Enhancement of Electron Transfer Between Fe/Mn Promotes Efficient Activation of Peroxomonosulfate by FeMn-NBC
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Catalysts
2.3. Characterization of Catalysts
2.4. Experimental Methods
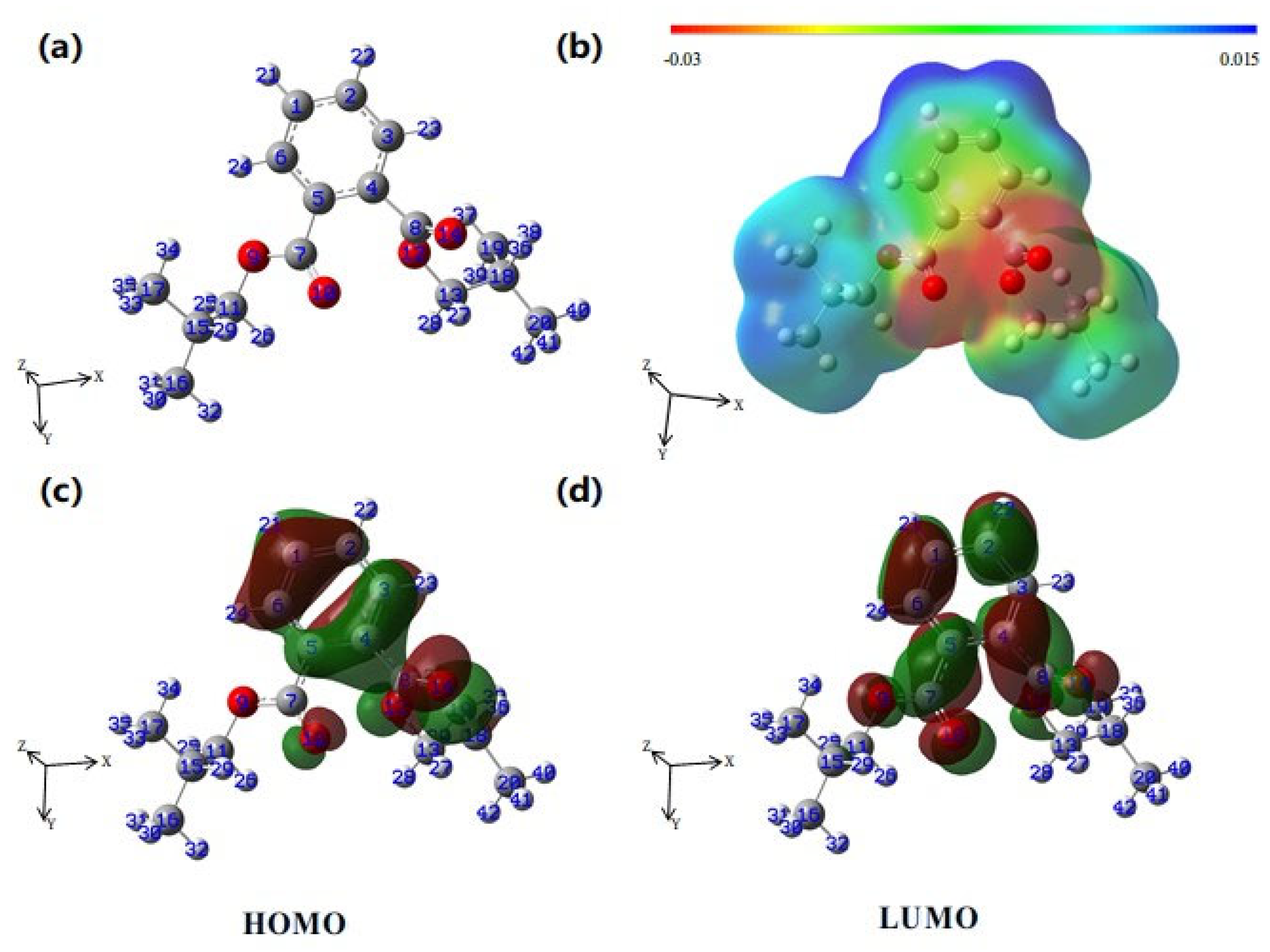
2.5. Toxicity Analysis and DFT Calculation
3. Results and Discussion
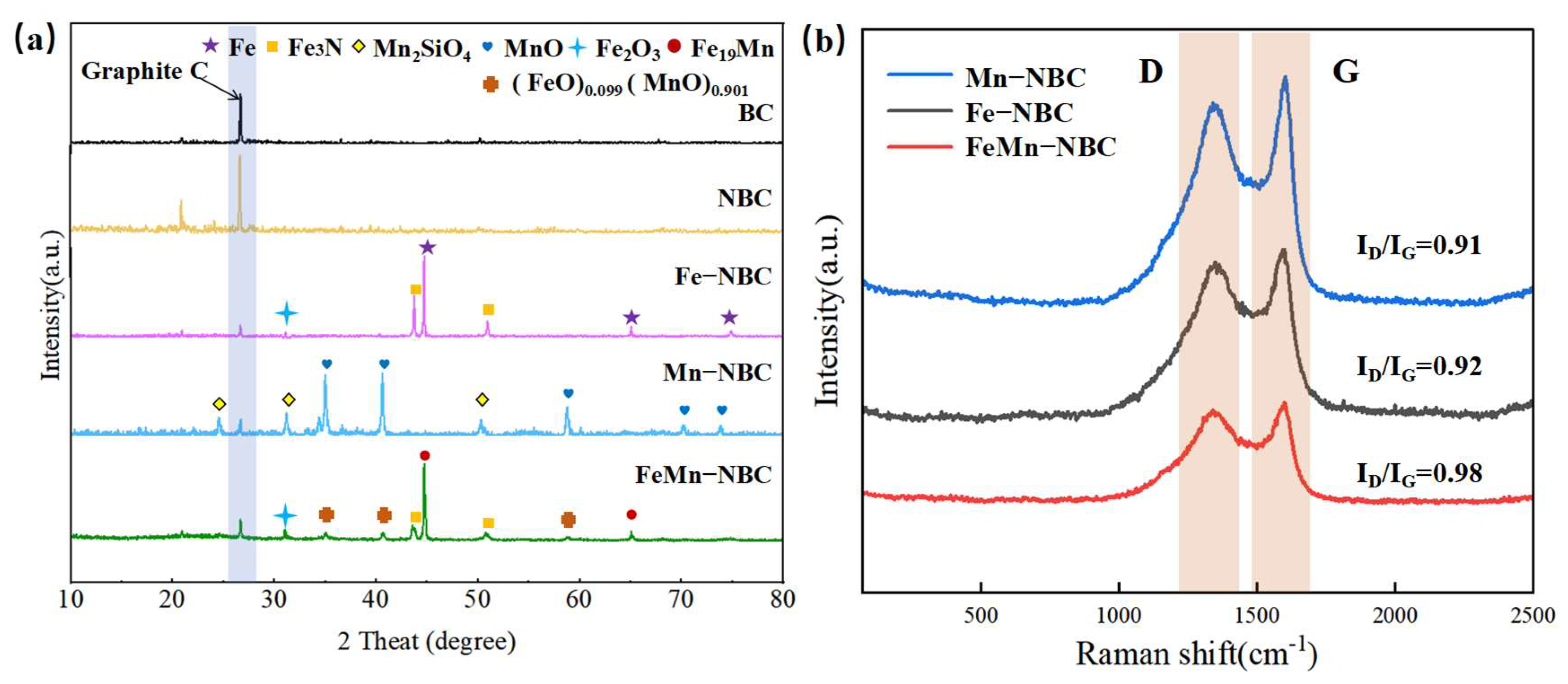
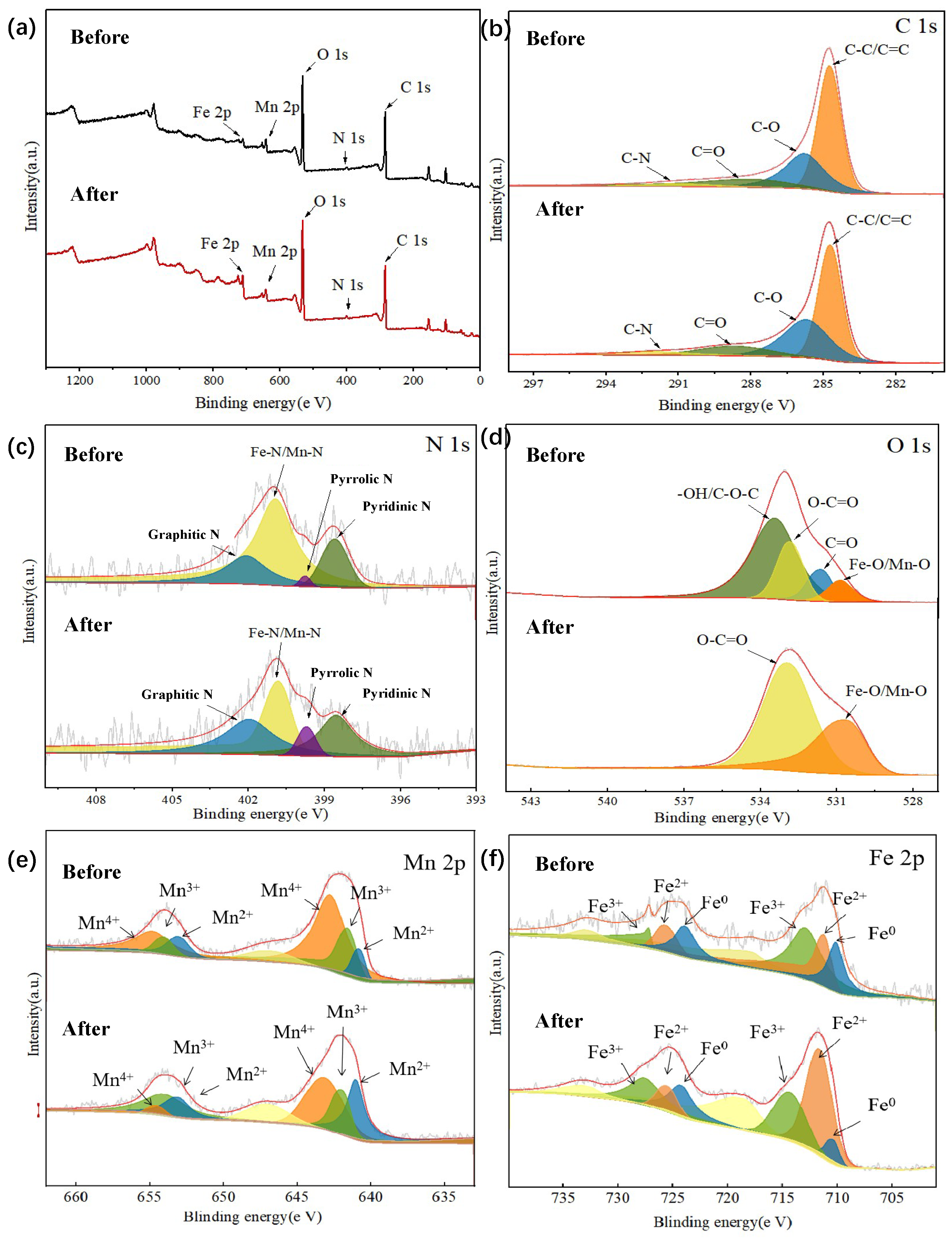
3.1. Catalyst Characterization
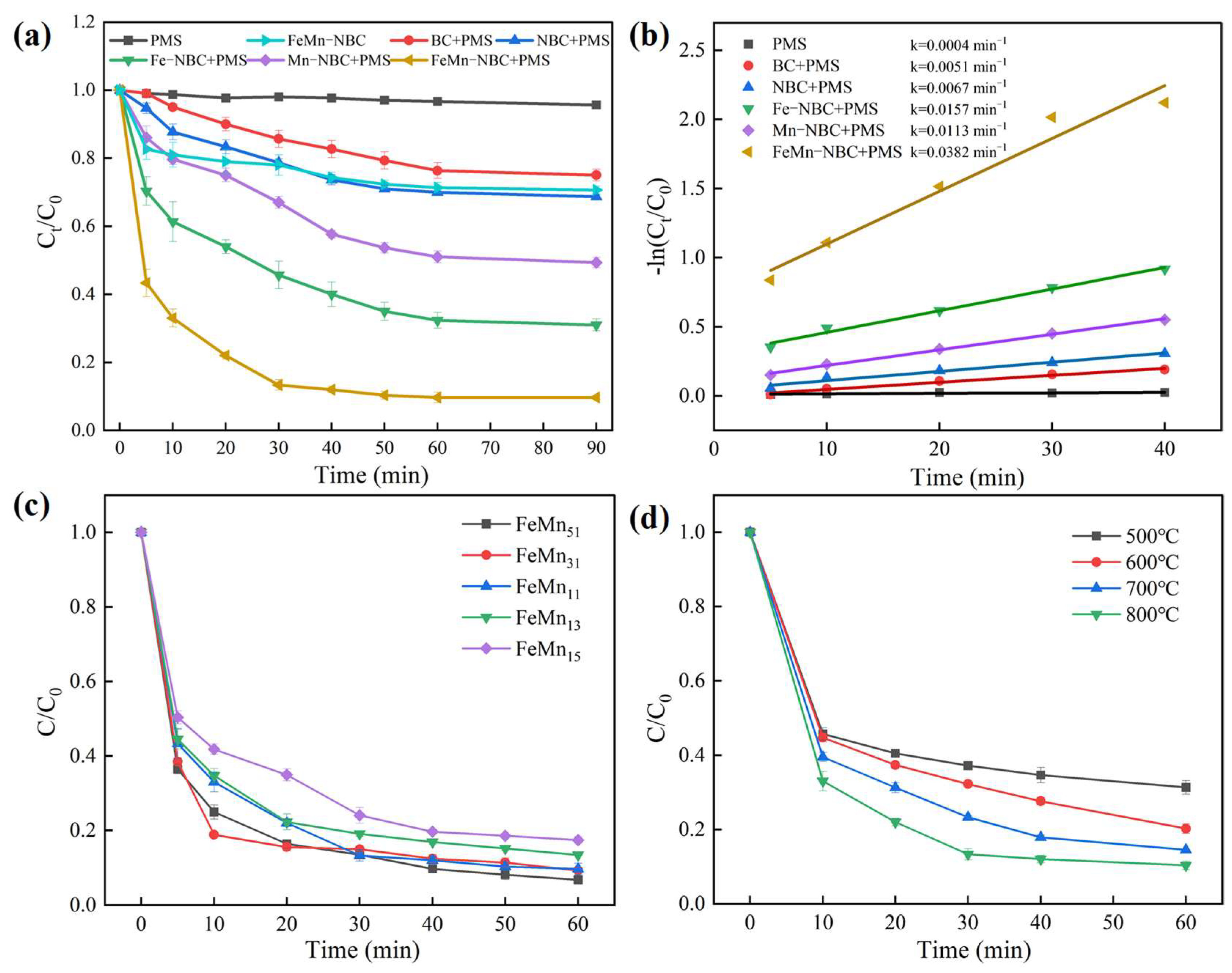
3.2. Catalytic Performance
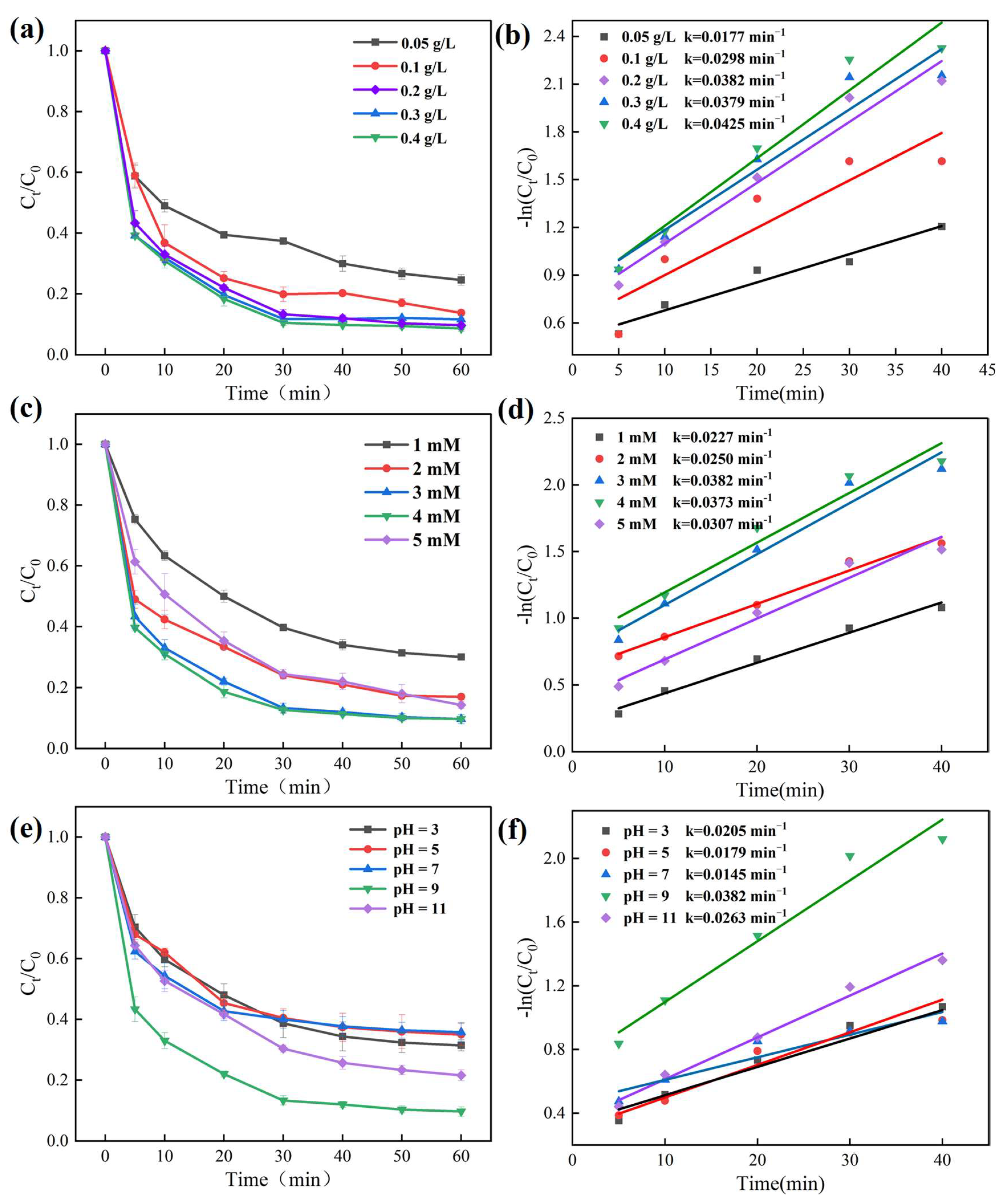
3.2.1. Effects of Operational Parameters
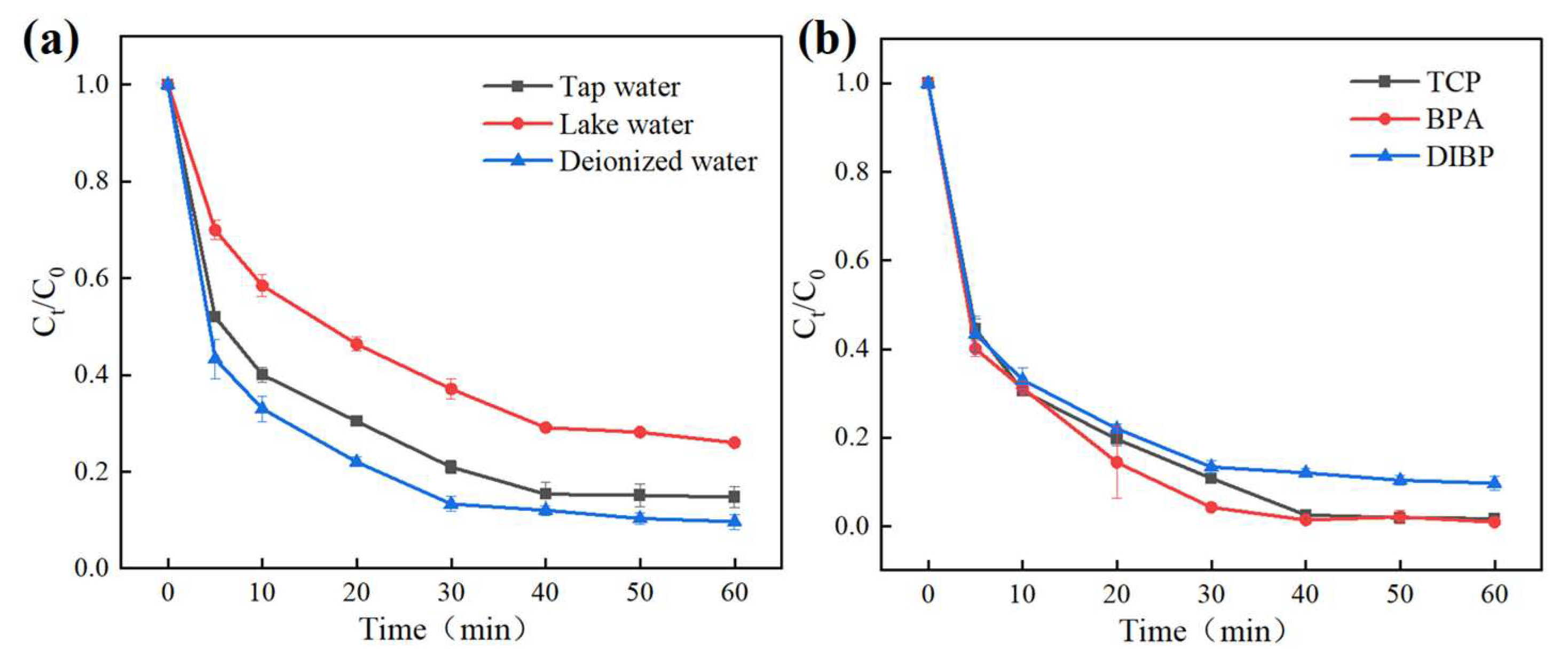
3.2.2. Inorganic Anions and Natural Organic Matters
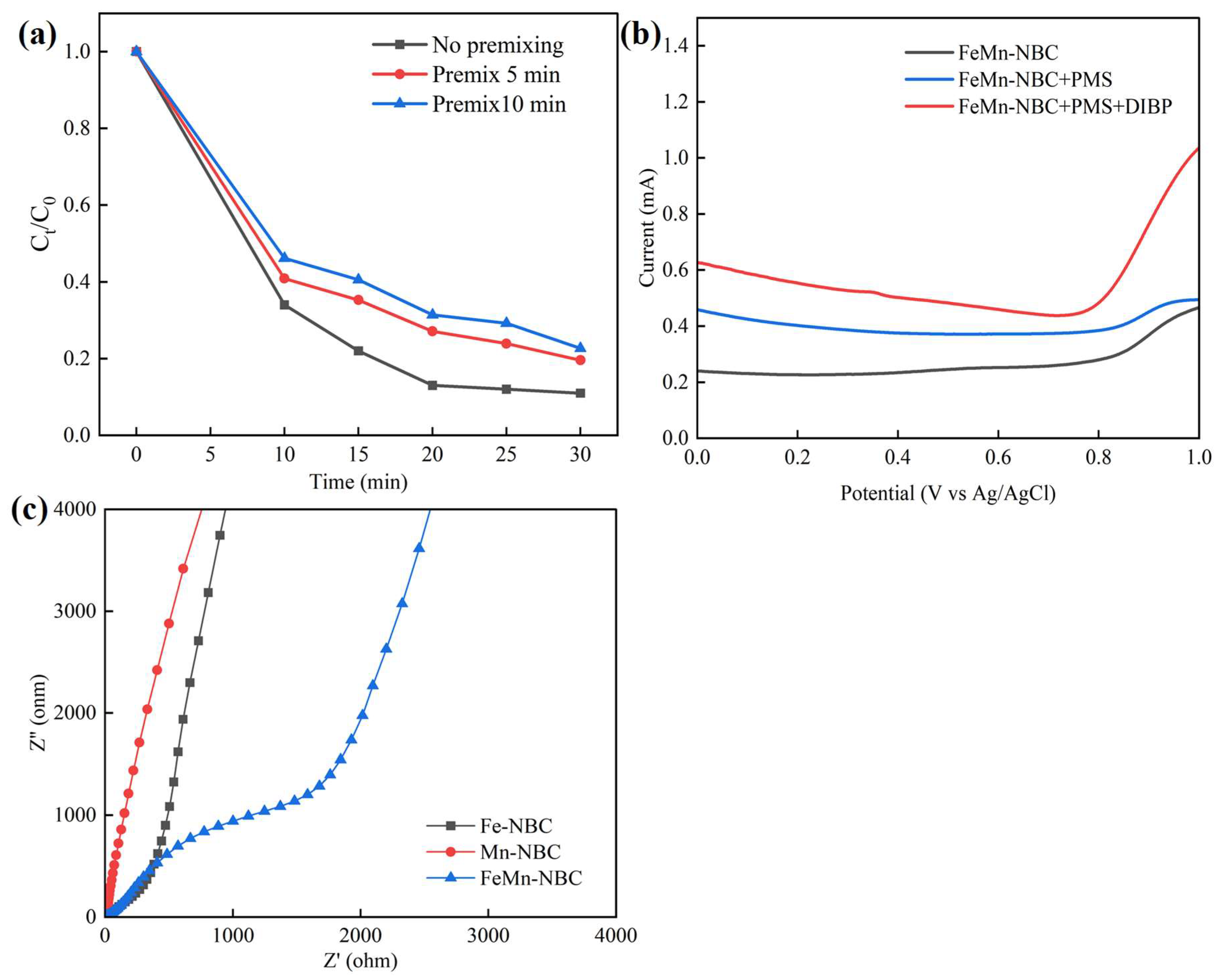
3.2.3. Stability and Practical Application
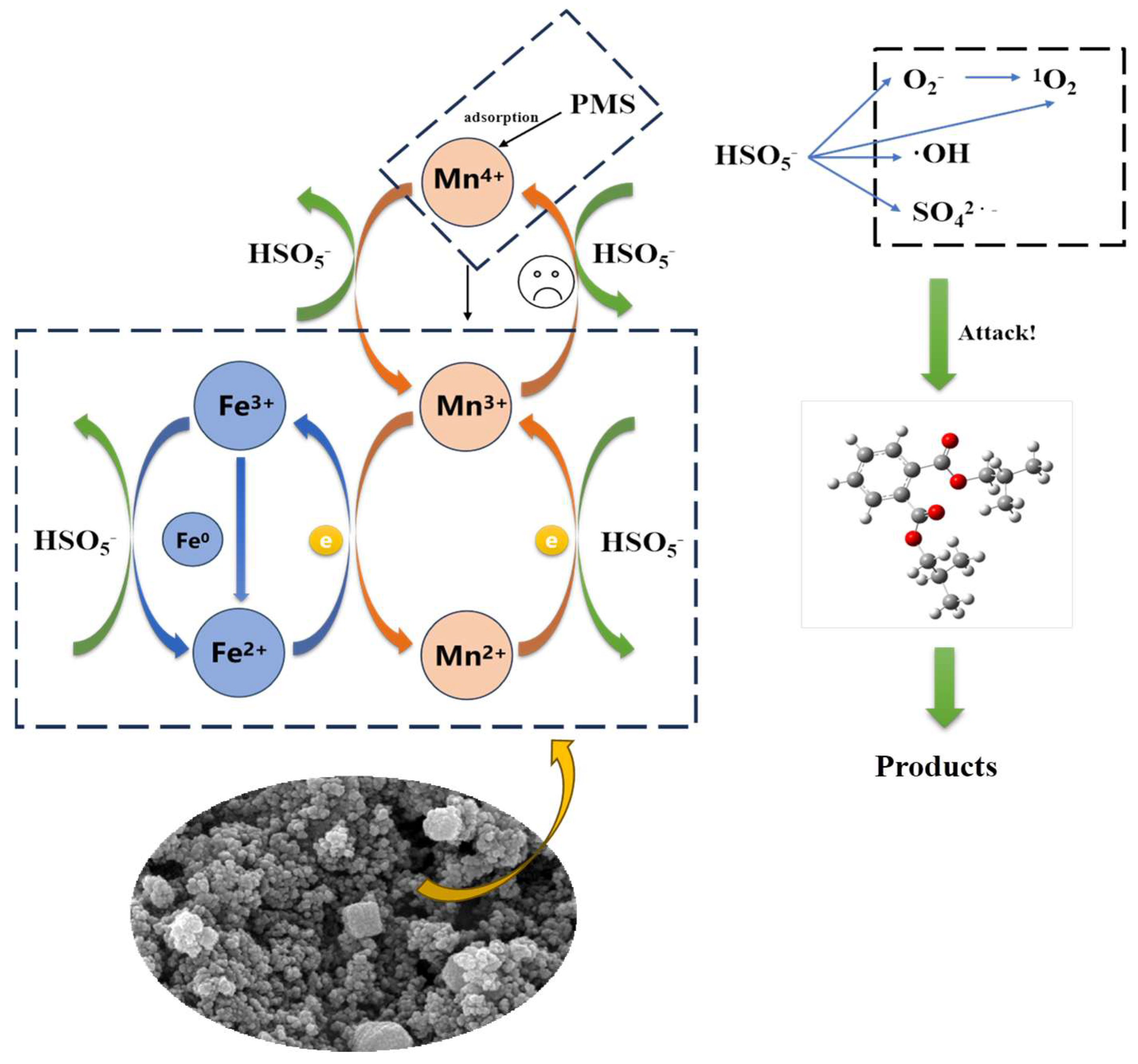
3.3. Mechanism of Catalysts System
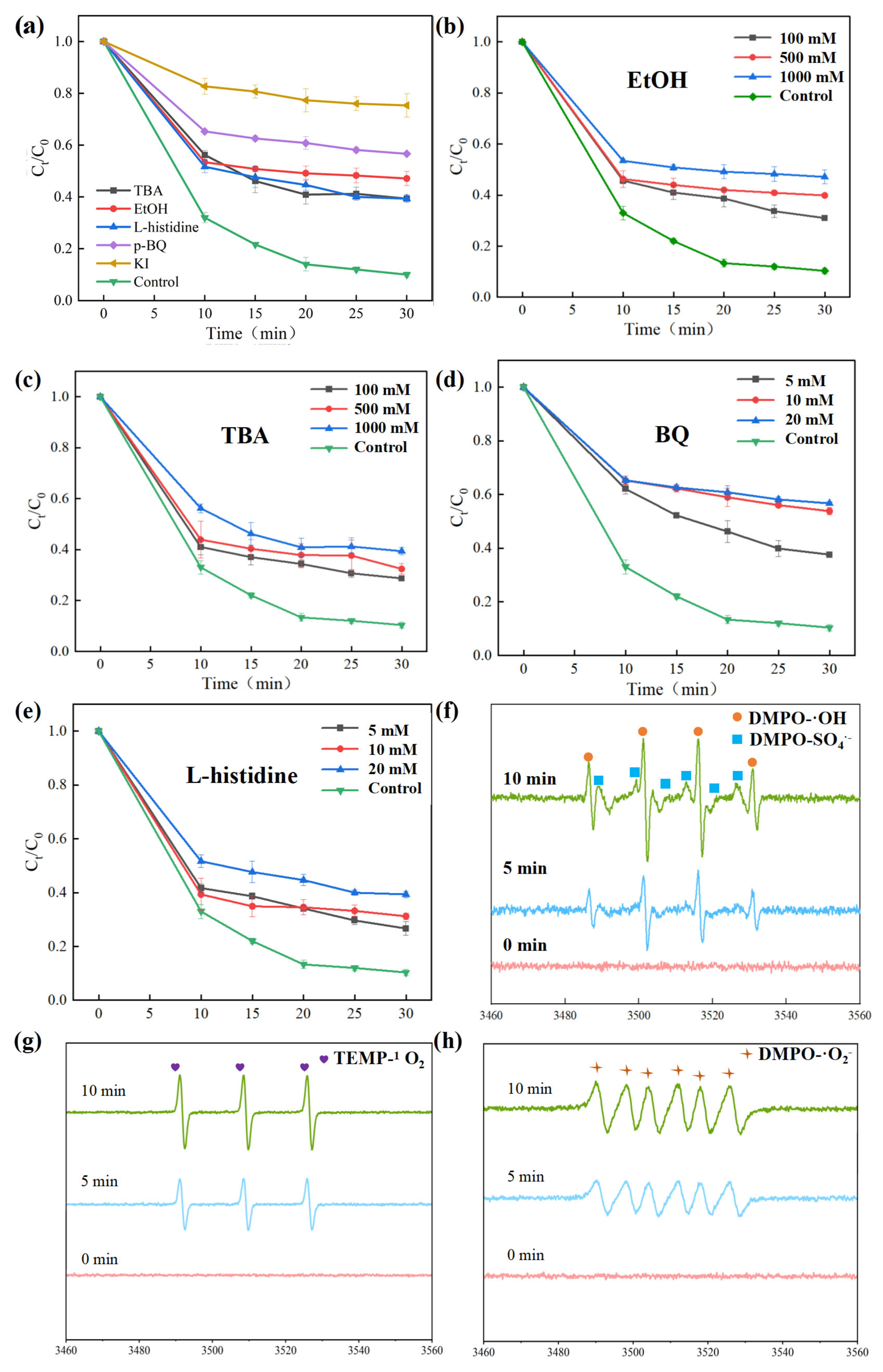
3.3.1. Identification of Active Species
3.3.2. Identification of High-Valent Active Species
3.3.3. Catalytic Mechanism of FeMn-NBC
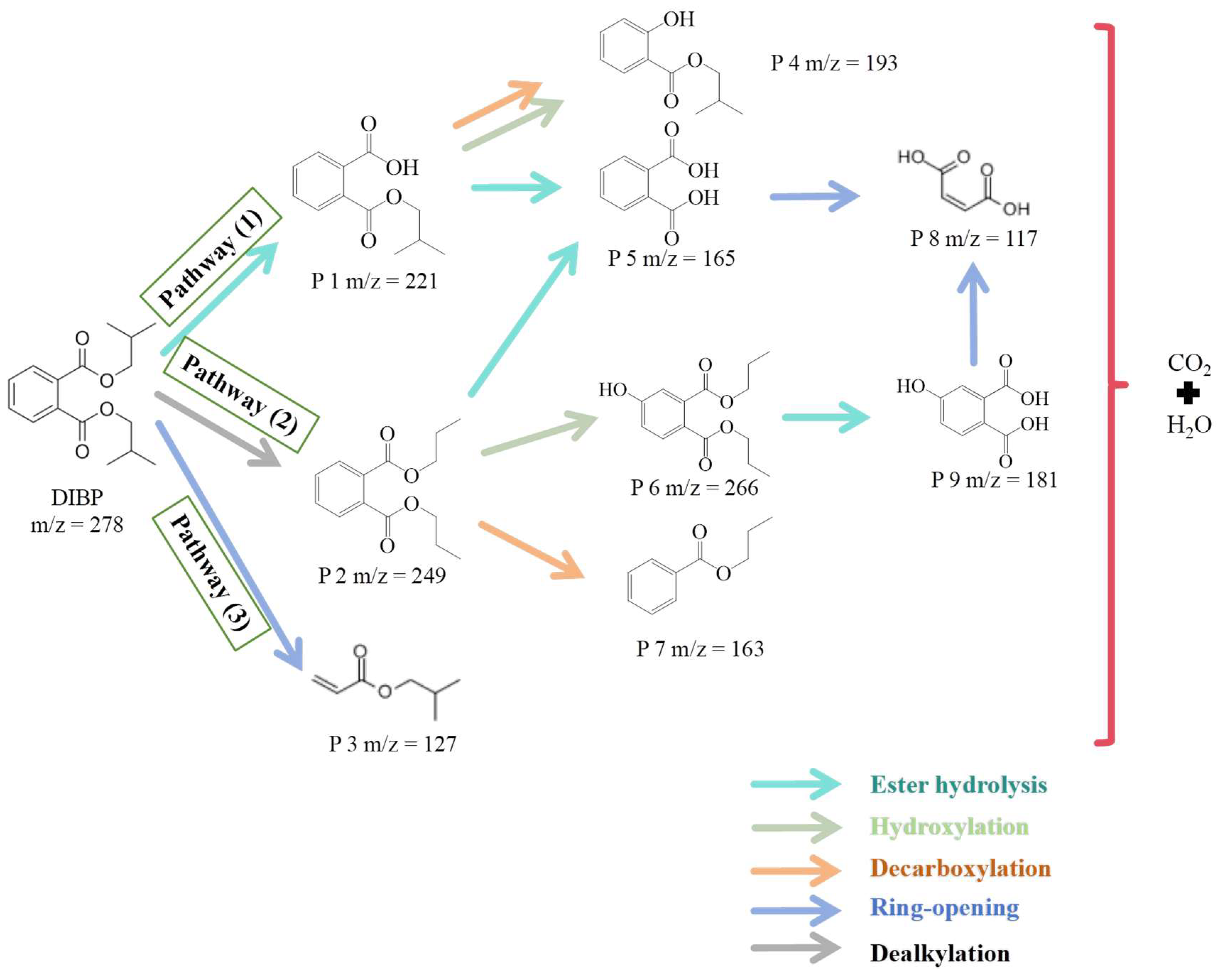
3.4. Degradation Pathways and Toxicity Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Lv, L.; Ding, L.; Gao, L.; Li, J.; Ma, X.; Yu, Y. Comparison of phthalate esters (PAEs) in freshwater and marine food webs: Occurrence, bioaccumulation, and trophodynamics. J. Hazard. Mater. 2024, 466, 133534. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Lu, G.; Liu, J.; Jiang, R. Phthalate esters in municipal sewage treatment plants: Occurrence level, removal rate and optimum combination technology. Front. Environ. Eng. 2023, 2, 1208689. [Google Scholar] [CrossRef]
- Belinda, M.; Chang, H.; Emma, K.; Mueller, J.F.; Benjamin, T. Bisphenols and phthalates in Australian wastewater: A statistical approach for estimating contributions from diffuse and point sources. Water Res. 2023, 246, 120680. [Google Scholar] [CrossRef]
- Liu, M.; Du, X.; Chen, H.; Bai, C.; Lan, L. Systemic investigation of di-isobutyl phthalate (DIBP) exposure in the risk of cardiovascular via influencing the gut microbiota arachidonic acid metabolism in obese mice model. Regen. Ther. 2024, 27, 290–300. [Google Scholar] [CrossRef]
- Safar, A.M.; Santacruz-Márquez, R.; Laws, M.J.; Meling, D.D.; Liu, Z.; Kumar, T.R.; Nowak, R.A.; Raetzman, L.T.; Flaws, J.A. Dietary exposure to an environmentally relevant phthalate mixture alters follicle dynamics, hormone levels, ovarian gene expression, and pituitary gene expression in female mice. Reprod. Toxicol. 2023, 122, 108489. [Google Scholar] [CrossRef]
- Tan, H.; Gao, P.; Luo, Y.; Gou, X.; Xia, P.; Wang, P.; Yan, L.; Zhang, S.; Guo, J.; Zhang, X.; et al. Are New Phthalate Ester Substitutes Safer than Traditional DBP and DiBP? Comparative Endocrine-Disrupting Analyses on Zebrafish Using In Vivo, Transcriptome, and In Silico Approaches. Environ. Sci. Technol. 2023, 57, 13744–13756. [Google Scholar] [CrossRef]
