Recent Advances in Antibiotic Degradation by Ionizing Radiation Technology: From Laboratory Study to Practical Application
Abstract
1. Introduction
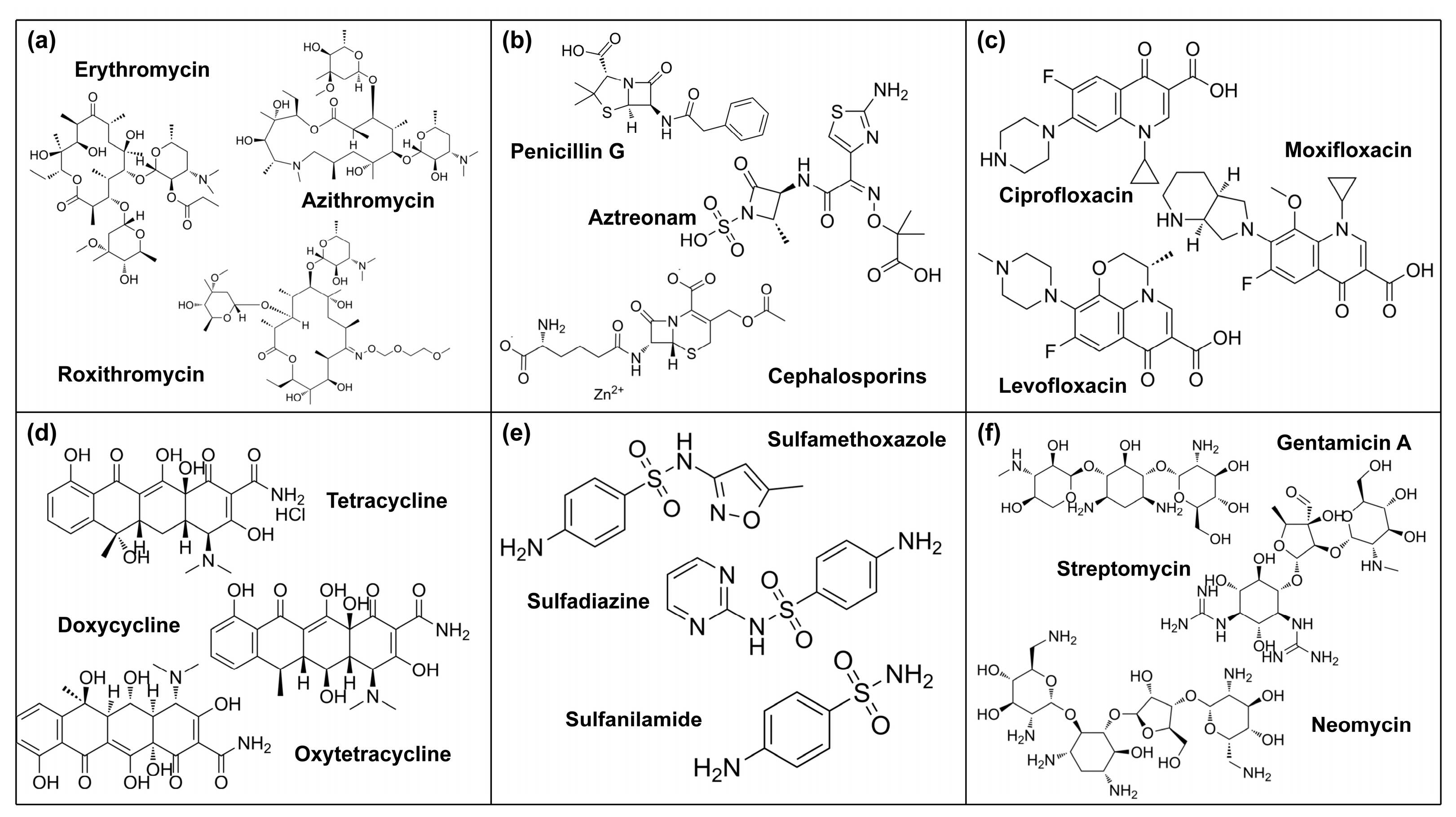
2. Commonly Used Antibiotics and Pollution
3. Ionizing Radiation Technology for Pollutant Degradation
4. Laboratory Studies of Antibiotic Degradation by Ionizing Radiation
4.1. Key Influencing Factors
4.1.1. Effect of Absorbed Dose
4.1.2. Effect of Initial Antibiotic Concentration
4.1.3. Effect of Solution pH
4.1.4. Effect of Inorganic Anions and Organic Compounds
4.1.5. Degradation Effect of Mixing Multiple Classes of Antibiotics
4.2. Removal of Antimicrobial Activity and Mineralization Effect
4.2.1. Removal of Antibacterial Activity
4.2.2. Mineralization Effects
4.3. Ionizing Radiation in Combination with Other AOPs
4.3.1. PMS-Assisted Processes
4.3.2. H2O2-Assisted Processes
4.3.3. O3-Assisted Processes
4.4. Degradation Pathways of Antibiotics
5. Practical Application of Antibiotics Degradation by Ionizing Radiation
6. Conclusions and Prospects
6.1. Conclusions
6.2. Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and Fate of Antibiotics, Antibiotic Resistant Genes (ARGs) and Antibiotic Resistant Bacteria (ARB) in Municipal Wastewater Treatment Plant: An Overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef]
- Jacobs, G.P. Irradiation of Pharmaceuticals: A Literature Review. Radiat. Phys. Chem. 2022, 190, 109795. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Ishaque, F.; Ahn, Y.-H. Fate of Antibiotic Resistant Genes in Wastewater Environments and Treatment Strategies—A Review. Chemosphere 2022, 298, 134671. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Shibata, K.; Fujimori, K.; Ohtani, Y. Rapid Removal of Tetracycline Antibiotics from Water by Coagulation-Flotation of Sodium Dodecyl Sulfate and Poly(Allylamine Hydrochloride) in the Presence of Al(III) Ions. Sep. Purif. Technol. 2017, 187, 76–83. [Google Scholar] [CrossRef]
- Mei, Y.; Zhuang, S.; Wang, J. Biochar: A Potential and Green Adsorbent for Antibiotics Removal from Aqueous Solution. Rev. Environ. Sci. Bio/Technol. 2024, 23, 1065–1103. [Google Scholar] [CrossRef]
- Ghalamara, S.; Coscueta, E.R.; Silva, S.; Brazinha, C.; Pereira, C.D.; Pintado, M.E. Integrated Ultrafiltration, Nanofiltration, and Reverse Osmosis Pilot Process to Produce Bioactive Protein/Peptide Fractions from Sardine Cooking Effluent. J. Environ. Manag. 2022, 317, 115344. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Metal Organic Framework with Coordinatively Unsaturated Sites as Efficient Fenton-like Catalyst for Enhanced Degradation of Sulfamethazine. Environ. Sci. Technol. 2018, 52, 5367–5377. [Google Scholar] [CrossRef]
- Lim, S.; Shi, J.L.; von Gunten, U.; McCurry, D.L. Ozonation of Organic Compounds in Water and Wastewater: A Critical Review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, G.; Shao, P.; Ren, W.; Li, M.; Hu, Y.; Yang, L.; Shi, H.; Luo, X. A Comparison of SMX Degradation by Persulfate Activated with Different Nanocarbons: Kinetics, Transformation Pathways, and Toxicity. Appl. Catal. B Environ. Energy 2022, 310, 121345. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of Antibiotics by Advanced Oxidation Processes: An Overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Multivalent Metal Catalysts in Fenton/Fenton-like Oxidation System: A Critical Review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wang, Y.; Wang, H.; Wu, H.; Gao, D. Efficient Photocatalytic Degradation of Sulfonamides in Wastewater Using G-C3N4 Heterostructures: A Critical Review. Environ. Technol. Innov. 2024, 36, 103854. [Google Scholar] [CrossRef]
- Ouyang, W.-Y.; Birkigt, J.; Richnow, H.H.; Adrian, L. Anaerobic Transformation and Detoxification of Sulfamethoxazole by Sulfate-Reducing Enrichments and Desulfovibrio Vulgaris. Environ. Sci. Technol. 2020, 55, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Kang, P.; Zhang, T.; Ma, Y.; Guo, X.; Wan, J.; Wang, Y. Effect of Minute Amounts of Arsenic on the Sulfamethoxazole Removal and Microbial Community Structure via the SBR System. Water Sci. Technol. 2023, 87, 423–435. [Google Scholar] [CrossRef]
- Huang, X.; Wen, D.; Wang, J. Radiation-Induced Degradation of Sulfonamide and Quinolone Antibiotics: A Brief Review. Radiat. Phys. Chem. 2024, 215, 111373. [Google Scholar] [CrossRef]
