Abstract
The Andean river basins of central–southern Chile face multiple anthropogenic disturbances, including water extraction, hydropower, mining, and industrial discharges, which affect their ability to adapt to new disturbances. Disturbance intensity forms a gradient from high (Maipo, Rapel, Biobío, Maule) through medium (Mataquito, Itata) to low (Imperial, Toltén). This study evaluated resilience in these eight river basins based on fish assemblages, using taxonomic and functional trait indices within the framework of the three Rs of resilience: resources, recruitment, and refugia. Taxonomic indices captured changes in species richness, abundance, diversity, evenness, and beta diversity, while functional traits reflected the fish species characteristics promoting resilience. Statistical tests revealed significant differences in resilience indices among basins. Recruitment was the most impacted resilience mechanism, with beta diversity revealing effects from river fragmentation in the Maipo, Rapel, and Biobío basins. The resources mechanism was also affected, primarily by land-use changes and water pollution, leading to low species richness in the Maipo, Rapel, Mataquito, and Maule river basins. Interestingly, basins with medium disturbance levels showed high resilience, indicating adaptive responses to moderate impacts. This study emphasizes the importance of multiple indicators to assess ecosystem resilience and calls for integrated strategies to address the complex challenges impacting freshwater biodiversity.
1. Introduction
Freshwater ecosystems present an alarming biodiversity decline caused by a combination of local effects and disturbances across catchments, which is challenging to manage [1]. Indeed, river ecosystems are often modified and impacted to the point that their contributions to society are severely affected [2]. Furthermore, anthropogenic modifications may prevent river ecosystems from adapting to new disturbances, causing shifts in the demographic rates of aquatic organisms [3]. Some anthropogenic disturbances can significantly erode the ecological resilience of river ecosystems, making them more vulnerable to new disturbances [4]. Resilience is an ecosystem’s ability to absorb disturbances and remain within the same regime, i.e., in terms of structure, function, and feedbacks [5,6,7,8]. As such, resilience also encompasses the ability of an ecosystem to reorganize itself and adapt to change [9]. In river ecosystems, resilience implies the persistence of biological communities in highly dynamic hydrological and geomorphological regimes [10]. Indeed, anthropogenic activities, such as fragmentation, flow regime alteration, water pollution, habitat degradation, species invasions, and climate change, pose significant challenges to the maintenance of river ecosystem resilience [10,11,12,13]. Evaluation of anthropogenic impacts on the biophysical components of ecosystems plays a vital role in identifying ecological resilience, and monitoring resilience indicators allows a better understanding of a river ecosystem’s capacity to adapt and transform in response to anthropogenic disturbances, informing adaptative management strategies [6].
To assess the resilience of river ecosystems, Van Looy et al. (2010) [10] proposed an integrated framework. This framework recognizes flow-related disturbances as dominant forces shaping riverine communities and suggests three major resilience mechanisms based on the responses of aquatic communities to disturbances in productivity (resources mechanism), spatial connectivity (recruitment mechanism), and habitat heterogeneity (refugia mechanism). For the resources mechanism, the potential recovery of an altered ecosystem relies on food availability and variables such as light, oxygen, substrate, and temperature [10]. Consequently, this mechanism operates at the community level, involving internal reorganization driven by biotic interactions such as competition and facilitation [14]. The recruitment mechanism is based on metacommunity dynamics and depends on habitat connectivity, species dispersal capacity, and regional species pool size [15]. As such, the river network configuration strongly affects community structure; i.e., site position in the river network affects post-disturbance recolonization or recruitment rates [16]. Therefore, community recovery is largely influenced by species dispersal capacity since it affects not only the rate of recruitment or recolonization after a disturbance but also dispersal to avoid disturbances [10]. The refugia mechanism operates through a mosaic of patches of habitats that confer heterogeneity to the ecosystem [10]. This habitat heterogeneity provides ecological resilience to communities through mechanisms of functional redundancy, driven simultaneously by the diversity of responses of organisms and habitat specialization [17]. The concept of functional redundancy implies that the loss of species is compensated for by others that contribute similarly to ecosystem functioning, i.e., the functional similarity of species in one trait and their differentiation in another [10,18,19,20]. This framework provides a concept of river resilience that can be evaluated using a set of indicators that together offer a comprehensive view of the strength of human impacts on the ecosystem and its capacity for recovery. Therefore, resilience can be inferred from the communities’ taxonomic characteristics and species functional traits that promote resilience rather than from a pre- versus post-impact comparison.
Fish are excellent indicators of the ecological status of river ecosystems, as they can integrate the effects of disturbances over long spatial and temporal scales since they are relatively long-lived and mobile organisms and their assemblages are functionally diverse [6]. Furthermore, as top predators in food webs, fish offer a comprehensive view of the ecological status of river ecosystems. Their survival, growth, and reproduction rely on a diversity of functions and processes [21,22]. For example, in the upper Mississippi River, dam construction not only altered river connectivity but also affected fish trophic positions and habitat heterogeneity, leading to a loss of functional diversity and, consequently, resilience [23]. This decline likely stemmed from diminishing habitat heterogeneity (refugia mechanism) near dams [10]. Long-term monitoring data further support these observations, demonstrating that regimen shifts in fish assemblages—evidenced by changes in body-size distributions and functional traits—are linked to non-native species dominance and shifts in resource availability. These findings highlight fish assemblages as integrative indicators of broader ecosystem change and the underlying processes governing ecological resilience [24].
The comprehension and management of river ecosystems requires an interdisciplinary and holistic approach that acknowledges the intricate interplay between their physical and biological components [25,26,27]. Furthermore, it necessitates an understanding of resilience and adaptive management strategies [6]. The interplay of physical and biological components within river ecosystems generates distinct spatial patterns noticeable across multiple scales [28,29]. The Riverine Ecosystem Synthesis (RES) has emerged as a framework to elucidate the hydrogeomorphological and ecological relationships that operate at these multiple scales [25,27]. This framework conceptualizes river networks as a series of river zones, named Functional Process Zones (FPZs). Each FPZ delineates a large section of river characterized by relatively uniform geological histories and channel morphologies, thereby promoting similar functional ecological organizations [30]. The physical attributes of FPZs influence ecological traits both within and across river sections [27]. Furthermore, geomorphological differences among FPZs have profound impacts on ecosystem structure and function [27]. Research has demonstrated that increased geomorphological heterogeneity, indicative of greater physical variability in an FPZ, is correlated with higher ecological complexity; e.g., biodiversity and food-chain length increase with the greater physical complexity of an FPZ [29,31].
The Andean rivers of central–southern Chile provide an excellent model for evaluating river resilience and its relationship with FPZs. These rivers are characterized by a diverse range of elevations across basins, supporting a diversity of FPZs [28,30]. Each Andean exorheic basin features short, steep rivers that flow from 3000 m.a.s.l. in the Andes Cordillera to the Pacific Ocean [32]. Furthermore, they remained isolated for more than 10,000 years [33] and are characterized by fish assemblages with low species diversity but high levels of endemism, explained by the basins’ geological history and geomorphological characteristics [34,35]. Presently, these rivers are affected by various anthropogenic disturbances, e.g., water extraction for human consumption, irrigation, domestic and industrial discharges, hydropower (flow regulation and physical fragmentation), mining, and recreation. Consequently, increasing anthropogenic disturbances threaten the ability of these rivers to continue providing contributions to society [30,32].
The aim of this study was to assess resilience in eight Andean river basins of central–southern Chile in two FPZs of contrasting geomorphological complexity based on an evaluation of fish assemblages as indicators of ecological resilience. We postulated that physically more complex FPZs are more resilient to anthropogenic disturbances than less complex FPZs. We described the composition and structure of fish assemblages in two FPZs of different levels of complexity in each basin. Subsequently, we assessed the resilience mechanisms (resources, recruitment, and refugia) of each basin through quantitative indices based on fish assemblages (taxonomic and functional traits) following the three Rs of river ecosystem resilience [10].
2. Materials and Methods
2.1. Study Site
This study was carried out across eight Andean river basins in central–southern Chile between 32°55′ and 39°40′ Lat. S. (Figure 1). These rivers are characterized by a relatively short length (<380 km), steep slopes, pluvio-nival flow regimes (i.e., fed by both rainfall and snowmelt), and average annual discharges ranging from 100 to 1000 m3/s [36]. The prevailing climate is a warm-summer Mediterranean climate (Csb), according to the Köppen–Geiger classification (Table 1). The studied river basins are located in the “Chilean winter rainfall–Valdivian forest” biodiversity hotspot [37,38] and belong to the Chilean ichthyogeographic province that accommodates the highest fish species richness and seven endemic species: Bullockia maldonadoi (Eigenmann, 1927), Cheirodon galusdae (Eigenmann, 1927), Diplomystes incognitus Arratia and Quezada-Romegialli, 2017, Diplomystes nahuelbutaensis Arratia, 1987, Diplomystes arratiae Muñoz-Ramírez, Colin, Canales-Aguirre, Manosalva, López-Rodriguez, Sukumaran, Górski, 2023, Percilia irwini (Eigenmann, 1927), and Trichomycterus chiltoni (Eigenmann, 1927). The latter three are endemic to the Biobío river basin, Chile’s most diverse river basin [39,40,41].
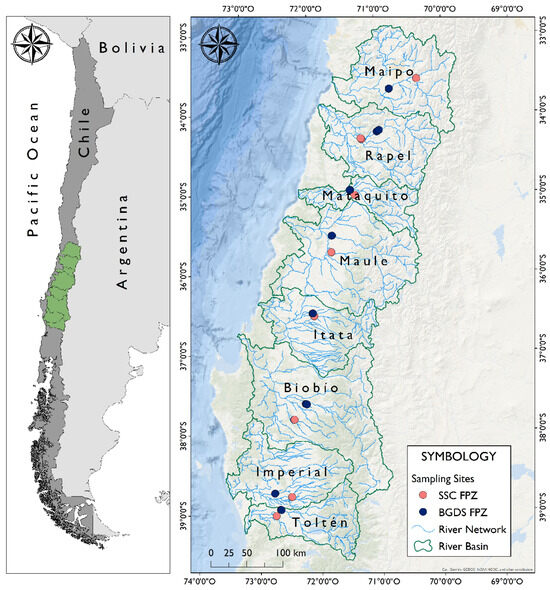
Figure 1.
