Dynamics of Nitrogen and Phosphorus Release from Submerged Soil–Plant Systems in the Three Gorges Reservoir
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Soil–Plant System
2.3. Experimental Setup and Sampling
2.4. Analysis Methods
3. Result
3.1. Initial Mass and Nutrient Content of the Soil–Plant System
3.2. The Nutrient Concentration of Overlying Water Changed During the Experiment
3.2.1. Nitrogen Form and Concentration Change of Overlying Water in Soil–Plant System
3.2.2. Phosphorus Form and Concentration Change of Overlying Water in Soil–Plant System
3.3. The Release Amount of Nutrients into Overlying Water After 200-d Submergence
3.3.1. Nitrogen Release
3.3.2. Phosphorus Release
3.3.3. Correlations of Nutrient Release and Initial Properties
4. Discussion
4.1. Nutrient Release and Pollution Loads via Submerged Soil–Plant System
4.2. Interaction of Soil and Plant in the Soil–Plant System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elliott, J.A. Predicting the impact of changing nutrient load and temperature on the phytoplankton of England’s largest lake, Windermere. Freshw. Biol. 2012, 57, 400–413. [Google Scholar] [CrossRef]
- Liu, S.S.; Zhu, Y.R.; Meng, W.; He, Z.Q.; Feng, W.Y.; Zhang, C.; Giesy, J.P. Characteristics and degradation of carbon and phosphorus from aquatic macrophytes in lakes: Insights from solid-state 13C NMR and solution 31P NMR spectroscopy. Sci. Total Environ. 2016, 543, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Li, H.; Xiao, J.; Zhou, Y.Y.; Song, C.L.; Bi, Y.H.; Cao, X.Y. Enhancement of sediment phosphorus release during a tunnel construction across an urban lake (Lake Donghu, China). Environ. Sci. Pollut. Res. 2016, 23, 17774–17783. [Google Scholar] [CrossRef]
- Meng, Z.J.; Yu, X.B.; Xia, S.X.; Zhang, Q.J.; Ma, X.; Yu, D.K. Effects of water depth on the biomass of two dominant submerged macrophyte species in floodplain lakes during flood and dry seasons. Sci. Total Environ. 2023, 877, 162690. [Google Scholar] [CrossRef]
- Lavoie, M.; Auclair, J.C. Phosphorus Mobilization at the Sediment-Water Interface in Softwater Shield Lakes: The Role of Organic Carbon and Metal Oxyhydroxides. Aquat. Geochem. 2012, 18, 327–341. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, B. Changes of riparian soil-plant system phosphorus responding to hydrological alternations of Three Gorges Reservoir. Sci. Rep. 2025, 15, 5629. [Google Scholar] [CrossRef]
- Zhang, S.M.; Wang, F.S.; Wang, Y.C.; Chen, X.P.; Xu, P.F.; Miao, H.C. Shifts of soil archaeal nitrification and methanogenesis with elevation in water level fluctuation zone of the three Gorges Reservoir, China. J. Environ. Manag. 2023, 339, 117871. [Google Scholar] [CrossRef]
- Guo, E.; Fan, Z.; Zhang, R.; Sun, J.; Yang, X. Research progresses on the response of vegetation and soil to hydrological changes in riparian ecosystems. Acta Ecol. Sin. 2021, 41, 9164–9173. [Google Scholar]