- Khan, N.A.; Ahmed, S.; Farooqi, I.H.; Ali, I.; Vambol, V.; Changani, F.; Yousefi, M.; Vambol, S.; Khan, S.U.; Khan, A.H. Occurrence, Sources and Conventional Treatment Techniques for Various Antibiotics Present in Hospital Wastewaters: A Critical Review. TrAC Trends Anal. Chem. 2020, 129, 115921. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Irradiation Treatment of Pharmaceutical and Personal Care Products (PPCPs) in Water and Wastewater: An Overview. Radiat. Phys. Chem. 2016, 125, 56–64. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R.; Chu, L. The Occurrence, Distribution and Degradation of Antibiotics by Ionizing Radiation: An Overview. Sci. Total Environ. 2019, 646, 1385–1397. [Google Scholar] [CrossRef]
- Huang, F.; An, Z.; Moran, M.; Liu, F. Recognition of Typical Antibiotic Residues in Environmental Media Related to Groundwater in China (2009–2019). J. Hazard. Mater. 2020, 399, 122813. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the Aquatic Environment—A Review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Guieu, B.; Jourdan, J.-P.; Dreneau, A.; Willand, N.; Rochais, C.; Dallemagne, P. Desirable Drug–Drug Interactions or When a Matter of Concern Becomes a Renewed Therapeutic Strategy. Drug Discov. Today 2020, 26, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Christian, J.S. The Quinolone Antibiotics. Prim. Care Update OB/GYNS 1996, 3, 87–92. [Google Scholar] [CrossRef]
- Puhlmann, N.; Olsson, O.; Kümmerer, K. Transformation Products of Sulfonamides in Aquatic Systems: Lessons Learned from Available Environmental Fate and Behaviour Data. Sci. Total Environ. 2022, 830, 154744. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Wu, Y.; Li, S.; Hu, J.; Sun, W.; Ni, J. Profiles, Drivers, and Prioritization of Antibiotics in China’s Major Rivers. J. Hazard. Mater. 2024, 477, 135399. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Ding, J.; Liu, X. Prioritization of Pharmaceuticals in Water Environment in China Based on Environmental Criteria and Risk Analysis of Top-Priority Pharmaceuticals. J. Environ. Manag. 2020, 253, 109732. [Google Scholar] [CrossRef]
- Lyu, J.; Yang, L.; Zhang, L.; Ye, B.; Wang, L. Antibiotics in Soil and Water in China—A Systematic Review and Source Analysis. Environ. Pollut. 2020, 266, 115147. [Google Scholar] [CrossRef] [PubMed]
- Townsend-Rose, C.; Buggey, T.; Ivory, J.; Stefanov, K.D.; Holland, A.D. Ionising Radiation Effects in a Soft X-Ray CMOS Image Sensor. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2024, 1059, 169011. [Google Scholar] [CrossRef]
- Chu, L.; Chen, D.; Wang, J.; Yang, Z.; Shen, Y. Degradation of Antibiotics and Antibiotic Resistance Genes in Erythromycin Fermentation Residues Using Radiation Coupled with Peroxymonosulfate Oxidation. Waste Manag. 2019, 96, 190–197. [Google Scholar] [CrossRef]
- Chu, L.; Zhuang, S.; Wang, J. Degradation Kinetics and Mechanism of Penicillin G in Aqueous Matrices by Ionizing Radiation. Radiat. Phys. Chem. 2018, 145, 34–38. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J. Degradation of Norfloxacin in Aqueous Solution by Ionizing Irradiation: Kinetics, Pathway and Biological Toxicity. Chem. Eng. J. 2020, 395, 125095. [Google Scholar] [CrossRef]
- Yao, B.; Qin, T.; Zhao, C.; Zhou, Y. Degradation of Sulfanilamide in Aqueous Solution by Ionizing Radiation: Performance and Mechanism. Environ. Pollut. 2023, 338, 122681. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J.; Chen, C.; Shen, Y.; He, S.; Wojnárovits, L.; Takács, E.; Zhang, Y. Abatement of Antibiotics and Antimicrobial Resistance Genes from Cephalosporin Fermentation Residues by Ionizing Radiation: From Lab-Scale Study to Full-Scale Application. J. Clean. Prod. 2021, 325, 129334. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J.; He, S.; Chen, C.; Wojnárovits, L.; Takács, E. Treatment of Pharmaceutical Wastewater by Ionizing Radiation: Removal of Antibiotics, Antimicrobial Resistance Genes and Antimicrobial Activity. J. Hazard. Mater. 2021, 415, 125724. [Google Scholar] [CrossRef] [PubMed]
- Ranković, B.; Sagatova, A.; Vujčić, I.; Mašić, S.; Veljović, Đ.; Pavićević, V.; Kamberović, Ž. Utilization of Gamma and E-Beam Irradiation in the Treatment of Waste Sludge from a Drinking Water Treatment Plant. Radiat. Phys. Chem. 2020, 177, 109174. [Google Scholar] [CrossRef]