- Sun, J.; Wei, B.; Mei, Q.; An, Z.; Wang, X.; Han, D.; Xie, J.; Zhan, J.; Zhang, Q.; Wang, W.; et al. Theoretical investigation on the degradation of dibutyl phthalate initiated byOH and SO 4 ·—In aqueous solution: Mechanism, kinetics and ecotoxicity assessment. Chem. Eng. J. 2020, 382, 122791. [Google Scholar] [CrossRef]
- Pang, X.; Sarvothaman, V.P.; Nathan, S.; Wang, Z.; Rooney, D.W.; Ranade, V.V.; Robertson, P.J. Kinetic modelling of the photocatalytic degradation of Diisobutyl phthalate and coupling with acoustic cavitation. Chem. Eng. J. 2022, 444, 136494. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A critical review of advanced oxidation processes for emerging trace organic contaminant degradation: Mechanisms, factors, degradation products, and effluent toxicity. J. Water Process Eng. 2020, 40, 101778. [Google Scholar] [CrossRef]
- Tang, M.; Wan, J.; Wang, Y.; Ye, G.; Yan, Z.; Ma, Y.; Sun, J. Overlooked role of void-nanoconfined effect in emerging pollutant degradation: Modulating the electronic structure of active sites to accelerate catalytic oxidation. Water Res. 2024, 249, 120950. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Sun, Z. A novel Co-N-doped carbon catalyst to activate peroxymonosulfate for efficient removal of sulfamethazine: Role of singlet oxygen and high-valent cobalt-oxo species. J. Water Process Eng. 2024, 63, 105398. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, C.; Wang, X.; Zhang, Q.; Yao, C.; Huang, D.; Liu, X.; Lu, Y.; Song, W.; Chen, S.; et al. A novel, low-cost, and efficient separation strategy based on S-doped C3N4 for the analysis of PCDD/Fs and dioxin-like PCBs. J. Environ. Chem. Eng. 2024, 12, 112529. [Google Scholar] [CrossRef]
- Long, L.; Wang, X.; Fu, H.; Qu, X.; Zheng, S.; Xu, Z. Robust Activity and Stability of P-Doped Fe-Carbon Composites Derived from MOF for Bromate Reduction. Acs Appl. Mater. Inter. 2024, 16, 21838–21848. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Q.; Zhang, X.; Shi, B.; Qin, D.; Wang, J.; Zhang, A.; Liu, Y. Degradation of phenol by Cu-Ni bimetallic-doped sludge biochar as a fenton-like catalyst: Mechanistic study and practical application. Sep. Purif. Technol. 2024, 347, 127554. [Google Scholar] [CrossRef]
- Zhao, Y.; An, H.; Dong, G.; Feng, J.; Ren, Y.; Wei, T. Elevated removal of di-n-butyl phthalate by catalytic ozonation over magnetic Mn-doped ferrospinel ZnFe2O4 materials: Efficiency and mechanism. Appl. Surf. Sci. 2020, 505, 144476. [Google Scholar] [CrossRef]
- Leal, T.W.; Lourenço, L.A.; de L Brandão, H.; da Silva, A.; de Souza, S.G.; de Souza, A. Low-cost iron-doped catalyst for phenol degradation by heterogeneous Fenton. J. Hazard. Mater. 2018, 359, 96–103. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, H.; Dong, W.; Du, T.; Li, X.; Wang, F.; Zhao, Z. Electron transfer-mediated peroxymonosulfate activation through monoatomic Fe–pyridinic N4 moiety in biochar-based catalyst for electron-rich pollutants degradation in groundwater. Chem. Eng. J. 2024, 492, 152158. [Google Scholar] [CrossRef]
