Abstract
Advanced oxidation processes (AOPs) show significant promise to degrade recalcitrant water contaminants, such as 1,4-dioxane, but slow degradation kinetics limit the energy efficiency of this technology. We realized substantial enhancements in the degradation of 1,4-dioxane (a suspected carcinogen) using gold-coated titanium dioxide (Au/TiO2) Janus nanoparticles (JNPs) irradiated with above-bandgap ultraviolet (UV) light (peak wavelength, 254 nm). To explain this result, we combined experimental measurements quantifying 1,4-dioxane degradation at varying UV wavelengths with finite-element simulations that provided explanatory insight into the light–matter interactions at play. The enhanced photocatalytic activity at the optimal condition (254 nm light, high intensity, Au/TiO2) resulted from a larger quantity of photogenerated holes in the TiO2 capable of reacting with water to form hydroxyl radicals that degrade 1,4-dioxane. This increased production of holes resulted from two sources: (1) more viable electron–hole pairs were created under 254 nm light owing to increased light absorption by the TiO2 that was localized near the surface; (2) the metal sequestered photogenerated electrons from the TiO2, which prevented electron–hole pairs from recombining, leaving more holes available to react with water. Our results motivate the exploration of different metal coatings (especially non-precious metals) and suggest a path toward broader implementation of TiO2-based photocatalytic AOPs, which can effectively remove many water pollutants that survive conventional treatment techniques.
1. Introduction
Contamination of water with 1,4-dioxane (C4H8O2) is a growing public health concern because of both its adverse health effects and the lack of methods to efficiently degrade it. A known carcinogen in animals [1,2], 1,4-dioxane is considered by the U.S. Environmental Protection Agency (EPA), the International Agency for Research on Cancer (IARC), and the Agency for Toxic Substances and Disease Registry (ATSDR) as a probable carcinogen in humans [3,4]; it has been blamed for increased incidences of miscarriages, premature births, maternal toxicosis, and decreased birth weight [5,6]. Owing to its many industrial uses (e.g., as a stabilizer for solvents used in the pharmaceutical industry, pesticides, metal degreasing, fabric cleaning, paper manufacturing, etc.), its high solubility in water, and accidental discharge into the environment, 1,4-dioxane has been detected in groundwater and drinking water supplies to a greater extent than many other synthetic chemicals [7,8,9,10,11]. Moreover, 1,4-dioxane resists many conventional water treatment methods, including carbon adsorption [12], air stripping [12], reverse osmosis [13], and chemical treatments such as chlorination [3,14,15,16,17]. It is also non-biodegradable, rendering bio-based treatment methods ineffective [10,18]; it is a representative example of so-called contaminants of emerging concern (CECs) [19], for which new treatment methods are urgently needed.
Advanced oxidation processes (AOPs) show significant promise to address the challenge of degrading CECs such as 1,4-dioxane [20,21,22,23]. AOPs employ various methods (e.g., ultraviolet light, photocatalysts, chemicals such as hydrogen peroxide or ozone, or a combination thereof) to generate reactive oxidizing species (ROS) that non-specifically attack and destroy a broad range of contaminants [21], often mineralizing them completely to carbon dioxide and water [24]. One popular AOP involves photocatalytic nanomaterials [21], which have several advantages. First, nanomaterials’ high surface-area-to-volume ratio enhances degradation kinetics compared to larger particles or continuous surfaces. Second, the use of photocatalysts enables the generation of ROS without chemical additives, bypassing a leading contributor to AOP costs. Third, the photocatalysts are essentially unaltered during treatment, enabling them to be recovered from the treated water and reused. Owing to these advantages, nanomaterial-based AOPs are expected to play a key role in the ongoing shift toward decentralized wastewater treatment plants, which operate at smaller scale and closer to the point of consumption than urban treatment centers prevalent today [21].
Among photocatalyst materials, titanium dioxide (TiO2) is one of the most common because it is stable, cost-effective, environmentally friendly, and tolerant of acidic and alkaline media. However, a key disadvantage of TiO2 is that most electron–hole pairs recombine before they can reach the particle surface to interact with solvent or pollutant molecules, which substantially reduces the rate at which pollutant-destroying ROS are ultimately produced. The documented tendency of electron–hole pairs to recombine within a few nanoseconds of their creation limits the quantum efficiency of bare TiO2 [3,4,25].
One approach to suppress electron–hole recombination is to decorate the photocatalyst nanoparticles with metals, such as gold (Au) [26,27,28,29,30,31,32,33,34,35,36,37,38]. The metal can take the form of discrete “islands” [31,33,35,39,40,41,42,43,44,45], two-faced “Janus” particles [39,46], or a “floating catalyst” consisting of Au and TiO2 patterned onto a porous polymer [47]. Au/TiO2 particles have been applied specifically for 1,4-dioxane degradation, such as by Youn et al. and Byrne et al. [48,49]. The metal can collect photogenerated electrons from the conduction band of the semiconductor, which leaves more photogenerated holes available in the TiO2 to react with water to produce ROS [50]. This approach has been exploited to accelerate artificial photosynthesis [51,52], hydrogen production [53], ammonia splitting [50], and nitric oxide degradation [54], among others.
Apart from the suppression of electron–hole recombination, metal decorations can enhance water decontamination performance in other ways. One prominent example is surface plasmon resonance (SPR) [40], in which plasmonic effects concentrate the electromagnetic field at the surface of the nanoparticle, which can boost excitation of electron–hole pairs and accelerate photocatalysis [30,32,34,42,55,56]. For Au nanostructures, the light wavelength that excites SPR is typically in the visible range, enabling the use of sunlight as an energy source. Another example is particle self-propulsion, which can enhance mass transport of pollutants to photocatalytic surfaces. Under certain conditions, metal-coated TiO2 micro- or nanoparticles can propel themselves through water using UV light as an energy source [57,58,59,60,61,62] by a mechanism known as “self-electrophoresis” [63,64]. However, the propulsive speed in self-electrophoresis is known to decrease sharply if salt is present [65,66]. Nonetheless, a growing body of literature supports the idea that particle self-propulsion is a viable route to accelerate nanoparticle-mediated degradation of pollutants [41,66,67,68,69,70,71,72] by overcoming mass transfer limitations.
Despite this progress, several knowledge gaps remain in the development of photocatalytic AOPs. For example, although light from many different sources has been used to fuel photocatalysis (e.g., sunlight, Xe lamps, ultraviolet light at different wavelengths), there has been little investigation of the effects of using different UV wavelengths on degradation. Additionally, the light–matter interactions (including the quantity and spatial dependence of light absorption at different wavelengths) have not been investigated in detail. Without a full understanding of the dependence of degradation on UV light wavelength, intensity, and metal coating, we will be unable to optimize the efficiency of such processes, so the true potential of photocatalytic nanomaterials to accelerate the removal of CECs will not be realized.
To close this knowledge gap, in this work, we performed a comprehensive experimental and computational study to characterize these dependencies. We demonstrated that photocatalytic degradation of 1,4-dioxane is dramatically accelerated using Au/TiO2 Janus nanoparticles (JNPs) irradiated by 254 nm UV light. This configuration outperformed both bare TiO2 NPs (under 254 nm or 365 nm light) and the same Au/TiO2 JNPs under 365 nm light. At the optimal condition (254 nm light, 320 µW/cm2, Au/TiO2 JNPs), the first-order rate constant for degradation (0.138 min−1) is, to our knowledge, the largest reported for 1,4-dioxane degradation methods that do not require chemical additives (Tables S2 and S3 in the Supplementary Information give a detailed comparison of our results with similar 1,4-dioxane degradation studies).
To explain our results, we combined thorough characterization of the Au/TiO2 JNPs, experimental data for 1,4-dioxane degradation with and without suitable controls (UV alone, TiO2 NPs, with and without salt and radical scavengers), and finite-element simulations of light–matter interactions. Our analysis revealed several important findings. First, stoichiometric changes in the TiO2 upon Au sputtering enable electrons to move more freely through the TiO2, which likely accelerates the collection of photogenerated electrons by the Au [50,59,73]. Second, the finite-element simulations revealed that 254 nm light was absorbed (and thus electron–hole pairs are created) preferentially near the TiO2/water interface, whereas most 365 nm light was absorbed near the particle’s core; this result implies that, under 365 nm light, holes must travel a larger distance before they can react with water molecules to form hydroxyl radicals, reducing degradation efficiency. Third, the 1,4-dioxane degradation rate was virtually unchanged in the presence of 10 mM sodium chloride (NaCl) salt but was significantly reduced in the presence of methanol. Methanol is a radical scavenger that preferentially scavenges hydroxyl radicals (●OH) [74]. These results imply that self-propulsion of particles (which is known to be almost eliminated in the presence of electrolytes such as NaCl [59]) is not essential to enhancing degradation.
Since SPR (which is not prevalent for Au nanostructures at UV wavelengths) and self-propulsion are not major factors in the Au-mediated degradation enhancement, the main function of the Au is instead to sequester photogenerated electrons from the conduction band of TiO2, while holes accumulate in the valence band of the semiconductor. Overall, we conclude that the combination of 254 nm UV light with Au/TiO2 JNPs led to the maximal production of ●OH radicals out of all the conditions tested, leading to efficient degradation of 1,4-dioxane. These results suggest that a judicious combination of metal coatings, above-bandgap UV light, and TiO2 particles provides a route to degrade 1,4-dioxane more efficiently than has been demonstrated before. Accordingly, our work suggests that a judicious combination of metallic coatings and 254 nm light is a viable pathway toward making TiO2-based AOPs more energy-efficient (and thus economically viable) for large-scale removal of CECs, such as 1,4-dioxane, from drinking water systems. Encouragingly, our results suggest that dramatic enhancements in degradation efficiency may be realized without SPR, and thus it may not be essential that the metal coating be Au, raising the possibility of realizing these advances with more cost-effective metals.
The article is arranged as follows. Section 2 describes the materials, preparation methods for the Au/TiO2 JNPs, as well as the procedures followed for characterizing the JNPs. Section 3 describes the results, including the XPS data, degradation kinetics, and simulation data. Section 4 presents a discussion of the results. Finally, Section 5 concludes the paper with the most significant findings and recommendations for future studies.
2. Materials and Methods
2.1. Materials and Reagents
Titanium (IV) oxide (TiO2, anatase) nanopowder (cat. No. 248576), 1,4-dioxane (C4H8O2, 99.8%), ethanol (C2H5OH, 99.9%), methanol (CH3OH, 99.8%), and sodium chloride (NaCl, >99%) were purchased from Sigma Aldrich (St. Louis, MO, USA). All chemicals were of analytical grade and used without further purification. Ultrapure, Milli-Q grade, deionized (DI) water (>18 MΩ·cm, MilliporeSigma, Burlington, MA, USA) was used to perform the experiments. Pre-cleaned glass slides (25.4 × 76.2 × 2 mm3) were purchased from AmScope (Irvine, CA, USA).