- Mazzei, H.G.; Specchia, S. Latest Insights on Technologies for the Treatment of Solid Medical Waste: A Review. J. Environ. Chem. Eng. 2023, 11, 109309. [Google Scholar] [CrossRef]
- Chen, D.; Chu, L.; Wang, J.; Yang, Z.; Yang, Q.; Shen, Y. Degradation of Antibiotic Cephalosporin c in Aqueous Solution and Elimination of Antimicrobial Activity by Gamma Irradiation. Chem. Eng. J. 2019, 374, 1102–1108. [Google Scholar] [CrossRef]
- Sági, G.; Bezsenyi, A.; Kovács, K.; Klátyik, S.; Darvas, B.; Székács, A.; Mohácsi-Farkas, C.; Takács, E.; Wojnárovits, L. Radiolysis of Sulfonamide Antibiotics in Aqueous Solution: Degradation Efficiency and Assessment of Antibacterial Activity, Toxicity and Biodegradability of Products. Sci. Total Environ. 2018, 622–623, 1009–1015. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Radiation-Induced Degradation of Sulfamethoxazole in the Presence of Various Inorganic Anions. Chem. Eng. J. 2018, 351, 688–696. [Google Scholar] [CrossRef]
- Alsager, O.A.; Alnajrani, M.N.; Alhazzaa, O. Decomposition of Antibiotics by Gamma Irradiation: Kinetics, Antimicrobial Activity, and Real Application in Food Matrices. Chem. Eng. J. 2018, 338, 548–556. [Google Scholar] [CrossRef]
- Takács, E.; Wang, J.; Chu, L.; Tóth, T.; Kovács, K.; Bezsenyi, A.; Szabó, L.; Homlok, R.; Wojnárovits, L. Elimination of Oxacillin, Its Toxicity and Antibacterial Activity by Using Ionizing Radiation. Chemosphere 2022, 286, 131467. [Google Scholar] [CrossRef]
- Jang, H.; Kim, T.-H.; Jeon, J. Degradation of Micropollutants by Gamma Irradiation: Insight of Transformation Product Formation Explored by Suspect and Non-Target Screening Using LC-HRMS. J. Environ. Chem. Eng. 2023, 12, 111659. [Google Scholar] [CrossRef]
- Chu, L.; Chen, D.; Wang, J.; Yang, Z.; Yang, Q.; Shen, Y. Degradation of Antibiotics and Inactivation of Antibiotic Resistance Genes (ARGs) in Cephalosporin c Fermentation Residues Using Ionizing Radiation, Ozonation and Thermal Treatment. J. Hazard. Mater. 2020, 382, 121058. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Chung, B.Y.; Lee, K.-B.; Lee, G.-H.; Hwang, S.A. Decomposition Reaction of the Veterinary Antibiotic Ciprofloxacin Using Electron Ionizing Energy. Chemosphere 2014, 117, 158–163. [Google Scholar] [CrossRef]
- Tominaga, F.K.; Boiani, N.F.; Silva, T.T.; Gomes, J.; Lebre, D.T.; Leo, P.; Borrely, S.I. Electron Beam Irradiation Applied for the Detoxification and Degradation of Single Ciprofloxacin Aqueous Solution and Multiclass Pharmaceutical Quaternary Mixture. Sep. Purif. Technol. 2022, 307, 122818. [Google Scholar] [CrossRef]
- Sayed, M.; Shah, L.A.; Khan, J.A.; Shah, N.S.; Khan, H.M.; Khan, R.A.; Khan, A.R.; Khan, A.M. Hydroxyl Radical Based Degradation of Ciprofloxacin in Aqueous Solution. Journal of the Chilean Chemical Society 2016, 61(2), 2949–2953. [Google Scholar] [CrossRef]
- Tegze, A.; Sági, G.; Kovács, K.; Tóth, T.; Takács, E.; Wojnárovits, L. Radiation Induced Degradation of Ciprofloxacin and Norfloxacin: Kinetics and Product Analysis. Radiat. Phys. Chem. 2019, 158, 68–75. [Google Scholar] [CrossRef]
- Salem, I.B.; Mezni, M.; Khamassi, M.A.; Lagha, A.; Hosni, F.; Saidi, M. Influence of Ionizing Radiation on the Stability of Clarithromycin Antibiotics. Radiat. Phys. Chem. 2017, 145, 143–147. [Google Scholar] [CrossRef]
- Bujak, I.T.; Pocrnić, M.; Blažek, K.; Bojanić, K.; Trebše, P.; Lebedev, A.T.; Galić, N. Radiation-Induced Degradation of Doxazosin: Role of Reactive Species, Toxicity, Mineralization and Degradation Pathways. J. Water Process Eng. 2022, 51, 103401. [Google Scholar] [CrossRef]
- Salem, S.B.; Mezni, M.; Errami, M.; Amine, K.M.; Salghi, R.; Ismat, H.A.; Chakir, A.; Hammouti, B.; Messali, M.; Fattouch, S. Degradation of Enrofloxacin Antibiotic under Combined Ionizing Radiation and Biological Removal Technologies. Int. J. Electrochem. Sci. 2015, 10, 3613–3622. [Google Scholar] [CrossRef]
- Szabó, L.; Szabó, J.; Illés, E.; Kovács, A.; Belák, Á.; Mohácsi-Farkas, C.; Takács, E.; Wojnárovits, L. Electron Beam Treatment for Tackling the Escalating Problems of Antibiotic Resistance: Eliminating the Antimicrobial Activity of Wastewater Matrices Originating from Erythromycin. Chem. Eng. J. 2017, 321, 314–324. [Google Scholar] [CrossRef]