- Lai, C.; Wang, N.; Xu, F.; Zhang, M.; Huang, D.; Ma, D.; Zhou, X.; Xu, M.; Li, L.; Yan, H.; et al. Pomelo peel biochar supported nZVI@Bi0 as a persulfate activator for the degradation of acetaminophen: Enhanced performance and degradation mechanism. Sep. Purif. Technol. 2024, 350, 127966. [Google Scholar] [CrossRef]
- Tong, S.; Chen, D.; Jiang, X.; Xu, Z.; Liu, X.; Shen, J. Persulfate activation by Fe3O4-doped biochar synthesized from Fenton sludge and sewage sludge for enhanced 1-H-1,2,4-triazole degradation. Chem. Eng. J. 2023, 461, 142075. [Google Scholar] [CrossRef]
- Hao, M.; Wang, Q.; Yu, F.; Guan, Z.; Zhang, X.; Sun, Y. Efficient degradation of 2,4-dichlorophphenol in groundwater using persulfate activated by nitrogen-doped biochar-supported nano zero-valent iron. J. Clean. Prod. 2024, 458, 142415. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, Q.; Wang, Z.; Gao, B.; Fan, Z.; Li, M.; Hao, H.; Wei, X.; Zhong, M. Degradation of anthraquinone dye reactive blue 19 using persulfate activated with Fe/Mn modified biochar: Radical/non-radical mechanisms and fixed-bed reactor study. Sci. Total Environ. 2021, 758, 143584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, X.; Yuan, J.; Wang, Z. Efficient degradation and toxicity reduction of tetracycline by recyclable ferroferric oxide doped powdered activated charcoal via peroxymonosulfate (PMS) activation. Chem. Eng. J. 2022, 441, 136061. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; She, Y.; Yu, Y.; Hong, J. High-efficiency degradation of bisphenol A by heterogeneous Mn–Fe layered double oxides through peroxymonosulfate activation: Performance and synergetic mechanism. Sep. Purif. Technol. 2021, 270, 118770. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Y.; Xu, M.; Zhu, Z.; Zhang, Q.; Hong, J. Efficient degradation of benzalkonium chloride by FeMn-CA300 catalyst activated persulfate process: Surface hydroxyl potentiation mechanism and degradation pathway. Environ. Pollut. 2023, 333, 121986. [Google Scholar] [CrossRef]
- Li, L.; Lai, C.; Huang, F.; Cheng, M.; Zeng, G.; Huang, D.; Li, B.; Liu, S.; Zhang, M.; Qin, L.; et al. Degradation of naphthalene with magnetic bio-char activate hydrogen peroxide: Synergism of bio-char and Fe–Mn binary oxides. Water Res. 2019, 160, 238–248. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Liu, P.; Bai, X.; Du, X.; Jin, P.; Yang, L.; Jin, X.; Shi, X.; Wang, Y. Peroxymonosulfate activation by nitrogen-doped biochar from sawdust for the efficient degradation of organic pollutants. Chem. Eng. J. 2020, 387, 124065. [Google Scholar] [CrossRef]
- Zhu, Q.; Xiang, T.; Chen, C.; Zhang, J.; Wu, Z.; Rao, S.; Li, B.; Yang, J. Enhancing activity and stability of FeNC catalysts through co incorporation for oxygen reduction reaction. J. Colloid. Interf. Sci. 2024, 663, 53–60. [Google Scholar] [CrossRef]
- Xiang, M.; Wang, Y.; Ren, X.; Yang, Z.; Huang, Y.; Zhu, S.; Chen, L.; Zhang, J.; Li, H. Efficient degradation of tetrabromobisphenol A by metal-organic framework-derived N-doped Fe/Fe3C@NC catalysts in sequential reduction-oxidation system. Sep. Purif. Technol. 2024, 340, 126672. [Google Scholar] [CrossRef]