2.2. Synthesis of Au/TiO2 JNPs
The synthesis process for Au/TiO2 JNPs is depicted in Figure S1 and consisted of drop casting of bare TiO2 particles onto glass slides, physical vapor deposition of Au, and the release of particles from the slides by ultrasonication [59]. Briefly, we prepared stock solutions (in batches of ~500 mL at a time) of TiO2 NPs in pure ethanol at mass fractions ranging from 0.033 to 0.33 mg/mL. The NPs were dispersed in ethanol in a bath ultrasonicator (Branson 3800 Series, Brookfield, CT, USA). Aliquots (~400 μL) of the TiO2/ethanol suspension were pipetted onto glass slides, and the sample was dried at room temperature, leading to the formation of a nanoparticle film. The nanoparticle-coated glass slide was then sputter-coated with a thin gold (Au) layer using an AJA ATC 1800 sputtering system (Hingham, MA, USA) at a rate of ~0.63 nm s−1 (~63 s deposition) at a constant power of 200 W. During the Au deposition process, the substrate was rotated at 60 revolutions per minute (RPM), and the Au target was held at a 15-degree angle to the substrate. The substrate was then immersed in a beaker of DI water and briefly sonicated to release the Au/TiO2 JNPs. The JNPs were recovered by centrifugation and dried for further experimental studies. Both the Au/TiO2 JNPs (without any light) and the same TiO2 NPs without the Au coating were used as controls in the experimental studies.
2.3. Characterization Methods
Field emission scanning electron microscopy (FESEM, Hitachi S-4800, Schaumburg, IL, USA) and energy-dispersive X-ray spectroscopy (EDS) were used to examine the morphology and surface composition of Au/TiO2 JNPs. For FESEM analysis, the samples were vacuum-dried and mounted on a sample holder with carbon tape. The elemental analysis of the sample was performed using an EDS spectrometer integrated with the FESEM instrument. The thickness of the Au coating on TiO2 particles was estimated using a Bruker Dektak XT profilometer (Bruker, Billerica, MA, USA). The crystallinity of the samples was confirmed by means of X-ray diffraction (Malvern PANalytical X’Pert Pro, Westborough, MA, USA). X-ray photoelectron spectroscopy (XPS) was performed using an ESCALAB 250Xi spectrometer (Thermo Scientific, Waltham, MA, USA) with a monochromatic Al Kα X-ray as the source (15 kV, 150 W). The intensity-weighted hydrodynamic diameter distribution of the particles was obtained with dynamic light scattering (Malvern Zetasizer Nano ZS, Westborough, MA, USA). A UV–visible spectrophotometer (Lambda 950 UV/Vis/NIR, Perkin Elmer, Springfield, IL, USA) was used to record the reflectance spectra of the samples. The concentration of 1,4-dioxane was quantitatively monitored by means of a high-performance liquid chromatography (HPLC) instrument (1220 LC, Agilent, Santa Clara, CA, USA) with diode-array detection (DAD) at 190 nm using a Waters C18 column (Milford, MA, USA), isocratic elution with an acetonitrile/DI water (5% acetonitrile in water) mobile phase at 0.8 mL min−1.
2.4. Photocatalytic Degradation Experiments
A 200 mg/L 1,4-dioxane stock solution was prepared in DI water and diluted to 20 mg/L for the degradation experiments. The pH of the solution was not adjusted, but was likely close to 7 for the duration of the experiment since the sample consisted of pure water with 1,4-dioxane added. The photocatalytic degradation of 1,4-dioxane was carried out in a 100 mL glass beaker containing 40 mL of 20 mg/L 1,4-dioxane solution. Then, 2 mg of Au/TiO2 JNPs (leading to a particle mass fraction of 50 mg/L) were introduced into the beaker, and the experiments were carried out for 3 h under UV irradiation. Stirring of the solution was avoided to minimize the effects of convective mass transfer (apart from any contributions resulting from self-propulsion of the particles as discussed below). To evaluate the degradation performance, a 600 µL aliquot was withdrawn from the beaker every 30 min, and the aliquot was filtered using a polycarbonate membrane (0.45 μm pore size) to remove the particles. Following this, the 1,4-dioxane concentration in the water sample was determined by HPLC.
A series of independent experiments was carried out under UV light irradiation at 254 and 365 nm. The control experiments eliminated the effects of adsorption by the filter membrane and evaporation in degradation studies. The high-intensity 254 nm UV light (320 µW/cm2) for photodegradation was generated by an Aquafine low-pressure mercury lamp (Trojan Technologies, London, ON, Canada), and the low-intensity 254 nm UV light (3.1 μW/cm2) was provided by the same lamp at a longer distance between the light source and the beaker. High-intensity 365 nm UV light (320 µW/cm2) was provided by a UVP B-100AP/R high-intensity medium-pressure UV lamp (analytikJena, Tewksbury, MA, USA). UV light intensity was measured by means of a UVX Radiometer UV light intensity meter (UVP) (analytikJena, Tewksbury, MA, USA). The low-pressure lamp emitted narrowband light centered at 254 nm, whereas the medium-pressure lamp is polychromatic, having its dominant emission at 365 nm accompanied by additional weaker features deeper in the UV. For conciseness, the medium-pressure lamp is referred to hereafter as the “365 nm lamp” and its low-pressure counterpart as the “254 nm lamp.” These names are merely for convenience and are not meant to indicate that either lamp is perfectly monochromatic. Representative spectral profiles provided by the manufacturers for each lamp are provided and/or referenced in the Supplementary Information (SI) (see Figure S9).
2.5. Computational Modeling
Two versions of COMSOL Multiphysics were used to perform simulation studies: version 5.4 for self-propulsion studies (methodology in the SI) and version 5.6 for electromagnetic simulations. The electromagnetic simulation methodology was verified and validated by ensuring numerical simulations of Au spherical nanoparticles matched the analytical solutions for Mie scattering. Quantitatively, mesh elements were stipulated to be smaller than a maximum size of λ/6.5, where λ is the incident wavelength.
In the simulations, the nanoparticle was assumed to be an ellipsoid having a size comparable to the average size distribution in Figure 1e. The ellipsoid was preferentially covered in gold on one side with a thickness that decreased from its maximum value of ~40 nm until it eventually became discontinuous on the uncoated TiO2 face. The discontinuities were modeled as truncated spheres representing the smaller gold “islands” that appeared on the JNPs (Figure 1a). Further simulation details are explained in the SI.
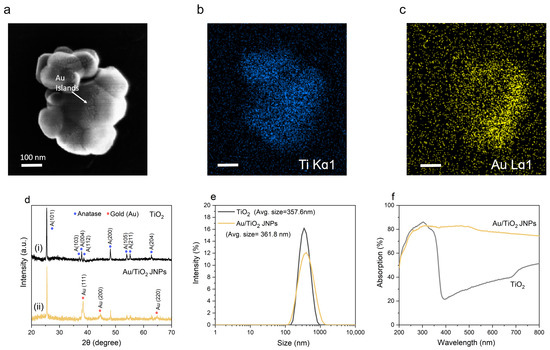
Figure 1.
Physical and chemical characterization of TiO2 NPs and Au/TiO2 JNPs. (a) Representative scanning electron micrograph of an Au/TiO2 JNP. (b,c) EDS maps of Ti (b) and Au (c) for the same image in panel (a), showing that Au deposition occurs preferentially on the right-hand side. All scale bars: 100 nm. (d) XRD data for (i) TiO2 (black) and (ii) Au/TiO2 JNPs (gold). (e) Intensity-weighted hydrodynamic diameter distribution of TiO2 NPs and Au/TiO2 JNPs, obtained by DLS. (f) UV–vis diffuse reflectance spectra of TiO2 NPs and Au/TiO2 JNPs.
3. Results
3.1. Characterization
3.1.1. Morphology and Elemental Composition of JNPs
An SEM micrograph of an Au/TiO2 Janus particle and false-color EDS elemental maps of Ti and Au are shown in Figure 1a–c, respectively. Additional SEM/TEM/EDS data are shown in Figures S3 and S4. The TiO2 particles were ellipsoidal, with major and minor axes in the range of 300–600 nm. EDS elemental maps associated with the Ti Kα1 (Figure 1b) and Au Lα1 (Figure 1c) X-rays indicate particles coated preferentially on one side with metal, commonly known as “Janus” particles [59,75]. Small fragments of Au were also irregularly scattered across the exposed TiO2 surface (Figure 1a), leading to isolated Au “islands.”
3.1.2. TiO2 Crystalline Phase
Figure 1d(i,ii) shows XRD data for as-received uncoated TiO2 nanopowder and as-synthesized Au/TiO2 JNPs, respectively. The XRD pattern in Figure 1d(i) exhibits characteristic diffraction peaks at 2θ = 25.4°, 37.08°, 37.98°, 38.64°, 48.1°, 54.09°, 55.11°, and 62.76°, attributed to (101), (103), (004), (112), (200), (105), (211), and (204) crystallographic planes of anatase TiO2 (JCPDS 21-1272), respectively [76]. In Figure 1d(ii), the XRD plot shows diffraction peaks at 38.25°, 44.53°, and 64.66°, which can be indexed to the (111), (200), and (220) planes of the face-centered cubic structure of elemental Au (JCPDS 002-1095) [77]. The presence of predominant Au peaks in Figure 1d(ii) indicates the formation of a metallic Au coating on the surface of the TiO2 NPs, which is consistent with the SEM micrograph of Au/TiO2 JNPs (Figure 1a).
3.1.3. Particle Size Distribution
Particle size distribution was quantified through dynamic light scattering (DLS), which showed (Figure 1e) that the average hydrodynamic diameters of the TiO2 and the Au/TiO2 NPs were 358 and 362 nm, respectively. These values are in the upper range of sizes in which the term “nanoparticle” is commonly applied, and they exceed the 100 nm threshold that is sometimes considered the maximum particle size for the term “nanoparticle” to be applicable. However, according to the International Union of Pure and Applied Chemistry (IUPAC) guidelines, the use of the prefix “nano” is acceptable for particles with sizes up to 500 nm, provided that reference to this definition is indicated. Since the DLS results showed that the average hydrodynamic diameter of both the TiO2 and Au/TiO2 Janus particles was below 500 nm, the use of the term “nanoparticle” was appropriate under this definition [78].
A comparison of the DLS results for TiO2 and Au/TiO2 indicates that the average size of TiO2 particles was not significantly altered by the Au coatings. We note that the particles were generally non-spherical (Figure 1a, Figures S3 and S4) and deposited in random orientations on the glass slides; therefore, the metal coating covered different regions of different particles. Additionally, it was likely that some TiO2 NPs were not coated with any Au at all, considering that multilayered coatings of particles sometimes formed in preparing the material for metal deposition, preventing particles in the underlying layers from being coated. These factors help explain why there was only a modest increase in the mean effective hydrodynamic diameter of the Au/TiO2 JNPs compared to the TiO2 NPs, even though with the deposition rate (0.63 nm s−1) and time (63 s), the nominal thickness of the Au coating on a flat surface would be approximately 40 nm.