- Shen, Y.; Chu, L.; Zhuan, Y.; Xiang, X.; Sun, H.; Wang, J. Degradation of Antibiotics and Antibiotic Resistance Genes in Fermentation Residues by Ionizing Radiation: A New Insight into a Sustainable Management of Antibiotic Fermentative Residuals. J. Environ. Manag. 2019, 232, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Muneer, M.; Kanjal, M.I.; Saeed, M.; Jamal, M.A.; ul Haq, A.; Iqbal, M.; ul Haq, E.; Ali, S. Degradation of Moxifloxacin by Ionizing Radiation and Toxicity Assessment. Z. Für Phys. Chem. 2021, 235, 1629–1643. [Google Scholar] [CrossRef]
- Sayed, M.; Khan, J.A.; Shah, L.A.; Shah, N.S.; Khan, H.M.; Rehman, F.; Khan, A.R.; Khan, A.M. Degradation of Quinolone Antibiotic, Norfloxacin, in Aqueous Solution Using Gamma-Ray Irradiation. Environ. Sci. Pollut. Res. 2016, 23, 13155–13168. [Google Scholar] [CrossRef]
- Tegze, A.; Sági, G.; Kovács, K.; Homlok, R.; Tóth, T.; Mohácsi-Farkas, C.; Wojnárovits, L.; Takács, E. Degradation of Fluoroquinolone Antibiotics during Ionizing Radiation Treatment and Assessment of Antibacterial Activity, Toxicity and Biodegradability of the Products. Radiat. Phys. Chem. 2018, 147, 101–105. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Homlok, R.; Kovács, K.; Bezsenyi, A.; Takács, E. Ionizing Radiation Induced Removal of Ofloxacin, Abatement of Its Toxicity and Antibacterial Activity in Various Water Matrices. Appl. Sci. 2023, 13, 7211. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Wang, J.; Chu, L.; Tóth, T.; Kovács, K.; Bezsenyi, A.; Szabó, L.; Homlok, R.; Takács, E. Matrix Effect in the Hydroxyl Radical Induced Degradation of β-Lactam and Tetracycline Type Antibiotics. Radiat. Phys. Chem. 2022, 193, 109980. [Google Scholar] [CrossRef]
- Ji, D.; Song, W.; Liu, X.; Cui, S.; Zhang, Y. Discussion on the Radiolysis Rate of Organic Substances in Oxytetracycline Pharmaceutical Wastewater. J. Radioanal. Nucl. Chem. 2023, 332, 2231–2237. [Google Scholar] [CrossRef]
- Ardestani, S.B.; Akhavan, A.; Kazeminejad, H.; Ahmadi-Roshan, M.; Roozbahani, A. Ionizing Radiation Effect on the Removing of Antibiotic Pollutants from Pharmaceutical Wastewater: Identification of Radio-Degradation Products. J. Radioanal. Nucl. Chem. 2024, 333, 4523–4541. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J. Degradation of Antibiotics in Activated Sludge by Ionizing Radiation: Effect of Adsorption Affinity of Antibiotics. Chem. Eng. J. 2023, 468, 143821. [Google Scholar] [CrossRef]
- Wei, L.; Chu, L.; Wang, J.; Yang, Q. Radiolytic Degradation of β-Lactam and Tetracycline Antibiotics in the Presence of Protein. J. Mol. Liq. 2022, 364, 119965. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J. Degradation Performance and Mechanism of Penicillin G in Aqueous Solution by Ionizing Radiation. J. Clean. Prod. 2021, 328, 129625. [Google Scholar] [CrossRef]
- Zhu, F.; Pan, J.; Zou, Q.; Wu, M.; Wang, H.; Xu, G. Electron Beam Irradiation of Typical Sulfonamide Antibiotics in the Aquatic Environment: Kinetics, Removal Mechanisms, Degradation Products and Toxicity Assessment. Chemosphere 2021, 274, 129713. [Google Scholar] [CrossRef] [PubMed]
- Zhuan, R.; Wang, J. Degradation of Sulfamethoxazole by Ionizing Radiation: Kinetics and Implications of Additives. Sci. Total Environ. 2019, 668, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Sági, G.; Csay, T.; Pátzay, G.; Csonka, E.; Wojnárovits, L.; Takács, E. Oxidative and Reductive Degradation of Sulfamethoxazole in Aqueous Solutions: Decomposition Efficiency and Toxicity Assessment. J. Radioanal. Nucl. Chem. 2014, 301, 475–482. [Google Scholar] [CrossRef]
- Sági, G.; Kovács, K.; Bezsenyi, A.; Csay, T.; Takács, E.; Wojnárovits, L. Enhancing the Biological Degradability of Sulfamethoxazole by Ionizing Radiation Treatment in Aqueous Solution. Radiat. Phys. Chem. 2016, 124, 179–183. [Google Scholar] [CrossRef]
- Sági, G.; Szabacsi, K.; Szabó, L.; Homlok, R.; Kovács, K.; Mohácsi-Farkas, C.; Pillai, S.D.; Takács, E.; Wojnárovits, L. Influence of Ionizing Radiation on the Antimicrobial Activity of the Antibiotics Sulfamethoxazole and Trimethoprim. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2018, 53, 687–693. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, T.-H.; Cha, S.M.; Yu, S. Degradation of Sulfamethoxazole by Ionizing Radiation: Identification and Characterization of Radiolytic Products. Chem. Eng. J. 2017, 313, 556–566. [Google Scholar] [CrossRef]
- He, H.; Wang, S.; Wang, J. Radiolytic Degradation of Thiophene: Performance, Pathway and Toxicity Evaluation. Radiat. Phys. Chem. 2021, 189, 109738. [Google Scholar] [CrossRef]