Geographic location of the eight studied basins and location of the sampling sites associated with two Functional Process Zones (FPZs). SSC, Sinuous Single Channel; BGDS, Braided Gentle Downstream Slope.

Table 1.
Geographic position, catchment area, predominant climate, flow regime, mean annual precipitation, mean annual discharge at the mouth of the eight river basins, and Fragmentation Index [30,36]. Csa, hot-summer Mediterranean climate; Csb, warm-summer Mediterranean climate; Cfb, oceanic climate (marine west coast).
2.2. Functional Process Zones
The characterization of the river landscape to identify groups of river sections with a similar hydrogeomorphic character (FPZs) was based on Habit et al. (2022) [30], using 16 hydrogeomorphic variables corresponding to three spatial scales: river basin, valley, and channel. Seven FPZs were identified, and these showed a patchy distribution within and among the river networks. Within the eight study basins, three FPZs were more abundant: Sinuous Single Channel (SSC), Sinuous Multi-Channel (SMC), and Braided Gentle Downstream Slope (BGDS). The SSC and BGDS FPZs are contrasting in terms of geomorphological complexity, differing mainly by the number of channels and the channel planform. SSC FPZs are characterized by sinuous rivers with a wide single channel and a narrow valley, whereas BGDS FPZs are characterized by braided gentle downstream slopes with a wide channel and valley.
2.3. Anthropogenic Disturbances
The study area is characterized by a gradient of anthropogenic disturbances from north to south, as quantified by Habit et al. (2024) [42]. A high level of disturbances due to fragmentation, land-use changes, and water pollution are present in the Maipo, Rapel, Biobío, and Maule rivers; moderate disturbances in the Mataquito and Itata rivers; and the Imperial and Toltén rivers are less altered and non-fragmented. Some of the basins are highly populated; e.g., the Metropolitan region in the Maipo river basin is inhabited by 7 million people, the Gran Concepción area in the Biobío river basin is inhabited by 1.5 million people, and the Maule region is inhabited by 1 million people [43]. Consequently, the Maipo, Biobío, and Maule rivers receive wastewater from multiple sources. The Maipo river basin has been described as the most polluted in Chile [44]. In addition, the Maipo and Rapel river basins accommodate copper mining in their headwaters, causing water extraction, damming and channelization, and changes in the physical and chemical water quality [32]. Forestry activity and pulp mills, with industrial effluents discharging directly into rivers, are present in the Biobío river basin (three pulp mills) and, to a lesser extent, in the Itata river basin (one pulp mill). The primary industrial discharge that flows into the Biobío River comes from the pulp mill industry, which generates 80% of the 5 billion tons per year of the pulp produced in Chile, with water consumption reaching up to 127 m3/ton of product [45]. Most of the studied basins have undergone a pervasive land-use change, especially the Mataquito, Maule, Itata, and Biobío river basins, with 22.3% of land use for forest plantations dominated by Pinus radiata D. Don, 1836, and Eucalyptus globulus Labill, 1800, and 20.7% for agriculture and livestock [42]. Large reservoirs that store water for irrigation are located mainly in the Rapel and Maule river basins [46]. In addition, the Maipo, Rapel, Biobío, and Maule river basins host between 9 and 19 hydropower plants, including large dams with reservoirs and hydropeaking as well as run-of-river dams [47]. Indeed, the Rapel river basin accommodates the oldest reservoir in Chile, built in 1968, and is the most fragmented basin in the country (Table 1). The Imperial and Toltén river basins are significantly less affected by anthropogenic activities, with primary disturbances being aquaculture farms and Chinook salmon (Oncorhynchus tshawytscha (Walbaum, 1792)) invasion, particularly in the Toltén River [42].
2.4. Fish Sampling
Between January and April 2021, 46 sites were sampled in the Maipo, Rapel, Mataquito, Maule, Itata, Biobío, Imperial, and Toltén river basins under low flow conditions (Figure 1). Of these, 23 sites were located in the SSC FPZ and 23 in the BGDS FPZ. Within each river basin, each FPZ had three sampling sites, except for the BGDS FPZ in the Maule river basin and the SSC FPZ in the Toltén river basin, each represented by two sampling sites. At each site, sampling was conducted using a Halltech HT-2000 (Halltech, Guelph, ON, Canada) backpack electrofisher for approximately 30 min, depending on the available habitat area. In addition, fish were captured in shallow water habitats (<1 m depth), characterized by gravel and sand patches, using 6 m long beach seines (1.5 m in height and 10 mm stretched mesh size), with between two and four seine hauls per sampling site. Hence, sampling was performed in every microhabitat within each site, covering riffles, pools, and runs. Captured specimens were anesthetized, identified to the species level using specialized identification keys [48,49], counted, weighed, and then returned to their habitats. The total number of fish captured at each site—combining electrofishing and seining events—was treated as the unit of effort. To account for potential differences in capture efficiency between sampling methods and to reduce the influence of highly abundant species, we applied standardization and square root transformation to the abundance data.
2.5. Data Analysis
Non-metric multidimensional scaling (NMDS) analysis was performed to explore changes in the composition and structure of fish assemblages between FPZs (SSC and BGDS). NMDS was based on the Bray–Curtis dissimilarity matrix of the square root-transformed abundance [50]. Subsequently, differences in the composition and structure of fish assemblages between FPZs and among basins were tested by permutational multivariate analysis of variance (PERMANOVA) [51]. The threshold was established at p < 0.05. Subsequently, an analysis of similarity percentages (SIMPER) was used to estimate the contribution of each fish species to the similarity among basins [52]. The PRIMER-E v7.0 program [51] was used for data treatment and analyses.
Using Van Looy’s river resilience framework [10], resilience for the eight river basins was assessed based on the taxonomic and functional trait indices derived from fish assemblages for each mechanism. For the classification of functional traits for each species, see Table S1 [47,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68]. Information on functional traits was obtained from the scientific literature and expert knowledge. Table 2 shows the expected trend for higher or lower resilience for each taxonomic index; for functional traits, see Table S2. For the resources mechanism, taxonomic indices capture changes in species richness, abundance, diversity, and evenness, which, in turn, allow disturbance consequences at the community level to be inferred. For the recruitment mechanism, taxonomic indices such as beta diversity, based on Sørensen, and the Bray–Curtis coefficient capture recruitment recovery at the basin level [10]. For the refugia mechanism, changes in taxonomic richness—in this case, richness by taxonomic order, diversity by taxonomic order, and evenness by taxonomic order—represent the redundancy response to disturbances, i.e., what is suitable for one species will also be suitable for closely related ones [3]. The community level analyses examined complete fish assemblages, including native and non-native species. The potential negative impact of non-native species on resilience are inferred from their functional traits.

Table 2.
Taxonomic and functional trait indices used for each resilience mechanism. ↑ indicates resilience increase as the value of the index increases; ↓ indicates resilience decrease as the value of the index increases. * indicates indices and traits proposed by Van Looy et al. (2019) [10]; † indicates indices and traits proposed in the present study.
The functional traits correspond to the species characteristics that promote resilience for each mechanism. For example, a fish species belonging to the omnivorous trophic guild adds more resilience to the assemblage than invertivore–piscivore, invertivore, or detritivore species, as it can feed on a variety of resources, providing greater flexibility in times of scarcity. Similarly, a species that uses the floodplain is more resilient than one that does not due to the higher number and diversity of resources available to cope with disturbances. Functional traits that facilitate recruitment or recolonization following disturbances, such as parental care, also contribute to resilience by promoting higher survival rates. Additionally, populations of migratory species are highly affected by river fragmentation; thus, non-migratory species may indicate higher resilience to potential fragmentation. Finally, traits that allow species to exploit different habitats in response to environmental changes increase their adaptability, enhancing the resilience of the assemblage. In this study, phylogenetic indices were not used due to the lack of information on the phylogeny of several native fish species. Taxonomic indices for the resources and refugia mechanisms were estimated using the Diverse routine of PRIMER-E v7.0 [69]. Taxonomic indices of the recruitment mechanism were based on a distance-based approach to obtain beta diversity (see [36]). The homogeneity dispersion test (PERMIDSP) was used to assess the multivariate dispersion within each river basin [70]. PERMDISP provided a measure of total beta diversity based on both presence–absence (Sørensen coefficient) and abundance data (Bray–Curtis coefficient).
Fish biomass was calculated for the functional trait that represents more resilience (indicated with ↑ in Table S2, i.e., omnivorous, frequent floodplain use, rapid sexual maturity, high fecundity, multiple spawning, small egg size, parental care, benthopelagic, eurytopic), resulting in a functional trait index based on biomass. For functional traits with more than two ordinal categories (Table S2), Principal Component Analysis (PCA) was performed using the first principal component as a proxy for the functional trait index in PRIMER-E v7.0 software (Table S3). Subsequently, statistically significant differences in taxonomic and functional trait indices among basins were assessed. One-way ANOVAs were conducted for normally distributed indices with homogenous variances. For indices with non-normal distribution but homogeneous variance, the Kruskal–Wallis test was used. Post hoc Tukey’s (HSD) and Dunn tests (Bonferroni method) were carried out in cases of significant differences. The normality distribution and variance homogeneity were assessed using the Shapiro–Wilk test and the Levene test, respectively. All analyses were performed in R version 4.0.3 [71]. For Beta diversity based on the Sørensen and Bray–Curtis coefficients, PERMANOVA was used to test significant differences among basins [51]. The differences were considered significant at p < 0.1. To identify the most affected mechanism, we determined the proportion of significant indices out of the total indices for each mechanism. Finally, to assess the resilience for each river basin, the post hoc analyses (significant differences) results were used to estimate the percentage of low-resilience indices out of the total significant indices. River basins were classified according to the proportion of significant low-resilience indices relative to the total number of significant indices: basins with 50–100% were categorized as having low resilience, those with 20–50% as having medium resilience, and those with 0–20% as having high resilience.