3.1.4. Light Absorption
Absorption of UV and visible light by the nanoparticles was measured by means of UV–vis diffuse reflectance spectroscopy. UV–vis spectra for Au/TiO2 JNPs and TiO2 NPs are shown in Figure 1f. We used a UV–visible spectrophotometer (Lambda 950 UV/Vis/NIR, Perkin Elmer) equipped with a 150 mm integrating sphere to record the reflectance and transmission. We then calculated the absorbance from the reflectance and transmission according to the following formula: , where the transmission was zero since the photocatalyst was loaded on an opaque plate. By itself, TiO2 only absorbs significantly at wavelengths below 400 nm, consistent with the magnitude of its bandgap (in Section S9 of the Supplementary Information, we present the results of a Tauc analysis that confirm the bandgap of the TiO2 to be 3.22 eV, in excellent agreement with the literature value of 3.2 eV). The Au/TiO2 JNP, in contrast, exhibited broadband absorption across the full visible range (Figure 1f), consistent with the broadband response of Au in this spectral window. Figure 1f shows that the absorption spectrum of Au/TiO2 below 400 nm was very similar to that of TiO2 below 400 nm, implying that absorption in the UV range was dominated by the TiO2. These spectra, coupled with the fact that all our experiments were carried out with UV light, imply that surface plasmon resonance (which, for Au nanostructures, requires light in the visible range) played a minimal role in the degradation of 1,4-dioxane in our experiments.
3.1.5. XPS Analysis
X-ray photoelectron spectroscopy (XPS) was performed on both uncoated TiO2 NPs and Au/TiO2 JNPs (Figure 2, Figures S5 and S6). Figure 2a shows the deconvoluted XPS spectrum of the Ti 2p orbital for TiO2, which exhibited two characteristic peaks corresponding to Ti4+ in the TiO2 lattice at 458.5 eV (Ti 2p3/2) and 464.2 eV (Ti 2p1/2) [34]. The Ti 2p3/2 and Ti 2p1/2 peaks of Ti4+ have a binding energy difference of 5.7 eV, which is consistent with previous literature for anatase TiO2 [34,79]. Additionally, a shoulder peak was observed at 459.9 eV, which is attributed to Ti3+ species in the Ti2O3 lattice [79]. The XPS spectrum of uncoated TiO2 (Figure 2a) indicated that the as-received anatase nanoparticles contained primarily Ti4+ species with relatively few Ti3+ defects. In the XPS spectrum of the TiO2/Au JNPs (Figure 2b), the binding energies of the Ti4+ peaks (Ti 2p3/2 and Ti 2p1/2) remained relatively unchanged and did not display any considerable shift compared to uncoated TiO2. However, the relative area of the Ti 2p3/2 peak (458.4 eV) exhibited a significant decrease (from 57.9% to 36.4%). Conversely, for the TiO2/Au JNPs, the peak associated with Ti3+ exhibited a negative shift in position (i.e., lower binding energy) of 0.6 eV and a marked increase in the relative area (from 9.1% to 27%) [80]. The decrease and increase in the relative areas of the Ti4+ and Ti3+ peaks, respectively, signified the reduction of Ti4+ to Ti3+ occurring on the TiO2 surface associated with the Au sputtering process [80,81].
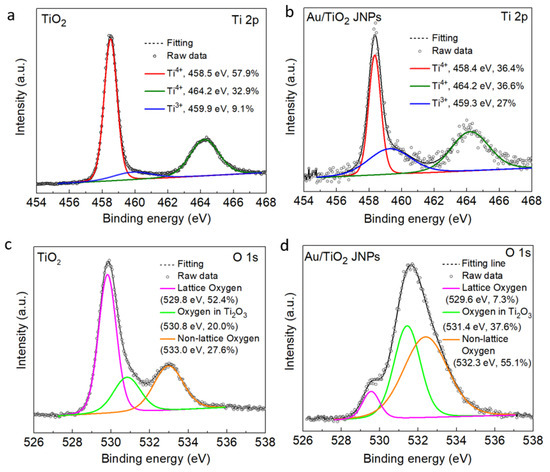
Figure 2.
High-resolution deconvoluted XPS spectra of the Ti 2p region for (a) the uncoated TiO2 NPs and (b) Au/TiO2 JNPs, and O 1s region for (c) the uncoated TiO2 NPs and (d) Au/TiO2 JNPs.
Figure 2c shows the XPS spectrum of the O 1s orbital of the uncoated TiO2 sample. In this spectrum, the characteristic peak at 529.8 eV corresponded to the crystal lattice oxygen (Ti–O–Ti), and the peak at 533 eV was associated with the so-called “non-lattice” oxygen of TiO2 [82]. Recent studies suggest that the peak at 530.8 eV can be attributed to the lattice oxygen in Ti2O3 [79,83]. In the O 1s XPS spectrum of the Au/TiO2 samples (Figure 2d), there was no significant shift in the position of the lattice oxygen peak in TiO2, but there was a considerable decrease in its relative area compared to Figure 2c. The binding energy of non-lattice oxygen underwent a negative shift of 0.8 eV, along with an increase in the relative peak area compared to bare TiO2. Recent findings by Bharti et al. suggested that an increase in the peak area of non-lattice oxygen is positively correlated with the formation of oxygen vacancies (Vo) in the TiO2 lattice [79]. For the Au/TiO2 JNPs, the oxygen peak signals from Ti2O3 showed a positive shift in the binding energy of 0.6 eV along with an increase in the peak area, as compared to the XPS spectra for uncoated TiO2. Taken together, the considerable variations in the Ti and O XPS spectra of Au/TiO2 JNPs compared to uncoated TiO2 imply that the Au deposition led to the formation of Vo-rich zones on the TiO2 surface layer [84]. Previous studies have shown that oxygen vacancies and Ti3+ species can suppress electron–hole recombination and facilitate photocatalysis [85,86]. XPS data for Au are given in Figure S6.
3.2. Factors Affecting Degradation Kinetics
Photocatalytic degradation performance is affected by many factors, including the presence or absence of radical scavengers and the intensity and wavelength of the UV light. To quantify these effects, we conducted a series of batch experiments under different conditions (UV alone, UV/uncoated TiO2, UV/Au/TiO2, dark/Au/TiO2). Figure 3 shows the normalized concentration of 1,4-dioxane as a function of time under high-intensity 254 nm UV light (Figure 3a); low-intensity 254 nm UV light (Figure 3b); high-intensity 365 nm UV light (Figure 3c); high-intensity 254 nm UV light with sodium chloride (NaCl) or methanol (MeOH) added to the water (Figure 3d).
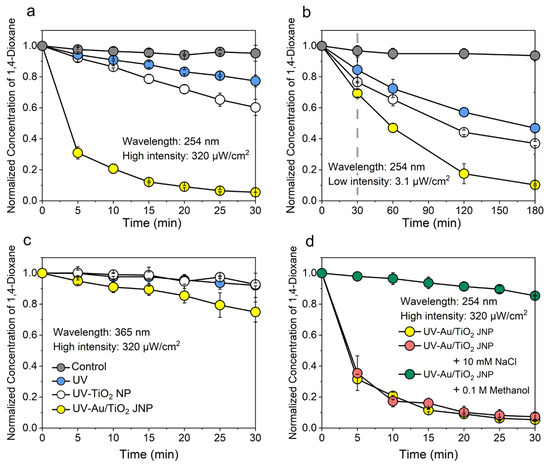
Figure 3.
Photocatalytic degradation of 1,4-dioxane under (a) 254 nm UV light at an intensity of 320 μW/cm2, (b) 3.1 μW/cm2; under a 365 nm UV lamp at (c) 320 μW/cm2; and under (d) a 254 nm UV lamp at 320 μW/cm2, with either 10 mmol/L NaCl or 0.1 mol/L MeOH added to the water. In all the four panels, the initial concentration of 1,4-dioxane was 20 mg/L, and the concentration (w/v) of TiO2 or Au/TiO2 JNPs was 50 mg/L. The total duration of the experiment depicted in (b) was 180 min, whereas the duration of the experiments depicted in panels (a,c,d) was 30 min (vertical dashed line at t = 30 min in panel (b)).
As shown in Figure 3a,b, Au/TiO2 JNPs did not degrade 1,4-dioxane without UV light. Illumination of water containing 1,4-dioxane with UV light alone led to minimal degradation. UV-illuminated uncoated TiO2 NPs degraded 1,4-dioxane more rapidly than UV alone, but they were wholly outperformed by the Au-coated TiO2 JNPs. Figure 3a demonstrates that, under a narrowband 254 nm lamp at high intensity, the degradation of 1,4-dioxane was substantially accelerated upon addition of the Au layer. For simplicity, we assumed first-order degradation kinetics, such that the 1,4-dioxane concentration followed . Under the 254 nm lamp at high intensity (320 µW/cm2), the reaction rate constant () for the Au/TiO2 JNPs was 138.0 × 10−3 min−1, a more than eightfold increase compared to the case of uncoated TiO2 NPs (kobs = 17.15 × 10−3 min−1) for the same experimental conditions. Table S1 includes the rate constants and percentage of the 1,4-dioxane degraded after 30 min for all the experimental conditions depicted in Figure 3. Table S3 presents a rough comparison of the rate constants measured in this work to those in previous studies in terms of photocatalytic 1,4-dioxane degradation and demonstrates that, of the studies surveyed, the degradation rate demonstrated in our work is among the faster rates in the absence of chemical additives. However, since the photon flux and light intensity varied significantly in these studies (some references do not report intensity at all), a direct apples-to-apples comparison of our work with these previous studies is not possible.
3.2.1. Effect of Wavelength
The Au/TiO2 JNPs showed a significantly enhanced photocatalytic degradation of 1,4-dioxane when exposed to the light whose dominant peak was at 254 nm, compared to the light whose dominant peak was at 365 nm. Both lamps that irradiated the JNPs are narrowband, and, thus, visible absorption was negligible. This enhanced catalytic activity also matched the absorption profile of pure TiO2 (Figure 1f), indicating that absorption within the TiO2 was a primary driver of photocatalysis.
Figure 1f demonstrates that absorption by the Au/TiO2 JNPs was comparable at the two wavelengths tested (254 nm and 365 nm), but the photocatalytic performance was substantially improved at 254 nm. To gain further insight into this dependence, we conducted finite-element simulations of light–matter interactions, which are presented in Section 4.
3.2.2. Effect of Light Intensity
Increasing the light intensity modestly enhances the photocatalytic degradation performance of uncoated TiO2 particles, but significantly enhances the performance of Au/TiO2 JNPs. In Figure 3a,b, it only takes approximately 30 min for over 90% of the 1,4-dioxane to be degraded with high-intensity UV light (320 µW/cm2), compared to 3 h for a lamp with an intensity 2 orders of magnitude lower (3.1 µW/cm2), in line with previous observations of intensity effects [87]. However, similar improvement was not observed with uncoated TiO2, which only demonstrated a small increase in the catalytic degradation rate under higher-intensity illumination. These results indicate that partial coverage of Au atop TiO2 substantially improved photocatalytic performance at high UV intensity. Intensity dependence in the degradation, in turn, is a signature of photocatalytic performance that is primarily dependent on the metal–semiconductor Schottky barrier [73].