- Percuoco, V.P.; Kalnejais, L.H.; Officer, L.V. Nutrient release from the sediments of the Great Bay Estuary, NH USA. Coast. Shelf Sci. 2015, 161, 76–87. [Google Scholar] [CrossRef]
- Shaughnessy, A.R.; Sloan, J.J.; Corcoran, M.J.; Hasenmueller, E.A. Sediments in Agricultural Reservoirs Act as Sinks and Sources for Nutrients over Various Timescales. Water Resour. Res. 2019, 55, 5985–6000. [Google Scholar] [CrossRef]
- Hale, R.; Reich, P.; Daniel, T.; Lake, P.S.; Cavagnaro, T.R. Assessing changes in structural vegetation and soil properties following riparian restoration. Agric. Ecosyst. Environ. 2018, 252, 22–29. [Google Scholar] [CrossRef]
- Huang, J.T.; Luo, Z.; Xu, Z.P.; Jiang, Y.X.; Guo, J.S. Interactions between N, P in the overlying water and flooding-induced decomposition of Cynodon dactylon in the water-level fluctuation zone. Front. Plant Sci. 2025, 16, 1526507. [Google Scholar] [CrossRef]
- Shi, P.; Schulin, R. Erosion-induced losses of carbon, nitrogen, phosphorus and heavy metals from agricultural soils of contrasting organic matter management. Sci. Total Environ. 2018, 618, 210–218. [Google Scholar] [CrossRef]
- Tang, X.Q.; Wu, M.; Li, R. Distribution, sedimentation, and bioavailability of particulate phosphorus in the mainstream of the Three Gorges Reservoir. Water Res. 2018, 140, 44–55. [Google Scholar] [CrossRef]
- Chlot, S.; Widerlund, A.; Öhlander, B. Interaction between nitrogen and phosphorus cycles in mining-affected aquatic systems-experiences from field and laboratory measurements. Environ. Sci. Pollut. Res. 2013, 20, 5722–5736. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Xin, X.K.; Li, J.; Jia, H.Y.; Wen, L.Q.; Yin, W. Water level-driven agricultural nonpoint source pollution dominated the ammonia variation in China’s second largest reservoir. Environ. Res. 2022, 215, 114367. [Google Scholar] [CrossRef]
- Ma, L.Y.; Qi, X.; Zhou, S.Q.; Niu, H.F.; Zhang, T.X. Spatiotemporal distribution of phosphorus fractions and the potential release risks in sediments in a Yangtze River connected lake: New insights into the influence of water-level fluctuation. J. Soils Sediments 2023, 23, 496–511. [Google Scholar] [CrossRef]
- Geurts, J.J.M.; Smolders, A.J.P.; Banach, A.M.; de Graaf, J.; Roelofs, J.G.M.; Lamers, L.P.M. The interaction between decomposition, net N and P mineralization and their mobilization to the surface water in fens. Water Res. 2010, 44, 3487–3495. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.Q.; Zhang, Y.L.; Zhu, G.W.; Gao, G. Eutrophication control of large shallow lakes in China. Sci. Total Environ. 2023, 881, 163494. [Google Scholar] [CrossRef]
- Blackwell, M.S.A.; Brookes, P.C.; de la Fuente-Martinez, N.; Murray, P.J.; Snars, K.E.; Williams, J.K.; Haygarth, P.M. Effects of soil drying and rate of re-wetting on concentrations and forms of phosphorus in leachate. Biol. Fertil. Soils 2009, 45, 635–643. [Google Scholar] [CrossRef]