- Lu, J.; Jv, A.; Zhao, H.; Che, G.; Feng, B.; Zhang, Y.; Liu, C.; Guan, R. Fc-COOH modified MIL-101(Cr) as enhanced photocatalyst to efficiently degrade tetracycline under visible light. Sep. Purif. Technol. 2024, 346, 127442. [Google Scholar] [CrossRef]
- Hu, C.; Xing, G.; Han, W.; Hao, Y.; Zhang, C.; Zhang, Y.; Kuo, C.H.; Chen, H.; Hu, F.; Li, L.; et al. Inhibiting demetalation of Fe-N-C via Mn sites for efficient oxygen reduction reaction in Zinc-Air batteries. Adv. Mater. 2024, 36, 2405763. [Google Scholar] [CrossRef]
- Bai, J.; Cheng, L.; Liu, S.; Lian, Y.; Deng, Y.; Zhou, Q.; Xiang, M.; Tang, Y.; Su, Y. Construct N-doped carbon anchored CoFe alloy nanoparticles with high content graphitic-N for electrocatalytic oxygen reduction. J. Colloid. Interf. Sci. 2024, 653, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Nie, C.; Li, W.; Duan, X.; Lai, B.; Ao, Z.; Wang, S.; An, T. Insight into the effect of lignocellulosic biomass source on the performance of biochar as persulfate activator for aqueous organic pollutants remediation: Epicarp and mesocarp of citrus peels as examples. J. Hazard. Mater. 2020, 399, 123043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, H.; Murugananthan, M.; Sun, P.; Dionysiou, D.D.; Zhang, K.; Khan, A.; Zhang, Y. Glucose and melamine derived nitrogen-doped carbonaceous catalyst for nonradical peroxymonosulfate activation. Carbon. 2020, 156, 399–409. [Google Scholar] [CrossRef]
- Xu, L.; Fu, B.; Sun, Y.; Jin, P.; Bai, X.; Jin, X.; Shi, X.; Wang, Y.; Nie, S. Degradation of organic pollutants by Fe/N co-doped biochar via peroxymonosulfate activation: Synthesis, performance, mechanism and its potential for practical application. Chem. Eng. J. 2020, 400, 125870. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Wu, Q.; Luo, H.; Zhao, Q.; Si, Q.; Sseguya, F.; Ren, N. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism. Water Res. 2019, 160, 405–414. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.; Hu, Z.; Sun, Y.; Webster, R.; Li, S.; Lim, T. Enhancing sulfacetamide degradation by peroxymonosulfate activation with N-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes. Appl. Catal. B Environ. 2018, 225, 243–257. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, C.; Zhang, W.; Liu, Z.; Li, Z.; Han, F.; Zhang, M.; Xu, F.; Zhou, W. Cu-doped CoOOH activates peroxymonosulfate to generate high-valent cobalt-oxo species to degrade organic pollutants in saline environments. Appl. Catal. B Environ. 2024, 340, 123224. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Xiong, Z.; Ao, Z.; Pu, S.; Yao, G.; Lai, B. Core-shell magnetic Fe3O4@Zn/Co-ZIFs to activate peroxymonosulfate for highly efficient degradation of carbamazepine. Appl. Catal. B Environ. 2020, 277, 119136. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.T.; Kwon, E.E.; Lee, J. Biochar as a catalytic material for the production of 1,4-butanediol and tetrahydrofuran from furan. Environ. Res. 2020, 184, 109325. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, Y.; Sun, Z.; Zhang, D.; Huang, Y.; Gu, S.; Chen, W. Understanding reactions and pore-forming mechanisms between waste cotton woven and FeCl3 during the synthesis of magnetic activated carbon. Chemosphere 2020, 241, 125120. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, Y.; Ye, X.; Lv, Y.; Liu, Y.; Liu, M. Enhanced persulfate activation for sulfadiazine degradation by N, S self-doped biochar from sludge and sulfonated lignin: Emphasizing the roles of graphitic nitrogen and thiophene sulfur. J. Environ. Chem. Eng. 2024, 12, 112407. [Google Scholar] [CrossRef]
- He, Y.; Qin, H.; Wang, Z.; Wang, H.; Zhu, Y.; Zhou, C.; Zeng, Y.; Li, Y.; Xu, P.; Zeng, G. Fe-Mn oxycarbide anchored on N-doped carbon for enhanced Fenton-like catalysis: Importance of high-valent metal-oxo species and singlet oxygen. Appl. Catal. B Environ. 2024, 340, 123204. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Tan, X.; Wu, H.; Liang, J.; Song, B.; Tang, N.; Zhang, P.; Yang, Y.; Chen, Q.; et al. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer. Appl. Catal. B Environ. 2020, 269, 118850. [Google Scholar] [CrossRef]
- Xie, D.; Guo, P.; Zhong, K.; Sheng, G. Highly dispersed Co/Fe bimetal in carbonaceous cages as heterogeneous Fenton nanocatalysts for enhanced sulfamethoxazole degradation. Appl. Catal. B Environ. 2022, 319, 121923. [Google Scholar] [CrossRef]
- El Batouti, M.; Al-Harby, N.F.; Elewa, M.M. A Review on Promising Membrane Technology Approaches for Heavy Metal Removal from Water and Wastewater to Solve Water Crisis. Water 2021, 13, 3241. [Google Scholar] [CrossRef]
- Punnoy, P.; Aryal, P.; Hefner, C.E.; Brack, E.; Rodthongkum, N.; Potiyaraj, P.; Potiyaraj, P.; Henry, C. Smartphone-assisted dual-sided capillary microfluidic device for multiplex detection of heavy metals and nutrients in drinking water. Anal. Chim. Acta 2025, 1356, 344031. [Google Scholar] [CrossRef]
- Yu, Y.; Li, N.; Lu, X.; Yan, B.; Chen, G.; Wang, Y.; Duan, X.; Cheng, Z.; Wang, S. Co/N co-doped carbonized wood sponge with 3D porous framework for efficient peroxymonosulfate activation: Performance and internal mechanism. J. Hazard. Mater. 2022, 421, 126735. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Z.; Zhu, Y.; Li, T.; Hu, C. New Insights into the Generation of Singlet Oxygen in the Metal-Free Peroxymonosulfate Activation Process: Important Role of Electron-Deficient Carbon Atoms. Environ. Sci. Technol. 2020, 54, 1232–1241. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Li, Q.; Zhao, Z.; Cheng, X. Non-Radical Activation of Peracetic Acid by Powdered Activated Carbon for the Degradation of Sulfamethoxazole. Environ. Sci. Technol. 2023, 57, 10478–10488. [Google Scholar] [CrossRef]
- Chen, D.; Bai, X.; Chen, Y.; Wang, Y.; Zhu, Y. Efficient removal of sulfamethoxazole by activated peroxodisulphates using Fe/N co-doped biochar: The minimal role of high-valent iron species. Sep. Purif. Technol. 2024, 343, 127078. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Wei, J.; Liu, Z.; Wang, H.; Zhu, Y.; Shao, Q.; Xie, P. Efficient bimetallic activation of sulfite by CoFe-LDH for bisphenol A removal: Critical role of Co/Fe ratio on ROS generation. Chem. Eng. J. 2024, 492, 152171. [Google Scholar] [CrossRef]