3.2.3. Effects of Solution Salinity and Radical Scavengers
Next, we sought to determine whether adding background electrolyte or radical scavengers to the feed affected the photocatalytic performance of the Au/TiO2 JNPs. As shown previously, particle self-propulsion is one viable approach to enhancing the efficiency of water decontamination by nanoparticles, as has been specifically described for metal/TiO2 micromotors. However, the self-propelled speed of Au/TiO2 micromotors decreases rapidly as the electrolyte concentration is increased, and is nearly zero for 10 mM NaCl [59]. Since we observed a negligible change in degradation rate with the same salt and same concentration (10 mM NaCl) (Figure 3d), we inferred that self-propulsion was not a primary enhancement mechanism of metal-coated JNPs under the conditions tested. Second, radical scavengers such as MeOH can preferentially react with hydroxyl radicals, and thus radical-mediated degradation should be slower in the presence of MeOH. Therefore, radical scavengers (0.1 M MeOH) were added into separate samples of 1,4-dioxane solution to examine the influence of self-propulsion and radical scavenging on degradation. Figure 3d shows appreciably slower degradation in the presence of MeOH. MeOH preferentially scavenges hydroxyls, and this property is sometimes used in photocatalytic processes [74]. Therefore, the dramatic reduction in degradation in the presence of MeOH can be explained by fewer ●OH being available to react with 1,4-dioxane. It is worth noting that superoxide radicals (●O2−) may also be produced, but do not react with MeOH readily [88]. Superoxide radicals have a lower oxidation potential compared to more powerful oxidants, including H2O2 and ●OH [21]; accordingly, superoxide radicals are weaker [89] and relatively unreactive [90,91,92,93]. Based on these observations, we conclude that superoxide radicals play at most a minor role in the degradation enhancement. Instead, we attributed the degradation of 1,4-dioxane in this system primarily to hydroxyl radicals, which can be formed in at least two plausible routes, as illustrated in Figure S10 of the Supplementary Information. Both routes require the presence of the Au layer directly or indirectly [94].
3.2.4. Role of Self-Propulsion
To determine the role of self-propulsion in degradation, we repeated the experiments with an inert salt at a concentration that has been demonstrated to suppress self-propulsion [59]. Figure 3d shows that in the presence of 10 mM NaCl, the degradation of 1,4-dioxane by Au/TiO2 JNPs was unchanged to within experimental uncertainty from the case without NaCl. Since this NaCl concentration has previously been reported to suppress the self-propulsion of Au/TiO2 micromotors entirely [59], this result supports the assertion that self-propulsion of particles is not important in enhancing 1,4-dioxane degradation. Further evidence of the minimal role of self-propulsion in this system comes from finite-element simulations (using COMSOL Multiphysics 5.4) of the self-propulsion phenomenon, whose results are reported in the Supplementary Information (Figures S7 and S8). These simulations predict that self-propulsion only exert a substantial effect on the surrounding distribution if the Péclet number, Pe = Ud/D (where U is the propulsive speed in m/s, d is the particle diameter in m, and D is the diffusivity of the pollutant in m2/s) is greater than 1 [59,95]. In the conditions tested here, the Peclet number was indicating that diffusion dominated over self-propulsion-induced advection in transporting pollutants to the particle surface. Taken together, the experimental and computational evidence presented here shows that self-propulsion plays, at most, a minor role in accelerating 1,4-dioxane degradation by Au/TiO2 JNPs. However, the enhancements could be substantial for different combinations of micromotors and pollutants for which the Péclet number is greater than unity, as exemplified by the high-Pe case in Figure S8c. For some pollutant/micromotor combinations, inhibited electron–hole recombination and self-propulsion could synergistically work in concert to enhance photocatalytic degradation further.
Since plasmons are excited in gold nanoparticles by visible light, we cannot attribute the observed enhancement to surface plasmon resonance either [96]. Therefore, the wavelength- and intensity-dependent degradation supports the hypothesis that improved degradation is due to an increase in the number of holes capable of reacting with water to produce ●OH through means other than SPR or self-propulsion. Thus, we make the case that (i) absorption within the TiO2 and (ii) efficient separation of electron–hole pairs promoted by the induced voltage between TiO2 and Au were driving the observed photocatalytic performance enhancement [97,98].
The UV–vis diffuse reflectance spectroscopy data in Figure 1f support the primacy of TiO2 absorption on the degradation characteristics. The spectrum of the Au-coated particles showed broad absorbance at wavelengths above 300 nm, while the uncoated particles experienced a steep drop-off in absorbance beyond the band gap of TiO2. However, although the coated particles absorbed a longer-wavelength (365 nm) light, both coated and uncoated particles had poor degradation with the said light, following the trends of standard TiO2 photocatalysis. This suggests that the absorption by the TiO2 is the main driving force for degradation, and that gold enhances the photocatalytic degradation not because of the increased light absorption or SPR, but because the presence of Au synergistically enhances the redox chemistry by sequestering photogenerated electrons, preventing them from recombining with holes in the TiO2. This leaves more electron holes available in the TiO2 to react with water and form pollutant-degrading ●OH. In the Supplementary Materials (see Section S8, Figure S10), we elaborate on two plausible routes by which ●OH can be generated at an enhanced rate by Au/TiO2 JNPs compared to TiO2 NPs alone [94]. Briefly, ●OH can either be generated directly on the TiO2 surface by reacting with water molecules or hydroxide ions or photogenerated electrons can react with oxygen in the solution to form superoxide radicals, which then form hydrogen peroxide as an intermediate before finally yielding ●OH. Figure S10 also gives estimates of the conduction band and valence band energies of the TiO2, which are calculated in Section S10 of the Supplementary Materials. Since the Fermi level of the Au lies between the VB and CB energies [94,99], it is reasonable to assume a driving force exists that drives an electron current from TiO2’s CB to the Au. Future studies could corroborate these estimates experimentally, e.g., via measurement of the work function of both Au and TiO2 via ultraviolet photoelectron spectroscopy (UPS) [100] in the presence and absence of UV light.
3.3. Simulations of Light Absorption
To elucidate the physical mechanisms governing the enhanced degradation by Au/TiO2 JNPs, we examined the spectral dependence of light absorption within the Au/TiO2 JNPs using full-wave electromagnetic simulations (see the Methods section). The total and relative amount of light each material absorbed changed significantly depending on the incident wavelength, as shown in Figure 4d,e.
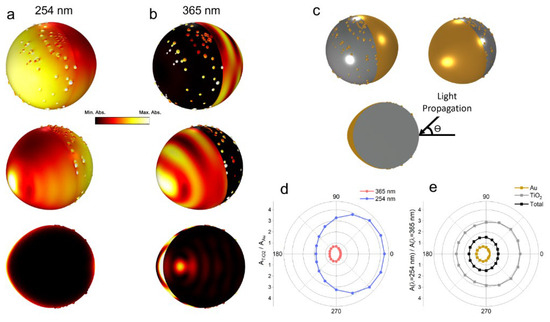
Figure 4.
Full-wave electromagnetic simulation (COMSOL Multiphysics 5.6) of light–Au/TiO2 JNP interactions. (a) Spatial distribution of absorption for 254 nm (a) and 365 nm (b) incident light viewed isometrically (top and middle) and with a cross-sectional view (bottom) across the major axis of the nanoparticle. In all cases, light was directly incident on the uncoated TiO2 side (). (c) Rendering of an Au-coated TiO2 JNP with randomly deposited Au asperities and the cross-section exhibiting thinning of the gold layer moving away from the metal-coated side. (d) Ratio of absorption within TiO2 to that within Au at 254 and 365 nm. (e) Ratio of absorption of 254 nm light to 365 nm light within Au and TiO2. All the results are averaged to simulate unpolarized light. The major axis of the ellipsoid-like particle was 520 nm.
Quantitatively, TiO2 exhibited greater absorption at all incident angles when irradiated with 254 nm light compared to 365 nm light. In contrast, 365 nm light was absorbed preferentially by Au (Figure 4d,e). This difference arose from the TiO2 bandgap (~3.2 eV, λ = 387 nm) [101], which resulted in greater absorption for the deeper UV (i.e., 254 nm) light. The diminished absorption of the TiO2 particle at longer wavelengths observed in UV–vis spectroscopy (Figure 1) testifies to this fact. Decreased TiO2 absorption at 365 nm ultimately means that less light was absorbed overall by the JNPs at the longer wavelength (Figure 4d). Simply put, more light was absorbed overall at 254 nm because much more of it was absorbed by the TiO2 at this wavelength. From the enhanced degradation rates of 1,4-dioxane upon exposure to 254 nm light (Figure 3), we conclude that absorption within TiO2 drives photocatalysis.
The volumetric distribution of absorption (i.e., where absorption occurs) provides further evidence for the primacy of TiO2 absorption. Figure 4 plots the spatial distribution of absorption when illuminated by the two wavelengths when light is incident on the uncoated TiO2 side of the nanoparticle. Under 365 nm illumination, absorption took place within the interior of the TiO2 and throughout the full thickness of the Au (Figure 4b, bottom). In contrast, 254 nm light induced absorption that was concentrated near the surface (Figure 4a, bottom). Holes generated near the surface drive photocatalytic reactions, so the increased surface absorption at 254 nm helps explain the increased degradation. The increased oxygen vacancy concentration in the gold-coated TiO2, which is correlated with the increased presence of non-lattice oxygen and Ti3+ ions (Figure 2), aids the process as well. Specifically, oxygen vacancies in the TiO2 result in shallow traps that can capture photogenerated electrons. This capture inhibits electron–hole recombination, thereby inducing a net increase in hole concentration and thus faster overall degradation [102].
The near-surface absorption of 254 nm light is not attributable to surface plasmons excited within the Au. Plasmon resonance occurs in Au nanostructures only for light in the visible range and does not extend into the UV region. The Au is, however, critical for the observed enhancement of photoinduced degradation for two primary reasons. First, as explained above, Au sequesters photogenerated electrons due to the driving potential difference between the TiO2 and Au. Mechanistically, the built-in potential field that exists within the so-called space charge region that forms near the Au/TiO2 interface efficiently drives electrons to the Au, thereby reducing the recombination rate of the holes within the TiO2. [73,98] The holes remaining in the space charge regions of the TiO2 are then available to participate in photocatalysis. Second, by creating discontinuous networks of “islands,” the total area of the space charge region increases, as does the volume of TiO2 that can participate in photocatalysis. Taken in aggregate, the utility of the metal in enhancing photodegradation of 1,4-dioxane is not in driving self-propulsion or plasmonic light absorption, but rather in the effects (increasing light absorption, separating charge) it has on the photocatalytic response of TiO2.