- Kovács, K.; Székely, D.; Tegze, A.; Homlok, R.; Bezsenyi, A.; Wojnárovits, L. Gamma and Pulse Radiolysis of Trimethoprim in Aqueous Solutions: Intermediate Radicals, Rate of Oxidation, Toxicity. J. Water Process Eng. 2024, 69, 106743. [Google Scholar] [CrossRef]
- Basfar, A.A.; Khan, H.M.; Alshahrani, A.A.; Cooper, W.J. Radiation Induced Decomposition of Methyl Tert-Butyl Ether in Water in Presence of Chloroform: Kinetic Modelling. Water Res. 2005, 39, 2085–2095. [Google Scholar] [CrossRef]
- Changotra, R.; Rajput, H.; Guin, J.P.; Dhir, A. Comparative Assessment of Application of Ionizing Radiations in Degradation of Amoxicillin Trihydrate (AMT) in Aqueous Solutions. Chem. Eng. J. 2021, 421, 127847. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J. Degradation of Antibiotic Cephalosporin c in Different Water Matrices by Ionizing Radiation: Degradation Kinetics, Pathways, and Toxicity. Sci. Total Environ. 2021, 791, 148253. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Wang, J.; Chen, C.; He, S.; Wojnárovits, L.; Takács, E. Advanced Treatment of Antibiotic Wastewater by Ionizing Radiation Combined with Peroxymonosulfate/H2O2 Oxidation. J. Clean. Prod. 2021, 321, 128921. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Shen, Y. Enhanced Performance of Anaerobic Digestion of Cephalosporin c Fermentation Residues by Gamma Irradiation-Induced Pretreatment. J. Hazard. Mater. 2020, 384, 121335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, H.; Wang, J.; Zhang, Y. Degradation of Carbamazepine by Combined Radiation and Persulfate Oxidation Process. Radiat. Phys. Chem. 2020, 170, 108639. [Google Scholar] [CrossRef]
- He, J.; Ye, Q.; Zhu, Y.; Yang, M.; Zhao, L. Enhanced Degradation Performance and Mineralization of Ciprofloxacin by Ionizing Radiation Combined with G-C3N4/CDs. Radiat. Phys. Chem. 2023, 208, 110958. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Enhanced Antibiotic Degradation and Hydrogen Production of Deacetoxycephalosporin c Fermentation Residue by Gamma Radiation Coupled with Nano Zero-Valent Iron. J. Hazard. Mater. 2021, 424, 127439. [Google Scholar] [CrossRef]
- Chu, L.; Zhuan, R.; Chen, D.; Wang, J.; Shen, Y. Degradation of Macrolide Antibiotic Erythromycin and Reduction of Antimicrobial Activity Using Persulfate Activated by Gamma Radiation in Different Water Matrices. Chem. Eng. J. 2019, 361, 156–166. [Google Scholar] [CrossRef]
- Zhuan, R.; Wang, J. Enhanced Mineralization of Sulfamethoxazole by Gamma Radiation in the Presence of Fe3O4 as Fenton-like Catalyst. Environ. Sci. Pollut. Res. 2019, 26, 27712–27725. [Google Scholar] [CrossRef]
- Zhuan, R.; Wang, J. Enhanced Degradation and Mineralization of Sulfamethoxazole by Integrating Gamma Radiation with Fenton-like Processes. Radiat. Phys. Chem. 2020, 166, 108457. [Google Scholar] [CrossRef]
- Chu, L.; Yin, W.; Dierxiati, A.; Dong, L.; Chen, Q. Degradation of Steroid Androgen in Aqueous Solution Using Ionizing Radiation and Thermal Activation Persulfate Processes. Radiat. Phys. Chem. 2024, 229, 112472. [Google Scholar] [CrossRef]
- Yao, B.; Zhou, Y. Enhanced Degradation of Sulfathiazole by Ionizing Radiation Activated Persulfate and Periodate: Performances and Mechanisms. Chem. Eng. J. 2024, 496, 154284. [Google Scholar] [CrossRef]
- Liu, N.; Lei, Z.-D.; Wang, T.; Wang, J.-J.; Zhang, X.-D.; Xu, G.; Tang, L. Radiolysis of Carbamazepine Aqueous Solution Using Electron Beam Irradiation Combining with Hydrogen Peroxide: Efficiency and Mechanism. Chem. Eng. J. 2016, 295, 484–493. [Google Scholar] [CrossRef]
- Kabasa, S.; Wang, S.; Sun, Y.; Wang, J.; Bulka, S. Degradation of Hydroxychloroquine from Aqueous Solutions under Fenton-Assisted Electron Beam Treatment. Processes 2024, 12, 2860. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Wang, J. Fe2+ Enhancing Sulfamethazine Degradation in Aqueous Solution by Gamma Irradiation. Radiat. Phys. Chem. 2013, 96, 81–87. [Google Scholar] [CrossRef]
- Lei, Y.; Zhou, Y.; Zhang, S.; Zhou, S.; Ma, N.; Liu, H.; Liu, Y.; Zhang, J.; Miao, H.; Cao, L. Nitrogen-Doped Bimetallic MOFs Derivatives for Efficient Ionizing Radiation Catalytic Degradation of Chloramphenicol. Sep. Purif. Technol. 2023, 326, 124785. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, D.; Chu, L.; Wang, J. Enhancement of Ionizing Radiation-Induced Catalytic Degradation of Antibiotics Using Fe/c Nanomaterials Derived from Fe-Based MOFs. J. Hazard. Mater. 2020, 389, 122148. [Google Scholar] [CrossRef]