3. Results
3.1. Fish Composition Variation Between Functional Process Zones Among River Basins
A total of 3094 fish specimens were captured, corresponding to fifteen native and five non-native species. Eighteen species were found in the SSC FPZ, while sixteen were captured in the BGDS FPZ (Table S4). Both evaluated FPZs were dominated by Basilichthys microlepidotus (Jenyns, 1841), Percilia gillissi Girard, 1855, and Trichomycterus areolatus (Valenciennes, 1948). Also, rare species were found in both FPZs, e.g., from the Diplomystidae family, D. incognitus was found in the BGDS FPZ in the Rapel river basin, D. arratiae in the SSC FPZ in the Biobío river basin, and Diplomystes camposensis Arratia, 1987, in the BGDS FPZ in the Toltén river basin. Regarding non-native species, Gambusia holbrooki Girard, 1959, Oncorhynchus mykiss (Walbaum, 1792), and Salmo trutta Linnaeus, 1758, were found in both FPZs in some basins (Table S4). It is noteworthy that in one replicate of the SSC FPZ of the Maipo river basin, no fish specimens were found. Species richness increased with increasing latitude, independently of the FPZ, from the northern Maipo river basin, with a median of two species in the SSC FPZ, to the southern Toltén river basin, with a median of seven species in both FPZs (Figure 2). NMDS, based on the Bray–Curtis dissimilarity matrix of fish abundance at each sampling site, did not present any clear similarity patterns for FPZ fish assemblages (Figure S1). Fish composition and abundance was significantly different among basins and FPZs nested within river basins (Table 3).
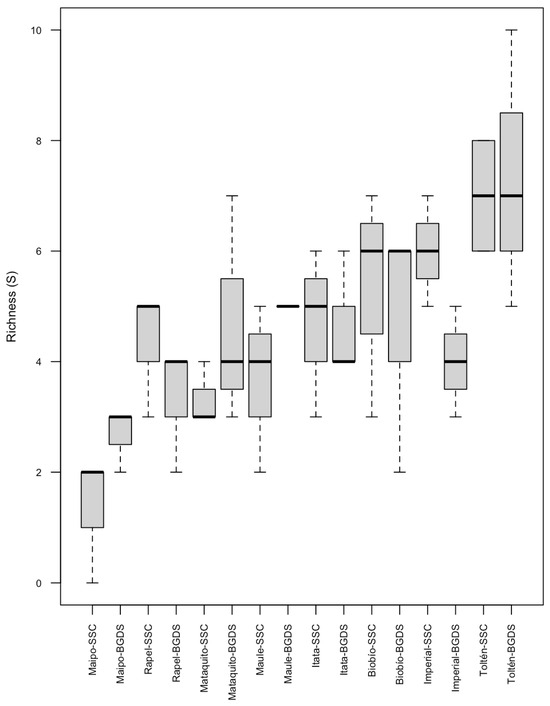
Figure 2.
Fish species richness in both FPZs in each river basin. SSC, Sinuous Single Channel; BGDS, Braided Gentle Downstream Slope.

Table 3.
Results of PERMANOVA conducted on the Bray–Curtis similarity matrix based on fish abundance data. df, degrees of freedom; SS, sums of squares; Pseudo-F, distance-based pseudo-F-statistic; p, probability values (obtained using 9999 permutations of residuals under a reduced model). Significant effects are indicated in bold.
These differences in assemblage structure were further supported by SIMPER analysis. Each basin significantly differed in fish assemblage composition and abundance (Table 3). The dominant species were T. areolatus and P. gillissi in the Rapel, Mataquito, Itata, Imperial, and Toltén river basins, while in the Biobío river basin, P. irwini and Percichthys trucha Cuvier and Valenciennes, 1840, were dominant (Figure 3). Percichthys trucha was also abundant in the Itata river basin and, to a lesser extent, in the Maipo and Mataquito river basins. Among non-native species, G. holbrooki was abundant in the Rapel and Maule river basins, whereas O. mykiss was abundant in the Maipo river basin and southern basins such as Imperial and Toltén (Figure 3). Native fish species richness exceeded non-native species richness in all basins (Table S4). The central–southern river basins, Biobío, Imperial, and Toltén, hosted the highest number of native species, accommodating nine, seven, and ten species, respectively. In contrast, the presence of non-native species appeared relatively uniform across the eight evaluated basins, ranging from one to two introduced species per basin (Table S4). The non-native species found correspond to Cnesterodon decemmaculatus Garman, 1895, Cyprinus carpio Linnaeus, 1758, G. holbrooki, O. mykiss, and S. trutta.
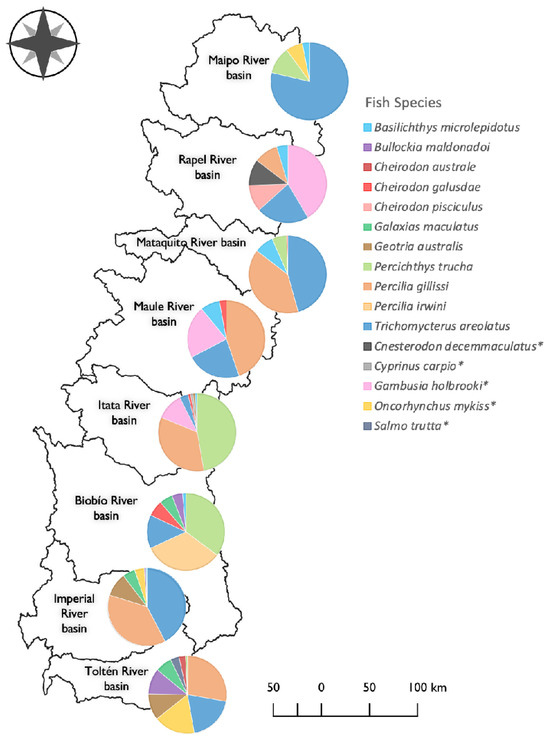
Figure 3.
Fish assemblage composition in eight Andean river basins. The pie chart represents the percentage of contribution of each species to the differences among basins (similarity percentage analysis). Species marked with * are non-native.
3.2. Resilience Mechanisms
3.2.1. Resources Mechanism
Fish species richness, total abundance, and the Shannon diversity index showed significant differences among the eight studied basins (Table 4; Figure 4; Tables S5 and S6). A clear pattern of richness increase with increasing latitude was observed, where the Maipo river basin had the lowest richness (median = 2) and the Toltén river basin the highest richness (median = 7; Figure 4a). Total abundance also increased with increasing latitude, and in the Mataquito river basin, it exhibited notably high variability in comparison with the rest of the basins (Figure 4b). The Shannon diversity index also increased with increasing latitude (Figure 4c). In contrast, the Pielou evenness index (J′), trophic guilds, and floodplain use did not show significant differences among the studied basins (Table 4).

Table 4.
Effects of basin factors on resources mechanism variables based on ANOVA and Kruskal–Wallis analyses (see Tables S5 and S6 for details). Significant differences (p < 0.1) are indicated in bold. F, F value; H, chi-squared.
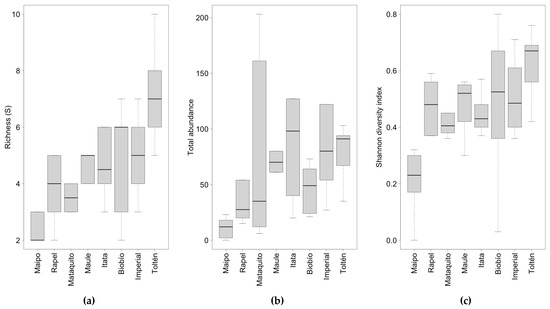
Figure 4.
Boxplot of (a) richness (S), (b) total abundance, and (c) Shannon diversity index (H′) for the eight studied basins. The lower end of the whisker represents the minimum value, the upper end the maximum value, the black line in the boxplot represents the median, and the dotted line represents the standard deviation.
3.2.2. Recruitment Mechanism
Beta diversity (Sørensen), abundance-weighted average size, rapid sexual maturity, high fecundity, multiple spawning, small egg size, and parental care showed significant differences among basins (Table 5). Beta diversity, based on presence–absence (Sørensen), was higher in the highly fragmented Maipo, Rapel, and Biobío river basins (Figure 5a; Table 1 and Table S7). The abundance-weighted average size generally increased with increasing latitude, except for in the Maipo river basin, which exhibited considerable variability (Figure 5b). No clear pattern of differences among basins was observed for rapid sexual maturity; however, the Rapel, Mataquito, and Maule river basins showed the same fish biomass values for this trait, which were higher than those of other basins (Figure 5c). Similarly, high fecundity showed no clear pattern of differences among basins (Figure 5d); however, the Biobío and Imperial river basins showed higher variation than other studied basins. The Maipo and Toltén river basins exhibited the lowest biomass for the multiple spawning trait (Figure 5e; Table S8). The Maipo river basin presented the highest variation in the small egg size trait, whereas egg size was significantly lower in the Toltén river basin (Figure 5f; Table S9). Lastly, all river basins were characterized by high variation in the parental care trait, except for the Maipo river basin, with zero biomass for this trait (Figure 5g). Beta diversity, based on the Bray–Curtis index, and migratory life history did not show significant differences among the studied basins (Table 5).

Table 5.
Effects of basin factors on recruitment mechanism variables based on ANOVA and Kruskal–Wallis analyses. Significant differences (p < 0.1) are indicated in bold (see Tables S5–S7 for details). Pseudo-F, Pseudo-F value; F, F value; H, chi-squared.