- Wang, Z.; Nengzi, L.; Zhang, X.; Zhao, Z.; Cheng, X. Novel NiCo2S4/CS membranes as efficient catalysts for activating persulfate and its high activity for degradation of nimesulide. Chem. Eng. J. 2020, 381, 122517. [Google Scholar] [CrossRef]
- Feng, Y.; Liao, C.; Kong, L.; Wu, D.; Liu, Y.; Lee, P.; Shih, K. Facile synthesis of highly reactive and stable Fe-doped g-C3N4 composites for peroxymonosulfate activation: A novel nonradical oxidation process. J. Hazard. Mater. 2018, 354, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; He, D.; Wang, X.; Yan, Y. The role of Fe(IV) in the zero-valent iron biochar activated persulfate system for treatment of contaminants of emerging concern. Chem. Eng. J. 2024, 487, 150553. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Zhan, X.; Gao, R.; Chen, L.; Yu, J.; Wang, H.; Shi, H. A ZIF-8-derived copper-nitrogen co-hybrid carbon catalyst for peroxymonosulfate activation to degrade BPA. Chemosphere 2022, 308, 136489. [Google Scholar] [CrossRef]
- Huang, M.; Wang, X.; Liu, C.; Fang, G.; Gao, J.; Wang, Y.; Zhou, D. Mechanism of metal sulfides accelerating Fe(II)/Fe(III) redox cycling to enhance pollutant degradation by persulfate: Metallic active sites vs. reducing sulfur species. J. Hazard. Mater. 2021, 404, 124175. [Google Scholar] [CrossRef]
- Guo, Z.; Li, C.; Gao, M.; Xiao, H.; Zhang, Y.; Zhang, W.; Li, W. Mn-O Covalency Governs the Intrinsic Activity of Co-Mn Spinel Oxides for Boosted Peroxymonosulfate Activation. Angew. Chem. Int. Ed. 2020, 60, 274–280. [Google Scholar] [CrossRef]
- Niu, H.; Dong, Z.; Guo, H.; Yang, Y.; Yang, W.; Yan, M.; Niu, C.; Li, J. Cu inanchored MnO/carbonaceous frameworks trigger peroxymonosulfate activation for enrofloxacin degradation: ROS generation and pollutant attacking processes. J. Environ. Chem. Eng. 2024, 12, 113243. [Google Scholar] [CrossRef]
- Yan, C.; Li, J.; Sun, Z.; Chen, L.; Sun, X.; Wang, X.; Xia, S. Engineering sulfur vacancies on Mo-doped FeS2 nanosheets grown on activated carbon fibers enhances peroxymonosulfate activation for efficient elimination of sulfamethazine, antibiotic resistant bacteria and antibiotic resistance genes: The dominant role of singlet oxygen. Chem. Eng. J. 2024, 493, 152643. [Google Scholar] [CrossRef]
- Yao, J.; Yu, Y.; Qu, R.; Chen, J.; Huo, Z.; Zhu, F.; Wang, Z. Fe-Activated Peroxymonosulfate Enhances the Degradation of Dibutyl Phthalate on Ground Quartz Sand. Environ. Sci. Technol. 2020, 54, 9052–9061. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Li, S.; Cheng, X.; Liu, D.; Huang, W. Unraveling the crucial role of Mo2N from Fe/Mo bimetal MOF-derived catalyst in initiating Fe3+/Fe2+ redox cycle to activate peroxymonosulfate for dibutyl phthalate degradation. Chem. Eng. J. 2023, 476, 146693. [Google Scholar] [CrossRef]