Considering that the main function of the metal coating is to aid in separating charge, these results imply that gold—a precious metal—is not essential for UV-based photocatalysis. Rather, metals having larger work function differences relative to the electron affinity of TiO2 may outperform Au as they would form larger space charge regions where recombination would be minimized. Therefore, future work should be undertaken to reassess the utility of TiO2 JNPs decorated with metals other than gold for use in UV-based photocatalysis.
4. Discussion
Partially Au-coated Janus nanoparticles were found to accelerate the degradation of a model pollutant—1,4-dioxane—when illuminated with ultraviolet light above the bandgap of the TiO2, and the primary mechanisms underlying this behavior were identified. The enhanced performance resulted from an Au-induced increase in the number of photogenerated electron holes at the TiO2/water interface, which react with water to produce pollutant-degrading hydroxyl radicals.
One of the primary findings of this work is the experimental demonstration that 1,4-dioxane degradation is significantly accelerated under UV light with a peak wavelength at 254 nm compared to a peak wavelength of 365 nm. To explain these experimental measurements, we complemented them with finite-element simulations that gave insight into the physics at play at each condition. The simulation data yielded several further insights:
- In the case of 254 nm light, light was preferentially absorbed close to the water/TiO2 interface, whereas 365 nm light was mostly absorbed near the particle’s core. This suggests that even though roughly the same amount of light was absorbed by the particles overall at the two wavelengths (gold data in Figure 1f, black data in Figure 4e), a significantly larger proportion of the electron–hole pairs created by 254 nm light resulted in viable ●OH radicals to degrade pollutants. This could explain why the reaction rate constant was nearly 15 times larger for 254 nm light than for 365 nm light for the Au/TiO2 JNPs at the same light intensity (see Table S1 in the Supplementary Information).
- For 254 nm light, more light was absorbed by the TiO2 overall, as evidenced by Figure 4d,e. The data in Figure 1f are consistent with this observation, since the absorbance for pure TiO2 was roughly 80% at 254 nm and roughly 62% at 365 nm. This observation suggests that more electron–hole pairs were created overall for 254 nm light, but the difference was not necessarily sufficient to explain the vast enhancement in the degradation rate that was observed between the two wavelengths.
- Although the total amount of light absorbed was similar for 254 nm and 365 nm light, a significantly greater portion was absorbed by the TiO2 rather than by the Au. This could explain why, even though the total absorption was similar for the Au/TiO2 nanostructures (Figure 1f), the rate of reaction was significantly higher (rate constant of 0.138 min−1 for 254 nm light and 0.009 min−1 for 365 nm light).
The metal coating benefits photocatalysis in two main ways. First, the concentration of oxygen vacancies increases in TiO2 during the Au deposition; these oxygen vacancies produce “electron traps” that prevent photogenerated electrons from recombining with nearby holes and increase the mobility of photogenerated electrons in TiO2, facilitating their sequestration by the metal [103]. Second, the potential difference between Au and TiO2 drives photogenerated electrons from TiO2 to Au. This reduces recombination, resulting in a net increase in the holes available to mediate the formation of hydroxyl radicals. Despite the Au-coated Janus morphology, self-propulsion and plasmonic absorption were shown to be of little consequence to degradation for the system studied here.
The extent to which the morphology of the Au coating affects the enhanced photocatalytic activity is unclear, since ●OH radicals are generated on the TiO2 surface. However, it is likely that the Au morphology indirectly affects the overall degradation rate. If the particle surface were completely covered in discrete Au nanostructures, the overall degradation rate may be increased since, in this scenario, a larger percentage of the exposed TiO2 surface area would be available for space charge regions (which are localized near Au and are important sites of hydroxyl radical generation) to develop. It is important to note that these arguments apply to UV-only experiments, such as those conducted in this work. The morphology of the Au coating will exert a more direct effect when visible light is used instead of or in addition to UV light. SPR depends heavily on the geometry and thickness of the Au coating [104,105]; thus, in experiments where SPR is important, the degradation rate would depend directly on the Au morphology. This was observed previously by Min et al. [42], who showed that by varying the number density and size of Au nanostructures supported on TiO2, the photocatalytic degradation rate of 1,4-dioxane could be controllably varied, and that maximizing surface plasmon absorption maximized the degradation rate.
5. Conclusions
In conclusion, we demonstrated that the photocatalytic degradation of 1,4-dioxane by Au/TiO2 JNPs shows a sensitive dependence on the UV wavelength that is selected, and we also provided explanatory insight into those results via finite-element simulations. Future work could build on our results in several ways, as described below.
First, it would be helpful to conduct photovoltage and photocurrent measurements of TiO2/Au nanoscale heterojunctions in the presence of UV light at both 254 and 365 nm and compare the results. Such measurements were beyond the scope of this work but would provide quantitative details as to the increase in photocurrent (and thus increased hydroxyl production) enabled under 254 nm light.
Second, although the primary focus of this study was detailed material characterization, future work should systematically identify (using, e.g., HPLC or NMR) the byproducts left behind after 1,4-dioxane degradation. Past studies on the degradation of 1,4-dioxane found that the main intermediate was 1,2-ethanediol diformate [14,106,107], along with ethylene glycol, glycolic acid, oxalate anion, and formic acid [108], with CO2 and H2O as the final products. As mentioned in the Introduction, one advantage of AOPs is that pollutants are typically completely mineralized into harmless byproducts. We expect, but have not verified, that the final degradation products in our case would be carbon dioxide and water.
Finally, another promising future direction would be to optimize the morphology of the Au coating. Our results imply that TiO2 nanoparticles decorated uniformly with Au “islands” may outperform Au/TiO2 Janus NPs, since these would increase the percentage of the TiO2 surface that contains the space charge regions that contain electron holes and produce pollutant-degrading hydroxyl radicals. This hypothesis could be tested by running a set of designed experiments with different Au coatings, including continuous Au “caps” and discrete Au “islands” with different sizes and number densities. One could then use a statistical technique, such as response surface methodology (RSM), to determine the optimum configuration of Au coatings to maximize the degradation rate. RSM could also be applied to optimize other conditions, such as the spectrum of the light source used. Such experiments were beyond the scope of this work.
Finally, since Au’s function is primarily to enable electron sequestration (rather than SPR, for which Au is often used), precious metals may not be needed, as it is work function differences between the metal and semiconductor that likely impact performance more than the conductivity of the metal. This observation suggests that the dramatic metal-associated performance increases observed in this work may be obtainable with more affordable metals, increasing the cost effectiveness and broadening the applicability of our approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17111708/s1. Contents of the Supporting Information include Au spectra of XPS data, computational modeling of particle transport, fluid flow, and degradation, Poisson–Nernst–Planck–Stokes equations, particle fabrication, particle and materials characterization, results of Poisson–Nernst–Planck–Stokes modeling, and photocatalysis experimental details. References [109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, D.M.W. and J.L.M.; methodology, Y.J., M.J.T., T.E.B., D.M.W., and J.L.M.; formal analysis, Y.J., M.J.T., L.O.M., A.K.S., Y.F., S.R., T.E.B., J.L.M., and D.M.W.; resources, D.M.W. and J.L.M.; writing—original draft preparation, M.J.T., Y.J., and A.K.S.; writing—review and editing, all authors; supervision, D.M.W. and J.L.M.; project administration, J.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
D.M.W. and J.L.M. acknowledge the Departments of Mechanical Engineering at Purdue University and George Mason University, respectively, for providing startup funds.
Data Availability Statement
Data will be made available by the corresponding authors upon reasonable request.
Acknowledgments
We appreciate thoughtful discussions with D. Houpt, J. Porter, T. Fu, and M. Ackerman during the experiments. We would like to thank H. Masand (Texas Instruments) for generating Figure S1. We acknowledge the support of the National Institute of Standards and Technology’s NanoFab and the University of Maryland NanoCenter and its FabLab for sputter coating facilities.
Conflicts of Interest
Author Lamar O. Mair was employed by the company Weinberg Medical Physics, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SPR | Surface plasmon resonance |
| JNP | Janus nanoparticle |
| AOP | Advanced oxidation process |
| RSM | Response surface methodology |
| DLS | Dynamic light scattering |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| EDS | Energy-dispersive X-ray spectroscopy |
| UV/Vis | Ultraviolet–visible spectroscopy |
| CEC | Contaminant of emerging concern |
| ROS | Reactive oxygen species |
| DI | Deionized (water) |
| HPLC | High-performance liquid chromatography |
| DAD | Diode-array detection |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| NaCl | Sodium chloride |
| MeOH | Methanol |
| UPS | Ultraviolet photoelectron spectroscopy |
| ●OH | Hydroxyl radical |
| CB | Conduction band |
| VB | Valence band |
References
- Stepanov, M.G.; Arutiunian, A.V.; Ailamazian, E.K. Disruption of Central Regulation of Reproductive Function under the Effect of Unfavorable Environmental Factors. Vopr. Med. Khimii 1995, 41, 33–35. [Google Scholar] [PubMed]
- 1,4-Dioxane Priority Existing Chemical No. 7 Full Public Report. 1998. Available online: https://www.industrialchemicals.gov.au/sites/default/files/PEC7-1-4-Dioxane.pdf (accessed on 19 May 2025).