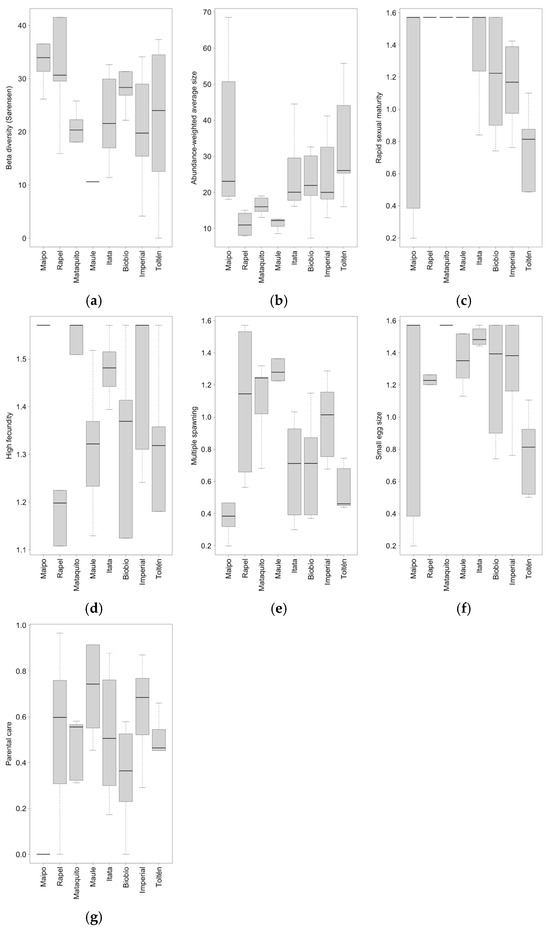
Figure 5.
Boxplot of (a) beta diversity (Sørensen), (b) abundance-weighted average size, (c) rapid sexual maturity, (d) high fecundity, (e) multiple spawning, (f) small egg size, and (g) parental care for the eight studied basins. The lower end of the whisker represents the minimum value, the upper end the maximum value, the black line in the boxplot represents the median, and the dotted line represents the standard deviation.
3.2.3. Refugia Mechanism
Richness by taxonomic order, the Shannon diversity index by taxonomic order, and velocity preference showed significant differences among the eight studied basins (Table 6). Similarly to species richness (resources mechanism), richness by taxonomic order showed a clear pattern of increase with increasing latitude (Figure 6a; Table S5). The Shannon diversity index by taxonomic order also showed a clear increase with increasing latitude (Figure 6b). Lastly, velocity preference showed a pattern of increase with increasing latitude, with the Maipo and Itata rivers being high-value outliers, indicating higher eurytopic fish biomass (Figure 6c). The Pielou evenness index by taxonomic order and vertical position did not show significant differences among the studied basins (Table 6).

Table 6.
Effects of basin factors on refugia mechanism variables based on ANOVA and Kruskal–Wallis analyses (see Tables S5 and S6 for details). Significant differences (p < 0.1) are indicated in bold. F, F value; H, chi-squared.
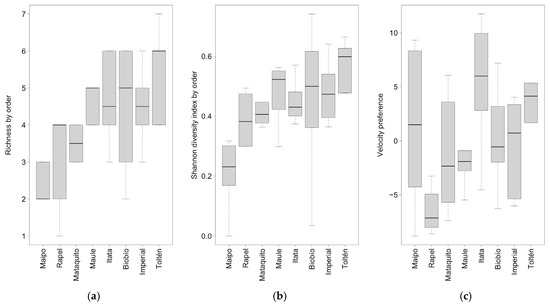
Figure 6.
Boxplot of (a) richness by taxonomic order, (b) diversity index by taxonomic order, and (c) velocity for the eight studied basins. The lower end of the whisker represents the minimum value, the upper end the maximum value, the black line in the boxplot represents the median, and the dotted line represents the standard deviation.
3.3. Resilience per Basin
Among the eight studied basins, the Maipo and Rapel river basins, which are characterized by a greater number of anthropogenic disturbances, exhibited the lowest resilience. The Biobío and Toltén river basins were classified as having medium resilience, whereas the Mataquito, Maule, Itata, and Imperial river basins showed the highest resilience (Figure 7a). The Maipo river basin showed ten significant indices—nine of these were of lower resilience, of which six indices corresponded to taxonomic and three to functional traits (Figure 7b,c). The Rapel river basin showed seven significant indices, of which four were of lower resilience (two taxonomic and two functional traits). The Mataquito and Maule river basins only exhibited one significant index of low resilience each, a taxonomic index (low richness; Figure 7b,c). For the Itata river basin, the significant index of low resilience corresponded to a functional trait (multiple spawning). The Biobío river basin showed two low-resilience indices out of five significant ones, one taxonomic (high beta diversity based on the Sørensen index), and one functional trait (multiple spawning). Notably, the Imperial river basin, characterized by low-level anthropogenic disturbances, presented all its significant indices as high resilience (Figure 7b). Lastly, the Toltén river basin, also with a low level of disturbances, was characterized by ten significant indices, among which four (corresponding to functional traits) represented low resilience (Figure 7b,c).
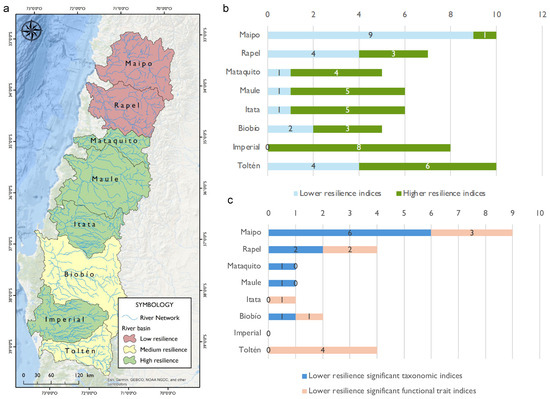
Figure 7.
(a) Resilience classification for the studied basins based on the evaluated indices; (b) the number of significant resilience indices per basin; (c) the number of significant lower resilience taxonomic and functional trait indices per basin.
4. Discussion
Fish assemblages identified in each of the eight river basins allowed an assessment of the resources, recruitment, and refugia resilience mechanisms, evaluated through taxonomic and functional trait indices. The most affected resilience mechanism was recruitment [linked to the beta diversity (Sørensen) indicator], related to the high fragmentation level of rivers such as the Rapel, Biobío, and Maipo [29,45]. Fragmentation in these river basins is caused primarily by hydropower plants, water-diverting structures, and reservoirs for irrigation. Furthermore, functional trait analyses revealed significant alteration of the recruitment mechanism by the invasion of non-native trout in the Toltén river basin, as indicated by trait shifts toward larger body size, late sexual maturity, single spawning, and larger egg size, which suggest a decrease in ecosystem resilience. The second most affected mechanism was resources (linked to the species richness indicator), potentially influenced by land-use changes and conditions associated with water pollution, as suggested by the patterns observed in the Maipo, Rapel, Mataquito, and Maule river basins. Lastly, the refugia mechanism was mostly affected in the most disturbed Maipo river basin.
Ecological resilience in river ecosystems, while theoretically well founded, faces substantial challenges in quantification due to the intricate and dynamic nature of these ecosystems [72,73,74]. Some of the most common difficulties are the presence of multiple environmental stressors, spatial and temporal variability, and the scarcity of long-term data [6,59]. Despite these difficulties, few empirical approaches that may allow the measurement of resilience in river ecosystems have been suggested [72,75,76,77,78].
Downing and Leibold (2010) [75] experimentally assessed resilience in aquatic food webs, evaluating how species richness and composition responded to a disturbance. Tracy et al. (2022) [76] focused on creating a river resilience index for a river basin that includes multiple relevant natural and anthropogenic impact factors, such as landscape features, connectivity, heterogeneity, land use, and water extraction. Bouska et al. (2019) [77] applied three general resilience principles—diversity and redundancy, connectivity, and controlling variables—to a large river ecosystem, using ecological indicators such as aquatic habitat diversity and redundancy, fish functional diversity and redundancy, longitudinal and lateral connectivity, and aquatic invasive species, among others, to assess an ecosystem’s capacity to cope with disturbance. Jaiswal et al. (2021) [72] and Jaiswal and Pandey 2019 [78] created a river resilience risk index focused on biochemical processes as resilience feedbacks to characterize tipping points in large, disturbed rivers. As such, there are different approaches to understanding river ecosystem resilience, some based on the ecological components and others based on biochemical processes.
In the present study, we employed an empirical approach based on fish assemblage taxonomic and functional trait indices adapted and expanded from the original proposal of Van Looy et al. (2019) [10]. Data availability is a common issue when working with functional traits, and there is a growing disconnect between trait-based ecology and the actual availability and interpretability of trait data [79]. To address this issue, we combined an extensive literature review and expert knowledge to obtain the most comprehensive trait database for Chilean river fish species. Despite the conceptual framework provided by Van Looy et al. 2019 [10], identifying and measuring ecologically relevant traits remains a challenge [79]. Not all functional traits are equally useful, and their utility depends on their ecological relevance [80]. Indeed, in this study, functional traits such as trophic guild, floodplain use, migratory life history, and vertical position were not significantly different among the river basins. Therefore, we suggest further exploration of functional traits that are ecologically meaningful for assessing resilience based on fish assemblages.
Another concern in measuring resilience is finding an appropriate spatial scale. The RES conceptual framework suggests the FPZ as an appropriate scale to assess river functioning processes and resilience [26,81]. Indeed, advances in understanding the critical factors influencing resilience in river ecosystems are often hindered by a limited focus on spatial dimensions [82]. Our results indicate that some elements of resilience operate at the river basin level, perhaps due to the specific context of isolation of the river basins evaluated with the presence of a high degree of endemism. Notably, the strong fish-assemblage river basin identity revealed in our study is associated with the geological isolation of the assessed basins for over 10,000 years [32], which resulted in native fish assemblages characterized by multiple early diverged and highly endemic species [34]. The river basins evaluated in the present study are in two of the three areas of endemism described for the Chilean ichthyographic province. The central area, which includes the Maipo and Rapel river basins, has two endemic species: Diplomystes chilensis (Molina, 1782) (extinct) and Cheirodon pisciculus Girard, 1855; and the south–central area, between the Maule and Imperial river basins, has six endemic species: B. maldonadoi, C. galusdae, D. nahuelbutaensis, D. arratiae, T. chiltoni, and P. irwini. The latter three species are endemic to the Biobío river basin [38,39,40,83]. However, these findings are based on limited samples for each FPZ in each basin, and, as such, we recognize the necessity for further research to elucidate the effects of type, size, and location (both longitudinal and lateral) of FPZs within each basin.