- Chen, X.; Zhuan, R.; Wang, J. Assessment of Degradation Characteristic and Mineralization Efficiency of Norfloxacin by Ionizing Radiation Combined with Fenton-like Oxidation. J. Hazard. Mater. 2021, 404, 124172. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (⋅OH/⋅O−) in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Degradation of Sulfamethazine by Gamma Irradiation in the Presence of Hydrogen Peroxide. J. Hazard. Mater. 2013, 250–251, 99–105. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Electron Beam Technology Coupled to Fenton Oxidation for Advanced Treatment of Dyeing Wastewater: From Laboratory to Full Application. ACS ES&T Water 2022, 2, 852–862. [Google Scholar]
- Wang, S.; Wang, J.; Chen, C.; He, S.; Hu, J.; Zhang, Y. First Full-Scale Application of Electron Beam Technology for Treating Dyeing Wastewater (30,000 M3/D) in China. Radiat. Phys. Chem. 2022, 196, 110136. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Chen, C.; Hu, J.; He, S.; Zhou, Y.; Zhu, H.; Wang, X.; Hu, D.; Lin, J. Treatment of Hospital Wastewater by Electron Beam Technology: Removal of COD, Pathogenic Bacteria and Viruses. Chemosphere 2022, 308, 136265. [Google Scholar] [CrossRef]
- Fang, L.; Chu, L.; Wang, J.; Yang, Q. Treatment of Polyacrylamide-Containing Wastewater by Ionizing Radiation: Efficient Reduction of Viscosity and Degradation of Polyacrylamide. Radiat. Phys. Chem. 2022, 202, 110547. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J. Treatment of Oil-Field Produced Wastewater by Electron Beam Technology: Demulsification, Disinfection and Oil Removal. J. Clean. Prod. 2022, 378, 134532. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J. Pretreatment of Alkali/Surfactant/Polymer (ASP)-Flooding Produced Wastewater by Electron Beam Radiation to Improve Oil-Water Separation. Chemosphere 2024, 351, 141252. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X. Removal of Antibiotic Resistance Genes (ARGs) in Various Wastewater Treatment Processes: An Overview. Crit. Rev. Environ. Sci. Technol. 2020, 52, 571–630. [Google Scholar] [CrossRef]
- Shen, Y.; Zhuan, R.; Chu, L.; Xiang, X.; Sun, H.; Wang, J. Inactivation of Antibiotic Resistance Genes in Antibiotic Fermentation Residues by Ionizing Radiation: Exploring the Development of Recycling Economy in Antibiotic Pharmaceutical Factory. Waste Manag. 2019, 84, 141–146. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J. Enhanced Medium-Chain Fatty Acids Production from Cephalosporin c Antibiotic Fermentation Residues by Ionizing Radiation Pretreatment. J. Hazard. Mater. 2022, 440, 129714. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chu, L.; Ding, M. Comparison of the Efficiency of Gamma Irradiation and Pyrolysis on the Reduction of Antibiotic and Cephalosporin Resistance Gene from Cephalosporin Fermentation Residues. Radiat. Phys. Chem. 2020, 176, 109059. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Sable, S.; Gamal El-Din, M. Advanced Oxidation Processes for the Degradation of Dissolved Organics in Produced Water: A Review of Process Performance, Degradation Kinetics and Pathway. Chem. Eng. J. 2022, 429, 132492. [Google Scholar] [CrossRef]

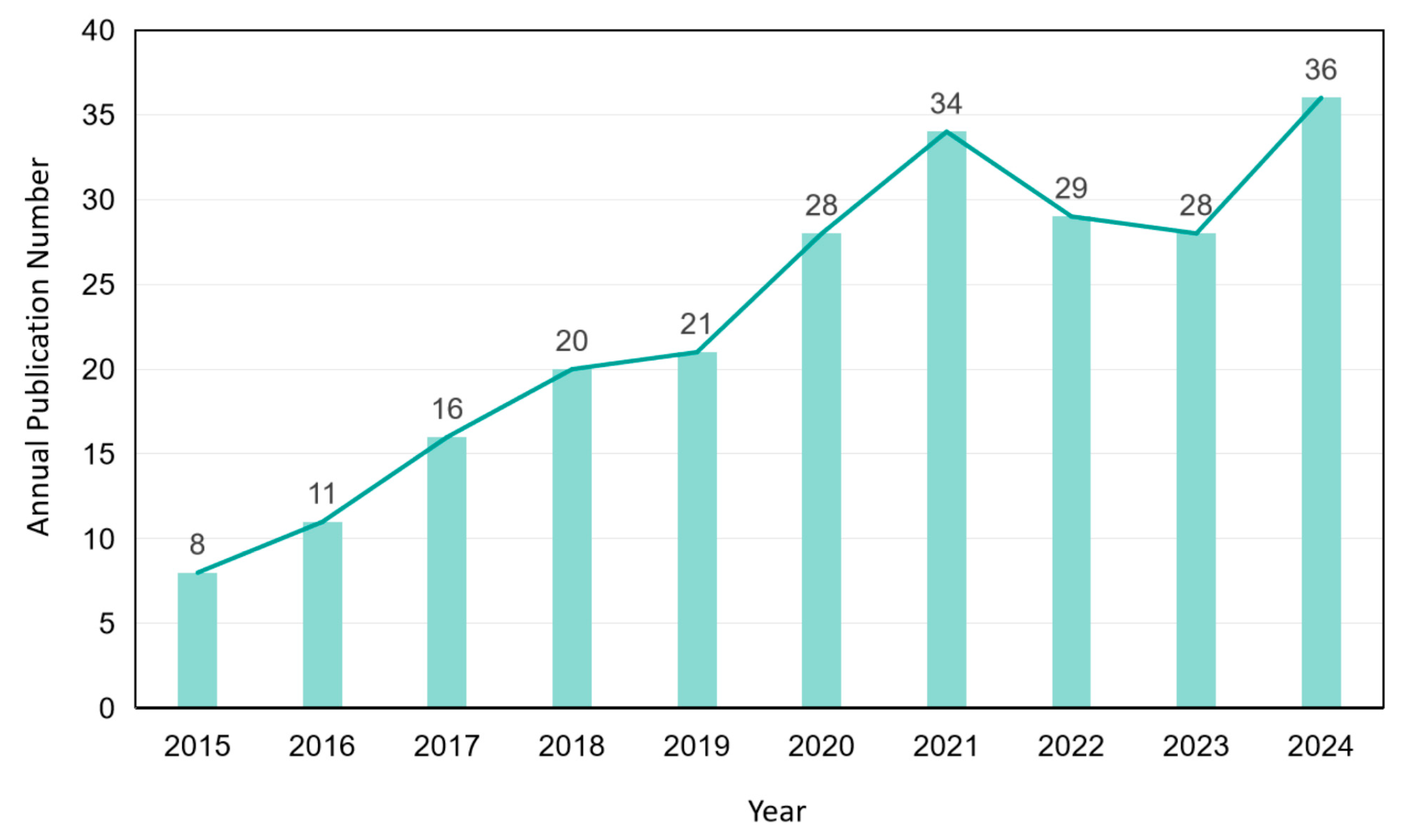



| Antibiotics | Initial Concentration | Radiation Source | Matrix | Removal Efficiency | Mineralization Efficiency | References |
|---|---|---|---|---|---|---|
| Amoxicillin, Doxycycline, Ciprofloxacin | 50 µM | 60Co | Aqueous solution; food matrix pH 6.8 0–7 kGy | ≥90% | Amoxicillin, Ciprofloxacin: 100% Doxycycline: 85% | [39] |
| Benzoxacillin, Oxacillin | 1.0 × 10−4 mol/L (40 mg/L) | 60Co, EB | purified water pH 7.7–9.2 0.5–4 kGy | ≈100% | TOC 25% COD 50% | [40] |
| Carbamazepine (CBZ) Cetirizine (CTZ) Tramadol (TRM) | 10 mg/L | 60Co | Aqueous solution, wastewater 0.1–5 kGy | 100% | 40% | [41] |
| Cephalosporin C (CEP-C) | 0.02–0.20 mM | 60Co | Aqueous solution; groundwater 0.5–20 kGy | 100% | 5–22% | [36] |
| Cephalosporin C (CEP-C) | 216 ± 18 mg/L | 60Co | pH 5.5 ± 0.1 25–150 kGy | 85.5% ARGs 74.2% | -- | [42] |
| Chloramphenicol (CAP) | 100 mg/L | EB | Aqueous solution 1, 5, 10 kGy | 32.4% (1 kGy) 86.9% (5 kGy) 100% (10 kGy) | 4.6% (1 kGy) 12.1% (5 kGy) 17.1% (10 kGy) | [43] |