- Lam, S.W.; Hermawan, M.; Coleman, H.M.; Fisher, K.; Amal, R. The Role of Copper(II) Ions in the Photocatalytic Oxidation of 1,4-Dioxane. J. Mol. Catal. A Chem. 2007, 278, 152–159. [Google Scholar] [CrossRef]
- Sittig, M. Handbook of Toxic and Hazardous Chemicals and Carcinogens: 1991—Third Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2009; ISBN -13-978-0815512868. [Google Scholar]
- Coleman, H.M.; Vimonses, V.; Leslie, G.; Amal, R. Degradation of 1,4-Dioxane in Water Using TiO2 Based Photocatalytic and H2O2/UV Processes. J. Hazard. Mater. 2007, 146, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Aǐlamazian, E.K. Effect of Ecological Factors on the Course of Pregnancy. Vestn. Akad. Med. Nauk SSSR 1990, 23–25. Available online: https://pubmed.ncbi.nlm.nih.gov/2220046/ (accessed on 19 May 2025). [PubMed]
- Godri Pollitt, K.J.; Kim, J.H.; Peccia, J.; Elimelech, M.; Zhang, Y.; Charkoftaki, G.; Hodges, B.; Zucker, I.; Huang, H.; Deziel, N.C.; et al. 1,4-Dioxane as an Emerging Water Contaminant: State of the Science and Evaluation of Research Needs. Sci. Total Environ. 2019, 690, 853–866. [Google Scholar] [CrossRef]
- Zhang, S.; Gedalanga, P.B.; Mahendra, S. Advances in Bioremediation of 1,4-Dioxane-Contaminated Waters. J. Environ. Manag. 2017, 204, 765–774. [Google Scholar] [CrossRef]
- Mohr, T.K.; Stickney, J.A.; DiGuiseppi, W.H.; Anderson, J. Environmental Investigation and Remediation 1,4-Dioxane and Other Solvent Stabilizers, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Stepien, D.K.; Diehl, P.; Helm, J.; Thoms, A.; Püttmann, W. Fate of 1,4-Dioxane in the Aquatic Environment: From Sewage to Drinking Water. Water Res. 2014, 48, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Adamson, D.T.; Mahendra, S.; Walker, K.L.; Rauch, S.R.; Sengupta, S.; Newell, C.J. A Multisite Survey to Identify the Scale of the 1,4-Dioxane Problem at Contaminated Groundwater Sites. Environ. Sci. Technol. Lett. 2014, 1, 254–258. [Google Scholar] [CrossRef]
- Reviews of environmental contamination and toxicology: Vol. 98 (Continuation of Residue Reviews). Edited by G.W. Ware, Springer-Verlag, Berlin, 1987. Pp. 166, ISBN 3 540 96448 7. Price: DM 92·00. Environ. Pollut. 1988, 50, 253–254. [CrossRef]
- Xu, X.; Liu, S.; Sun, P.; Guo, Z.; Smith, K.; Zhang, D.; Li, H.; Bedia, J.; Belver, C. Iron Tungstate on Nano-γ-Alumina as Photocatalyst for 1,4-Dioxane Solar Degradation in Water. J. Clean. Prod. 2022, 377, 134232. [Google Scholar] [CrossRef]
- Maurino, V.; Calza, P.; Minero, C.; Pelizzetti, E.; Vincenti, M. Light-Assisted 1,4-Dioxane Degradation. Chemosphere 1997, 35, 2675–2688. [Google Scholar] [CrossRef]
- Klečka, G.M.; Gonsior, S.J. Removal of 1,4-Dioxane from Wastewater. J. Hazard. Mater. 1986, 13, 161–168. [Google Scholar] [CrossRef]
- Adams, C.D.; Scanlan, P.A.; Secrist, N.D. Oxidation and Biodegradability Enhancement of 1,4-Dioxane Using Hydrogen Peroxide and Ozone. Environ. Sci. Technol. 1994, 28, 1812–1818. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H. Rate Constants of Reactions of Ozone with Organic and Inorganic Compounds in Water-I. Non-Dissociating Organic Compounds. Water Res. 1983, 17, 173–183. [Google Scholar] [CrossRef]
- Howard, P.H. Handbook of Environmental Fate and Exposure Data for Organic Chemicals; Taylor & Francis: New York, NY, USA, 1991. [Google Scholar]
- Sauvé, S.; Desrosiers, M. A Review of What Is an Emerging Contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef]
- Kamat, P.V.; Meisel, D. Nanoparticles in Advanced Oxidation Processes. Curr. Opin. Colloid Interface Sci. 2002, 7, 282–287. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.H. Challenges and Prospects of Advanced Oxidation Water Treatment Processes Using Catalytic Nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Araki, S.; Yamamoto, H. Evaluation of Advanced Oxidation Processes (AOP) Using O3, UV, and TiO2 for the Degradation of Phenol in Water. J. Water Process Eng. 2015, 7, 54–60. [Google Scholar] [CrossRef]
- Fernandes, A.; Makoś, P.; Wang, Z.; Boczkaj, G. Synergistic Effect of TiO2 Photocatalytic Advanced Oxidation Processes in the Treatment of Refinery Effluents. Chem. Eng. J. 2020, 391, 123488. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New Perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef]
- Venkatachalam, N.; Palanichamy, M.; Murugesan, V. Sol-Gel Preparation and Characterization of Alkaline Earth Metal Doped Nano TiO2: Efficient Photocatalytic Degradation of 4-Chlorophenol. J. Mol. Catal. A Chem. 2007, 273, 177–185. [Google Scholar] [CrossRef]
- Prakash, J.; Sun, S.; Swart, H.C.; Gupta, R.K. Noble Metals-TiO2 Nanocomposites: From Fundamental Mechanisms to Photocatalysis, Surface Enhanced Raman Scattering and Antibacterial Applications. Appl. Mater. Today 2018, 11, 82–135. [Google Scholar] [CrossRef]
- Chen, Y.; Bian, J.; Qi, L.; Liu, E.; Fan, J. Efficient Degradation of Methylene Blue over Two-Dimensional Au/TiO2 Nanosheet Films with Overlapped Light Harvesting Nanostructures. J. Nanomater. 2015, 2015, 905259. [Google Scholar] [CrossRef]
- Pradhan, S.; Ghosh, D.; Chen, S. Janus Nanostructures Based on Au-TiO2 Heterodimers and Their Photocatalytic Activity in the Oxidation of Methanol. ACS Appl. Mater. Interfaces 2009, 1, 2060–2065. [Google Scholar] [CrossRef]
- Mrowetz, M.; Villa, A.; Prati, L.; Selli, E. Effect of Au Nanoparticles on TiO2 in the Photocatalytic Degradation of an Azo Dye. Gold Bull. 2007, 40, 154–160. [Google Scholar] [CrossRef]
- Orlov, A.; Jefferson, D.A.; Tikhov, M.; Lambert, R.M. Enhancement of MTBE Photocatalytic Degradation by Modification of TiO2 with Gold Nanoparticles. Catal. Commun. 2007, 8, 821–824. [Google Scholar] [CrossRef]
- Bumajdad, A.; Madkour, M.; Abdel-Moneam, Y.; El-Kemary, M. Nanostructured Mesoporous Au/TiO2 for Photocatalytic Degradation of a Textile Dye: The Effect of Size Similarity of the Deposited Au with That of TiO2 Pores. J. Mater. Sci. 2014, 49, 1743–1754. [Google Scholar] [CrossRef]
- Dang, X.; Zhang, X.; Lu, Z.; Yang, Z.; Dong, X.; Zhang, X.; Ma, C.; Ma, H.; Xue, M.; Shi, F. Construction of Au@TiO2/Graphene Nanocomposites with Plasmonic Effect and Super Adsorption Ability for Enhanced Visible-Light-Driven Photocatalytic Organic Pollutant Degradation. J. Nanopartic. Res. 2014, 16, 2–9. [Google Scholar] [CrossRef]
- Do, T.C.M.V.; Nguyen, D.Q.; Nguyen, K.T.; Le, P.H. TiO2 and Au-TiO2 Nanomaterials for Rapid Photocatalytic Degradation of Antibiotic Residues in Aquaculture Wastewater. Materials 2019, 12, 2434. [Google Scholar] [CrossRef]
- Seh, Z.W.; Liu, S.; Low, M.; Zhang, S.Y.; Liu, Z.; Mlayah, A.; Han, M.Y. Janus Au-TiO2 Photocatalysts with Strong Localization of Plasmonic near-Fields for Efficient Visible-Light Hydrogen Generation. Adv. Mater. 2012, 24, 2310–2314. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Chang, I.G.; Tai, Y.; Wu, K.W. Effect of Surface Plasmon Resonance on the Photocatalytic Activity of Au/TiO2 under UV/Visible Illumination. J. Nanosci. Nanotechnol. 2012, 12, 416–422. [Google Scholar] [CrossRef]
- Yasmeen, H.; Zada, A.; Ali, S.; Khan, I.; Ali, W.; Khan, W.; Khan, M.; Anwar, N.; Ali, A.; Huerta-Flores, A.M.; et al. Visible Light-Excited Surface Plasmon Resonance Charge Transfer Significantly Improves the Photocatalytic Activities of ZnO Semiconductor for Pollutants Degradation. J. Chin. Chem. Soc. 2020, 67, 1611–1617. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, W.; Qian, X.Y.; Liu, J.B.; Wu, J.M. UV and Visible Light Photocatalytic Activity of Au/TiO2 Nanoforests with Anatase/Rutile Phase Junctions and Controlled Au Locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef] [PubMed]
- Jose, D.; Sorensen, C.M.; Rayalu, S.S.; Shrestha, K.M.; Klabunde, K.J. Au-TiO2 Nanocomposites and Efficient Photocatalytic Hydrogen Production under UV-Visible and Visible Light Illuminations: A Comparison of Different Crystalline Forms of TiO2. Int. J. Photoenergy 2013, 2013, 685614. [Google Scholar] [CrossRef]
- Chauhan, A.; Rastogi, M.; Scheier, P.; Bowen, C.; Kumar, R.V.; Vaish, R. Janus Nanostructures for Heterogeneous Photocatalysis. Appl. Phys. Rev. 2018, 5, 041111. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and Applications of Plasmon-Induced Charge Separation at TiO2 Films Loaded with Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef]
- Soler, L.; Magdanz, V.; Fomin, V.M.; Sanchez, S.; Schmidt, O.G. Self-Propelled Micromotors for Cleaning Polluted Water. ACS Nano 2013, 7, 9611–9620. [Google Scholar] [CrossRef]
- Min, B.K.; Heo, J.E.; Youn, N.K.; Joo, O.S.; Lee, H.; Kim, J.H.; Kim, H.S. Tuning of the Photocatalytic 1,4-Dioxane Degradation with Surface Plasmon Resonance of Gold Nanoparticles on Titania. Catal. Commun. 2009, 10, 712–715. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Shiraishi, Y.; Sugano, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Gold Nanoparticles Located at the Interface of Anatase/Rutile TiO2 Particles as Active Plasmonic Photocatalysts for Aerobic Oxidation. J. Am. Chem. Soc. 2012, 134, 6309–6315. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Hong, F.; Gao, Y.; Zeng, B.; Haider, R.S.; Fan, F.; Huang, J.; Li, C. Effects of the Interfacial Defects in Au/TiO2on Plasmon-Induced Water Oxidation. J. Chem. Phys. 2020, 152, 194702. [Google Scholar] [CrossRef]
- Primo, A.; Corma, A.; García, H. Titania Supported Gold Nanoparticles as Photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 886–910. [Google Scholar] [CrossRef] [PubMed]