In this study, recruitment appeared to be the resilience mechanism most affected by anthropogenic disturbances. The beta diversity index was an effective indicator of lower resilience related to the recruitment mechanism and strongly associated with the high fragmentation of several of the studied basins. Indeed, beta diversity has been shown to be a good predictor of river fragmentation [84,85,86]. Barriers cause fish assemblages to become increasingly different because of the loss of longitudinal connectivity, causing a higher turnover of species or higher beta diversity between the local fish assemblages [35,87]. Fragmentation is one of the major threats to riverine ecosystems, and this is most explicitly expressed by the decline in numbers of migratory fish species [88]. In fact, the most fragmented river basins—Maipo, Rapel, and Biobío—were characterized by the highest beta diversity indices [45]. Indeed, the fragmentation-driven loss of resilience linked to the recruitment mechanism in the Maipo and Rapel river basins seems to be related to impediments to recolonization after disturbance and the resulting extirpation of migratory species such as Mordacia lapicida Gray, 1851, G. australis, and Galaxias maculatus (Jenyns, 1842) [89].
An interesting finding was that the recruitment mechanism appears to also be affected by lower resilience functional traits, such as large body size, late sexual maturity, single spawning, and large egg size, in less impacted and non-fragmented basins. These functional traits are dominant in the non-native trout O. mykiss and S. trutta [57,90,91,92]. The Toltén river basin is an example of this effect because the recruitment mechanism is altered by the high abundance of non-native salmonids. Salmonid invasion is the most important threat to native biodiversity in southern Chilean river basins [41]. Indeed, trout negatively impact native fish assemblages, leading to a decline in their abundances due to predation and competition [93,94]. Furthermore, trout affect the trophic position of native fish, changing natural food webs [95,96,97]. Here, the effects of trout and the lower resilience traits that trout contribute to fish assemblage are reflected in lower resilience in the recruitment mechanism, specifically in functional trait indicators. In this way, in the less disturbed Toltén river basin, the functional trait indices captured the effects of biological disturbances that in other river basins with a higher number of physical disturbances did not emerge.
The resources mechanism, linked to the species richness indicator, was the second most affected by anthropogenic disturbances in the studied basins. A major driver of species richness decline worldwide is land-use change [98,99,100,101,102,103]. The conversion of the natural landscape into other productive systems affects the integrity of river ecosystems in terms of nutrient cycling, increased surface runoff, and reduced water quality [104,105,106]. Therefore, land-use changes can modify consumer–resource interactions and reconfigure the flow of energy through the entire river ecosystem food web, altering the resources mechanism [107]. Water pollution from domestic and industrial effluents is also a major threat to biodiversity in river ecosystems [12,98,99,100,101,102,103,104,105,106,107,108,109,110], reducing the abundance and diversity of native fish fauna and other organisms [111,112,113,114]. Loss of resilience in relation to the resources mechanism due to extensive land-use changes in the Maipo, Rapel, Mataquito, and Maule river basins was reflected in significantly lower species richness than the rest of the studied basins. During the last decades, land use has changed from native forest to agriculture and urbanization, as well as exotic plantations of P. radiata and E. globulus for pulp production in the central–southern basins (Mataquito and Maule river basins) [115,116,117,118]. For example, in the Rapel river basin, land-use changes have caused high loads of organic matter and nutrients in the Rapel reservoir located in the lower basin, causing several algal blooms, hypoxia and anoxia events, and associated fish mortalities [119]. The Maipo river basin is the most disturbed among the studied basins, and this is reflected in other resources mechanism indicators, such as low abundance and low Shannon’s diversity. It is also the only river basin within the study area with low-resilience taxonomic indicators for the refugia mechanism, such as low richness by taxonomic order and low Shannon’s diversity by taxonomic order. Indeed, apart from fragmentation, land-use changes, and pollution, the Maipo river basin accommodates 40% of the Chilean population (7 million people) [42], and it has been described as one of the most contaminated river basins in Chile [41]. Indeed, these anthropogenic disturbances seem to have a profound impact on the availability and quality of refuges for fish assemblages. Therefore, it seems to have reduced ability to facilitate the survival of fish species, and possibly other aquatic organisms, under these adverse conditions [10,120].
The relationships between anthropogenic disturbances and river resilience assessed by fish assemblage indicators showed some non-linear responses across river basins, i.e., a highly disturbed river basin did not always result in low resilience. These results have different local explanations, such as the highly disturbed Biobío river basin still hosting a naturally highly diverse fish assemblage, indicating high resilience [41,121]. Meanwhile, unexpectedly, the Toltén river basin is a representation of the significance of biological invasions in physically well-conserved ecosystems [122,123,124]. Also, the Itata and Mataquito river basins, with medium levels of disturbances, showed high resilience. These basins possibly exemplify that at a medium level of disturbance, fish assemblages can adapt to disturbances and the resilience of the river ecosystem can be maintained. This diversity and non-linearity of responses highlights the complexity of river resilience mechanisms, where the influence of disturbances varies across basins, challenging assumptions about disturbance–resilience patterns.
The resilience assessment based on fish assemblages of Andean river basins of central–southern Chile provided insights into resources, recruitment, and refugia resilience mechanisms in river ecosystems. Fish assemblages were suitable indicators of river ecosystem resilience, which could be related to different degrees of anthropogenic disturbances at the basin scale. The use of several resilience indicators revealed the different responses of fish assemblages to anthropogenic disturbances associated with each resilience mechanism. Recruitment indices (beta diversity Sørensen and functional traits) revealed low resilience due to fragmentation and the presence of non-native trout. Meanwhile, resource indices (richness, abundance, and Shannon’s diversity) revealed the effects of land-use changes and water pollution. Thus, these indices emerged as key indicators of river resilience in the studied basins. Integrated analysis approaches, such as the one used here, are necessary since the interplay between climate change, land-use intensification, species invasion, and human population growth bring new challenges to the global management and conservation of freshwater ecosystems. This highlights the need to raise understanding of adaptive management approaches for ecosystem resilience and measures to improve the resilience of river ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17121749/s1, Figure S1: Non-metric multidimensional scaling (NMDS) based on the Bray-Curtis dissimilarity matrix of fish abundance at each sampling site. SSC, sinuous single channel; BGDS, braided gentle downstream slope, Table S1: Classification of functional traits for each species. Information was extracted from different sources [47,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68] and expert knowledge. *Non-native species, Table S2: Functional traits used to assess fish assemblages’ resilience mechanisms in each basin. These traits were selected based on the available knowledge about fish species captured in the eight basins (Table S1). ↑ indicates resilience increase as biomass of fish in a particular trait category increase. ↓ indicates resilience decrease as the biomass of fish in a particular trait category increase, Table S3: Scores of principal coordinate analysis (PCA) for trophic guilds, migratory life history, vertical position and velocity preference, Table S4: Fish composition in each FPZ by basin. SSC, sinuous single channel; BGDS, braided gentle downstream slope, Table S5: ANOVA results for richness, Shannon diversity index, trophic guilds, migratory life history, multiple spawning, richness by taxonomic order, Shannon diversity index by taxonomic order, vertical position, velocity preference, Table S6: Kruskal-Wallis results for total abundance, Pielou evenness index, floodplain use, abundance—weighted average size, rapid sexual maturity, high fecundity, small egg size, parental care and Pielou by taxonomic order, Table S7: PERMANOVA results for beta diversity (Sørensen) and beta diversity (Bray-Curtis), Table S8: Post-hoc Tukey test results for richness, Shannon diversity index, multiple spawning, richness by taxonomic order, Shannon diversity index by taxonomic order, velocity preference, Table S9: Post-hoc Dunn test results for total abundance, abundance—weighted average size, rapid sexual maturity, high Fecundity, small egg size and parental care.
Author Contributions
Conceptualization: P.V., K.G. and E.H.; developing methods: P.V., K.G. and E.H.; conducting the research: P.V., K.G. and E.H.; data analysis: P.V.; data interpretation: P.V., K.G. and E.H.; preparation of figures and tables: P.V.; writing: P.V., K.G. and E.H. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this work was received from FONDECYT by Evelyn Habit (Project 1190647) and Konrad Górski (Project 1230617).
Data Availability Statement
Data are available from the authors upon reasonable request.
