W. Diversity of wintering waterbirds enhanced by restoring aquatic vegetation at Shengjin Lake, China. Sci. Total Environ. 2020, 737, 140190. [Google Scholar] [CrossRef]
- Takemaru, K. Birds of Lake Izunuma-Uchinuma. In Lake Izunuma-Uchinuma Environmental Conservation Science Research Report; Izunuma-Uchinuma Environmental Conservation Academic Investigation Committee: Sendai, Japan, 1988; pp. 271–301. [Google Scholar]
- Takahashi, M.; Miya, A.; Ebina, J.; Sannohe, S.; Mugisawa, T.; Tsumagari, T. Wintering record of Japanese marsh warbler at Lake Izunuma-Uchinuma. Izunuma-Uchinuma Wetl. Res. 2017, 11, 1–5. [Google Scholar] [CrossRef]
- Gilbert, G.; Tyler, G.A.; Dunn, C.J.; Smith, K.W. Nesting habitat selection by bitterns Botaurus stellaris in Britain and the implications for wetland management. Biol. Conserv. 2005, 124, 547–553. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Nishigaki, M.; Sekioka, H.; Yoshida, T. Coastal restoration in Mikata-goko: Beach nourishment and conservation guidance. Jpn. J. Conserv. Ecol. 2022, 27, 107–118. [Google Scholar] [CrossRef]
- Fujimoto, Y. Influence of cutting leaves of lotus plants Nelumbo nucifera on their survival. Ecol. Civ. Eng. 2018, 21, 37–43. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujimoto, Y.; Takahashi, Y.; Hayami, H.; Nomura, M.; Yokoyama, J.; Shimada, T.; Nishimura, O. Restoring Lakeshore Vegetation in the Face of Hysteresis: A Water-Level and Sediment-Based Strategy for Shallow Lakes. Water 2025, 17, 1760. https://doi.org/10.3390/w17121760
Fujimoto Y, Takahashi Y, Hayami H, Nomura M, Yokoyama J, Shimada T, Nishimura O. Restoring Lakeshore Vegetation in the Face of Hysteresis: A Water-Level and Sediment-Based Strategy for Shallow Lakes. Water. 2025; 17(12):1760. https://doi.org/10.3390/w17121760
Chicago/Turabian StyleFujimoto, Yasufumi, Yusuke Takahashi, Hiroki Hayami, Munehiro Nomura, Jun Yokoyama, Tetsuo Shimada, and Osamu Nishimura. 2025. "Restoring Lakeshore Vegetation in the Face of Hysteresis: A Water-Level and Sediment-Based Strategy for Shallow Lakes" Water 17, no. 12: 1760. https://doi.org/10.3390/w17121760
APA StyleFujimoto, Y., Takahashi, Y., Hayami, H., Nomura, M., Yokoyama, J., Shimada, T., & Nishimura, O. (2025). Restoring Lakeshore Vegetation in the Face of Hysteresis: A Water-Level and Sediment-Based Strategy for Shallow Lakes. Water, 17(12), 1760. https://doi.org/10.3390/w17121760