- Klatt, S.; Kraus, D.; Kraft, P.; Breuer, L.; Wlotzka, M.; Heuveline, V.; Haas, E.; Kiese, R.; Butterbach-Bahl, K. Exploring impacts of vegetated buffer strips on nitrogen cycling using a spatially explicit hydro-biogeochemical modeling approach. Environ. Model. Softw. 2017, 90, 55–67. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Zhang, Y.W.; Liu, H.; Xiong, S.; Li, B.; Deng, W. The littoral zone in the Three Gorges Reservoir, China: Challenges and opportunities. Environ. Sci. Pollut. Res. 2013, 20, 7092–7102. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Guo, P.; Zhang, Q.H.; Hu, H.; Hong, H.; Zhang, L.; Yang, Y.S.; Xia, Z.Y.; Li, M.Y.; Kang, H.L.; et al. Variation in soil properties and its influence on the dynamic change of soil erosion resistance to overland flow in the water-level fluctuation zone of the Three Gorges Reservoir, China. Catena 2022, 213, 106141. [Google Scholar] [CrossRef]
- Xiao, L.W.; Zhu, B.; Kumwimba, M.N.; Jiang, S.W. Plant soaking decomposition as well as nitrogen and phosphorous release in the water-level fluctuation zone of the Three Gorges Reservoir. Sci. Total Environ. 2017, 592, 527–534. [Google Scholar] [CrossRef]
- Li, Y.Y.; Gao, B.; Xu, D.Y.; Lu, J.; Zhou, H.D.; Gao, L. Heavy metals in the water-level-fluctuation zone soil of the three Gorges Reservoir, China: Remobilization and catchment-wide transportation. J. Hydrol. 2022, 612, 128108. [Google Scholar] [CrossRef]
- Guo, P.; Xiao, H.; Gao, F.; Li, Z.J.; Hu, H.; Zhang, Q.H.; Xia, Z.Y.; Li, M.Y.; Yang, Y.S. The vertical heterogeneity of soil detachment by overland flow on the water-level fluctuation zone slope in the Three Gorges Reservoir, China The vertical heterogeneity of soil detachment capacity. Hydrol. Process. 2021, 35, e14282. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, L.; Zhu, B. Characteristics of Greenhouse Gases Emission under Inundation from Dominant Herbaceous Plants in the Water-level Fluctuation Zone of the Three Gorges Reservoir. Resour. Environ. Yangtze Basin 2020, 29, 1965–1974. [Google Scholar]
- Su, Y.M.; Liu, W.B.; Rahaman, M.H.; Chen, Z.B.; Zhai, J. Methane emission from water level fluctuation zone of the Three Gorges Reservoir: Seasonal variation and microbial mechanism. Sci. Total Environ. 2024, 912, 168935. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Cheng, X.L.; Zhang, Q.F. Recovery approach affects soil quality in the water level fluctuation zone of the Three Gorges Reservoir, China: Implications for revegetation. Environ. Sci. Pollut. Res. 2014, 21, 2018–2031. [Google Scholar] [CrossRef]
- Chen, C.D.; Cheng, H.; Jia, J.S.; Wang, X.Y.; Zhao, J.J. Use it or not: An agro-ecological perspective to flooded riparian land along the Three Gorges Reservoir. Sci. Total Environ. 2019, 650, 1062–1072. [Google Scholar] [CrossRef]