| Ciprofloxacin (CIP) | 11.4 mg/L 0.789 mg/L (mixed system) | EB | Ultrapure water, natural water pH 4.22–7.00 1.0–5.0 kGy | ≥95.86%, 81.25–99.48% (natural water) | 19.04–37.62% | [44] |
| Ciprofloxacin (CIP) | 4.6–17.9 mg/L | 60Co | 0–870 Gy | 90–100% (0.5–25 kGy) | -- | [45] |
| Ciprofloxacin, Norfloxacin | 0.1 mmol/L | 60Co | Aqueous solution 0.2–8 kGy | 70% (0.5 kGy), 100% (2 kGy) | -- | [46] |
| Clarithromycin (CLA) | 25 mg/mL (as Zeclar® powder) | 60Co | 2, 5, 25 kGy | significant decrease in antibacterial activity (25 kGy) | -- | [47] |
| Doxazosin (DOX) | 10 mg/L | 60Co | Aqueous solution 200 Gy | ≈100% | ≈60% | [48] |
| Enrofloxacin | 0.02 mg/mL | 60Co | Aqueous solution | -- | 68.2–98% | [49] |
| Erythromycin (ERY) | 0.5 mM, 1 mg/mL | EB | Aqueous solution pH 9.0 0.4–200 kGy | ≈100% | Antimicrobial activity was eliminated at 80 Gy | [50] |
| Erythromycin (ERY) Sulfamethoxazole (SMX) Tetracycline (TC) | ERY: 25.1 mg/L SMX: 33 µg/L TC: 14 µg/L | 60Co | Pharmaceutical wastewater pH 7.6 1.0–50 kGy | 82.6–100% | 10.3% (COD) | [33] |
| Erythromycin A | 60Co | Antibiotic fermentation residues (containing erythromycin A, ermB/ermF genes, resistant bacteria) 5–10 kGy | 86% (10 kGy) | Resistance genes (ermB/ermF): 89–98% (10 kGy) Resistant bacteria: >99% inactivation (10 kGy) | [51] | |
| Moxifloxacin | 50–100 mg/L | 137Cs | Aqueous solution 0.3–4 kGy | 94.01% (50 mg/L), 88.30% (100 mg/L) | 65–73% (COD) | [52] |
| Norfloxacin | 3.4–16.1 mg/L | 60Co | Aqueous solution pH 6.5 Dose rate: 290, 212, 97 Gy/h | 76.10–91.0% | -- | [53] |
| Norfloxacin (NOR) Ciprofloxacin (CIP) | 0.1 mmol/L | 60Co | Aqueous solution, 0.5–6 kGy | ≥99% | 40% | [54] |
| Ofloxacin | 0.1 mmol/L | 60Co | Aqueous solution, pH 7.6 0–4 kGy | Loss of antimicrobial activity at 2 kGy | -- | [55] |
| Oxacillin, Cloxacillin; Tetracycline, Chlortetracycline | 0.1 mmol/L (40–48 mg/L) | 60Co | Pure water, tap water, synthetic wastewater, purified wastewater; pH ~7 0.5–4 kGy | Achieve biodegradable products (2–2.5 kGy) | ~40–50% (COD) 15% (TOC) | [56] |
| Oxytetracycline (OTC) | 1367 ± 16 mg/L | EB | Pharmaceutical wastewater pH 5–7 0–300 kGy | 96.4% (0–20 kGy) | -- | [57] |
| Oxytetracycline, Enrofloxacin, Erythromycin, Sulfamethoxazole | Synthetic wastewater (10–100 ppm), actual wastewater (unspecified) | EB | Aqueous solution 10–50 kGy | Synthesis effluent (25 kGy): >90% Actual wastewater (25 kGy): erythromycin 100%, enrofloxacin 90% | -- | [58] |
| Penicillin (PEG), Sulfonamide (SMX) Norfloxacin (NOF) Oxytetracycline (OTC) | 20 mg/L | 60Co | 10–50 kGy sludge pH 7 | SMX, PEG ≥97% NOF 62% OTC 77% | [59] | |
| Penicillin G (PEG), Cephalosporin C, Oxytetracycline, Tetracycline, Hydrochloride | 0.05–0.1 mM | 60Co | Aqueous solution + Bovine serum albumin (BSA) 0.1–2.0 kGy | 100% | - | [60] |
| Penicillin G (PG) | 25–200 mg/L | 60Co | Aqueous solution, wastewater pH 3.38–9.53 0.2–2 kGy | 80.2–100% | 8.8–16.2% | [61] |
| Sulfamerazine (SMR) Sulfadiazine (SDZ) Sulfapyridine (SPD) | 50 mg/L | EB | Ultrapure water (UW), surface water (SW), gray water (GW) pH 3.0–10.0 0–8 kGy | ≈100% | SMR, SDZ < 10% SPD 45.2% | [62] |
| Sulfamethoxazole (SMX) | 5–40 mg/L | 60Co | Aqueous solution, wastewater pH 3.09–10.96 0.2–1.5 kGy | 76.2–100% | 12.4–21.4% | [63] |
| Sulfamethoxazole (SMX) | 0.1 mmol/L (25.3 mg/L) | 60Co | Aqueous solution pH 4 and natural pH (~5.8) 5 kGy | ≈100% (5 kGy) | Complete mineralized (COD & TOC) | [64] |
| Sulfamethoxazole (SMX) | 0.04 mM | 60Co | Aqueous solution pH 7.11 1 kGy | 99.6% (1 kGy) | 12.8% (1 kGy) | [38] |
| Sulfamethoxazole (SMX) | 0.1 mmol/L (25.3 mg/L) | 60Co | Aqueous solution 0.4–10 kGy | 90% 0.4 kGy | 15% (2.5 kGy) | [65] |
| Sulfamethoxazole, Trimethoprim | 500 mmol/L (SMX), 100 mmol/L (TMP) | 60Co | Aqueous solution | 100% (SMX, 0.2 kGy) (TMP, 0.8 kGy) (SMX + TMP, 10 kGy) | 5–10% (TOC) 15–37% (COD) | [66] |
| Sulfamethoxazole, SMX | 394.82 μM | 60Co | Aqueous solution 0.1–5.0 kGy | 88.6% (5 kGy) | 17.6% (5 kGy) | [67] |
| Sulfanilamide (SA), Sulfaguanidine (SGD), Sulfathiazole (STZ), Sulfamethoxazole (SMX) | 100 μM | 60Co | Aqueous solution 0.5–2.5 kGy | 100% (1.5 kGy) | 8% (1.5 kGy) | [37] |
| Thiophene | 5 mg/L | 60Co | coal chemical waste water pH 9 5 kGy | 100% | - | [68] |
| Trimethoprim (TMP) | 0.1–0.3 mmol/L | 60Co | Pure water, tap water, synthetic wastewater, purified wastewater pH 9–9.5 0.25–6.0 kGy | 15–100% | 3–30% | [69] |