Acknowledgments
This study constitutes part of the thesis presented by Paulina Vega as a partial requirement of the MSc in Environmental Sciences at the University of Concepción (Chile), available at: https://repositorio.udec.cl/ (accessed on 16 April 2024). The authors wish to express their gratitude to Aliro Manosalva, Bárbara Toledo, Gustavo Díaz, Joaquín Cárcamo, and Laura Habit for their help during the fieldwork and Camila Bañales-Seguel for her help in the literature review related to fish species traits.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Linke, S.; Turak, E.; Nel, J. Freshwater conservation planning: The case for systematic approaches. Freshwater Biol. 2011, 56, 6–20. [Google Scholar] [CrossRef]
- Wohl, E. Rivers in the Landscape: Science and Management; Wiley-Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.S.; Furukawa, T.; Sasaki, T. Response diversity determines the resilience of ecosystems to environmental change. Biol. Rev. 2013, 88, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Holling, C.S. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. Available online: http://www.jstor.org/stable/2096802 (accessed on 9 June 2021). [CrossRef]
- Parsons, M.; Thoms, M.C.; Flotemersch, J.; Reid, M. Monitoring the resilience of rivers as social-ecological systems: A paradigm shift for river assessment in the twenty-first century. In River Science: Research and Management for the 21st Century, 1st ed.; Gilvear, D.J., Greenwood, M.T., Thoms, M.C., Wood, P.J., Eds.; Wiley-Blackwell: Chichester, UK, 2016; pp. 197–220. [Google Scholar]
- Walker, B.; Salt, D. Resilience thinking: Sustaining ecosystems and people in a changing world. In Coral Reefs; Island Press: Washington, DC, USA, 2006. [Google Scholar]
- Westman, W.E. Measuring the inertia and resilience of ecosystems. BioScience 1978, 28, 705–710. [Google Scholar] [CrossRef]
- Elmqvist, T.; Folke, C.; Nyström, M.; Peterson, G.; Bengtsson, J.; Walker, B.; Norberg, J. Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 2003, 1, 488–494. [Google Scholar] [CrossRef]
- Van Looy, K.; Tonkin, J.D.; Floury, M.; Leigh, C.; Soininen, J.; Larsen, S.; Heino, J.; Poff, N.L.; Delong, M.; Jähnig, S.C.; et al. The three Rs of river ecosystem resilience: Resources, recruitment, and refugia. River Res. Appl. 2019, 35, 107–120. [Google Scholar] [CrossRef]
- Arthington, A.H.; Finlayson, C.M.; Pittock, J. Freshwater ecological principles. In Freshwater Ecosystems in Protected Areas: Conservation and Management, 1st ed.; Finlayson, C.M., Arthington, A.H., Pittock, J., Eds.; Routledge: London, UK, 2018; pp. 34–53. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status & conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Jaiswal, D.; Pandey, J. River ecosystem resilience risk index: A tool to quantitatively characterize resilience and critical transitions in human-impacted large rivers. Environ. Pollut. 2021, 268, 115771. [Google Scholar] [CrossRef]
- Connell, S.D.; Ghedini, G. Resisting regime-shifts: The stabilising effect of compensatory processes. Trends Ecol. Evol. 2015, 30, 513–515. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Stoll, S.; Jähnig, S.C.; Haase, P. Contrasting metacommunity structure and beta diversity in an aquatic-floodplain system. Oikos 2016, 125, 686–697. [Google Scholar] [CrossRef]
- Angeler, D.G.; Allen, C.R. Quantifying resilience. J. Appl. Ecol. 2016, 53, 617–624. [Google Scholar] [CrossRef]
- Biggs, C.R.; Yeager, L.A.; Bolser, D.G.; Bonsell, C.; Dichiera, A.M.; Hou, Z.; Keyser, S.R.; Khursigara, A.J.; Lu, K.; Muth, A.F.; et al. Does functional redundancy affect ecological stability and resilience? A review and meta-analysis. Ecosphere 2020, 11, e03184. [Google Scholar] [CrossRef]
- Fetzer, I.; Johst, K.; Schawea, R.; Banitz, T.; Harms, H.; Chatzinotas, A. The extent of functional redundancy changes as species’ roles shift in different environments. Proc. Natl. Acad. Sci. USA 2015, 112, 14888–14893. [Google Scholar] [CrossRef]
- Nash, K.L.; Graham, N.A.J.; Jennings, S.; Wilson, S.K.; Bellwood, D.R. Herbivore cross-scale redundancy supports response diversity and promotes coral reef resilience. J. Appl. Ecol. 2016, 53, 646–655. [Google Scholar] [CrossRef]
- Karr, J.R. Assessment of biotic integrity using fish communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Schiemer, F. Fish as indicators for the assessment of the ecological integrity of large rivers. Hydrobiologia 2000, 422–423, 271–278. [Google Scholar] [CrossRef]
- Delong, M.D.; Thoms, M.C. Changes in the trophic status of fish feeding guilds in response to flow modification. J. Geophys. Res. Biogeosci. 2016, 121, 949–964. [Google Scholar] [CrossRef]
- Delong, M.D.; Thoms, M.C. An Ecosystem Framework for River Science and Management. In River Science: Research and Management for the 21st Century, 1st ed.; Gilvear, D.J., Greenwood, M.T., Thoms, M.C., Wood, P.J., Eds.; Wiley-Blackwell: Chichester, UK, 2016; pp. 12–36. [Google Scholar] [CrossRef]
- Dollar, E.S.J.; James, C.S.; Rogers, K.H.; Thoms, M.C. A framework for interdisciplinary understanding of rivers as ecosystems. Geomorphology 2007, 89, 147–162. [Google Scholar] [CrossRef]
- Thorp, J.H.; Thoms, M.C.; Delong, M.D. The Riverine Ecosystem Synthesis. In The Riverine Ecosystem Synthesis: Toward Conceptual Cohesiveness in River Science, 1st ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar] [CrossRef]
- Elgueta, A.; Thoms, M.C.; Górski, K.; Díaz, G.; Habit, E.M. Functional process zones and their fish communities in temperate Andean river networks. River Res. Appl. 2019, 35, 1702–1711. [Google Scholar] [CrossRef]
- Thoms, M.C.; Delong, M.D.; Flotemersch, J.E.; Collins, S.E. Physical heterogeneity and aquatic community function in river networks: A case study from the Kanawha River Basin, USA. Geomorphology 2017, 290, 277–287. [Google Scholar] [CrossRef]
- Habit, E.; Zurita, A.; Díaz, G.; Manosalva, A.; Arriagada, P.; Link, O.; Górski, K. Latitudinal & altitudinal gradients of riverine landscapes in Andean rivers. Water 2022, 14, 2614. [Google Scholar] [CrossRef]
- Maasri, A.; Pyron, M.; Arsenault, E.R.; Thorp, J.H.; Mendsaikhan, B.; Tromboni, F.; Minder, M.; Kenner, S.J.; Costello, J.; Chandra, S.; et al. Valley-scale hydrogeomorphology drives river fish assemblage variation in Mongolia. Ecol. Evol. 2021, 11, 6527–6535. [Google Scholar] [CrossRef] [PubMed]
- Habit, E.; Górski, K.; Alò, D.; Ascencio, E.; Astorga, A.; Colin, N.; Contador, T.; de los Ríos, P.; Delgado, V.; Dorador, C.; et al. Biodiversidad de ecosistemas de agua dulce. In Mesa Biodiversidad-Comité Científico COP25; Ministerio de Ciencia, Tecnología, Conocimiento e Innovación: Santiago, Chile, 2019; p. 64. [Google Scholar]
- Charrier, R.; Ramos, V.A.; Tapia, F.; Sagripanti, L. Tectono-stratigraphic evolution of the Andean Orogen between 31 and 37°S (Chile and Western Argentina). Geol. Soc. Spec. Publ. 2015, 399, 13–61. [Google Scholar] [CrossRef]
- Campos, H.; Dazarola, G.; Dyer, B.S.; Fuentes, L.; Gavilán, J.F.; Huaquín, L.; Martínez, G.; Meléndez, R.; Pequeño, G.; Ponce, F.; et al. Categorías de conservación de peces nativos de aguas continentales de Chile. Boletín Mus. Nac. Hist. Nat. Chile 1998, 47, 101–122. [Google Scholar] [CrossRef]
- Habit, E.; Belk, M.C.; Tuckfield, R.C.; Parra, O. Response of the fish community to human-induced changes in the Biobío River in Chile. Freshwater Biol. 2006, 51, 1–11. [Google Scholar] [CrossRef]
- Díaz, G.; Górski, K.; Heino, J.; Arriagada, P.; Link, O.; Habit, E. The longest fragment drives fish beta diversity in fragmented river networks: Implications for river management and conservation. Sci. Total Environ. 2021, 766, 144323. [Google Scholar] [CrossRef]
- Arroyo, M.T.K.; Marquet, P.; Marticorena, C.; Simonetti, J.; Lohengrin, C.; Squeo, F.; Rozzi, R. Chilean Winter Rainfall-Valdivian Forest. Hotspots Revisit. 2004, 99–103. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Dyer, B. Systematic review and biogeography of the freshwater fishes of Chile. Estud. Oceanol. 2000, 19, 77–98. [Google Scholar]
- Muñoz-Ramírez, C.P.; Colin, N.; Canales-Aguirre, C.B.; Manosalva, A.; López-Rodríguez, R.; Sukumaran, J.; Górski, K. Species tree analyses and speciation-based species delimitation support new species in the relict catfish family Diplomystidae and provide insights on recent glacial history in Patagonia. Mol. Phylogenet. Evol. 2023, 189, 107932. [Google Scholar] [CrossRef] [PubMed]
- Vila, I.; Habit, E. Current situation of the fish fauna in the Mediterranean region of Andean river systems in Chile. Fishes in Mediterr. Environ. 2015, 2015. [Google Scholar] [CrossRef]
- Habit, E.; Górski, K.; Vila, I.; Manosalva, A.; Díaz, G.; Toledo, B.; Rojas, P.; Zurita, A. The effects of anthropogenic pressures on native Chilean fish and lamprey fauna. Gayana 2024, 88, 76–104. [Google Scholar]
- INE. Resultados CENSO 2017. Available online: http://resultados.censo2017.cl (accessed on 12 January 2024).
- Vega-Retter, C.; Muñoz-Rojas, P.; Vila, I.; Copaja, S.; Véliz, D. Genetic effects of living in a highly polluted environment: The case of the silverside Basilichthys microlepidotus (Jenyns) (Teleostei: Atherinopsidae) in the Maipo River basin, central Chile. Popul. Ecol. 2014, 56, 569–579. [Google Scholar] [CrossRef]
- Figueroa, R.; Parra, O.; Díaz, M.E. La cuenca hidrográfica del río Biobío. In EULA-CHILE Evolución y Perspectivas a 30 Años de su Creación; Universidad de Concepción: Concepción, Chile, 2020; pp. 91–137. [Google Scholar]
- Díaz, G.; Arriagada, P.; Górski, K.; Link, O.; Karelovic, B.; González, J.; Habit, E. Fragmentation of Chilean Andean rivers: Expected effects of hydropower development. Rev. Chil. Hist. Nat. 2019, 92, 1–13. [Google Scholar] [CrossRef]
- Ministerio de Energía. Infraestructura de Datos Espaciales. Available online: https://ide-energia.minenergia.cl/portal/apps/webappviewer/index.html?id=5c526a138b1449458e0667b2235d2b19 (accessed on 12 January 2024).