- Hanlon, R.D.G. The Breakdown and Decomposition of Allochthonous and Autochthonous Plant Litter in an Oligotrophic Lake Frongoch, Llyn. Hydrobiologia 1982, 88, 281–288. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Zak, J.C. Effects of Gap Size on Litter Decomposition and Microbial Activity in a Subtropical Forest. Ecology 1995, 76, 2196–2204. [Google Scholar] [CrossRef]
- Jacobson, T.K.B.; Bustamante, M.M.D.; Kozovits, A.R. Diversity of shrub tree layer, leaf litter decomposition and N release in a Brazilian Cerrado under N, P and N plus P additions. Environ. Pollut. 2011, 159, 2236–2242. [Google Scholar] [CrossRef]
- Xie, Y.H.; Qin, H.Y.; Yu, D. Nutrient limitation to the decomposition of water hyacinth (Eichhornia crassipes). Hydrobiologia 2004, 529, 105–112. [Google Scholar] [CrossRef]
- Lu, J.; Bunn, S.E.; Burford, M.A. Nutrient release and uptake by littoral macrophytes during water level fluctuations. Sci. Total Environ. 2018, 622, 29–40. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.S.; Zhang, Y.; Liu, B.Y.; Zhou, Q.H.; Zeng, L.; He, F.; Wu, Z.B. Synergistic removal effect of P in sediment of all fractions by combining the modified bentonite granules and submerged macrophyte. Sci. Total Environ. 2018, 626, 458–467. [Google Scholar] [CrossRef]
- Yao, X.D.; Zhang, N.L.; Zeng, H.; Wang, W. Effects of soil depth and plant-soil interaction onmicrobial community in temperate grasslands of northern China. Sci. Total Environ. 2018, 630, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Smolders, A.J.P.; Lamers, L.P.M.; den Hartog, C.; Roelofs, J.G.M. Mechanisms involved in the decline of Stratiotes aloides L. in The Netherlands: Sulphate as a key variable. Hydrobiologia 2003, 506, 603–610. [Google Scholar] [CrossRef]
- Tan, S.D.; Zhu, M.Y.; Zhang, Q.F. Physiological responses of bermudagrass (Cynodon dactylon) to submergence. Acta Physiol. Plant. 2010, 32, 133–140. [Google Scholar] [CrossRef]
- Jiang, X.J.; Liu, W.J.; Chen, C.F.; Liu, J.Q.; Yuan, Z.Q.; Jin, B.C.; Yu, X.Y. Effects of three morphometric features of roots on soil water flow behavior in three sites in China. Geoderma 2018, 320, 161–171. [Google Scholar] [CrossRef]
- Liu, J.H.; Zeng, B.; Lin, F.; Ayi, Q.L. Effects of water level regulation on the seed germination and production of annual plant Xanthium sibiricum in the water-level-fluctuating-zone of Three Gorges Reservoir. Sci. Rep. 2017, 7, 5056. [Google Scholar] [CrossRef] [PubMed]
- Fraaije, R.G.A.; ter Braak, C.J.F.; Verduyn, B.; Breeman, L.B.S.; Verhoeven, J.T.A.; Soons, M.B. Early plant recruitment stages set the template for the development of vegetation patterns along a hydrological gradient. Funct. Ecol. 2015, 29, 971–980. [Google Scholar] [CrossRef]
- Garssen, A.G.; Baattrup-Pedersen, A.; Riis, T.; Raven, B.M.; Hoffman, C.C.; Verhoeven, J.T.A.; Soons, M.B. Effects of increased flooding on riparian vegetation: Field experiments simulating climate change along five European lowland streams. Global Change Biol. 2017, 23, 3052–3063. [Google Scholar] [CrossRef] [PubMed]