- Lattuada, M.; Hatton, T.A. Synthesis, Properties and Applications of Janus Nanoparticles. Nano Today 2011, 6, 286–308. [Google Scholar] [CrossRef]
- Yaou Balarabe, B.; Maity, P. A Polymer-Au/TiO2 Nano-Composite Based Floating Catalyst for Photocatalytic Dye Degradation under Natural Sunlight. J. Photochem. Photobiol. A Chem. 2024, 449, 115405. [Google Scholar] [CrossRef]
- Youn, N.K.; Heo, J.E.; Joo, O.S.; Lee, H.; Kim, J.; Min, B.K. The effect of dissolved oxygen on the 1,4-dioxane degradation with TiO2 and Au-TiO2 photocatalysts. J. Hazard. Mater. 2010, 177, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Ganguly, P.; Maccioni, M.B.; Nolan, M.; Hermosilla, D.; Merayo, N.; Blanco, A.; Hinder, S.; Pillai, S.C. Impact of Au on the transition temperature and photocatalytic activity of TiO2. J. Photochem. Photobiol. A 2024, 456, 115848. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Toi, S.; Ichikawa, S.; Hirai, T. Photocatalytic NH3 Splitting on TiO2 Particles Decorated with Pt-Au Bimetallic Alloy Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 1612–1620. [Google Scholar] [CrossRef]
- Collado, L.; Reynal, A.; Coronado, J.M.; Serrano, D.P.; Durrant, J.R.; De la Peña O’Shea, V.A. Effect of Au Surface Plasmon Nanoparticles on the Selective CO2 Photoreduction to CH4. Appl. Catal. B Environ. 2015, 178, 177–185. [Google Scholar] [CrossRef]
- Collado, L.; Reynal, A.; Fresno, F.; Barawi, M.; Escudero, C.; Perez-Dieste, V.; Coronado, J.M.; Serrano, D.P.; Durrant, J.R.; de la Peña O’Shea, V.A. Unravelling the Effect of Charge Dynamics at the Plasmonic Metal/Semiconductor Interface for CO2 Photoreduction. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Fontelles-Carceller, O.; Muñoz-Batista, M.J.; Rodríguez-Castellón, E.; Conesa, J.C.; Fernández-García, M.; Kubacka, A. Measuring and Interpreting Quantum Efficiency for Hydrogen Photo-Production Using Pt-Titania Catalysts. J. Catal. 2017, 347, 157–169. [Google Scholar] [CrossRef]
- Hernández Rodríguez, M.J.; Pulido Melián, E.; García Santiago, D.; González Díaz, O.; Navío, J.A.; Doña Rodríguez, J.M. NO Photooxidation with TiO2 Photocatalysts Modified with Gold and Platinum. Appl. Catal. B Environ. 2017, 205, 148–157. [Google Scholar] [CrossRef]
- Kowalska, E.; Abe, R.; Ohtani, B. Visible Light-Induced Photocatalytic Reaction of Gold-Modified Titanium(IV) Oxide Particles: Action Spectrum Analysis. Chem. Commun. 2009, 9, 241–243. [Google Scholar] [CrossRef]
- Ghasemi, S.; Hashemian, S.J.; Alamolhoda, A.A.; Gocheva, I.; Rahman Setayesh, S. Plasmon Enhanced Photocatalytic Activity of Au@TiO2-Graphene Nanocomposite under Visible Light for Degradation of Pollutants. Mater. Res. Bull. 2017, 87, 40–47. [Google Scholar] [CrossRef]
- Xu, L.; Mou, F.; Gong, H.; Luo, M.; Guan, J. Light-Driven Micro/Nanomotors: From Fundamentals to Applications. Chem. Soc. Rev. 2017, 46, 6905–6926. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Cai, Y.; Yang, Y.; Gao, W.; Ren, B. Photocatalytic Micro/Nanomotors: From Construction to Applications. Acc. Chem. Res. 2018, 51, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, Q.; Gao, W.; Pei, A.; Ren, B. Highly Efficient Light-Driven TiO2-Au Janus Micromotors. ACS Nano 2016, 10, 839–844. [Google Scholar] [CrossRef]
- Maric, T.; Nasir, M.Z.M.; Webster, R.D.; Pumera, M. Tailoring Metal/TiO2 Interface to Influence Motion of Light-Activated Janus Micromotors. Adv. Funct. Mater. 2020, 30, 1908614. [Google Scholar] [CrossRef]
- Moran, J.L.; Wheat, P.M.; Posner, J.D. Locomotion of Electrocatalytic Nanomotors Due to Reaction Induced Charge Autoelectrophoresis. Phys. Rev. E Stat. Nonlinear, Soft Matter Phys. 2010, 81, 065302. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, J.; Duan, S.; Lv, X.; Wang, J.; Ma, X.; Tang, J.; Wang, W. Bimetallic Coatings Synergistically Enhance the Speeds of Photocatalytic TiO2 Micromotors. Chem. Commun. 2020, 56, 4728–4731. [Google Scholar] [CrossRef]
- Paxton, W.F.; Sen, A.; Mallouk, T.E. Motility of Catalytic Nanoparticles through Self-Generated Forces. Chem. Eur. J. 2005, 11, 6462–6470. [Google Scholar] [CrossRef]
- Mitchell, P. Self-Electrophoretic Locomotion in Microorganisms: Bacterial Flagella as Giant Ionophores. FEBS Lett. 1972, 28, 1–4. [Google Scholar] [CrossRef]
- Paxton, W.F.; Baker, P.T.; Kline, T.R.; Wang, Y.; Mallouk, T.E.; Sen, A. Catalytically Induced Electrokinetics for Motors and Micropumps. J. Am. Chem. Soc. 2006, 128, 14881–14888. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Ng, D.H.L.; Liu, J.; Liu, Y.; Yang, W. 3D Hierarchical ACFs-Based Micromotors as Efficient Photo-Fenton-like Catalysts. Carbon 2020, 158, 738–748. [Google Scholar] [CrossRef]
- Vilela, D.; Parmar, J.; Zeng, Y.; Zhao, Y.; Sánchez, S. Graphene-Based Microbots for Toxic Heavy Metal Removal and Recovery from Water. Nano Lett. 2016, 16, 2860–2866. [Google Scholar] [CrossRef]
- Wang, H.; Gu, X.; Wang, C. Self-Propelling Hydrogel/Emulsion-Hydrogel Soft Motors for Water Purification. ACS Appl. Mater. Interfaces 2016, 8, 9413–9422. [Google Scholar] [CrossRef]
- Soler, L.; Sánchez, S. Catalytic Nanomotors for Environmental Monitoring and Water Remediation. Nanoscale 2014, 6, 7175–7182. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Kierulf, A.; Abbaspourrad, A. Nano- and Micromotors for Cleaning Polluted Waters: Focused Review on Pollutant Removal Mechanisms. Nanoscale 2017, 9, 13850–13863. [Google Scholar] [CrossRef] [PubMed]
- Parmar, J.; Vilela, D.; Villa, K.; Wang, J.; Sánchez, S. Micro- and Nanomotors as Active Environmental Microcleaners and Sensors. J. Am. Chem. Soc. 2018, 140, 9317–9331. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, R.; Zhang, Q.; Ren, B. Dye-Enhanced Self-Electrophoretic Propulsion of Light-Driven TiO2-Au Janus Micromotors. Nano-Micro Lett. 2017, 9, 30. [Google Scholar] [CrossRef]
- Luna, M.; Barawi, M.; Gómez-Moñivas, S.; Colchero, J.; Rodríguez-Peña, M.; Yang, S.; Zhao, X.; Lu, Y.H.; Chintala, R.; Reñones, P.; et al. Photoinduced Charge Transfer and Trapping on Single Gold Metal Nanoparticles on TiO2. ACS Appl. Mater. Interfaces 2021, 13, 50531–50538. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Falaras, P.; Likodimos, V.; O’Shea, K.; de la Cruz, A.A.; Dunlop, P.S.M.; Byrne, J.A.; Dionysiou, D.D. Use of Selected Scavengers for the Determination of NF-TiO2 Reactive Oxygen Species during the Degradation of Microcystin-LR under Visible Light Irradiation. J. Mol. Catal. A Chem. 2016, 425, 183. [Google Scholar] [CrossRef]
- Granick, S.; Jiang, S.; Chen, Q. Janus Particles. Phys. Today 2009, 62, 68. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Zhu, X.; Kong, X.Z. Preparation of Hollow TiO2 Nanoparticles through TiO2 Deposition on Polystyrene Latex Particles and Characterizations of Their Structure and Photocatalytic Activity. Nanoscale Res. Lett. 2012, 7, 646. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Ahmad, M.; Nisar, A.; Sun, H.; Karim, S.; Khan, M.; Khan, S.D.; Iqbal, M.; Hussain, S.Z. Enhanced Photocatalytic and Electrochemical Properties of Au Nanoparticles Supported TiO2 Microspheres. New J. Chem. 2014, 38, 1424–1432. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of Oxygen Vacancies and Ti3+ State in TiO2 Thin Film and Enhanced Optical Properties by Air Plasma Treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, R.; Kait, C.F.; Chia, H.Y.; Isa, M.H.; Huei, L.W. Glycerol-Mediated Facile Synthesis of Colored Titania Nanoparticles for Visible Light Photodegradation of Phenolic Compounds. Nanomaterials 2019, 9, 1586. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, S.; Wan, P.; Sun, J.; Hood, Z.D. Introducing Ti3+ Defects Based on Lattice Distortion for Enhanced Visible Light Photoreactivity in TiO2 Microspheres. RSC Adv. 2017, 7, 32461–32467. [Google Scholar] [CrossRef]
- Veziroglu, S.; Ullrich, M.; Hussain, M.; Drewes, J.; Shondo, J.; Strunskus, T.; Adam, J.; Faupel, F.; Aktas, O.C. Plasmonic and Non-Plasmonic Contributions on Photocatalytic Activity of Au-TiO2 Thin Film under Mixed UV–Visible Light. Surf. Coatings Technol. 2020, 389, 125613. [Google Scholar] [CrossRef]
- Bertóti, I.; Mohai, M.; Sullivan, J.L.; Saied, S.O.; Bertóti, I.; Mohai, M.; Sullivan, J.L.; Saied, S.O. Surface Characterisation of Plasma-Nitrided Titanium: An XPS Study. Appl. Surf. Sci. 1995, 84, 357–371. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, H.X.; Qiu, Y.; Liu, S.Q.; Diao, J.X.; Chang, C.R.; Si, R.; Guo, X.H. An Oxygen Vacancy-Rich Two-Dimensional Au/TiO2 Hybrid for Synergistically Enhanced Electrochemical N2 Activation and Reduction. J. Mater. Chem. A 2020, 8, 6586–6596. [Google Scholar] [CrossRef]
- Liu, X.; Gao, S.; Xu, H.; Lou, Z.; Wang, W.; Huang, B.; Dai, Y. Green Synthetic Approach for Ti3+ Self-Doped TiO2-x Nanoparticles with Efficient Visible Light Photocatalytic Activity. Nanoscale 2013, 5, 1870–1875. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Pumera, M. Light-Powered Self-Adaptive Mesostructured Microrobots for Simultaneous Microplastics Trapping and Fragmentation via in Situ Surface Morphing. Small 2023, 19, 2301467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dong, R.; Wu, Y.; Gao, W.; He, Z.; Ren, B. Light-Driven Au-WO3@C Janus Micromotors for Rapid Photodegradation of Dye Pollutants. ACS Appl. Mater. Interfaces 2017, 9, 4674–4683. [Google Scholar] [CrossRef] [PubMed]
- Manion, J.A.; Huie, R.E.; Levin, R.D.; Burgess, D.R., Jr.; Orkin, V.L.; Tsang, W.; McGivern, W.S.; Hudgens, J.W.; Knyazev, V.D.; Atkinson, D.B.; et al. NIST Chemical Kinetics Database (Web Version). Available online: http://kinetics.nist.gov/ (accessed on 25 April 2025).