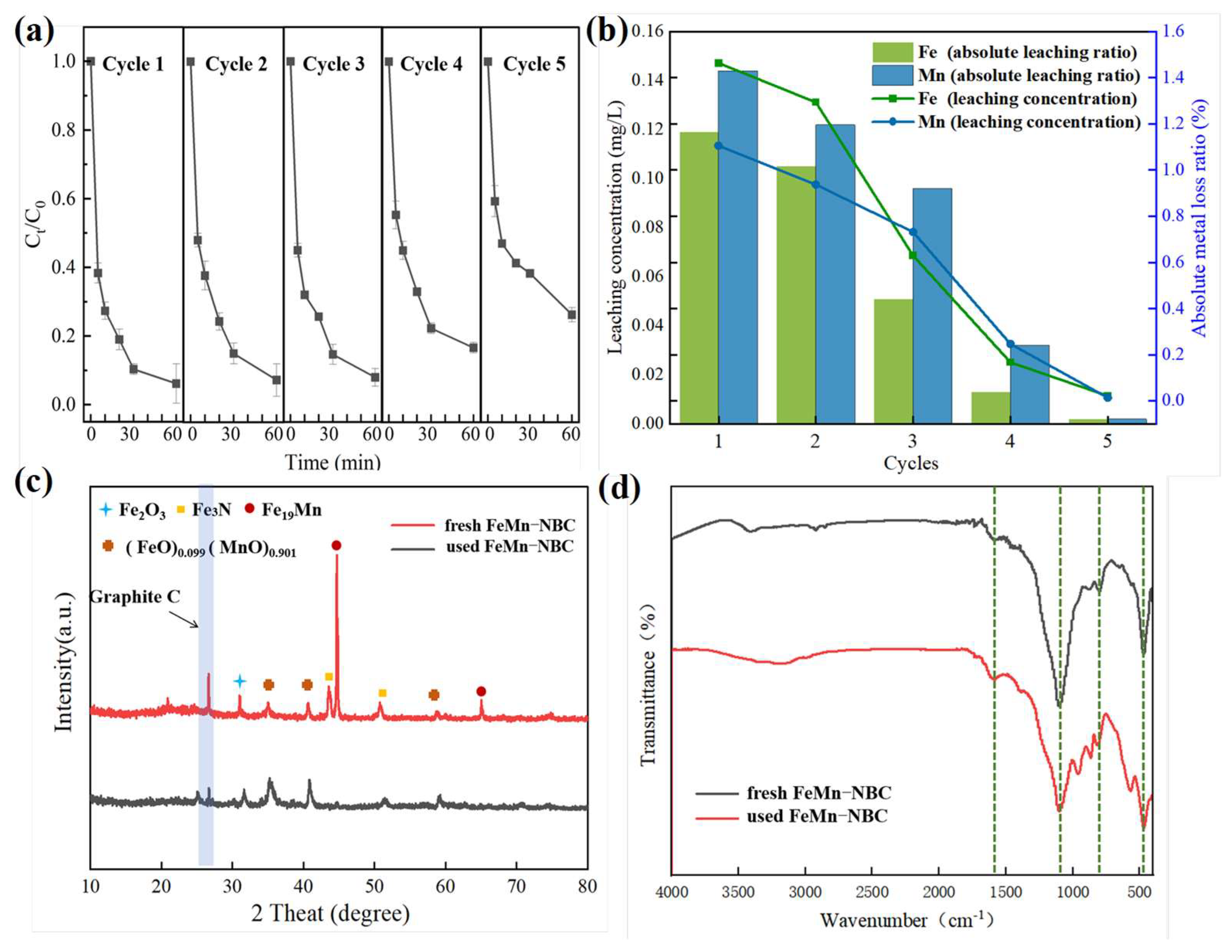


| Cycles | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Fe | Leaching concentration (mg/L) | 1.464 | 1.294 | 0.629 | 0.167 | 0.021 |
| Absolute metal loss ratio (%) | 0.119 | 0.105 | 0.051 | 0.013 | 0.002 | |
| Mn | Leaching concentration (mg/L) | 1.106 | 0.937 | 0.732 | 0.246 | 0.013 |
| Absolute metal loss ratio (%) | 0.144 | 0.122 | 0.096 | 0.032 | 0.002 | |
| Catalysts | Element | ||||||
|---|---|---|---|---|---|---|---|
| Fresh FeMn-NBC | N 1s (%) | Pyridinic N | Pyrrolic N | Graphic N | Fe-N/Mn-N | ||
| 1.36 | 15.56 | 19.78 | 63.34 | ||||
| Fe 2p (%) | Fe 0 | Fe2+ | Fe3+ | ||||
| 21.22 | 26.06 | 30.65 | |||||
| Mn 2p (%) | Mn2+ | Mn3+ | Mn IV | ||||
| 16.42 | 42.81 | 35.63 | |||||
| Used FeMn-NBC | N 1s (%) | Pyridinic N | Pyrrolic N | Graphic N | Fe-N/Mn-N | ||
| 28.36 | 40.54 | 6.41 | 24.69 | ||||
| Fe 2p (%) | Fe 0 | Fe2+ | Fe3+ | ||||
| 14.54 | 32.7 | 27.22 | |||||
| Mn 2p (%) | Mn2+ | Mn3+ | Mn IV | ||||
| 30.84 | 32.09 | 22.84 | |||||
| Atom | q (N) | q (N + 1) | q (N − 1) | f− | f+ | f0 | Δf |
|---|---|---|---|---|---|---|---|
| 1 (C) | −0.0286 | −0.1002 | 0.0585 | 0.0872 | 0.0716 | 0.0794 | −0.0156 |
| 2 (C) | −0.0234 | −0.1165 | 0.0329 | 0.0563 | 0.0931 | 0.0747 | 0.0368 |
| 3 (C) | −0.0243 | −0.0636 | 0.0119 | 0.0362 | 0.0393 | 0.0377 | 0.0031 |
| 4 (C) | 0.0029 | −0.0618 | 0.064 | 0.0611 | 0.0648 | 0.0629 | 0.0037 |
| 5 (C) | −0.0087 | −0.0786 | 0.0346 | 0.0433 | 0.0699 | 0.0566 | 0.0266 |
| 6 (C) | −0.0273 | −0.0749 | 0.0095 | 0.0369 | 0.0476 | 0.0422 | 0.0107 |
| 7 (C) | 0.2148 | 0.1429 | 0.2327 | 0.018 | 0.0719 | 0.0449 | 0.0539 |
| 8 (C) | 0.2165 | 0.1837 | 0.2484 | 0.0319 | 0.0328 | 0.0323 | 0.0009 |
| 9 (O) | −0.1157 | −0.1457 | −0.0833 | 0.0324 | 0.03 | 0.0312 | −0.0023 |
| 10 (O) | −0.261 | −0.3434 | −0.1948 | 0.0662 | 0.0824 | 0.0743 | 0.0162 |
| 11 (C) | 0.0398 | 0.03 | 0.0505 | 0.0106 | 0.0098 | 0.0102 | −0.0008 |
| 12 (O) | −0.12 | −0.1314 | −0.0785 | 0.0415 | 0.0114 | 0.0264 | −0.0301 |
| 13 (C) | 0.041 | 0.0338 | 0.0508 | 0.0097 | 0.0072 | 0.0085 | −0.0025 |
| 14 (O) | −0.2666 | −0.3274 | −0.1256 | 0.141 | 0.0609 | 0.1009 | −0.0801 |
| 15 (C) | −0.011 | −0.0124 | −0.0067 | 0.0044 | 0.0014 | 0.0029 | −0.003 |
| 16 (C) | −0.076 | −0.083 | −0.0691 | 0.0069 | 0.007 | 0.007 | 0.0001 |
| 17 (C) | −0.0829 | −0.0858 | −0.0786 | 0.0043 | 0.0028 | 0.0036 | −0.0015 |
| 18 (C) | −0.0114 | −0.0129 | −0.0061 | 0.0053 | 0.0015 | 0.0034 | −0.0038 |
| 19 (C) | −0.0839 | −0.0861 | −0.0788 | 0.0051 | 0.0022 | 0.0037 | −0.0029 |
| 20 (C) | −0.0768 | −0.0829 | −0.0687 | 0.0081 | 0.0061 | 0.0071 | −0.0021 |
| 21 (H) | 0.0443 | 0.0019 | 0.0833 | 0.039 | 0.0424 | 0.0407 | 0.0034 |
| 22 (H) | 0.0457 | −0.0024 | 0.0796 | 0.034 | 0.0481 | 0.041 | 0.0141 |
| 23 (H) | 0.0465 | 0.0153 | 0.0725 | 0.026 | 0.0312 | 0.0286 | 0.0053 |
| 24 (H) | 0.0407 | 0.0099 | 0.0674 | 0.0268 | 0.0308 | 0.0288 | 0.004 |
| 25 (H) | 0.0352 | 0.0178 | 0.0522 | 0.017 | 0.0174 | 0.0172 | 0.0004 |