- Lu, R. Soil Agricultural Chemistry Analysis; Chinese Agriculture and Technology Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Müller, S.; Mitrovic, S.M.; Baldwin, D.S. Oxygen and dissolved organic carbon control release of N, P and Fe from the sediments of a shallow, polymictic lake. J. Soils Sediments 2016, 16, 1109–1120. [Google Scholar] [CrossRef]
- Chao, C.X.; Lv, T.; Wang, L.G.; Li, Y.; Han, C.; Yu, W.C.; Yan, Z.W.; Ma, X.W.; Zhao, H.C.; Zuo, Z.J.; et al. The spatiotemporal characteristics of water quality and phytoplankton community in a shallow eutrophic lake: Implications for submerged vegetation restoration. Sci. Total Environ. 2022, 821, 153460. [Google Scholar] [CrossRef]
- Beumer, V.; van Wirdum, G.; Beltman, B.; Griffioen, J.; Verhoeven, J.T.A. Biogeochemical consequences of winter flooding in brook valleys. Biogeochemistry 2007, 86, 105–121. [Google Scholar] [CrossRef]
- Qinfei, B.; Yuhai, B.; Yantong, Y.; Jie, Y.; Yanqi, W.; Jie, W. Effects of different vegetation restoration models on soil nutrients in the water level fluctuation zone of a large reservoir. Ecol. Indic. 2024, 169, 112955. [Google Scholar] [CrossRef]
- Ren, S.X.; Huo, T.C.; Wang, D.J.; Liang, J.Y. Losses of native mineral-associated organic nitrogen through microbial mineralization and gaseous emissions induced by ammonium and nitrate addition. Soil Biol. Biochem. 2024, 193, 109420. [Google Scholar] [CrossRef]
- Lu, J.; Faggotter, S.J.; Bunn, S.E.; Burford, M.A. Macrophyte beds in a subtropical reservoir shifted from a nutrient sink to a source after drying then rewetting. Freshw. Biol. 2017, 62, 854–867. [Google Scholar] [CrossRef]
- Zhang, B.; Fang, F.; Guo, J.S.; Chen, Y.P.; Li, Z.; Guo, S.S. Phosphorus fractions and phosphate sorption-release characteristics relevant to the soil composition of water-level-fluctuating zone of Three Gorges Reservoir. Ecol. Eng. 2012, 40, 153–159. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Yan, D.J.; Zhu, Y.G.; Zhang, Y.H.; Zhang, Z.B. Effects of wet-dry alternation on organic phosphorus dynamics and sediment characteristics in the intertidal zone of Nansi Lake. Ecotox. Environ. Safe. 2024, 281, 116668. [Google Scholar] [CrossRef] [PubMed]
- Rydin, E.; Huser, B.; Welch, E.B. Amount of phosphorus inactivated by alum treatments in Washington lakes. Limnol. Oceanogr. 2000, 45, 226–230. [Google Scholar] [CrossRef]
- Walls, R.L.; Wardrop, D.H.; Brooks, R.P. The impact of experimental sedimentation and flooding on the growth and germination of floodplain trees. Plant Ecolog. 2005, 176, 203–213. [Google Scholar] [CrossRef]
- Rao, K.; Zhang, X.; Yi, X.J.; Li, Z.S.; Wang, P.; Huang, G.W.; Guo, X.X. Interactive effects of environmental factors on phytoplankton communities and benthic nutrient interactions in a shallow lake and adjoining rivers in China. Sci. Total Environ. 2018, 619, 1661–1672. [Google Scholar] [CrossRef]