| Antibiotics | Initial Concentration | Radiation Source | Synergistic Technology | Experimental Substrate | Removal Efficiency | Mineralization Efficiency | References |
|---|---|---|---|---|---|---|---|
| Carbamazepine (CBZ) | 42.32 μM | 60Co | persulfate (PS) | CBZ solution, pH 6.5–8.5, PS 0.5–2.0 mM, 1 kGy | -- | 34.1% pH 6.5 with 1.5 mM PS | [75] |
| Cephalosporin C (CEP-C) | 10–200 mg/L | 60Co | H2O2 and PMS | Aqueous solution, groundwater; wastewater pH 3.5–9.2 0.2–2 kGy | 100% | 8.0–46.2% | [72] |
| Ciprofloxacin (CIP) | 50–200 mg/L | EB | g-C3N4/CDs | Deionized water, tap water, lake water 0–25 kGy | 63.8–77.4% | 18.1–69.0% | [76] |
| Deacetoxycephalosporin C (DAOC) | 871.7 mg/L in antibiotic fermentation residue (AFR) | 60Co | nano zero-valent iron (nZVI) | AFR pH 7.0 50 kGy | 98.60% | 9.1–19.7% | [77] |
| Deacetyloxy cephalosporin C (DOCPC) | 810–920 mg/kg wet residue | 60Co, EB | H2O2 | High concentration of organic matter, about 91% water. pH 3.5–4.0 5–50 kGy | 92.9–97.5% | 61.5 ± 10.3% (first) 75–84% (secondary radiation) | [32] |
| Erythromycin (ERY) | 599 ± 58 mg/L | 60Co | PMS | Erythromycin fermentation residue pH 5.2–6.8 25, 50 kGy | ERY 49–55% ARGs 96.3–99.6% | -- | [78] |
| Erythromycin (ERY) | 538 mg/kg wet residue (7.5 mg/g total solids, TS) | 60Co | H2O2 | Erythromycin thiocyanate fermentation residue pH 5.86–9.13 10, 30 kGy | ERY 56% ARGs 90–95% (1.0–1.3 log) | -- | [51] |
| Erythromycin (ERY) | 0.1 mM (20 mg/L) | 60Co | Activated persulfate (PS) | Deionized water, groundwater, treated wastewater, pH 6.5–8.5 10 kGy | antimicrobial activity 100% eliminated | 3.4–52% | [28] |
| Sulfamethoxazole (SMX) | 5–30 mg/L | 60Co | Fenton-like process with Fe3O4 | SMX solution pH 3.01–10.96 2 kGy | >98% (pH 3.0–11.0) | increased by 200% with Fe3O4 addition | [79] |
| Sulfamethoxazole (SMX) | 5, 10, 20, 30 mg/L | 60Co | goethite (α-FeOOH) catalyzed Fenton-like process | SMX solution, pH 3.06–10.98, absorbed dose up to 2 kGy | 100% (1.5 kGy, 0.1 g/L goethite) | 26.7% (1 g/L goethite) | [80] |
| Antibiotics | Initial Concentration | Radiation Source | Experimental Substrate | Removal Efficiency | Mineralization Efficiency | References |
|---|---|---|---|---|---|---|
| Erythromycin (ERY) | 599 ± 58 mg/L | 60Co | Erythromycin fermentation residue pH 5.2–6.8 25–50 kGy | ERY 49–55% ARGs 96.3–99.6% | -- | [28] |
| Erythromycin (ERY) | 538 mg/kg wet residue (7.5 mg/g total solids, TS) | 60Co | Erythromycin thiocyanate fermentation residue pH 5.86–9.13 10–30 kGy | ERY 56% ARGs 90–95% (1.0–1.3 log) | -- | [51,98] |
| Cephalosporin C (CEP-C) | - | 60Co | Cephalosporin C fermentation residue pH 7.2 10–50 kGy | 27.7–77.9% tolC gene 100% | 30% (Volatile solids, VS) | [74] |
| Cephalosporin C (CEP-C) | 10–200 mg/L | 60Co | Aqueous solution, groundwater; wastewater pH 3.5–9.2 0.2–2 kGy | 100% | 8.0–46.2% | [88] |
| Deacetyloxy cephalosporin C (DOCPC) | 810–920 mg/kg | 60Co | High concentration of organic matter pH 3.5–4.0 5–50 kGy | 97.5% | 61.5 ± 10.3% (first) 75–84% (secondary radiation) | [32] |
| EB | 92.9% | |||||
| Cephalosporin C (CEP-C) | 15.11 mg/L | 60Co | Cephalosporin C fermentation residue 0–50 kGy | 80.01% (γ pretreatment) 100% (two-stage fermentation) | 35.03–49.36% (Volatile solids, VS) | [99] |
| Cephalosporin C (CEP-C) | 300–400 mg/L | 60Co | fermentation residue | 92–96% | -- | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Wang, Y.; Wang, J. Recent Advances in Antibiotic Degradation by Ionizing Radiation Technology: From Laboratory Study to Practical Application. Water 2025, 17, 1719. https://doi.org/10.3390/w17121719
Song Y, Wang Y, Wang J. Recent Advances in Antibiotic Degradation by Ionizing Radiation Technology: From Laboratory Study to Practical Application. Water. 2025; 17(12):1719. https://doi.org/10.3390/w17121719
Chicago/Turabian StyleSong, Yuening, Yulin Wang, and Jianlong Wang. 2025. "Recent Advances in Antibiotic Degradation by Ionizing Radiation Technology: From Laboratory Study to Practical Application" Water 17, no. 12: 1719. https://doi.org/10.3390/w17121719
APA StyleSong, Y., Wang, Y., & Wang, J. (2025). Recent Advances in Antibiotic Degradation by Ionizing Radiation Technology: From Laboratory Study to Practical Application. Water, 17(12), 1719. https://doi.org/10.3390/w17121719