- Ruiz, V.; Marchant, M. Ictiofauna de Aguas Continentales de Chile; Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción: Concepción, Chile, 2004; 356p. [Google Scholar]
- Salas, D.; Véliz, D.; Scott, S. Morphological differentiation in the genus Cheirodon (Ostariophysi: Characidae) using both traditional and geometric morphometrics. Gayana 2012, 76, 142–152. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E: Plymouth, UK, 2001; p. 172. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ Primer V7: User Manual; Primer-E Ltd.: Plymouth, UK, 2008; p. 214. [Google Scholar]
- Anderson, M.J.; Willis, T.J. Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Baker, C.F.; Jellyman, D.J.; Reeve, K.; Crow, S.; Stewart, M.; Buchinger, T.; Li, W. First observations of spawning nests in the pouched lamprey (Geotria australis). Can. J. Fish. Aquat. Sci. 2017, 74, 1603–1611. [Google Scholar] [CrossRef]
- Campos, H. Reproducción del Aplochiton taeniatus Jenyns. Bol. Mus. Nac. Hist. Nat. 1969, 29, 207–222. [Google Scholar]
- Campos, H. Galaxias maculatus (Jenyns) en Chile, con especial referencia a su reproducción. Bol. Mus. Nac. Hist. Nat. 1970, 31, 5–20. [Google Scholar] [CrossRef]
- Chiang, G.; Munkittrick, K.R.; McMaster, M.E.; Tucca, F.; Saavedra, M.F.; Ancalaf, A.; Gavilán, J.F.; Unzueta, L.; Barra, R. Seasonal changes in oocyte development, growth and population size distribution of Percilia gillissi and Trichomycterus areolatus in the Itata basin, Chile. Gayana 2012, 76, 131–141. [Google Scholar] [CrossRef][Green Version]
- Estay, F.J.; Colihueque, N.; Yáñez, M. Reproductive performance assessed during three spawning seasons in a naturalized rainbow trout population from southern Chile. Fish. Res. 2021, 244, 106107. [Google Scholar] [CrossRef]
- Ferriz, R.A.; Bentos, C.A.; Gómez, S.E. Fecundidad en Jenynsia lineata y Cnesterodon decemmaculatus (Pisces, Cyprinodontiformes) de la Pampasia Argentina. Acta Biol. Venez. 1999, 19, 33–39. [Google Scholar]
- Golusda, P. Aclimatación y cultivo de especies salmonídeas en Chile. Bol. Soc. Biol. Concepción 1927, 1, 80–100. [Google Scholar]
- Habit, E.; Victoriano, P. Peces de agua dulce de la Cordillera de la Costa. In Historia, Biodiversidad y Ecología de la Cordillera de la Costa de Chile; Smith-Ramírez, C., Armesto, J., Valdovinos, C., Eds.; Editorial Universitaria: Santiago, Chile, 2005; pp. 392–406. [Google Scholar]
- Habit, E.; Jara, A.; Colin, N.; Oyanedel, A.; Victoriano, P.; Gonzalez, J.; Solis-Lufí, K. Threatened fishes of the world: Diplomystes camposensis Arratia, 1987 (Diplomystidae). Environ. Biol. Fish. 2009, 84, 393–394. [Google Scholar] [CrossRef]
- Manriquez, A.; Huaqúin, L.; Arellano, M.; Arratia, G. Aspectos Reproductivos de Trichomycterus areolatus Valenciennes, 1846 (Pisces: Teleostei: Siluriformes) en Río Angostura, Chile. Stud. Neotrop. Fauna Environ. 1988, 23, 89–102. [Google Scholar] [CrossRef]
- Montoya, G.; Jara, A.; Solis-Lufí, K.; Colin, N.; Habit, E. Primeros estadios del ciclo de vida de peces nativos del río San Pedro (cuenca del río Valdivia, Chile). Gayana 2012, 76, 86–100. [Google Scholar] [CrossRef]
- Oyanedel, A.; Habit, E.; Belk, M.C.; Solis-Lufí, K.; Colin, N.; Gonzalez, J.; Jara, A.; Muñoz-Ramírez, C.P. Movement patterns and home range in Diplomystes camposensis (Siluriformes: Diplomystidae), an endemic and threatened species from Chile. Neotrop. Ichthyol. 2018, 16, e170134. [Google Scholar] [CrossRef]
- Patimar, R.; Ghorbani, M.; Gol-Mohammadi, A.; Azimi-Glugahi, H. Life history pattern of mosquitofish Gambusia holbrooki (Girard, 1859) in the Tajan River (Southern Caspian Sea to Iran). Chin. J. Oceanol. Limnol. 2011, 29, 167–173. [Google Scholar] [CrossRef]
- Prochelle, O.; Campos, H. The biology of the introduced carp Cyprinus carpio L., in the river Cayumapu, Valdivia, Chile. Stud. Neotrop. Fauna Environ. 1985, 20, 65–82. [Google Scholar] [CrossRef]
- Ruiz, V. Ictiofauna del río Andalién (Concepción, Chile). Gayana Zool. 1993, 57, 109–278. [Google Scholar]
- Serezli, R.; Guzel, S.; Kocabas, M. Fecundity and egg size of three salmonid species (Oncorhynchus mykiss, Salmo labrax, Salvelinus fontinalis) cultured at the same farm condition in North-Eastern, Turkey. J. Anim. Vet. Adv. 2010, 9, 576–580. [Google Scholar] [CrossRef][Green Version]
- Clarke, K.R.; Gorley, R.N. Getting started with PRIMER v7. Primer-E 2015, 1, 20. Available online: https://www.primer-e.com (accessed on 14 December 2022).
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 19 July 2022).
- Jaiswal, D.; Pandey, U.; Mishra, V.; Pandey, J. Integrating resilience with functional ecosystem measures: A novel paradigm for management decisions under multiple-stressor interplay in freshwater ecosystems. Glob. Chang. Biol. 2021, 27, 3699–3717. [Google Scholar] [CrossRef]
- Quinlan, A.E.; Berbés-Blázquez, M.; Haider, L.J.; Peterson, G.D. Measuring and assessing resilience: Broadening understanding through multiple disciplinary perspectives. J. Appl. Ecol. 2016, 53, 677–687. [Google Scholar] [CrossRef]
- Standish, R.J.; Hobbs, R.J.; Mayfield, M.M.; Bestelmeyer, B.T.; Suding, K.N.; Battaglia, L.L.; Eviner, V.; Hawkes, C.V.; Temperton, V.M.; Cramer, V.A.; et al. Resilience in ecology: Abstraction, distraction, or where the action is? Biol. Conserv. 2014, 177, 43–51. [Google Scholar] [CrossRef]
- Downing, A.L.; Leibold, M.A. Species richness facilitates ecosystem resilience in aquatic food webs. Freshwater Biol. 2010, 55, 2123–2137. [Google Scholar] [CrossRef]
- Tracy, E.E.; Infante, D.M.; Cooper, A.R.; Taylor, W.W. An ecological resilience index to improve conservation action for stream fish habitat. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 951–966. [Google Scholar] [CrossRef]
- Bouska, W.W.; Houser, J.N.; De Jager, N.R. Applying concepts of general resilience to large river ecosystems: A case study from the Upper Mississippi and Illinois rivers. Ecol. Indic. 2019, 101, 785–796. [Google Scholar] [CrossRef]
- Jaiswal, D.; Pandey, J. Hypoxia and associated feedbacks at sediment-water interface as an early warning signal of resilience shift in an anthropogenically impacted river. Environ. Res. 2019, 178, 108712. [Google Scholar] [CrossRef]
- Kremer, C.T.; Williams, A.K.; Finiguerra, M.; Fong, A.A.; Kellerman, A.; Paver, S.F.; Tolar, B.B.; Toscano, B.J. Realizing the potential of trait-based aquatic ecology: New tools and collaborative approaches. Limnol. Oceanogr. 2017, 62, 253–271. [Google Scholar] [CrossRef]
- Streit, R.P.; Bellwood, D.R. To harness traits for ecology, let’s abandon ‘functionality’. Trends Ecol. Evol. 2023, 38, 402–411. [Google Scholar] [CrossRef]
- Parsons, M.; Thoms, M.C. From academic to applied: Operationalising resilience in river systems. Geomorphology 2018, 305, 242–251. [Google Scholar] [CrossRef]
- Thorp, J.H.; Thoms, M.C.; Delong, M.D.; Maasri, A. The ecological nature of whole river macrosystems: New perspectives from the riverine ecosystem synthesis. Front. Ecol. Evol. 2023, 11, 1184433. [Google Scholar] [CrossRef]
- Arratia, G.; Quezada-Romegialli, C. The South American and Australian percichthyids and perciliids. What is new about them? Neotrop. Ichthyol. 2020, 17, e180102. [Google Scholar] [CrossRef]
- Baldan, D.; Cunillera-Montcusí, D.C.; Funk, A.; Piniewski, M.; Cañedo-Argüelles, M.; Hein, T. The effects of longitudinal fragmentation on riverine beta diversity are modulated by fragmentation intensity. Sci. Total Environ. 2023, 903, 166703. [Google Scholar] [CrossRef]
- Edge, C.B.; Fortin, M.J.; Jackson, D.A.; Lawrie, D.; Stanfield, L.; Shrestha, N. Habitat alteration and habitat fragmentation differentially affect beta diversity of stream fish communities. Landsc. Ecol. 2017, 32, 647–662. [Google Scholar] [CrossRef]
- Gauthier, M.; Launay, B.; Le Goff, G.; Pella, H.; Douady, C.J.; Datry, T. Fragmentation promotes the role of dispersal in determining intermittent headwater stream metacommunities. Freshwater Biol. 2020, 65, 2169–2185. [Google Scholar] [CrossRef]
- Xia, Z.; Heino, J.; Yu, F.; He, Y.; Liu, F.; Wang, J. Spatial patterns of site and species contributions to β diversity in riverine fish assemblages. Ecol. Indic. 2022, 145, 109728. [Google Scholar] [CrossRef]
- van Puijenbroek, P.J.T.M.; Buijse, A.D.; Kraak, M.H.S.; Verdonschot, P.F.M. Species and river specific effects of river fragmentation on European anadromous fish species. River Res. Appl. 2019, 35, 68–77. [Google Scholar] [CrossRef]
- Díaz, G.; Górski, K.; Manosalva, A.; Toledo, B.; Habit, E. Fragmentation level drives local fish assemblage diversity patterns in fragmented river basins. Diversity 2023, 15, 352. [Google Scholar] [CrossRef]
- Arismendi, I.; Sanzana, J.; Soto, D. Seasonal age distributions and maturity stage in a naturalized rainbow trout (Oncorhynchus mykiss Walbaum) population in southern Chile reveal an ad-fluvial life history. Ann. Limnol. 2011, 47, 133–140. [Google Scholar] [CrossRef]
- González, C.; Gortázar, J.; García De Jalón, D. Trucha común—Salmo trutta. In Enciclopedia Virtual de los Vertebrados Españoles, 1st ed.; Salvador, A., Elvira, B., Eds.; Museo Nacional de Ciencias Naturales: Chamartín, Madrid, 2012; Available online: https://digital.csic.es/bitstream/10261/107793/1/saltru_v2.pdf (accessed on 11 June 2022).