- Li, H.; Cai, Y.Y.; Song, C.L.; Cao, Z.; Li, J.; Qin, Z.H.; Zhang, S.X.; Li, J.F.; Cao, X.Y.; Zhou, Y.Y. Comparison of phosphorus migration and transformation characteristics in sediments of lakes within the Yangtze River basin and reservoirs on the Wujiang River. J. Soils Sediments 2024, 24, 991–1001. [Google Scholar] [CrossRef]
- Wang, Y.K.; Ji, Z.H.; Li, X.Q.; Long, Z.W.; Pei, Y.S. Comprehensive analysis of the migration and transformation of nutrients between sediment and overlying water in complex habitat systems. Sci. Total Environ. 2022, 852, 158433. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.F.; Tong, Y.; Ao, W.; Wang, Z.L.; Zhu, S.L.; Wang, Y.P. Quantification of Nutrient Fluxes from Sediments of Lake Hulun, China: Implications for Plateau Lake Management. Sustainability 2023, 15, 8680. [Google Scholar] [CrossRef]
- Miller, R.B.; Fox, G.A.; Penn, C.J.; Wilson, S.; Parnell, A.; Purvis, R.A.; Criswell, K. Estimating sediment and phosphorus loads from streambanks with and without riparian protection. Agric. Ecosyst. Environ. 2014, 189, 70–81. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, T.; Chen, L.Z.; Liu, W.H.; Zhang, M.T.; Shang, R.H. Multimedia Nitrogen and Phosphorus Migration and Source Control Using Multivariate Analysis and XGBoost: The Case Study in a Typical Agricultural Basin, Danjiangkou Reservoir. Water 2024, 16, 1936. [Google Scholar] [CrossRef]
- Haldna, M.; Milius, A.; Laugaste, R.; Kangur, K. Nutrients and phytoplankton in Lake Peipsi during two periods that differed in water level and temperature. Hydrobiologia 2008, 599, 3–11. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Chen, L.; Hong, Q.; Qiu, J.L.; Xie, H.; Liu, R.M. Assessment of nitrogen and phosphorus loads and causal factors from different land use and soil types in the Three Gorges Reservoir Area. Sci. Total Environ. 2013, 454, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Hu, H.Q.; Wan, C.Y.; Peng, J.H.; Xu, F.L.; Shi, F. Lateral and longitudinal variation in phosphorus fractions in surface sediment and adjacent riparian soil in the Three Gorges Reservoir, China. Environ. Sci. Pollut. Res. 2018, 25, 31262–31271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, Z.H.; Wang, T.; Dong, Z.X. Non-Point-Source Nitrogen and Phosphorus Loadings from a Small Watershed in the Three Gorges Reservoir Area. J. Mountain Sci. 2012, 9, 10–15. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Qiu, J.L.; Hong, Q.; Chen, L. Simulation of spatial and temporal distributions of non-point source pollution load in the Three Gorges Reservoir Region. Sci. Total Environ. 2014, 493, 138–146. [Google Scholar] [CrossRef]
- Yang, H.C.; Wang, G.Q.; Wang, L.J.; Zheng, B.H. Impact of land use changes on water quality in headwaters of the Three Gorges Reservoir. Environ. Sci. Pollut. Res. 2016, 23, 11448–11460. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, D.; Fan, X.; Liu, C.; Zhao, G. Landscape dynamics facilitated non-point source pollution control and regional water security of the Three Gorges Reservoir area, China. Environ. Impact Assess. Rev. 2022, 92, 106696. [Google Scholar] [CrossRef]