| 26 (H) | 0.0331 | 0.0226 | 0.042 | 0.0089 | 0.0105 | 0.0097 | 0.0017 |
| 27 (H) | 0.0356 | 0.0273 | 0.0458 | 0.0101 | 0.0083 | 0.0092 | −0.0019 |
| 28 (H) | 0.0347 | 0.0231 | 0.0536 | 0.0189 | 0.0116 | 0.0152 | −0.0073 |
| 29 (H) | 0.0287 | 0.0296 | 0.0304 | 0.0017 | −0.0009 | 0.0004 | −0.0026 |
| 30 (H) | 0.0318 | 0.0186 | 0.0449 | 0.0132 | 0.0132 | 0.0132 | 0 |
| 31 (H) | 0.026 | 0.016 | 0.0359 | 0.0099 | 0.01 | 0.01 | 0.0001 |
| 32 (H) | 0.0311 | 0.025 | 0.0366 | 0.0056 | 0.0061 | 0.0058 | 0.0006 |
| 33 (H) | 0.0293 | 0.0162 | 0.0429 | 0.0136 | 0.0132 | 0.0134 | −0.0004 |
| 34 (H) | 0.0248 | 0.0307 | 0.0208 | −0.004 | −0.0059 | −0.0049 | −0.002 |
| 35 (H) | 0.0249 | 0.0153 | 0.0349 | 0.0101 | 0.0096 | 0.0098 | −0.0005 |
| 36 (H) | 0.027 | 0.0259 | 0.0314 | 0.0044 | 0.0011 | 0.0028 | −0.0033 |
| 37 (H) | 0.0254 | 0.0312 | 0.0229 | −0.0025 | −0.0058 | −0.0042 | −0.0033 |
| 38 (H) | 0.0273 | 0.015 | 0.0429 | 0.0156 | 0.0122 | 0.0139 | −0.0034 |
| 39 (H) | 0.0241 | 0.0168 | 0.0357 | 0.0116 | 0.0073 | 0.0095 | −0.0043 |
| 40 (H) | 0.0303 | 0.018 | 0.0458 | 0.0156 | 0.0123 | 0.0139 | −0.0033 |
| 41 (H) | 0.0307 | 0.0249 | 0.0378 | 0.0071 | 0.0058 | 0.0065 | −0.0013 |
| 42 (H) | 0.0255 | 0.0175 | 0.0369 | 0.0115 | 0.0079 | 0.0097 | −0.0035 |
| Products | Mass-to-Charge Ratio (m/z) | Formula | Chemical Structure | |
|---|---|---|---|---|
| 1 | DIBP | 278 | C16H22O4 | 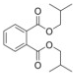 |
| 2 | P1 | 221 | C12H14O4 | 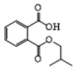 |
| 3 | P2 | 249 | C14H18O4 |  |
| 4 | P3 | 127 | C7H12O2 | 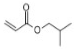 |
| 5 | P4 | 193 | C11H14O3 | 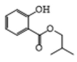 |
| 6 | P5 | 165 | C8H6O4 | 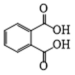 |
| 7 | P6 | 266 | C14H18O5 | 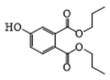 |
| 8 | P7 | 163 | C10H12O2 | 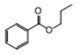 |
| 9 | P8 | 117 | C4H4O4 | 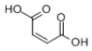 |
| 10 | P9 | 181 | C8H6O5 | 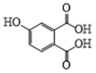 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Ge, Q.; Zhou, X.; Wang, Y.; Zhu, C.; Liu, K.; Wan, J. Enhancement of Electron Transfer Between Fe/Mn Promotes Efficient Activation of Peroxomonosulfate by FeMn-NBC. Water 2025, 17, 1700. https://doi.org/10.3390/w17111700
Lin X, Ge Q, Zhou X, Wang Y, Zhu C, Liu K, Wan J. Enhancement of Electron Transfer Between Fe/Mn Promotes Efficient Activation of Peroxomonosulfate by FeMn-NBC. Water. 2025; 17(11):1700. https://doi.org/10.3390/w17111700
Chicago/Turabian StyleLin, Xiaoni, Qiang Ge, Xianbo Zhou, Yan Wang, Congyun Zhu, Kuanyong Liu, and Jinquan Wan. 2025. "Enhancement of Electron Transfer Between Fe/Mn Promotes Efficient Activation of Peroxomonosulfate by FeMn-NBC" Water 17, no. 11: 1700. https://doi.org/10.3390/w17111700
APA StyleLin, X., Ge, Q., Zhou, X., Wang, Y., Zhu, C., Liu, K., & Wan, J. (2025). Enhancement of Electron Transfer Between Fe/Mn Promotes Efficient Activation of Peroxomonosulfate by FeMn-NBC. Water, 17(11), 1700. https://doi.org/10.3390/w17111700