- Tyler, C.R.; Pottinger, T.G.; Santos, E.; Sumpter, J.P.; Price, S.A.; Brooks, S.; Nagler, J.J. Mechanisms controlling egg size and number in the rainbow trout, Oncorhynchus mykiss. Biol. Reprod. 1996, 54, 8–15. [Google Scholar] [CrossRef][Green Version]
- Arismendi, I.; Soto, D.; Penaluna, B.; Jara, C.; Leal, C.; León-Muñoz, J. Aquaculture, non-native salmonid invasions and associated declines of native fishes in Northern Patagonian lakes. Freshwater Biol. 2009, 54, 1135–1147. [Google Scholar] [CrossRef]
- Habit, E.; González, J.; Ortiz-Sandoval, J.; Elgueta, A.; Sobenes, C. Efectos de la invasión de salmónidos en ríos y lagos de Chile. Ecosistemas 2015, 24, 43–51. [Google Scholar] [CrossRef]
- Belk, M.C.; Habit, E.; Ortiz-Sandoval, J.J.; Sobenes, C.; Combs, E.A. Ecology of Galaxias platei in a depauperate lake. Ecol. Freshwater Fish 2014, 23, 615–621. [Google Scholar] [CrossRef]
- Correa, C.; Bravo, A.P.; Hendry, A.P. Reciprocal trophic niche shifts in native and invasive fish: Salmonids and galaxiids in Patagonian lakes. Freshwater Biol. 2012, 57, 1769–1781. [Google Scholar] [CrossRef]
- Habit, E.; Victoriano, P. Composición, origen y valor de conservación de la ictiofauna del río San Pedro (cuenca del río Valdivia, Chile). Gayana 2012, 76, 10–23. [Google Scholar] [CrossRef]
- Allan, E.; Manning, P.; Alt, F.; Binkenstein, J.; Blaser, S.; Blüthgen, N.; Böhm, S.; Grassein, F.; Hölzel, N.; Klaus, V.H.; et al. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 2015, 18, 834–843. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; González, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; MacE, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Gossner, M.M.; Lewinsohn, T.M.; Kahl, T.; Grassein, F.; Boch, S.; Prati, D.; Birkhofer, K.; Renner, S.C.; Sikorski, J.; Wubet, T.; et al. Land-use intensification causes multitrophic homogenization of grassland communities. Nature 2016, 540, 266–269. [Google Scholar] [CrossRef]
- Murphy, G.E.P.; Romanuk, T.N. A meta-analysis of declines in local species richness from human disturbances. Ecol. Evol. 2014, 4, 91–103. [Google Scholar] [CrossRef]
- Powers, R.P.; Jetz, W. Global habitat loss and extinction risk of terrestrial vertebrates under future land-use-change scenarios. Nat. Clim. Chang. 2019, 9, 323–329. [Google Scholar] [CrossRef]
- Simkin, R.D.; Seto, K.C.; McDonald, R.I.; Jetz, W. Biodiversity impacts and conservation implications of urban land expansion projected to 2050. Proc. Natl. Acad. Sci. USA 2022, 119, e2117297119. [Google Scholar] [CrossRef] [PubMed]
- Esse, C.; Ríos, N.; Saavedra, P.; Fonseca, D.; Encina-Montoya, F.; Santander-Massa, R.; De los Ríos-Escalante, P.; Figueroa-Muñoz, G.; López-Pérez, A.; Correa-Araneda, F. Effects of land use change on water availability and water efficiency in the temperate basins of south-central Chile. J. King Saud Univ. Sci. 2021, 33, 101650. [Google Scholar] [CrossRef]
- Fierro, P.; Valdovinos, C.; Arismendi, I.; Díaz, G.; Ruiz De Gamboa, M.; Arriagada, L. Assessment of anthropogenic threats to Chilean Mediterranean freshwater ecosystems: Literature review and expert opinions. Environ. Impact Assess. Rev. 2019, 77, 114–121. [Google Scholar] [CrossRef]
- Miserendino, M.L.; Casaux, R.; Archangelsky, M.; Di Prinzio, C.Y.; Brand, C.; Kutschker, A.M. Assessing land-use effects on water quality, in-stream habitat, riparian ecosystems, and biodiversity in Patagonian northwest streams. Sci. Total Environ. 2011, 409, 612–624. [Google Scholar] [CrossRef]
- Price, E.L.; Sertić Perić, M.; Romero, G.Q.; Kratina, P. Land use alters trophic redundancy and resource flow through stream food webs. J. Anim. Ecol. 2019, 88, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Bassem, S.M. Water pollution and aquatic biodiversity. Biodivers. Int. J. Rev. 2020, 4, 10–16. [Google Scholar] [CrossRef]
- Gómez, J.; De La Maza, C.; Melo, Ó. Restoring environmental flow: Buy-back costs and pollution-dilution as a compliance with water quality standards. Water Policy 2014, 16, 864–879. [Google Scholar] [CrossRef]
- Groh, K.; vom Berg, C.; Schirmer, K.; Tlili, A. Anthropogenic chemicals as underestimated drivers of biodiversity loss: Scientific and societal implications. Environ. Sci. Technol. 2022, 56, 707–710. [Google Scholar] [CrossRef]
- Magurran, A.E.; Phillip, D.A.T. Implications of species loss in freshwater fish assemblages. Ecography 2001, 24, 645–650. Available online: https://www.jstor.org/stable/3683766 (accessed on 6 March 2024). [CrossRef]
- Ngor, P.B.; Uy, S.; Sor, R.; Chan, B.; Holway, J.; Null, S.E.; So, N.; Grenouillet, G.; Chandra, S.; Hogan, Z.S.; et al. Predicting fish species richness and abundance in the Lower Mekong Basin. Front. Ecol. Evol. 2023, 11, 1131142. [Google Scholar] [CrossRef]
- Paruch, L.; Paruch, A.M.; Eiken, H.G.; Sørheim, R. Faecal pollution affects abundance and diversity of aquatic microbial community in anthropo-zoogenically influenced lotic ecosystems. Sci. Rep. 2019, 9, 19469. [Google Scholar] [CrossRef]
- Sun, Z.; Sokolova, E.; Brittain, J.E.; Saltveit, S.J.; Rauch, S.; Meland, S. Impact of environmental factors on aquatic biodiversity in roadside stormwater ponds. Sci. Rep. 2019, 9, 5994. [Google Scholar] [CrossRef]
- Aguayo, M.; Pauchard, P.; Azócar, G.; Parra, O. Cambio del uso del suelo en el centro sur de Chile a fines del siglo XX. Entendiendo la dinámica espacial y temporal del paisaje. Rev. Chil. Hist. Nat. 2009, 82, 361–374. [Google Scholar] [CrossRef]
- Henríquez-Dole, L.; Usón, T.J.; Vicuña, S.; Henríquez, C.; Gironás, J.; Meza, F. Integrating strategic land use planning in the construction of future land use scenarios and its performance: The Maipo River Basin, Chile. Land Use Policy 2018, 78, 353–366. [Google Scholar] [CrossRef]
- Hermosilla-Palma, K.; Pliscoff, P.; Folchi, M. Sixty years of land-use and land-cover change dynamics in a global biodiversity hotspot under threat from global change. J. Land Use Sci. 2021, 16, 467–478. [Google Scholar] [CrossRef]
- Puertas, O.L.; Henríquez, C.; Meza, F.J. Assessing spatial dynamics of urban growth using an integrated land use model. Application in Santiago Metropolitan Area, 2010–2045. Land Use Policy 2014, 38, 415–425. [Google Scholar] [CrossRef]
- Vila, I.; Contreras, M.; Montecino, V.; Pizarro, J.; Adams, D.D. Rapel: A 30 years temperate reservoir. Eutrophication or contamination? Spec. Issues Adv. Limnol. 2000, 55, 31–44. [Google Scholar]
- Keppel, G.; Van Niel, K.P.; Wardell-Johnson, G.W.; Yates, C.J.; Byrne, M.; Mucina, L.; Schut, A.G.T.; Hopper, S.D.; Franklin, S.E. Refugia: Identifying and understanding safe havens for biodiversity under climate change. Glob. Ecol. Biogeogr. 2012, 21, 393–404. [Google Scholar] [CrossRef]
- Habit, E.; Belk, M.C.; Parra, O. Response of the riverine fish community to the construction and operation of a diversion hydropower plant in central Chile. Aquat. Conserv. Mar. Freshw. Ecosyst. 2007, 17, 37–49. [Google Scholar] [CrossRef]
- Bernery, C.; Bellard, C.; Courchamp, F.; Brosse, S.; Gozlan, R.E.; Jarić, I.; Teletchea, F.; Leroy, B. Freshwater Fish Invasions: A Comprehensive Review. Annu. Rev. Ecol. Evol. Syst. 2022, 53, 427–456. [Google Scholar] [CrossRef]
- Costantini, M.L.; Kabala, J.P.; Sporta Caputi, S.; Ventura, M.; Calizza, E.; Careddu, G.; Rossi, L. Biological Invasions in Fresh Waters: Micropterus salmoides, an American Fish Conquering the World. Water 2023, 15, 3796. [Google Scholar] [CrossRef]
- Hou, G.; Bai, L.; Si, S. Ecosystem resilience and stability analysis against alien species invasion patterns. Physica A Stat. Mech. Appl. 2023, 619, 128728. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).