- Smits, E.; Freeman, J.L. Environmental cleanup using plants: Biotechnological advances and ecological considerations. Front. Ecol. Environ. 2006, 4, 203–210. [Google Scholar] [CrossRef]
- Qiu, S.R.; Wang, Y.C.; Wang, J.F.; Li, S.Z.; Ji, Y.X.; Jin, Z.X.; Chen, J.A. Effects of artificial vegetation restoration on the fractions and availability of soil phosphorus in the water-level-fluctuating zone of Three Gorges Reservoir, China. J. Soils Sediments 2023, 24, 1826–1837. [Google Scholar] [CrossRef]
- Lu, B.; Xu, Z.S.; Li, J.G.; Chai, X. Removal of water nutrients by different aquatic plant species: An alternative way to remediate polluted rural rivers. Ecol. Eng. 2018, 110, 18–26. [Google Scholar] [CrossRef]
- Mito, M.S.; Silva, A.A.; Kagami, F.L.; Almeida, J.D.; Mantovanelli, G.C.; Barbosa, M.C.; Kern-Cardoso, K.A.; Ishii-Iwamoto, E.L. Responses of the weed Bidens pilosa L. to exogenous application of the steroidal saponin protodioscin and plant growth regulators 24-epibrassinolide, indol-3-acetic acid and abscisic acid. Plant Biol. 2019, 21, 326–335. [Google Scholar] [CrossRef]
- Forsyth, C.; Brown, N.A.C. Germination of the Dimorphic Fruits of Bidens-Pilosa L. New Phytol. 1982, 90, 151–164. [Google Scholar] [CrossRef]
- Garssen, A.G.; Baattrup-Pedersen, A.; Voesenek, L.; Verhoeven, J.T.A.; Soons, M.B. Riparian plant community responses to increased flooding: A meta-analysis. Global Change Biol. 2015, 21, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Lorah, M.M.; Olsen, L.D. Natural attenuation of chlorinated volatile organic compounds in a freshwater tidal wetland: Field evidence of anaerobic biodegradation. Water Resour. Res. 1999, 35, 3811–3827. [Google Scholar] [CrossRef]
- Verma, S.K.; Kingsley, K.; Bergen, M.; English, C.; Elmore, M.; Kharwar, R.N.; White, J.F. Bacterial endophytes from rice cut grass (Leersia oryzoides L.) increase growth, promote root gravitropic response, stimulate root hair formation, and protect rice seedlings from disease. Plant Soil 2018, 422, 223–238. [Google Scholar] [CrossRef]
- Wiessner, A.; Kuschk, P.; Kästner, M.; Stottmeister, U. Abilities of helophyte species to release oxygen into rhizospheres with varying redox conditions in laboratory-scale hydroponic systems. Int. J. Phytorem. 2002, 4, 1–15. [Google Scholar] [CrossRef]



| Characteristics | 150 m System | 160 m System | 170 m System |
|---|---|---|---|
| Initial mass (kg) | 14.01 ± 0.41 b | 15.30 ± 0.55 a | 15.72 ± 0.85 a |
| Plant species (Coverage) | C. dactylon (100%) | C. dactylon and X. sibiricum (80%, 20%) | C. Dactylon and B. pilosa (70%, 30%) |
| Total nitrogen content of the soil (g·kg−1) | 1.26 ± 0.11 a | 1.10 ± 0.07 a | 0.72 ± 0.17 b |