- Yan, N.; Liu, F.; Liu, B.; Brusseau, M.L. Treatment of 1,4-Dioxane and Trichloroethene Co-Contamination by an Activated Binary Persulfate-Peroxide Oxidation Process. Environ. Sci. Pollut. Res. 2018, 25, 32088–32095. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.M.; Chiesa, M. EPR of Paramagnetic Centres on Solid Surfaces. In Electron Paramagnetic Resonance; Royal Society of Chemistry: London, UK, 2009; Volume 21, pp. 105–130. [Google Scholar]
- Bockris, J.O.M.; Oldfield, L.F. The Oxidation-Reduction Reactions of Hydrogen Peroxide at Inert Metal Electrodes and Mercury Cathodes. Trans. Faraday Soc. 1955, 51, 249–259. [Google Scholar] [CrossRef]
- Buettner, G.R. The Pecking Order of Free Radicals and Antioxidants: Lipid Peroxidation, α-Tocopherol, and Ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef]
- Heck, K.N.; Wang, Y.; Wu, G.; Wang, F.; Tsai, A.L.; Adamson, D.T.; Wong, M.S. Effectiveness of Metal Oxide Catalysts for the Degradation of 1,4-Dioxane. RSC Adv. 2019, 9, 27042–27049. [Google Scholar] [CrossRef]
- Feng, C.; Yu, Z.; Liu, H.; Yuan, K.; Wang, X.; Zhu, L.; Zhang, G.; Xu, D. Enhanced Photocatalytic Performance of Au/TiO2 Nanofibers by Precisely Manipulating the Dosage of Uniform-Sized Au Nanoparticles. Appl. Phys. A Mater. Sci. Process. 2017, 123, 519. [Google Scholar] [CrossRef]
- Winkelmann, J. Diffusion Coefficient of 1,4-Dioxane in Water. In Diffusion in Gases, Liquids and Electrolytes; Springer: Berlin/Heidelberg, Germany, 2018; pp. 547–549. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface Plasmon Resonance in Gold Nanoparticles: A Review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Majeed, I.; Ali, H.; Idrees, A.; Arif, A.; Ashraf, W.; Rasul, S.; Khan, M.A.; Nadeem, M.A.; Nadeem, M.A. Understanding the Role of Metal Supported on TiO2 in Photoreforming of Oxygenates. Energy Adv. 2022, 1, 842–867. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T. Band Bending in Semiconductors: Chemical and Physical Consequences at Surfaces and Interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/Gold Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.H.; Cheng, W.H.; Crumlin, E.J.; Drisdell, W.S.; Atwater, H.A.; Schmeißer, D.; Lewis, N.S.; Brunschwig, B.S. X-Ray Photoelectron Spectroscopy and Resonant X-Ray Spectroscopy Investigations of Interactions between Thin Metal Catalyst Films and Amorphous Titanium Dioxide Photoelectrode Protection Layers. Chem. Mater. 2021, 33, 1265–1275. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.Q.; Fu, X.; Zhang, N.; Xu, Y.J. Defective TiO2 with Oxygen Vacancies: Synthesis, Properties and Photocatalytic Applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, A.C.; Beglitis, N.S.; Pang, C.L.; Teobaldi, G.; Cabailh, G.; Chen, Q.; Fisher, A.J.; Hofer, W.A.; Thornton, G. Electron Traps and Their Effect on the Surface Chemistry of TiO2(110). Proc. Natl. Acad. Sci. USA 2010, 107, 2391–2396. [Google Scholar] [CrossRef]
- Sun, H.; Yu, M.; Wang, G.; Sun, X.; Lian, J. Temperature-Dependent Morphology Evolution and Surface Plasmon Absorption of Ultrathin Gold Island Films. J. Phys. Chem. C 2012, 116, 9000–9008. [Google Scholar] [CrossRef]
- Juvé, V.; Cardinal, M.F.; Lombardi, A.; Crut, A.; Maioli, P.; Pérez-Juste, J.; Liz-Marzán, L.M.; Del Fatti, N.; Vallée, F. Size-Dependent Surface Plasmon Resonance Broadening in Nonspherical Nanoparticles: Single Gold Nanorods. Nano Lett. 2013, 13, 2234–2240. [Google Scholar] [CrossRef]
- Hill, R.R.; Jeffs, G.E.; Roberts, D.R. Photocatalytic Degradation of 1,4-Dioxane in Aqueous Solution. J. Photochem. Photobiol. A Chem. 1997, 108, 55–88. [Google Scholar] [CrossRef]
- Yamazaki, S.; Yamabe, N.; Nagano, S.; Fukuda, A. Adsorption and Photocatalytic Degradation of 1,4-Dioxane on TiO2. J. Photochem. Photobiol. A Chem. 2007, 185, 150–155. [Google Scholar] [CrossRef]
- Tawfik, A. Degradation Pathways of 1,4-Dioxane in Biological and Advanced Oxidation Processes. Desalin. Water Treat. 2020, 178, 360–386. [Google Scholar] [CrossRef]
- Padikkaparambil, S.; Narayanan, B.; Yaakob, Z.; Viswanathan, S.; Tasirin, S.M. Au/TiO2 Reusable Photocatalysts for Dye Degradation. Int. J. Photoenergy 2013, 2013, 752605. [Google Scholar] [CrossRef]
- Dorfman, L.E.; Adams, G.E. Reactivity of the Hydroxyl Radical in Aqueous Solutions; National Bureau of Standards: Gaithersburg, MD, USA, 1972.
- Schmid, J.; Hoenes, K.; Rath, M.; Vatter, P.; Hessling, M. Antimicrobial efficacy of 222 nm UVC light in the hospital environment. GMS Hyg. Infect. Control 2017, 12, Doc06. [Google Scholar] [PubMed]
- Chitra, S.; Paramasivan, K.; Cheralathan, M.; Sinha, P.K. Degradation of 1,4-dioxane using advanced oxidation processes. Environ. Sci. Pollut. Res. 2012, 19, 871–878. [Google Scholar] [CrossRef]
- Barndõk, H.; Hermosilla, D.; Han, C.; Dionysiou, D.D.; Negro, C.; Blanco, Á. Degradation of pharmaceuticals in water by advanced oxidation processes: A review. Appl. Catal. B Environ. 2016, 180, 44–52. [Google Scholar] [CrossRef]
- Seshadri, H.; Cheralathan, M.; Sinha, P.K. Photocatalytic performance of combustion-synthesized β and γ-Ga2O3 in the degradation of 1,4-dioxane in aqueous solution. Res. Chem. Intermed. 2013, 39, 991–1001. [Google Scholar] [CrossRef]
- Wang, W.; Qiao, Z.; Lee, G.J.; Chen, H.; Ding, L.; Zhu, M.; Liu, N.; Wu, J.J. In-situ anodic precipitation process for highly efficient separation of aluminium from impurities. Sep. Purif. Technol. 2020, 235, 116194. [Google Scholar] [CrossRef]
- Wang, W.M.; Zhang, L.; Wang, W.L.; Huang, J.Y.; Wu, Q.Y.; Wu, J.J. Photocatalytic Degradation of 1,4-Dioxane by Heterostructured Bi2O3/Cu-MOF with Oxygen Vacancies. Catalysts 2023, 13, 1211. [Google Scholar] [CrossRef]
- Son, H.S.; Im, J.K.; Zoh, K.D. A Fenton-like degradation mechanism for 1,4-dioxane using zero-valent iron (Fe⁰) and UV light. Water Res. 2009, 43, 1457–1463. [Google Scholar] [CrossRef]
- Xu, X.; Liu, S.; Smith, K.; Wang, Y.; Hu, H. Photocatalytic degradation of 1,4-dioxane using TiO2 modified with W: Influence of W content and calcination temperature. Chem. Eng. J. 2019, 373, 508. [Google Scholar]
- Mehrvar, M.; Anderson, W.A.; Moo-Young, M. Photocatalytic degradation of aqueous organic contaminants by UV/H2O2: Process optimization using response surface methodology. Int. J. Photoenergy 2000, 2, 67–80. [Google Scholar] [CrossRef]
- Mehrvar, M.; Anderson, W.A.; Moo-Young, M. Photocatalytic degradation of aqueous organic contaminants by UV/H2O2: A kinetic approach. Int. J. Photoenergy 2002, 4, 141–146. [Google Scholar] [CrossRef]
- Park, Y.K.; Chung, K.H.; Park, I.S.; Kim, S.C.; Kim, S.J.; Jung, S.C. Photocatalytic degradation of 1,4-dioxane using TiO2 under UV irradiation: Kinetics and mechanism. J. Hazard. Mater. 2020, 399, 123087. [Google Scholar] [CrossRef]
- Byrne, C.; Dervin, S.; Hermosilla, D.; Merayo, N.; Blanco, Á.; Hinder, S.; Harb, M.; Dionysiou, D.D.; Pillai, S.C. A review of heterogeneous photocatalysis for water and wastewater treatment: Fundamentals, mechanisms, and applications. Catal. Today 2021, 380, 199–208. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Coulter, J.B.; Birnie, D.P. Determining direct and indirect optical band gap energies from UV–Vis absorption spectra. Phys. Status Solidi Basic Res. 2018, 255, 1700393. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Band Gap Analysis Through UV-Visible Spectroscopy; Thermo Fisher Scientific: Waltham, MA, USA, 2023. [Google Scholar]
- Zhao, Y.; Jia, X.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; O’Hare, D.; Zhang, T. Layered double hydroxide nanostructured photocatalysts for water splitting: A review. Adv. Energy Mater. 2016, 6, 1501974. [Google Scholar] [CrossRef]
- Miao, L.; Jin, P.; Kaneko, K.; Terai, A.; Nabatova-Gabain, N.; Tanemura, S. Preparation and characterization of TiO2 thin films on Si substrates by sol–gel method. Appl. Surf. Sci. 2003. [Google Scholar] [CrossRef]
- Nagaraj, G.; Brundha, D.; Chandraleka, C.; Arulpriya, M.; Kowsalya, V.; Sangavi, S.; Jayalakshmi, R.; Tamilarasu, S.; Murugan, R. Green synthesis of zinc oxide nanoparticles using plant extracts and their antimicrobial activity: A review. SN Appl. Sci. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, X.; Chen, S.; Ashok, J.; Kawi, S. Oxygen-Deficient WO3/TiO2/CC Nanorod Arrays for Visible-Light Photocatalytic Degradation of Methylene Blue. Catalysts 2021, 11, 1349. [Google Scholar] [CrossRef]
- Senthil, R.A.; Theerthagiri, J.; Selvi, A.; Madhavan, J. Synthesis and characterization of low-cost g-C3N4/TiO2 composite with enhanced photocatalytic performance under visible-light irradiation. Opt. Mater. 2017, 64, 533–539. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).