| Total phosphorus content of the soil (g·kg−1) | 0.61 ± 0.02 a | 0.60 ± 0.03 a | 0.54 ± 0.02 b |
| Total nitrogen content of the aboveground plant (g·kg−1) | 10.06 ± 1.82 b | 10.88 ± 0.24 b | 10.89 ± 2.40 a |
| Total phosphorus content of the aboveground plant (g·kg−1) | 2.09 ± 0.49 b | 2.06 ± 0.08 b | 2.66 ± 0.12 a |
| Release Amounts | TN | TDN | PN | AN | NN |
|---|---|---|---|---|---|
| 150 m system | 69.54 ± 3.93 a,* | 0.61 ± 0.28 c | 69.08 ± 3.75 a | -- | 6.17 ± 0.71 c |
| 160 m system | 58.91 ± 4.40 b | 19.30 ± 1.39 a | 39.76 ± 4.64 c | -- | 14.79 ± 1.37 a |
| 170 m system | 61.20 ± 3.79 b | 14.85 ± 1.33 b | 46.50 ± 3.23 b | 0.73 ± 0.08 | 8.98 ± 0.95 b |
| Release Amounts | TP | TDP | PP | OPP |
|---|---|---|---|---|
| 150 m system | 10.30 ± 1.26 a,* | 0.66 ± 0.13 a | 9.40 ± 1.32 a | 0.51 ± 0.09 a |
| 160 m system | 5.75 ± 0.63 b | 0.08 ± 0.07 b | 5.45 ± 0.66 b | 0.11 ± 0.02 c |
| 170 m system | 6.07 ± 0.49 b | 0.00 ± 0.07 c | 5.85 ± 0.51 b | 0.20 ± 0.06 b |
| Properties | TN | TDN | PN | TP | TDP | PP |
|---|---|---|---|---|---|---|
| Initial mass | −0.832 ** | 0.694 * | −0.780 ** | −0.803 ** | −0.759 ** | −0.796 ** |
| Soil total nitrogen content | 0.434 | −0.515 | 0.504 | 0.626 * | 0.710 ** | 0.603 * |
| Soil total phosphorus content | 0.366 | −0.301 | 0.340 | 0.443 | 0.532 | 0.421 |
| Plant total nitrogen content | 0.434 | −0.515 | 0.504 | 0.626 * | 0.710 ** | 0.603 * |
| Plant total phosphorus content | 0.366 | −0.301 | 0.340 | 0.443 | 0.532 | 0.421 |
| Soil N/P | 0.403 | −0.519 | 0.493 | 0.612 * | 0.690 * | 0.591 * |
| Plant N/P | 0.185 | −0.078 | 0.125 | 0.169 | 0.240 | 0.154 |
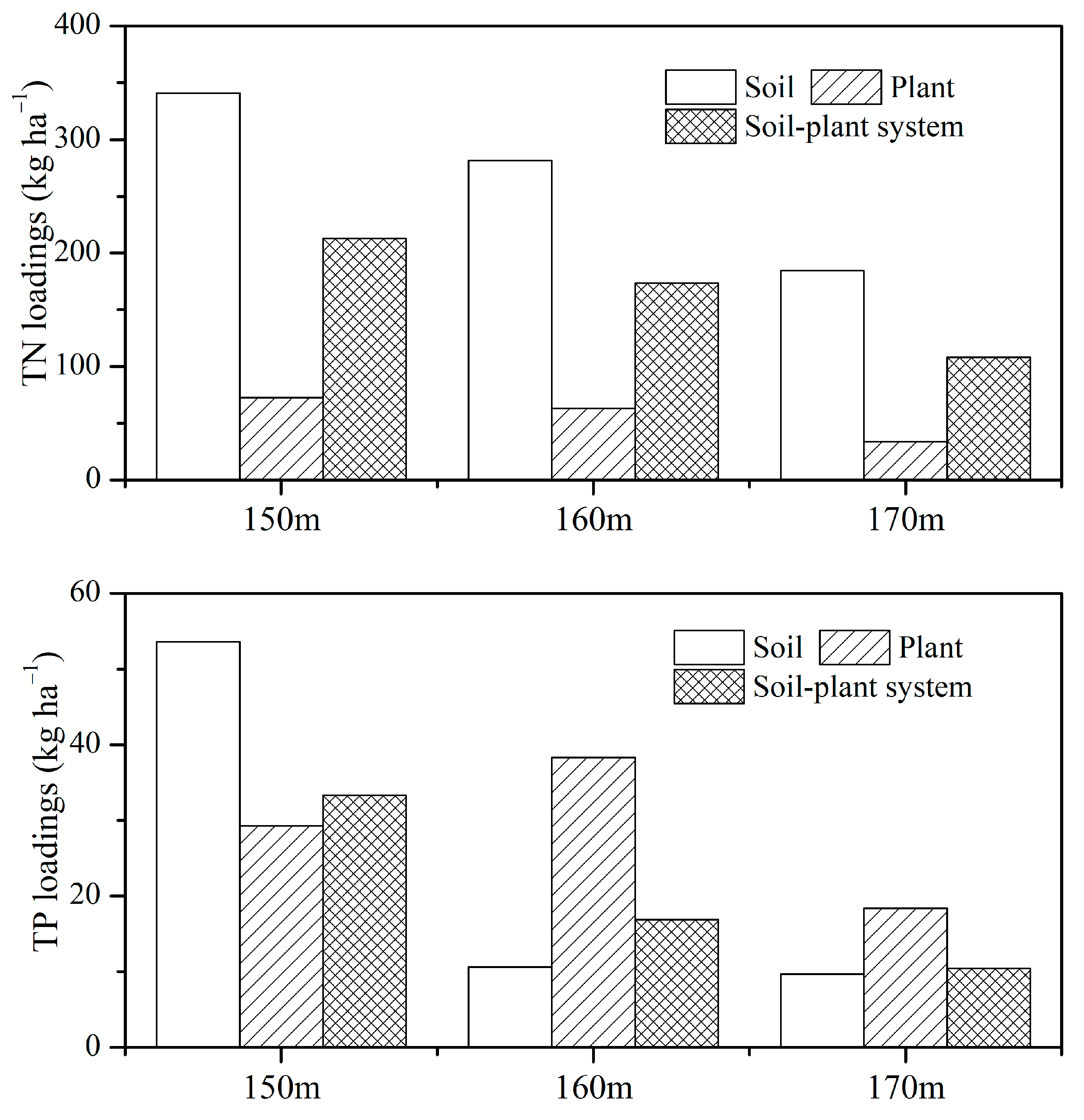
| Elevation | 150 m (145–155 m) | 160 m (155–165 m) | 170 m (165–175 m) | Total (Weighted Average) |
|---|---|---|---|---|
| Area of WLFZ (km2) | 110.30 | 112.23 | 126.47 | 349.00 |
| Weighted value (%) | 31.60 | 32.16 | 36.24 | 100 |
| Flooding time (d) | 240 | 200 | 120 | -- |
| Pollution load of TN (kg·ha−1) | 210.53 | 171.68 | 105.12 | (159.83) |
| Pollution load of TP (kg·ha−1) | 30.96 | 17.76 | 10.51 | (19.30) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Xiao, L.; Wang, T. Dynamics of Nitrogen and Phosphorus Release from Submerged Soil–Plant Systems in the Three Gorges Reservoir. Water 2025, 17, 1701. https://doi.org/10.3390/w17111701
Hu L, Xiao L, Wang T. Dynamics of Nitrogen and Phosphorus Release from Submerged Soil–Plant Systems in the Three Gorges Reservoir. Water. 2025; 17(11):1701. https://doi.org/10.3390/w17111701
Chicago/Turabian StyleHu, Lei, Liwei Xiao, and Tao Wang. 2025. "Dynamics of Nitrogen and Phosphorus Release from Submerged Soil–Plant Systems in the Three Gorges Reservoir" Water 17, no. 11: 1701. https://doi.org/10.3390/w17111701
APA StyleHu, L., Xiao, L., & Wang, T. (2025). Dynamics of Nitrogen and Phosphorus Release from Submerged Soil–Plant Systems in the Three Gorges Reservoir. Water, 17(11), 1701. https://doi.org/10.3390/w17111701







